Significance
The stimulation of certain surface receptors on immune cells triggers the release of calcium (Ca2+) stored in the endoplasmic reticulum (ER). This Ca2+ flux is required for efficient activation and function of immune cells, and involves the ER membrane Ca2+ channel, the inositol 1,4,5-triphosphate receptor (IP3R). We found that stable expression of IP3R requires the addition of a fatty acid through a process called palmitoylation catalyzed by an enzyme complex composed of DHHC6 (letters represent the amino acids aspartic acid, histidine, histidine, and cysteine in the catalytic domain) and selenoprotein K (Selk) proteins. These findings provide new mechanistic insight into the selenium-sensitive fine-tuning of immune cell activation through posttranslational modification of the IP3R Ca2+ channel. This study also reveals a novel DHHC6/Selk enzyme complex responsible for regulating stable expression of the IP3R.
Keywords: selenium, calcium, immune, endoplasmic reticulum, palmitoylation
Abstract
Calcium (Ca2+) is a secondary messenger in cells and Ca2+ flux initiated from endoplasmic reticulum (ER) stores via inositol 1,4,5-triphosphate (IP3) binding to the IP3 receptor (IP3R) is particularly important for the activation and function of immune cells. Previous studies demonstrated that genetic deletion of selenoprotein K (Selk) led to decreased Ca2+ flux in a variety of immune cells and impaired immunity, but the mechanism was unclear. Here we show that Selk deficiency does not affect receptor-induced IP3 production, but Selk deficiency through genetic deletion or low selenium in culture media leads to low expression of the IP3R due to a defect in IP3R palmitoylation. Bioinformatic analysis of the DHHC (letters represent the amino acids aspartic acid, histidine, histidine, and cysteine in the catalytic domain) family of enzymes that catalyze protein palmitoylation revealed that one member, DHHC6, contains a predicted Src-homology 3 (SH3) domain and DHHC6 is localized to the ER membrane. Because Selk is also an ER membrane protein and contains an SH3 binding domain, immunofluorescence and coimmunoprecipitation experiments were conducted and revealed DHHC6/Selk interactions in the ER membrane that depended on SH3/SH3 binding domain interactions. DHHC6 knockdown using shRNA in stably transfected cell lines led to decreased expression of the IP3R and impaired IP3R-dependent Ca2+ flux. Mass spectrophotometric and bioinformatic analyses of the IP3R protein identified two palmitoylated cysteine residues and another potentially palmitoylated cysteine, and mutation of these three cysteines to alanines resulted in decreased IP3R palmitoylation and function. These findings reveal IP3R palmitoylation as a critical regulator of Ca2+ flux in immune cells and define a previously unidentified DHHC/Selk complex responsible for this process.
Immune cell activation relies on receptor-mediated increases in cellular calcium (Ca2+) concentrations, which is an indispensable step in proliferation, differentiation, migration, and effector functions during immune responses (1, 2). A rapid influx of Ca2+ has been shown to be important during the activation of lymphocytes through the T- and B-cell receptors, macrophages through Fcγ receptors, and mast cells through Fcε receptors and stimulation of various immune cells through chemokine receptors (3). Engagement of these receptors at the plasma membrane leads to the activation of phosphoinositide-specific phospholipase C, which catalyzes the degradation of phosphatidylinositol-4,5-bisphosphate to generate inositol 1,4,5-triphosphate (IP3) and diacylglycerol (4). IP3 binds to the IP3 receptor (IP3R) in the endoplasmic reticulum (ER) membrane leading to Ca2+ mobilization from the ER, which is the main Ca2+ store in immune cells (5). The release of Ca2+ from the ER lumen to the cytosol causes structural changes and oligomerization of the ER transmembrane protein, stromal interaction molecule 1 (STIM1) (6, 7). These changes allow the cytosolic domain of STIM1 to directly interact with the pore-forming unit, Orai1, of Ca2+ channels on the plasma membrane called Ca2+ release-activated Ca2+ (CRAC) channels. These steps are collectively referred to as store-operated Ca2+ entry (SOCE) and defects in any of the factors involved in SOCE significantly impair immune cell function (8).
The IP3R family includes three isoforms with multiple splice variants, and all IP3R isoforms share a tetrameric architecture consisting of an N-terminal ligand binding domain and a C-terminal channel domain containing six transmembrane domains (9, 10). The intervening region is referred to as the regulatory or coupling domain and structure/function studies have revealed important insights into how IP3 binding at the N-terminal region leads to channel opening in the C-terminal region (11). There is evidence to suggest that the IP3Rs are arranged in the ER membrane in a nonrandom distribution and that dynamic clustering of these receptors may be functionally important (12, 13). Phosphorylation and dephosphorylation of the IP3R also regulates Ca2+ flux through this channel (14), but it remains to be determined whether the IP3R is regulated by other posttranslational modifications. Given the central role that the IP3R plays in SOCE in so many immune cell types, expression of this Ca2+ channel protein represents a crucial point of regulating immune responses.
Our previous studies have demonstrated that low levels of dietary selenium as well as a deficiency in a specific ER-localized selenium-containing protein, selenoprotein K (Selk), led to impaired SOCE (15, 16). The specific mechanism by which Selk deficiency affected Ca2+ flux was not apparent, but the fact that Ca2+ induced with thapsigargin was not altered in Selk-deficient immune cells suggested Selk may affect SOCE through the actions of IP3R. Subsequently, we found that Selk expression was required for the stable expression of a different membrane receptor, the low-density lipoprotein receptor CD36 (17). This study revealed that Selk was required for palmitoylation of CD36, and Selk-deficient macrophages exhibited lower levels of CD36 due to impaired palmitoylation.
Palmitoylation is a posttranslational modification involving the reversible addition of the 16-carbon fatty acid, palmitate, to cysteine residues through a thioester bond (18), and this modification can facilitate membrane association of proteins or stable expression of transmembrane proteins (19, 20). The requirement of Selk for palmitoylation of CD36 and the impaired Ca2+ flux in Selk-deficient immune cells led us to explore the possibility that the IP3R is palmitoylated in a Selk-dependent manner and that this is required for its stable expression. The data presented in the current study reveal that IP3R does indeed require palmitoylation for stable expression, and our data identify DHHC6 (letters represent the amino acids aspartic acid, histidine, histidine, and cysteine in the catalytic domain) as a palmitoyl acyl transferase enzyme that interacts with Selk to carry out the palmitoylation of specific cysteine residues within the IP3R in immune cells. These findings provide previously unidentified mechanistic insight into the fine tuning of immune cell activation and immune responses through posttranslational modification of the IP3R Ca2+ channel and uncover a novel enzyme complex responsible for regulating stable expression of the IP3R.
Results
Selk Deficiency Does Not Affect IP3 Production but Impairs IP3R Function.
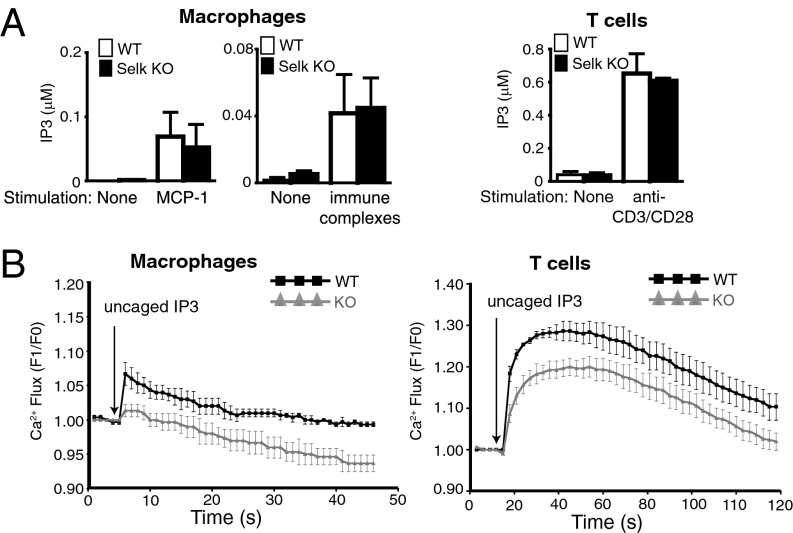
Because our previous work suggested that Selk was required for optimal receptor-mediated Ca2+ flux and that STIM-1/CRAC function was not involved (16), we hypothesized that either IP3 production or IP3R function may be affected by Selk deficiency in receptor-stimulated immune cells. Using cells from Selk KO mice and WT controls, we measured IP3 production in macrophages stimulated through chemokine or Fγc receptors with either MCP-1 or immune complexes, respectively, as well as T cells stimulated through the T-cell receptor with anti-CD3/CD28. Results showed that Selk deficiency did not affect IP3 production in stimulated macrophages or T cells (Fig. 1A). We next evaluated IP3R function by loading cells with a caged IP3 compound that is released upon UV photoactivation. This approach bypasses production of IP3 through receptor stimulation and allows uncaged IP3 to bind to the IP3R and induce Ca2+ flux. For both macrophages and T cells, Ca2+ flux induced with uncaged IP3 was impaired in cells from Selk KO mice compared with WT controls (Fig. 1B). Therefore, the reduced Ca2+ flux in Selk-deficient immune cells does not involve impaired production of IP3, but does involve impaired function of IP3R.
Fig. 1.
Selk deficiency does not affect IP3 production but decreases IP3R function. (A) BMDMs or T cells from WT or Selk KO mice were analyzed for levels of IP3 after stimulation with either 100 ng/mL MCP-1 or immune complexes (BMDM, Left) or plate-bound CD3/CD28 (T cells, Right). IP3 after stimulation was similar between WT and Selk KO for both BMDMs and T cells. (B) BMDM (Left) or T cells (Right) from WT or Selk KO mice were loaded with fluo4-acetoxymethyl ester (fluo4-AM) and caged IP3 and change in fluorescence was measured after flash UV-induced uncaging of the IP3. For both BMDMs and T cells, Selk KO cells exhibited decreased Ca2+ flux compared with WT cells. Data represent mean + SE from three experiments.
Selk Deficiency Decreases IP3R Expression Due to Reduced Palmitoylation.
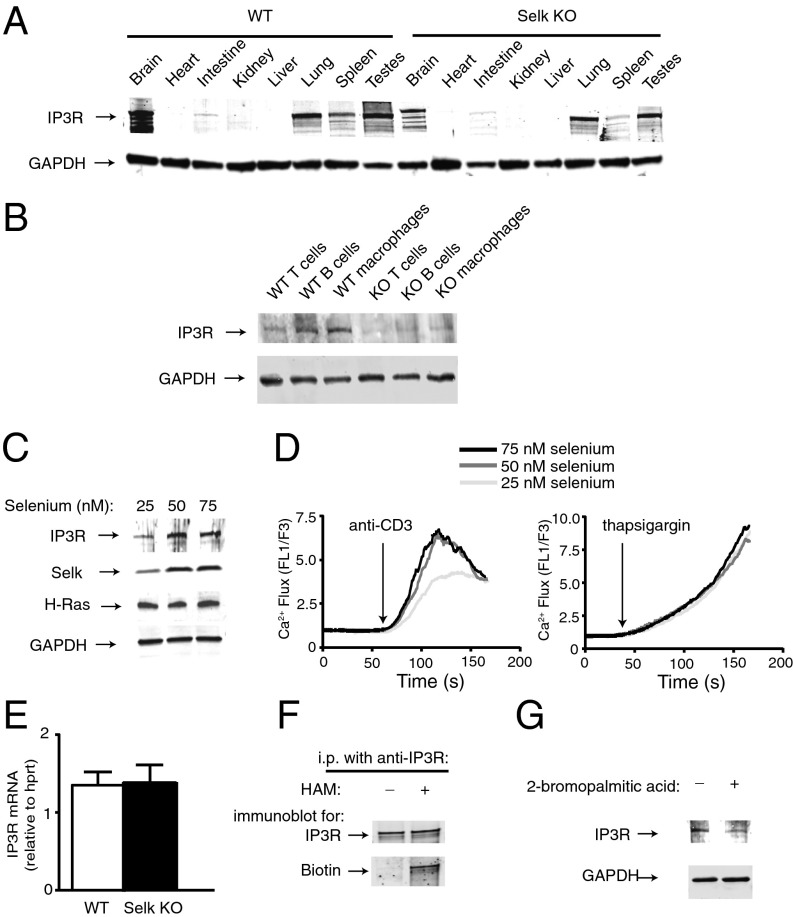
The Selk-dependent IP3R function shown above may be due to reduced or unstable expression of the IP3R mRNA or protein. Thus, protein levels of the IP3R were compared in different tissues from WT and Selk KO. Results demonstrated that Selk deficiency led to lower IP3R levels in certain tissues, with a striking reduction in IP3R in Selk KO spleen tissues compared with WT controls (Fig. 2A). We isolated T and B cells as well as macrophages from spleens to determine how IP3R protein levels were impacted by Selk deficiency in these different types of immune cells. Results showed that all three cell types exhibited reduced IP3R levels in the absence of Selk (Fig. 2B). In addition to genetically induced Selk deficiency, we found that Jurkat T cells cultured in low selenium conditions (25 nM) that reduced Selk protein expression also reduced IP3R expression and anti–CD3-induced Ca2+ flux that depends on IP3R function (Fig. 2 C and D). Importantly, low selenium did not affect thapsigargin-induced Ca2+ flux that bypasses the IP3R by shutting down the ER SERCA pump leading to SOCE.
Fig. 2.
Selk deficiency leads to decreased palmitoylation and stability of the IP3R. (A) Tissue blots from WT and Selk KO mice demonstrate decreased levels of the IP3R1 in certain tissues from KO mice. (B) IP3R1 levels are decreased in T and B cells and macrophages from spleens of Selk KO compared with WT controls. (C) Jurkat T cells cultured in media with 25, 50, or 75 nM selenium were analyzed by Western blot for levels of IP3R1, Selk, and H-Ras (as a control protein that is palmitoylated but not affected by selenium levels) with GAPDH as a loading control. (D) Anti-CD3 and thapsigargin stimulation of Jurkat T cells induced Ca2+ flux as measured by Ca2+ sensing fluorochromes (FL1, Fluo4-AM; FL3, fura red). (E) IP3R1 mRNA levels are similar between splenocytes from Selk KO and WT mice, with IP3R1 mRNA normalized to the housekeeping mRNA for hypoxanthine phosphoribosyltransferase (HPRT). Data represent mean + SE from three experiments. (F) Immunoprecipition (IP) of IP3R1 from HEK293 cell lysates was followed by hydroxyalmine (HAM) treatment, which removes palmitate from palmitoylated cysteines. BMCC-biotin [(1-biotinamido)-4[4′-(maleimidomethyl)-cyclohexanecarboxamido]hexane-biotin] was then conjugated to the available cysteine residues and detected with streptavidin secodary reagents. Results demonstrate palmitoylation of the IP3R1, and immunoprecipitated IP3R1 that was not treated with HAM did not contain biotin. (G) Jurkat T cells were or were not treated with an inhibitor of palmitoylation, 2-bromopalmitic acid. Lysates were then analyzed by Western blot for levels of IP3R1 with GAPDH as a loading control.
Interestingly, IP3R mRNA levels were similar between Selk KO splenocytes and WT controls (Fig. 2E), suggesting that Selk deficiency influenced IP3R protein at a posttranscriptional level. Because we recently found that Selk was required for posttranslational palmitoylation and stable levels of the scavenger receptor, CD36 (17), we explored whether IP3R was also palmitoylated in a Selk-dependent manner. Using an acyl-biotin exchange approach that identifies palmitoylated proteins in immunoprecipitation reactions, IP3R was clearly demonstrated to be palmitoylated (Fig. 2F). Treating Jurkat T cells with an inhibitor of palmitoylation, 2-bromopalmitic acid, reduced IP3R levels (Fig. 2G). Finally, Selk deficiency did not impact IP3R localization to the ER, but levels of protein were found to decrease in a manner that depended on proteosome function (Fig. S1). Together, these data suggest that IP3R is palmitoylated in a Selk-dependent manner and that this posttranslational modification is required for stable levels of IP3R protein.
Selk Complexes with the Palmitoyl Acyl Transferase DHHC6.
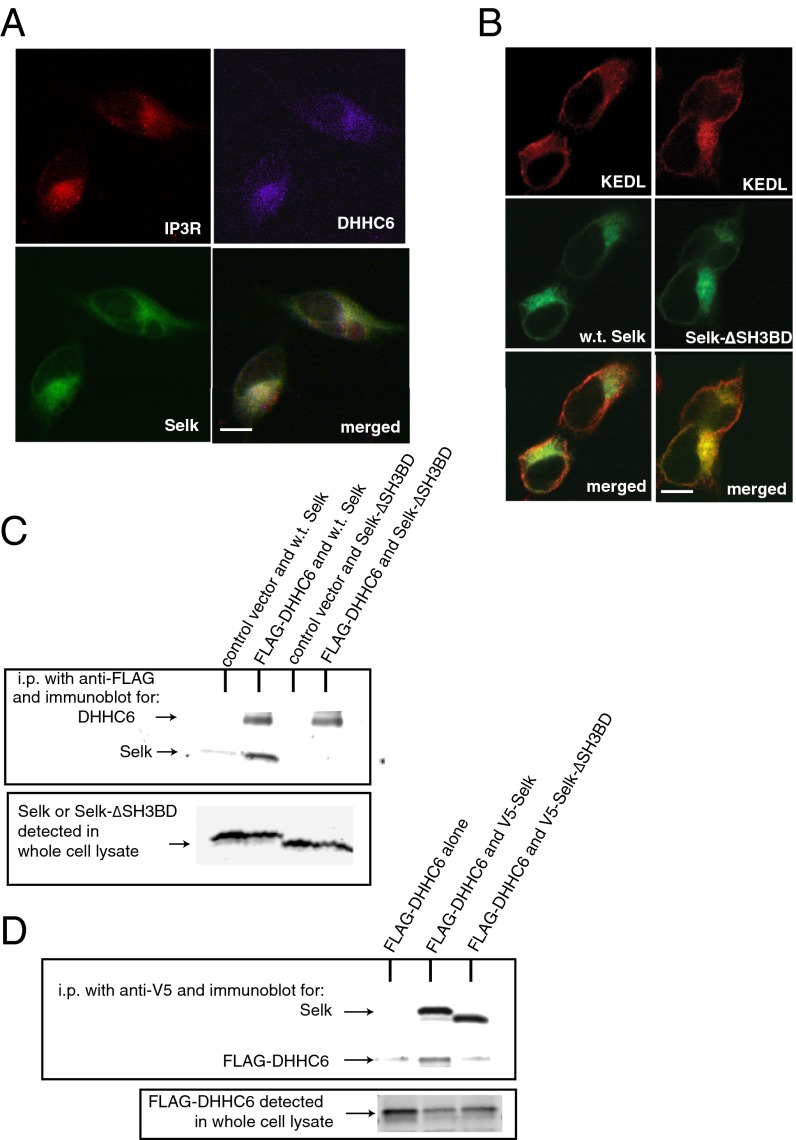
Palmitoylation may be carried out by 23 palmitoyl acyl transferase enzymes in mammals, each of which contains a common DHHC motif. Selk is an ER membrane protein and only two DHHC enzymes are localized to the ER membrane: DHHC4 and 6 (21). Furthermore, Selk contains a Src-homology 3 (SH3)-binding domain and the only palmitoyl acyl transferase that contains an SH3 domain is DHHC6. Thus, we investigated the possibility that Selk and DHHC6 may colocalize and found that these proteins showed strongly overlapping localization in immunofluorescence studies (Fig. 3A and Fig. S2). The IP3R exhibited perinuclear staining consistent with its reported ER membrane localization, and Selk and DHHC6 colocalized in similar regions of the ER membrane. To further explore a potential interaction between Selk and DHHC6, we coexpressed FLAG-tagged DHHC6 together with either wild-type Selk (WT Selk) or Selk lacking the SH3-binding domain (Selk-ΔSH3BD). The deletion of the SH3 binding domain in Selk did not affect its ER localization, nor did it affect the ER localization of DHHC6, and similar results were obtained when using Jurkat T cells (Fig. 3B and Fig. S2). Coimmunoprecipitation (coIP) experiments showed that FLAG-tagged DHHC6 interacted with WT Selk, but not with Selk-ΔSH3BD (Fig. 3 C and D). These data suggest that DHHC6 and Selk interact through SH3 domain and SH3-binding domains, respectively, to form a complex in the ER membrane.
Fig. 3.
Selk colocalizes and interacts with DHHC6 enzyme in the ER membrane. (A) HEK293 cells were simultaneously transfected with plasmids encoding IP3R1, FLAG-DHHC6, and GFP-Selk and immunofluorescence microscopy performed to detect each protein with representational colors in merged image as follows: IP3R1 (red), FLAG-DHHC6 (magenta), and GFP-Selk (green). (B) Deletion of the SH3 binding region in Selk (Selk-ΔSH3BD) did not alter ER localization compared with WT Selk. KDEL (Lys-Asp-Glu-Leu) was detected as an ER marker (red) and colocalized with WT Selk and Selk-SH3BD (both green) equivalently. (Scale bar for A and B, 10 μm.) Detailed methods and results of colocalization are included in Supporting Information. (C) Coimmunoprecipitation assays in HEK293 transfected cells in which FLAG-DHHC6 was immunoprecipitated with anti-FLAG-beads and detected by immunoblotting with anti-DHHC6. Only WT Selk coimmunoprecipitated with FLAG-DHHC6, whereas Selk-ΔSH3BD did not coimmunoprecipitate with FLAG-DHHC6 despite confirmed expression of both WT and mutant Selk in the whole cell lysate. (D) FLAG-DHHC6 coimmunoprecipitated with V5-Selk but not with V5-Selk-ΔSH3BD (V5 represents 95 GKPIPNPLLGLDST 108 of RNA polymerase α subunit of simian parainfluenza virus type 5 and serves as a tag), despite detectable levels of FLAG-DHHC6 in both whole cell lysates.
DHHC6 Deficiency Leads to Reduced IP3R Palmitoylation, Expression, and Function.
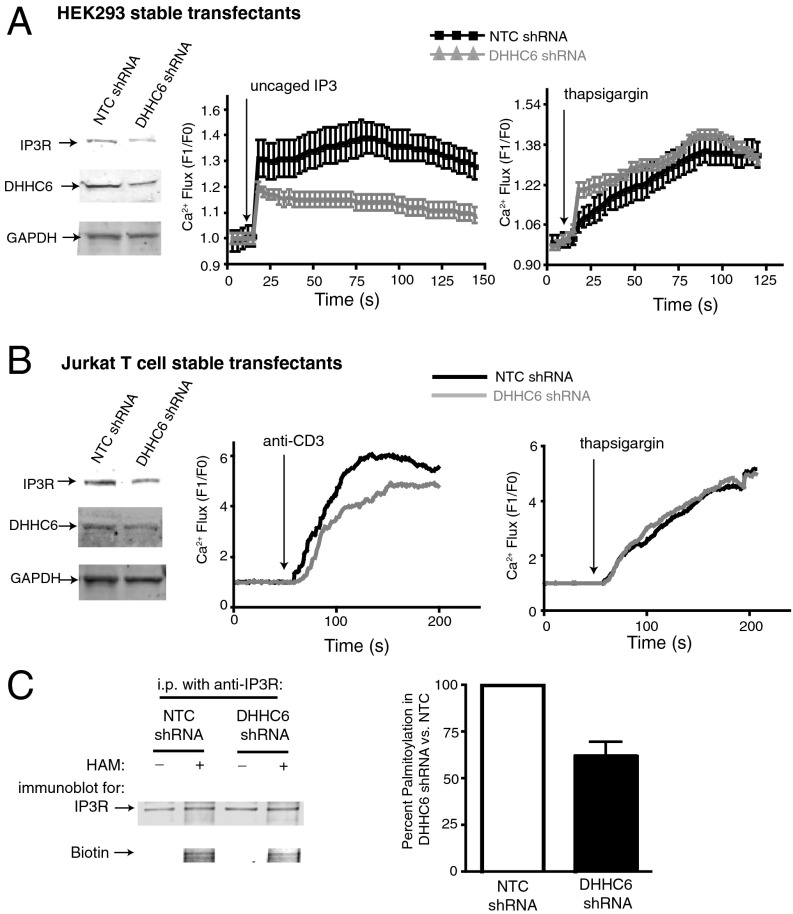
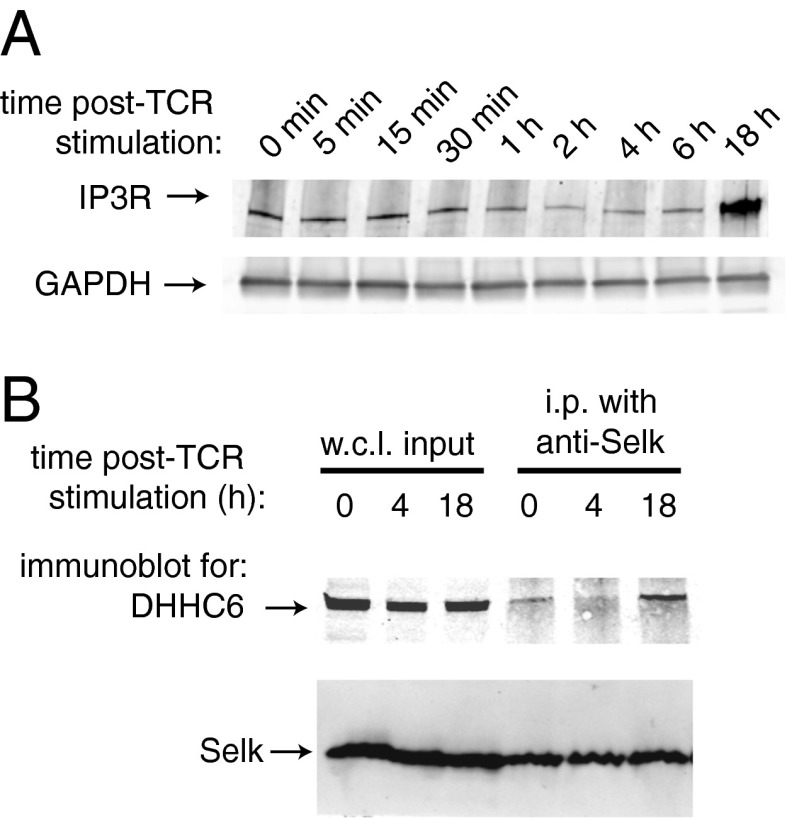
To determine if DHHC6 deficiency influenced IP3R expression and function, HEK293 and Jurkat T cells were stably transfected with shRNA targeting DHHC6 or nontargeting shRNA (NTC shRNA) as controls. We were not able to identify clones with 100% knockdown of DHHC6, perhaps due to an essential role of the DHHC6 enzyme in cell survival. However, clones were identified in which the DHHC6 shRNA reduced levels of both DHHC6 and IP3R proteins in HEK293 cells to ∼50% of controls (Fig. 4A). The functional consequences of reduced DHHC6 and IP3R were investigated in HEK293 cells using caged IP3 loaded into cells that was uncaged upon UV exposure to trigger Ca2+ flux through the IP3R. Results showed that DHHC6 knockdown in HEK293 cells reduced Ca2+ flux to ∼50% compared with NTC shRNA. Ca2+ flux induced by thapsigargin treatment that bypasses the IP3R was not affected by DHHC6 knockdown. DHHC6 shRNA in Jurkat T cells led to reduced levels of both DHHC6 and IP3R proteins, although not to levels as low as with HEK293 DHHC6 knockdown cells (Fig. 4B). Ca2+ flux in Jurkat T cells, which is dependent on IP3R, can be induced using anti-CD3 antibody that engages the T-cell receptor, and DHHC6 knockdown reduced anti–CD3-induced Ca2+ flux compared with NTC shRNA. Importantly, thapsigargin-induced Ca2+ flux in Jurkat T cells was not affected by DHHC6 knockdown, demonstrating the effect of DHHC6 knockdown on IP3R-dependent Ca2+ flux. Acyl-biotin exchange experiments were used to determine if the levels of IP3R palmitoylation were affected by DHHC6 deficiency, using the stably transfected Jurkat T cells. Results showed that DHHC6 knockdown led to reduced palmitoylation of the IP3R protein to ∼60% compared with controls (Fig. 4C). In contrast, we found that DHHC4 knockdown did not affect IP3R palmitoylation or Ca2+ flux (Fig. S3). Also, the effects of Selk or DHHC6 deficiency did not alter early T-cell receptor signaling events such as phospholipase C (PLC)γ phosphorylation (Fig. S4), supporting the notion that the Selk/DHHC6 complex regulates Ca2+ flux through IP3R function at the ER membrane. Interestingly, engagement of the T-cell receptor on Jurkat T cells led to lower levels of IP3R that are restored to high levels after 18 h (Fig. 5A). Consistent with these results, T-cell receptor stimulation caused a temporary uncoupling of Selk and DHHC6 and after 18 h, Selk and DHHC6 strongly reassociated (Fig. 5B). These data suggest that DHHC6 dynamically interacts with Selk to play an important role in IP3R palmitoylation, stability, and function.
Fig. 4.
DHHC6 deficiency reduces IP3R levels and IP3-dependent Ca2+ flux. (A) Western blot analyses of stable HEK293 cell lines transfected with DHHC6 shRNA showed reduced DHHC6 and IP3R compared with nontargeting control shRNA, with GAPDH included as a loading control. DHHC6 knockdown cells exhibited reduced uncaged IP3-induced Ca2+ flux as measured by fluo4-AM and flow cytometry. In contrast, thapsigargin-induced Ca2+ flux was similar between DHHC6 knockdown cells and nontargeting controls. (B) Western blot analyses of stable Jurkat T-cell lines transfected with DHHC6 shRNA showed reduced DHHC6 and IP3R compared with nontargeting control shRNA, with GAPDH included as a loading control. DHHC6 knockdown cells exhibited reduced anti–CD3-induced Ca2+ flux as measured by fluo4-AM and flow cytometry. In contrast, thapsigargin-induced Ca2+ flux was similar between DHHC6 knockdown cells and nontargeting controls. (C) Acyl-biotin exchange analyses of lysates from stable Jurkat T cells transfected with either nontargeting control shRNA or DHHC6 shRNA. Excess lysate was incubated with anti-IP3R beads to ensure IP of equivalent amounts of IP3R from DHHC6 knockdown cells and controls. HAM treatment of immunoprecipitated IP3R and biotin exchange reaction led to reduced biotin incorporation in DHHC6 knockdown cells compared with controls, suggesting a role for DHHC6 in the palmitoylation of IP3R. A representative Western blot image is shown (Left) and a compilation of densitometry from three independent experiments (mean + S.E.) is shown (Right). Percent palmitoylation was determined by measuring densitometry of biotin signal normalized to corresponding IP3R signal and comparing normalized biotin signal for DHHC6 vs. NTC shRNA.
Fig. 5.
The interaction between Selk and DHHC6 is dynamic and correlates with IP3R levels after T-cell receptor stimulation. (A) Jurkat T cells were stimulated through the T-cell receptor with anti-CD3/CD28, and at different timepoints lysates were analyzed by Western blot for levels of IP3R1 with GAPDH serving as a loading control. (B) CoIP of DHHC6 with immunoprecipitated Selk was evident before T-cell receptor stimulation, decreased after 4 h stimulation, and then strongly recovered 18 h after stimulation. Input whole cell lysate (WCL) is included to demonstrate equivalent levels of DHHC6 and Selk incubated with IP beads.
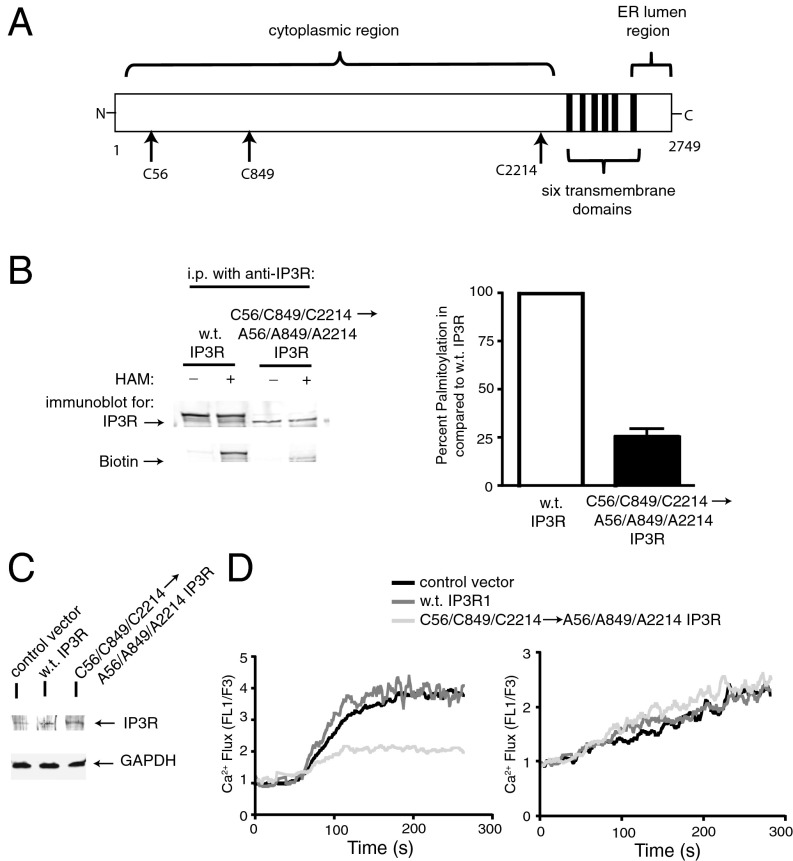
IP3R Is Palmitoylated on Specific Cysteine Residues.
Palmitoylation of proteins occurs through the attachment of the 16-carbon, saturated fatty acid moiety to cysteine residues of target proteins via a thioester bond (22). Mass spectrophotometry was used to analyze the 313-kDa IP3R protein for cysteines that may be palmitoylated. Thirteen IP3R peptides containing cysteine residues were identified and 2 of these 13 peptides were found to contain palmitoylated cysteine residues (Fig. S5). This led to the identification of C(56) and C(849) as palmitoylated residues, and a third palmitoylated cysteine (C2214) was strongly predicted using the CSS-Palm 4.0 prediction program (23). Overexpression of IP3R1 with these three cysteine residues mutated to alanines reduced palmitoylation to 25% of wild-type IP3R1 and led to impaired Ca2+ flux (Fig. 6).
Fig. 6.
The IP3R is palmitoylated on specific cysteine residues. (A) Bar diagram of IP3R1 protein (reference sequence, National Center for Biotechnology Information: NP_001007236.2) illustrates palmitoylated cysteine residues in the cytoplasmic region: C56, C849, and C2214. (B) HEK293 cells were transfected with a plasmid encoding wild-type rat IP3R1 or rat IP3R1 in which three cysteine residues were mutated to alanines (C56/C849/C2214→A56/A849/A2214) and acyl-biotin exchange analyses carried out using excess lysate proteins incubated with anti–IP3R1-coated beads. Note that upon IP, the mutated IP3R1 band was lower than WT IP3R, which may be due to reduced palmitoylation. Mutated IP3R1 showed reduced incorporation of biotin, indicative of lower palmitoylation, which was demonstrated using densitometric analyses of three independent acyl-biotin exchange experiments showing a 75% reduction in palmitoylation of the IP3R1 with C56, C849, and C2214 mutated to alanines. (C) In Jurkat T cells, overexpression of WT rat IP3R1 or rat IP3R1 with three Cys→Ala mutations led to similar levels of exogenous protein detected by Western blot. (D) Overexpression of the mutated rat IP3R1 in Jurkat T cells reduced Ca2+ flux in response to T-cell receptor stimulation with anti-CD3, but did not alter thapsigargin-induced Ca2+ flux.
Discussion
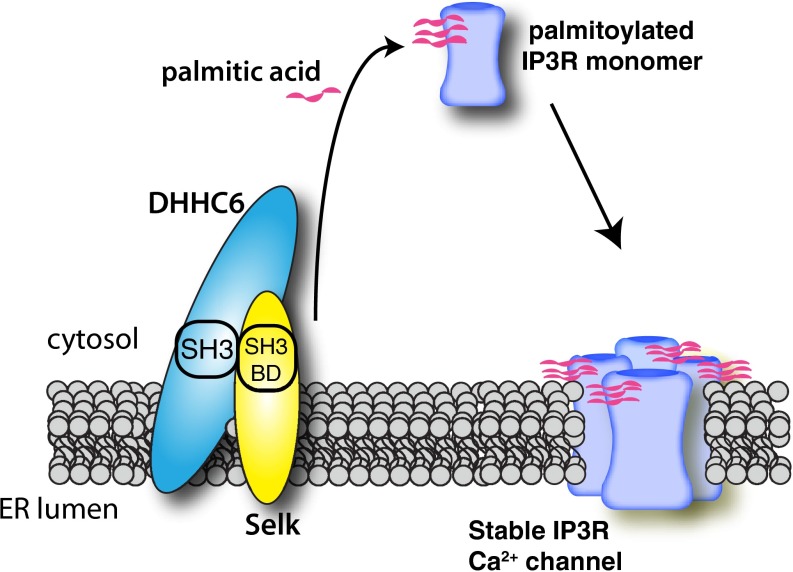
Palmitoylation has emerged as an important posttranslational modification that regulates expression, localization, and function of a wide variety of proteins. The addition of palmitate to target proteins differs from the other fatty acid modifications such as prenylation or N-myristoylation in that it is a reversible modification (18). This has led to the notion that palmitoylation serves to regulate protein function in a manner similar to phosphorylation or ubiquitination (24). Reversible palmitoylation regulates trafficking of proteins like H-Ras (25), G-coupled protein receptors (26), estrogen receptor α (27), and many others. A different role of palmitoylation has been demonstrated for regulating stabilization/degradation of cellular proteins like CD36 (28), CUB domain-containing protein 1 (29), regulator of G-protein signaling 4 (30), and others. Similar to the latter type of regulation, DHHC6/Selk-dependent palmitoylation of the IP3R regulates the stability and function of this Ca2+ channel protein (Fig. 7). The IP3R channels within the ER membrane are composed of four IP3R subunits that form a complex architecture and each subunit includes multiple transmembrane domains, pore-forming helices, hinge regions, and coupling domains that require assembly into a functioning channel. The addition of fatty acid residues to the cytosolic region of the IP3R subunits may facilitate assembly of the subunits or stabilize the assembled complex within the ER membrane. This study, to our knowledge, is the first to describe palmitoylation of the IP3R as well as the enzyme complex consisting of DHHC6 and Selk, which provides mechanistic data to explain previous reports of regulation of Ca2+ flux by dietary selenium and Selk expression in immune cells (15, 16, 31).
Fig. 7.
A model illustrating the role of the DHHC6–Selk complex in the palmitoylation of the IP3R. Palmitoylation is catalyzed by DHHC6 together with Selk, resulting in the addition of palmitic acid to at least three cysteine residues in the cytosolic portion of each IP3R protein. This stabilizes expression and/or assembly into a functional tetrameric Ca2+ channel in the ER membrane.
Whereas the list of palmitoylated proteins has rapidly increased, surprisingly limited information is available regarding the members of the palmitoyl acyl transferase (PAT) family that carry out this posttranslational modification in mammalian cells. The PAT family members were only recently characterized in yeast (32) and subsequently the 23 genes encoding human PATs were identified in the human genome (33). All PATs contain a zinc finger and common DHHC motif (Asp-His-His-Cys) within the catalytic domain. However, DHHC enzymes show wide diversity at the N- and C-terminal cytosolic domains that participate in protein–protein interactions and regulation of their activity is not well characterized (24). In regards to these issues, our data demonstrate that DHHC6 is regulated by its SH3 domain-dependent interactions with Selk at the ER membrane. DHHC9 is another PAT shown to interact with a cofactor, G-protein–coupled receptor protein (GCP) GCP16, to catalyze palmitoylation of its substrate proteins (34). The yeast orthologs of DHHC9 and GCP16 (ethylene-responsive factor-2 and -4, respectively) bind in a manner that stabilizes the palmitoyl-PAT intermediate (35). In a similar manner, the binding of Selk to DHHC6 may stabilize the palmitoyl-DHHC6 intermediate and thus increase its catalytic efficiency. Selk may serve as a coenzyme similar to vitamins such as riboflavin, thiamine, and folic acid that cannot be synthesized by the body and must be acquired from the diet. Investigations are currently underway to determine the chemistry involved in the catalytic reaction and to identify potential roles for cysteine–selenocysteine interactions between DHHC6, Selk, and substrates.
Our data highlight the notion that interactions of DHHCs with cofactors may be a common mode of regulation of palmitoylation and further investigation of DHHC binding partners is needed. We also are investigating whether the addition of palmitate to the IP3R by DHHC6/Selk is reversible. This is of interest because removal of the palmitate moieties from the IP3R may serve to increase the turnover of this Ca2+ channel protein. S-palmitoylation is reversed by acyl protein thioesterases (APTs), mainly the cytosolic enzymes APT1 and APT2 (36, 37). In this manner, palmitoylation/depalmitoylation through PATs/APTs may represent an enzymatic process that serves to fine tune the level of Ca2+ in activated immune cells and thereby regulate immune responses. Levels of dietary selenium that influence Selk expression levels may add another level of Ca2+ flux regulation.
Although our studies have focused on Ca2+ flux in immune cells, the IP3R plays a role in Ca2+ flux in other tissues. The protein expression pattern shown in Fig. 2 for the IP3R in mouse tissues shows high expression of multiple isoforms of IP3R in brain that is clearly decreased in Selk knockout mice. We have not detected any neurological problems or learning or behavioral defects in the Selk knockout mice compared with wild-type controls, and the only evident impairment related to the brain was the increased neuropathology induced by West Nile virus infection (16). This was shown to be related to the reduced clearance of virus in the periphery in Selk knockout mice, but our new data raise the possibility that low IP3R expression in the brain may also contribute to viral encephalitis. The IP3R has been implicated in staurosporine-induced apoptosis (38) as well as neurodegenerative disorders (39), and our data showing reduced IP3R in brains of Selk knockout mice suggest that neurodegeneration warrants further investigation in these mice. Also of interest is the resilience of IP3R expression to Selk knockout in the testes. Palmitoylation of the IP3R1 by the DHHC6/Selk complex may not be important for stable expression in this tissue or another PAT may be responsible for palmitoylation. Finally, our studies have focused on palmitoylation of IP3R1, but two of the Cys residues shown to be involved in its palmitoylation (C56 and C2214) are also present in IP3R2 and 3. Further studies are required to identify all potential sites of palmitoylation in the three different IP3R and their corresponding isoforms and to understand how this might regulate the functions of the IP3R family members. Altogether, our study provides novel data showing the importance of palmitoylation of the Ca2+ channel protein, IP3R, and the role of the DHHC6/Selk complex in this process. Efficient catalysis of this posttranslational modification by DHHC6/Selk may represent a key regulation point for a variety of physiological processes that rely on effective Ca2+ flux from the ER.
Materials and Methods
Mice.
C57BL/6J wild-type controls were generated from mice originally purchased from The Jackson Laboratory. Generation of selk−/− mice on a C57BL/6J background was previously described (16). All animal protocols were approved by the University of Hawaii Institutional Animal Care and Use Committee.
Jurkat T Cells and ex Vivo Cells.
Jurkat T cells clone E6.1 purchased from ATCC (Monassas) were cultured in RPMI with 5% (vol/vol) FBS containing a final concentration of 25, 50, or 75 nM selenium for 1 wk before experiments. Purification of splenic T cells, B cells, and macrophages was performed using a Miltenyi magnetic separator and purity of cells was determined by evaluation of each cell-type marker via flow cytometry on a FACScaliber (BD Biosciences) and found to be >90%. Bone marrow-derived macrophages (BMDMs) were cultured as previously described (31).
Acyl-Biotin Exchange Analysis and Mass Spectrophotometry.
Acyl-biotin exchange experiments to visualize protein palmitoylation were conducted following previously published studies (40). Detailed methods are provided in Supporting Information. For mass spectrophotometric analyses of palmitoylated cysteines in the IP3R, a band was excised from a polyacrylamide gel corresponding to rat IP3R overexpressed in HEK293 cells. The IP3R protein band was eluted from the gel slice, trypsin digested, and peptides sequenced and analyzed for modified amino acids by Applied Biomics.
IP3 Measurement.
IP3 was measured in BMDMs and T cells using a HitHunter IP3 FP Assay (DiscoveRx). T cells isolated from WT or Selk KO mice using a Miltenyi untouched mouse T-cell kit were plated at 4 × 104 cells per well in 20 μL PBS in a 96-well plate precoated with anti-CD3 (1 μg/mL) and anti-CD28 (10 μg/mL). For BMDMs, the same density of cells was stimulated with 20 μL of BSA/anti-BSA complexes as previously described (31). After 10 min of stimulation, the cells were lysed with 10 μL of 0.2 N perchloric acid and IP3 measured per manufacturer's protocol. Polarized fluoresence was measured using a Beckman Coulter DTX 880 plate reader and data were analyzed using a standard curve per protocol instructions.
Ca2+ Flux Assays.
Ca2+ flux was assayed for BMDMs, primary T cells, and HEK293 stable transfected cells via time-lapse video microscopy or via flow cytometry for Jurkat T-cell stable transfectants. A detailed description of Ca2+ flux assays is provided in Supporting Information.
Statistical Analyses.
Means between groups were compared using a Student t test, and standard curves were generated using logistic regression (GraphPad Prism).
Supplementary Material
Acknowledgments
We thank Ann Hashimoto for her help in animal studies and Susanne Bohl for her contributions to experiments. This research was supported by National Institutes of Health Grant R01AI089999, and core facilities were supported by Grants P20GM103516, P20RR016453, G12RR003061, G12MD007601.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417176111/-/DCSupplemental.
References
- 1.Quintana A, Griesemer D, Schwarz EC, Hoth M. Calcium-dependent activation of T-lymphocytes. Pflugers Archiv. 2005;450(1):1–12. doi: 10.1007/s00424-004-1364-4. [DOI] [PubMed] [Google Scholar]
- 2.Shaw PJ, Qu B, Hoth M, Feske S. Molecular regulation of CRAC channels and their role in lymphocyte function. Cell Mol Life Sci. 2013;70(15):2637–2656. doi: 10.1007/s00018-012-1175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feske S. Immunodeficiency due to defects in store-operated calcium entry. Ann N Y Acad Sci. 2011;1238:74–90. doi: 10.1111/j.1749-6632.2011.06240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454(7203):538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85(2):757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 8.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7(9):690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 9.Parys JB, De Smedt H. Inositol 1,4,5-trisphosphate and its receptors. Adv Exp Med Biol. 2012;740:255–279. doi: 10.1007/978-94-007-2888-2_11. [DOI] [PubMed] [Google Scholar]
- 10.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009;1793(6):933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Bhanumathy C, da Fonseca PC, Morris EP, Joseph SK. Identification of functionally critical residues in the channel domain of inositol trisphosphate receptors. J Biol Chem. 2012;287(52):43674–43684. doi: 10.1074/jbc.M112.415786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahman T. Dynamic clustering of IP3 receptors by IP3. Biochem Soc Trans. 2012;40(2):325–330. doi: 10.1042/BST20110772. [DOI] [PubMed] [Google Scholar]
- 13.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87(2):593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanderheyden V, et al. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim Biophys Acta. 2009;1793(6):959–970. doi: 10.1016/j.bbamcr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann FW, et al. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J Nutr. 2010;140(6):1155–1161. doi: 10.3945/jn.109.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma S, et al. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol. 2011;186(4):2127–2137. doi: 10.4049/jimmunol.1002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meiler S, et al. Selenoprotein K is required for palmitoylation of CD36 in macrophages: Implications in foam cell formation and atherogenesis. J Leukoc Biol. 2013;93(5):771–780. doi: 10.1189/jlb.1212647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bijlmakers MJ, Marsh M. The on-off story of protein palmitoylation. Trends Cell Biol. 2003;13(1):32–42. doi: 10.1016/s0962-8924(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain LH, et al. Palmitoylation and the trafficking of peripheral membrane proteins. Biochem Soc Trans. 2013;41(1):62–66. doi: 10.1042/BST20120243. [DOI] [PubMed] [Google Scholar]
- 20.Salaun C, Greaves J, Chamberlain LH. The intracellular dynamic of protein palmitoylation. J Cell Biol. 2010;191(7):1229–1238. doi: 10.1083/jcb.201008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorleku OA, Barns AM, Prescott GR, Greaves J, Chamberlain LH. Endoplasmic reticulum localization of DHHC palmitoyltransferases mediated by lysine-based sorting signals. J Biol Chem. 2011;286(45):39573–39584. doi: 10.1074/jbc.M111.272369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sefton BM, Buss JE. The covalent modification of eukaryotic proteins with lipid. J Cell Biol. 1987;104(6):1449–1453. doi: 10.1083/jcb.104.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren J, et al. CSS-Palm 2.0: An updated software for palmitoylation sites prediction. Protein Eng Des Sel. 2008;21(11):639–644. doi: 10.1093/protein/gzn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaskovic S, Blanc M, van der Goot FG. What does S-palmitoylation do to membrane proteins? FEBS J. 2013;280(12):2766–2774. doi: 10.1111/febs.12263. [DOI] [PubMed] [Google Scholar]
- 25.Rocks O, et al. The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell. 2010;141(3):458–471. doi: 10.1016/j.cell.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Jia L, et al. A mechanism regulating G protein-coupled receptor signaling that requires cycles of protein palmitoylation and depalmitoylation. J Biol Chem. 2014;289(9):6249–6257. doi: 10.1074/jbc.M113.531475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedram A, Razandi M, Lewis M, Hammes S, Levin ER. Membrane-localized estrogen receptor α is required for normal organ development and function. Dev Cell. 2014;29(4):482–490. doi: 10.1016/j.devcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorne RF, et al. Palmitoylation of CD36/FAT regulates the rate of its post-transcriptional processing in the endoplasmic reticulum. Biochim Biophys Acta. 2010;1803(11):1298–1307. doi: 10.1016/j.bbamcr.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Adams MN, et al. EGF inhibits constitutive internalization and palmitoylation-dependent degradation of membrane-spanning procancer CDCP1 promoting its availability on the cell surface. Oncogene. 2014 doi: 10.1038/onc.2014.88. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Xie Y, Wolff DW, Abel PW, Tu Y. DHHC protein-dependent palmitoylation protects regulator of G-protein signaling 4 from proteasome degradation. FEBS Lett. 2010;584(22):4570–4574. doi: 10.1016/j.febslet.2010.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Z, et al. Stimulation of unprimed macrophages with immune complexes triggers a low output of nitric oxide by calcium-dependent neuronal nitric-oxide synthase. J Biol Chem. 2012;287(7):4492–4502. doi: 10.1074/jbc.M111.315598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roth AF, et al. Global analysis of protein palmitoylation in yeast. Cell. 2006;125(5):1003–1013. doi: 10.1016/j.cell.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J Lipid Res. 2006;47(6):1118–1127. doi: 10.1194/jlr.R600007-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Swarthout JT, et al. DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J Biol Chem. 2005;280(35):31141–31148. doi: 10.1074/jbc.M504113200. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell DA, et al. The Erf4 subunit of the yeast Ras palmitoyl acyltransferase is required for stability of the Acyl-Erf2 intermediate and palmitoyl transfer to a Ras2 substrate. J Biol Chem. 2012;287(41):34337–34348. doi: 10.1074/jbc.M112.379297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duncan JA, Gilman AG. A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS) J Biol Chem. 1998;273(25):15830–15837. doi: 10.1074/jbc.273.25.15830. [DOI] [PubMed] [Google Scholar]
- 37.Tomatis VM, Trenchi A, Gomez GA, Daniotti JL. Acyl-protein thioesterase 2 catalyzes the deacylation of peripheral membrane-associated GAP-43. PLoS ONE. 2010;5(11):e15045. doi: 10.1371/journal.pone.0015045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alzayady KJ, Chandrasekhar R, Yule DI. Fragmented inositol 1,4,5-trisphosphate receptors retain tetrameric architecture and form functional Ca2+ release channels. J Biol Chem. 2013;288(16):11122–11134. doi: 10.1074/jbc.M113.453241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan CC. Modulating Ca(2+) release by the IP3R/Ca(2+) channel as a potential therapeutic treatment for neurological diseases. Pharm Pat Anal. 2013;2(5):629–636. doi: 10.4155/ppa.13.42. [DOI] [PubMed] [Google Scholar]
- 40.Drisdel RC, Green WN. Labeling and quantifying sites of protein palmitoylation. Biotechniques. 2004;36(2):276–285. doi: 10.2144/04362RR02. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.