Significance
We have shown previously that oxytocin (Oxt), other than regulating lactation and social bonding, is a potent stimulator of bone formation by the osteoblast. Here, we present evidence that this action is exerted through the nuclear localization of the Oxt receptor (Oxtr). Our findings prompt additional studies into the contribution of nuclear Oxtr signaling in regulating lactation and social bonding.
Keywords: nuclear translocation, G protein-coupled receptor, transcriptional regulation
Abstract
We report that oxytocin (Oxt) receptors (Oxtrs), on stimulation by the ligand Oxt, translocate into the nucleus of osteoblasts, implicating this process in the action of Oxt on osteoblast maturation. Sequential immunocytochemistry of intact cells or isolated nucleoplasts stripped of the outer nuclear membrane showed progressive nuclear localization of the Oxtr; this nuclear translocation was confirmed by monitoring the movement of Oxtr–EGFP as well as by immunogold labeling. Nuclear Oxtr localization was conclusively shown by Western immunoblotting and MS of nuclear lysate proteins. We found that the passage of Oxtrs into the nucleus was facilitated by successive interactions with β-arrestins (Arrbs), the small GTPase Rab5, importin-β (Kpnb1), and transportin-1 (Tnpo1). siRNA-mediated knockdown of Arrb1, Arrb2, or Tnpo1 abrogated Oxt-induced expression of the osteoblast differentiation genes osterix (Sp7), Atf4, bone sialoprotein (Ibsp), and osteocalcin (Bglap) without affecting Erk phosphorylation. Likewise and again, without affecting pErk, inhibiting Arrb recruitment by mutating Ser rich clusters of the nuclear localization signal to Ala abolished nuclear import and Oxtr-induced gene expression. These studies define a previously unidentified mechanism for Oxtr action on bone and open possibilities for direct transcriptional modulation by nuclear G protein-coupled receptors.
The oxytocin (Oxt) receptor (Oxtr), a member of the rhodopsin-type (class I) family of G protein-coupled receptors (GPCRs), responds to the neurohypophyseal hormone Oxt to stimulate lactation and social bonding (1, 2). More recently, we and others have shown that Oxt also stimulates bone formation directly by interacting with an osteoblastic Oxtr (3, 4). The genetic deletion of Oxt or Oxtr in mice thus decreases osteoblast differentiation and bone formation, causing osteopenia (5, 6). We believe that this action supports maternal skeletal remineralization after the intergenerational transfer of calcium during pregnancy and lactation (5). However, the precise mechanism through which Oxtr activation translates into increased bone formation remains unclear.
Oxt binding to Oxtrs is known to activate both G protein Gαi- and Gαq/11-mediated phospholipase C pathways (7). However, in common with other GPCRs, prolonged or repetitive agonist stimulation by Oxt results in Oxtr internalization through β-arrestin (Arrb). GPCR internalization can activate signaling pathways quite distinct from those activated by the same receptors resident at the cell surface. Hence, desensitization of primary G protein-dependent signaling can be followed by a second wave of Arrb–mediated signaling (8). β-Arrestins can also recruit signaling proteins that connect GPCRs to various cytoplasmic effector pathways, such as mitogen-activated protein kinase (MAPK) and protein kinase B/phosphatidylinositol-4,5-bisphosphate 3-kinase (Akt/PI3K) (9, 10). Such mechanisms can elicit delayed genomic responses.
After being internalized, GPCRs are either recycled back to the plasma membrane to resensitize the cell to ligand action or transported to lysosomes for degradation. Although G proteins have been found in the Golgi body, endoplasmic reticulum, and cytoskeleton (11–13), GPCRs can localize to the nucleus or nuclear membrane (14–16). For example, on treatment with sphingosine-1-phosphate (S1P), the S1P receptor subtype 1 can translocate to endothelial cell nuclei to activate transcription (17). Likewise, chemokine receptor 2 (CCR2), expressed in monocyte, has been shown to interact directly with transportin-1 (Tnpo1) to undergo nuclear localization (18). Cysteine (C)-X-C Receptor 4 (CXCR4) similarly responds to stromal cell-derived factor-1α (SDF-1α) to undergo internalization and Tnpo1-mediated nuclear targeting in prostate cancer cells (19).
Human osteosarcoma and breast cancer cell lines display both cell membrane and nuclear Oxtrs (20). However, the functional significance of the nuclear localization of the Oxtr or indeed, any GPCR continues to unravel. Here, we have combined proteomic, molecular, and cell biological approaches to confirm the translocation of the Oxtr to the nucleus in osteoblasts after Oxt binding and to study the putative function of this pathway in Oxt-induced bone formation. We find that Oxtr nuclear localization is Arrb- and Tnpo1-dependent. Thus, siRNA knockdown of Arrb or Tnpo1 inhibits not only nuclear localization but also, the proosteoblastic action of Oxt, interestingly without affecting Erk phosphorylation. These findings represent a hitherto unrecognized pathway for Oxt action.
Results
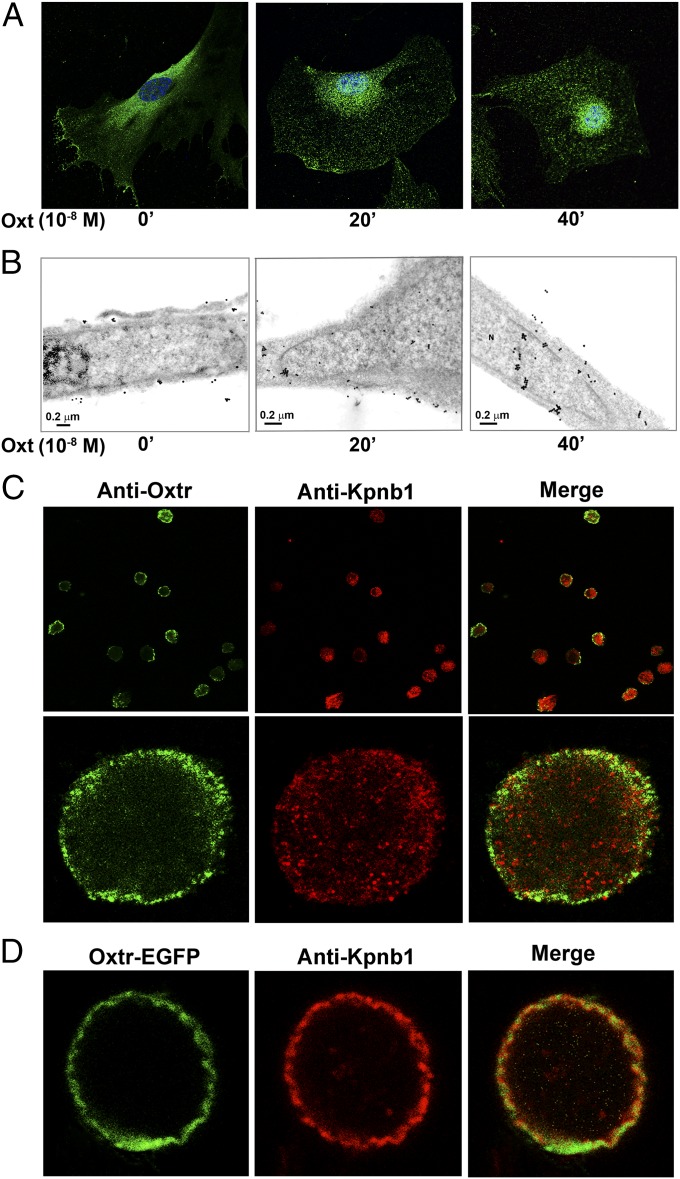
Considering the newly discovered anabolic function of Oxt in skeletal regulation, we sought to investigate the fate of the osteoblast Oxtr after Oxt binding. Confocal immunofluorescence microscopy showed that, in primary murine osteoblasts and MC3T3.E1 preosteoblastic cells, exposure to Oxt triggered the nuclear localization of Oxtrs. Under basal unstimulated conditions, Oxtrs appeared mainly at the plasma membrane and in the cytoplasm, whereas 20–40 min after the addition of Oxt (10−8 M), a clear nuclear localization was observed (Fig. 1A). This effect was definitively verified by transmission electron microscopy using an immunogold-labeled secondary antibody. Again, under basal conditions, immunogold particles corresponding to the anti-Oxtr were observed mainly on the plasma membrane, whereas after Oxt treatment, they progressively moved to the cytoplasm (∼20 min) and then, the nuclear membrane and nucleus (∼40 min) (Fig. 1B).
Fig. 1.
Oxtr translocates into the nucleus on Oxt binding. Primary mouse calvarial osteoblasts were stimulated with Oxt (10−8 M for 40 min) and stained with anti-Oxtr antibody (green); nuclei were counterstained with TOPRO (blue). (A) Midsectional confocal microscopy shows that, at time 0 min, Oxtr appears mainly in the cytoplasm and plasma membrane; at 20 min, it is seen close to or inside the nucleus, and at 40 min, there is complete nuclear localization. Oxtr was also detected by transmission EM using a secondary gold-conjugated antibody. (B) In untreated cells, gold particles are evident on the plasma membrane; 20 min post-Oxt, they are cytoplasmic (with some particles observed in the nuclear compartment and on the nuclear envelope), and at 40 min, they are noted in the nuclear compartment. The outer nuclear membrane was removed from isolated nuclei to produce nucleoplasts (33). Nuclei were either (C) stained with anti-Oxtr antibody or (D) transfected with Oxtr–EGFP. Oxtrs were visualized intranuclearly (green) after 40 min post-Oxt (counterstaining with anti-Kpnb1 antibody is in red). Higher magnification of a single nucleoplast shows intense Oxtr immunofluorescence on the nuclear membrane. (C and D) Merged micrographs show partial Oxtr colocalization with Kpnb1, confirming the presence of the receptors at the nuclear membrane. ’, minute.
To exclude potential artifacts arising from the extreme flatness of cultured cells or possible localization of Oxtrs to membrane structures close to the nuclear compartment, we isolated intact nucleoplasts from primary murine osteoblasts. Our technique removes the outer nuclear membrane, yielding a pure fraction of nucleoplasts that are surrounded by the inner but not outer membrane. Nucleoplasts were isolated after stimulation of intact osteoblasts with Oxt (40 min) and stained for Oxtr and importin-β (Kpnb1); the latter is a known inner nuclear membrane and nucleoplasm marker. Confocal microscopy revealed intense Oxtr immunofluorescence localized mainly within the inner nuclear membrane (Fig. 1C). Merged micrographs showed that Oxtr colocalized with Kpnb1, thus confirming the presence of the receptors at the inner nuclear membrane (Fig. 1C). Nuclear localization was further evaluated independently by transfecting osteoblasts derived from Oxtr−/− mice with Oxtr–EGFP fusion plasmid (gift from Bice Chini, The National Research Council, Milan, Italy) with EGFP tagged to the Oxtr carboxyl terminus. After exposure to Oxt (10−8 M), nucleoplasts were again isolated and marked with the anti-Kpnb1 antibody. Exogenous Oxtr–EGFP was found to localize to the inner nuclear membrane in a pattern that overlapped to an extent with Kpnb1 (Fig. 1D).
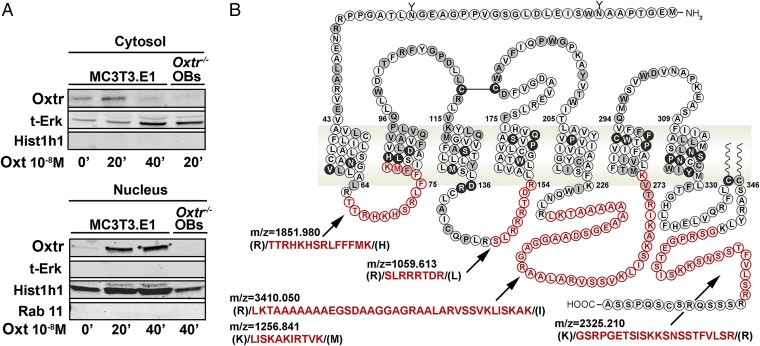
We also used biochemical and proteomic approaches to definitively confirm the nuclear localization observed on microscopy. Western immunoblotting was used to study the presence of Oxtrs in the cytosolic and nuclear compartments of osteoblasts exposed to Oxt (10−8 M) for up to 40 min. To assess the purity of subcellular fractions, we first probed for cytosolic, perinuclear, and nuclear markers. Total Erk was detected only in the cytosolic fraction, which did not have histone-H1 (Hist1h1). In contrast, the Hist1h1-rich nuclear fraction was negative for not only Erk but also, Rab11, a typical Rab located in perinuclear recycling endosomes. The latter data attested to the purity of the respective cytosolic and nuclear fractions. At 20–40 min, Oxtrs were localized to the nuclear fraction, with correspondingly reduced cytosolic protein at 40 min. Of note is that Oxtr−/− cells, with equally pure nuclear and cytosolic fractions, did not contain Oxtr protein, testifying to the specific detection of Oxtrs by our antibody (Fig. 2A).
Fig. 2.
Biochemical and proteomic detection of Oxtrs in osteoblast nuclei. MC3T3.E1 osteoblasts and Oxtr−/− primary osteoblasts (OBs) (negative control) were treated with Oxt (10−8 M) for 20 and 40 min. (A) Western blots show Oxtrs in the cytosolic and nuclear fractions at 40 min. Markers included t-Erk (cytosol), Rab11 (perinuclear), and Hist1h1 (nuclear). Proteins in the immunoprecipitate (with anti-Oxtr antibody) were identified using MS-Fit software (http://prospector.ucsf.edu/prospector/cgi-bin/msform.cgi?form=msfitstandard), and all other proteins were recognized as nuclear proteins. (B) Analysis of the spectra (FindPept database) revealed five peptides corresponding to Oxtr intracellular loops in the in-gel band (38 kDa) at m/z of 1,851.98 [(R)/TTRHKHSRLFFFMK/(H); residues 66–79], 1,059.61 [(R)/SLRRRTDR/(L); residues 146–153], 3,410.05 [(R)/LKTAAAAAAAEGSDAAGGAGRAALARVSSVKLISKAK/(I); residues 232–268], 1,256.84 [(K)/LISKAKIRTVK/(M); residues 263–273], and 2,325.21 [(K)/GSRPGETSISKKSNSSTFVLSR/(R); residues 353–374]. ’, minute.
Nuclear proteins were also immunoprecipitated with anti-Oxtr antibody and subjected to matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry analysis. Separation by SDS/PAGE was followed by in-gel digestion using trypsin and RapiGest SF (Waters). This approach allowed almost all proteins in the immunoprecipitate to be identified (using Aldente software); all other proteins were recognized as nuclear proteins. Analysis of spectra (FindPept database) in the excised band (38 kDa) revealed the presence of five peptides corresponding to the Oxtr intracellular loops (Fig. 2B).
Complementary data from intact cells and isolated nucleoplasts together with biochemical and proteomic detection thus show that, after ligand activation, Oxtrs move from the plasma membrane to the inner nuclear membrane. We, therefore, next explored the intracellular path used for Oxtr translocation to the nucleus. To analyze Oxtr trafficking, we stably expressed the Oxtr–EGFP fusion plasmid in Oxtr−/− primary osteoblasts. Functionality of the Oxtr–EGFP fusion protein was verified by examining its binding to an Oxt analog conjugated with Alexa-546 and the subsequent translocation of the ligand–receptor complex into the cytoplasm (Fig. 3A and SI Materials and Methods). The time-lapse confocal microphotographs show that the fluorescent agonist first appeared diffuse in the medium and eventually, colocalized with the Oxtr–EGFP on the membrane surface, giving rise to a yellow vesicles. Within 2 min, these vesicles moved into cells, indicating internalization of ligand–receptor complex; 15 min after treatment, almost all of Oxtr–EGFP was sequestered in cytoplasmic vesicles. This experiment established that the Oxtr–EGFP fusion protein was functionally responsive to agonist stimulation and trafficked intracellularly (Fig. 3A).
Fig. 3.
Oxtr intracellular trafficking mediated by Arrb, Rab5, and Tnpo1. (A) Shown are time-lapse confocal micrographs of primary Oxtr−/− osteoblasts transfected with Oxtr–EGFP, which appeared at the cell surface and in the cytosol (Movie S1). When a red fluorescent agonist of Oxtr (dLVT–Alexa-546) was added, diffuse fluorescence was detectable in the medium. On agonist binding to Oxtr on the membrane surface, yellow vesicles were visible in the cells within 2 min, indicating the internalization of dLVT–Alexa-546–bound Oxtr–EGFP. At 10 min, all of Oxtr–EGFP (green) was (A) sequestered into cytoplasmic vesicles and (B) colocalized with Arrb1/2 (red) within 2–3 min in proximity of the plasma membrane. (C) Oxtr also colocalized with Rab5 (red) 5 min after Oxt. Stimulation by Oxt for 5, 10, or 20 min resulted in colocalization of Oxtr (green) and Tnpo1 (red). In the absence of stimulation, Oxtr and Tnpo1 appeared apart, but within 5 min, they localized in vesicle-like structures and at 10 min, became crowded in proximity of the nucleus. (D) At 20 min, Oxtr and Tnpo1 again appeared separated, with Oxtr accumulating in the nuclear compartment and Tnpo1 accumulating in the cytoplasm. ’, minute.
Internalization and inactivation of GPCRs are classically mediated by the protein Arrb. After agonist addition, Arrb is recruited from the cytosol to the plasma membrane, where it binds to the Oxtr (21). Oxtr−/− osteoblasts stably transfected with Oxtr–EGFP, when stimulated with Oxt, displayed a colocalization with arrb1/2 in proximity to the plasma membrane within 2–3 min (Fig. 3B). No colocalization was observed at later time points, confirming rapid receptor endocytosis. Similar results were obtained in WT osteoblasts, where the endogenous Oxtr was labeled with an anti-Oxtr antibody.
It is known that, after receptor endocytosis, Arrb rapidly detaches from the vesicle, which then associates with Rab proteins; the latter proteins are small GTPases that regulate intracellular trafficking of several GPCRs (22). We investigated Oxtr interaction with Rab5, a GTPase specifically involved in endosome sorting of clathrin-coated vesicles. We noted that the Oxtr–EGFP fusion protein colocalized with Rab5 in ∼5 min (Fig. 3C). Importantly, no such interaction was observed between Oxtr–EGFP and Rab7, indicating that the receptor complex was not sorted toward degradation. This observation is not inconsistent with Oxtr–Rab7 interactions noted in cells that do not normally express the Oxtr (23).
Nuclear transport of proteins and RNA occurs through the nuclear pore complex and is mediated by a superfamily of transport receptors known collectively as karyopherins or importins (24). Karyopherins bind to their cargoes by recognizing specific nuclear localization signals or nuclear export signals. Transport through the nuclear pore complex is facilitated by transient interactions between the karyopherins and the nuclear pore complex. Of these molecules, Tnpo1 escorts proteins into the nucleus through interactions with a nuclear localization signal, which is composed of short stretches of basic amino acids (25). We studied the subcellular distribution of Tnpo1 and the Oxtr–EGFP in Oxt−/− osteoblasts; use of the latter cells avoids receptor activation by cell-secreted Oxt. Without Oxt stimulation, Oxtr and Tnpo1 were found to not colocalize. However, significant colocalization was noted beginning 5 min after exogenous Oxt (10−8 M). This cytosolic colocalization was in vesicle-like structures that eventually were found to be clustered in the perinuclear region. Dramatically, at 20 min post-Oxt, whereas Tnpo1 remained cytosolic, the Oxtr–EGFP fusion protein was almost exclusively nuclear. These experiments clearly indicate Tnpo1 as a primary carrier for Oxtr nuclear transport.
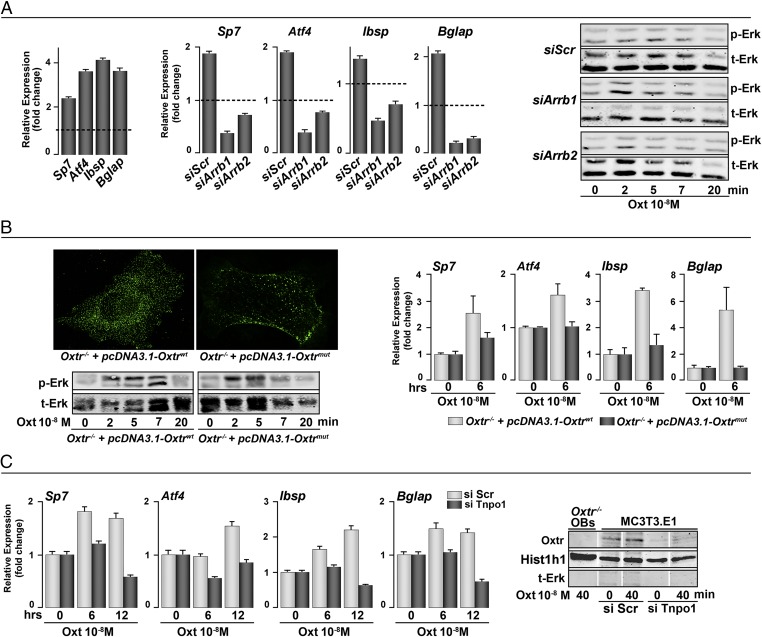
We examined whether various steps of Oxtr internalization were required for the anabolic action of Oxt. For this experiment, primary mouse calvarial osteoblasts were transfected with arrb1, arrb2, or Tnpo1 siRNA (50 nM). The silenced cells were then stimulated with Oxt (10−8 M) and lysed for RNA and proteins extraction. Of note was that the stimulation of Erk remained unchanged on Arrb1 or Arrb2 knockdown, which is different from that noted by others in murine uterine myocytes (26). However, the Oxt-induced induction of osteoblast differentiation genes, namely osterix (Sp7), Atf4, bone sialoprotein (Ibsp), and osteocalcin (Bglap), was dramatically suppressed in Arrb1/2 siRNA-transfected cells at 6 h (Fig. 4A). This result suggested that the effects of Oxt on osteoblast differentiation were Arrb1/2-dependent but independent of Erk activation.
Fig. 4.
Nuclear Oxtr modulates osteoblastic gene expression through nuclear trafficking. In control primary osteoblasts, Oxt up-regulates the expression of osterix (Sp7), Atf4, bone sialoprotein (Ibsp), and osteocalcin (Bglap). (A) siRNA knockdown of Arrb1/2 abolished this up-regulation without affecting Erk phosphorylation. We created an Oxtr construct (pcDNA3.1–Oxtrmut) that was unable to bind stably to Arrb for subsequent endocytosis. Oxtr−/− osteoblasts were stably transfected with pcDNA3.1–Oxtrwt or pcDNA3.1–Oxtrmut and stimulated with Oxt (10−8 M) for 15 min. (B) Immunostaining showed that mutant receptor appears mostly at the plasma membrane compared with WT receptor. Erk phosphorylation was not affected; however, gene up-regulation was dampened in the absence of endocytosis. (C) Nuclear membrane translocation of Oxtr was Tnpo1-dependent, because Oxtr was not detected in the nucleus after siRNA knockdown of Tnpo1. This knockdown also abolished the up-regulation of osteoblastic gene expression. Note that only relevant lanes in the Western blots are shown. Intervening lanes are replaced with white gaps. siScr, scrambled siRNA (control).
In complementary experiments, rather than inhibiting Arrb expression, we prevented its recruitment by the Oxtr. The Oxtr has three Ser clusters in the C-terminal tail that are targets for phosphorylation by GPCR kinases, an event that recruits Arrb. Mutations that prevent Ser phosphorylation, thus, inhibit Oxtr–Arrb interactions and receptor endocytosis (21). We generated an Oxtr mutant, in which two of three Ser clusters were mutated to Ala residues (Oxtrmut). Oxtr−/− osteoblasts were then transfected with WT or mutated Oxtr. Fig. 4B shows that the Oxtrmut is partially retained at the plasma membrane after Oxt stimulation, indicating the dependence of receptor internalization on Ser phosphorylation and Arrb recruitment. This effect was also independent on Erk phosphorylation, which remained unchanged in Oxtrmut-transfected osteoblasts compared with cells transfected with WT Oxtr. Importantly, however, transfection with Oxtrmut inhibited the induction of osteoblast gene expression by Oxt. This experiment together with the Arrb1/2 siRNA data (Fig. 4A) establish that Oxtr internalization followed by Arrb recruitment is required for Oxt-induced osteoblast differentiation.
Having established Tnpo1 as a primary carrier for the nuclear transport of Oxtrs, we evaluated the significance of this interaction in Oxt-induced osteoblast differentiation. Attenuated osteoblastic gene expression was noted in cells transfected with Tnpo1 siRNA at 6- and/or 12-h time points (Fig. 4C). Using the nuclear subfraction of MC3T3.E1 cells, we found no Oxtr localization in the Tnpo1 siRNA transfectants, compared with those transfected with scrambled siRNA (48 h) (Fig. 4C). Oxtr−/− osteoblasts expectedly showed no nuclear Oxtr localization. The data indicate that association with Tnpo1 is necessary for Oxtr nuclear localization and that this process is required for Oxt-induced osteoblast differentiation.
Discussion
We have shown previously that osteoblasts express abundant plasma membrane Oxtrs that drive cell differentiation to a mature, mineralizing phenotype in response to pituitary- and bone marrow-derived Oxt (4, 6). However, the mechanism of this effect has remained uncertain. Here, we show that the skeletal action of Oxt is mediated by and dependent on the internalization of Oxtrs and their subsequent translocation to the nucleus. Nuclear localization of the Oxtr on ligand binding has previously been reported in breast cancer cells and primary fibroblasts (20). In osteosarcoma cells, however, the process is constitutively active (20). Our data support these original observations but in addition, establish the precise mechanism of nuclear import. Notably, we find that, after Arrb-mediated internalization, Oxtrs interact with Rab5 and then binds to the karyopherin Tnpo1, which facilitates nuclear transport. Knockdown of either Arrb or Tnpo1 or the inhibition of Arrb recruitment abrogates the action of Oxt on gene expression.
The effects of Oxt on osteoblast differentiation, exerted through its nuclear translocation, seem independent of its effects on activating the MAPK pathway. Notably, despite knocking down Arrb isoforms, which abrogate the effects of Oxt on gene expression, the pErk signal persists. Likewise, blocking the recruitment of Arrb by mutating Oxtrs, which prevents their internalization, fails to inhibit Oxt-induced Erk phosphorylation. In osteoblasts, therefore, the rapid Erk phosphorylation seems separate from relatively protracted effects of internalized Oxtrs on gene expression. The Erk response could arise from an interaction of Oxt with the vasopressin receptor Avpr1, the activation of which, as we have shown, also causes Erk phosphorylation (27). However, this effect is in contrast to uterine myocytes, wherein pErk is reduced in mice lacking either Arrb1 or Arrb2 (26). The intactness of the pErk response in osteoblasts could be explained by the known activation of MAPKs by Gαi and Gαq (28); this actin could account for the positive effect of Oxt on osteoblast viability that we have shown earlier (4).
Our data are fully consistent with growing evidence of the presence of GPCRs within the nucleus (14, 15). In fact, molecules that have traditionally been considered part of the plasma membrane and cytosol have also been widely described in the nucleus. Nuclear localization has been documented for the CCR2, VPAC1, S1P1, and CXCR4 receptors, which in certain cases, are mediated by Tnpo1 (17–19). In contrast, GnRHR, mGluR5, and βARs have been found in the nuclear membrane. Importantly, these GPCRs have been shown to be signaling-efficient (29–31). The activation of GnRHR, for example, induces Hist1h3 acetylation and phosphorylation, whereas mGluR5 activation triggers nuclear Ca2+ signals and CREB phosphorylation (29, 30). Likewise, G proteins have been found in the nucleus. Immunoprecipitation studies show that the CXCR4 binds to a nuclear Gαi protein (19). Its activation by the ligand SDF-1α elicits intranuclear Ca2+ signals that are sensitive to inhibition by the Gαi inhibitor pertussis toxin—this effect testifies to the presence of a fully functional CXCR4/Gαi apparatus within the nucleus (19). Similarly, we found that the enzyme CD38, hitherto considered an extracellular cyclase that generates the second messenger cADPr, was localized to the inner nuclear membrane (32). At this location, it triggered Ca2+ signals through ryanodine receptor-gated Ca2+ channels, which we and others found were also resident within the inner nuclear membrane (33, 34). It has been shown recently that insulin triggers the nuclear localization of an otherwise cytosolic protein, caveolin-2; this effect, in turn, prevents histone methylation (35). We remain unsure whether a fully functional Oxtr/G protein exists in the nucleus.
Our data raise the possibility that membrane GPCRs, although signaling relatively rapidly through traditional phosphorylation cascades, may interact directly with the transcription machinery of a cell after nuclear localization. Furthermore, it might be worthwhile examining whether nuclear Oxtrs, in addition to mediating the skeletal actions of Oxt, also contribute to other more delayed actions of Oxt in social bonding. Likewise and more generally, it would be meaningful to further study whether certain unexplained actions of GPCRs, particularly long-term responses to agonist stimulation, are mediated by a nuclear mechanism.
At the physiologic level, albeit speculatively, these differences might translate into distinct regulatory actions of Oxt on calcium homeostasis during pregnancy and lactation. Oxtrs are present on osteoclast precursors and osteoclasts (36), and their stimulation results in increased osteoclastogenesis but inhibited bone resorption (4). Rapid rises in Oxt during periods of calcium stress (1) might enable calcium mobilization followed by the rapid cessation of bone resorption through a brake on active osteoclasts (4). Thereafter, the maternal skeleton recovers, and we speculate that the proosteoblastic action of the hormone mediates bone rebuilding (5, 37, 38). We find that bone formation during pregnancy is inhibited in Oxtr−/− mice and that Oxtr−/− pups display reduced trabecular mineralization (5). The extent to which nuclear Oxtr actions mediate these putative physiological effects requires additional studies. It is also important to determine if osteoclasts and osteoblasts respond differently to the range of concentrations of circulating Oxt during pregnancy and lactation. For example, whereas higher plasma Oxt levels may permit calcium mobilization by the osteoclast, falling concentrations postlactation may trigger reparative anabolic response.
Materials and Methods
Osteoblast-enriched cultures were performed using calvaria from newborn mice after sequential collagenase digestion (details in SI Materials and Methods). Intact nuclei were isolated as described in the work by Adebanjo et al. (32). The outer nuclear membrane and nucleoplasts (nuclei without outer membranes) were separated (32). For immunofluorescence microscopy, cells or nucleoplasts were fixed and incubated with antibodies to Oxtr (gift from Fabio Malavasi, University of Turin, Turin, Italy), Arrb1/2 (Santa Cruz), Tpno1, Kpnb1 (Abnova), and Rab5 (Cell Signaling). Fluorescent-labeled goat anti-mouse or anti-rabbit secondary antibodies (Alexa-488 and Alexa-568, respectively; Invitrogen) were used for detection. Nuclei were counterstained with Quinolinium 4-[3-(3-methyl-2(3H)-benzothiazolylidene)-1-propenyl]-1-[3-(trimethylammonio)propyl]-diiodide (TO-PRO). For immunogold staining, we followed the protocol detailed in SI Materials and Methods and examined the sections using a Zeiss EM 109 electron microscope and a Gatan CMS camera. MS analysis of nuclear extracts is detailed in SI Materials and Methods. For mutagenesis, we created pcDNA3.1–Oxtrmut, a mouse Oxtr mutant construct with two clusters of Ser residues mutated to Ala residues (S365A, S366A, and S367A and S376A, S377A, and S378A). Oxtr−/− mouse osteoblasts were stably transfected with pcDNA3.1–Oxtrmut or pcDNA3.1–Oxtrwt after geneticin selection. Western immunoblotting and quantitative PCR were performed using standard methods.
Supplementary Material
Acknowledgments
This study was supported by National Institute on Aging of the National Institutes of Health (NIH) Grant AG40132 (to M.Z.). This study was also supported by NIH Grants DK80459 (to L.S. and M.Z.), AG23176 (to M.Z.), AR06592 (to M.Z.), and AR06066 (to M.Z.). A.Z. is supported by the Italian Space Agency and the Italian Ministry of Education, Universities and Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419349111/-/DCSupplemental.
References
- 1.Kroeger M. Oxytocin: Key hormone in sexual intercourse, parturition, and lactation. Birth Gaz. 1996;13(1):28–30. [PubMed] [Google Scholar]
- 2.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50(4):506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Elabd SK, Sabry I, Hassan WB, Nour H, Zaky K. Possible neuroendocrine role for oxytocin in bone remodeling. Endocr Regul. 2007;41(4):131–141. [PubMed] [Google Scholar]
- 4.Tamma R, et al. Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci USA. 2009;106(17):7149–7154. doi: 10.1073/pnas.0901890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, et al. Oxytocin deficiency impairs maternal skeletal remodeling. Biochem Biophys Res Commun. 2009;388(1):161–166. doi: 10.1016/j.bbrc.2009.07.148. [DOI] [PubMed] [Google Scholar]
- 6.Colaianni G, et al. Bone marrow oxytocin mediates the anabolic action of estrogen on the skeleton. J Biol Chem. 2012;287(34):29159–29167. doi: 10.1074/jbc.M112.365049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 8.Shenoy SK, Lefkowitz RJ. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32(9):521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: Not just for seven-transmembrane receptors. Mol Cell. 2006;24(5):643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ. Arrestin development: Emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell. 2009;17(4):443–458. doi: 10.1016/j.devcel.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stow JL, et al. A heterotrimeric G protein, G alpha i-3, on Golgi membranes regulates the secretion of a heparan sulfate proteoglycan in LLC-PK1 epithelial cells. J Cell Biol. 1991;114(6):1113–1124. doi: 10.1083/jcb.114.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Audigier Y, Nigam SK, Blobel G. Identification of a G protein in rough endoplasmic reticulum of canine pancreas. J Biol Chem. 1988;263(31):16352–16357. [PubMed] [Google Scholar]
- 13.Carlson KE, Woolkalis MJ, Newhouse MG, Manning DR. Fractionation of the beta subunit common to guanine nucleotide-binding regulatory proteins with the cytoskeleton. Mol Pharmacol. 1986;30(5):463–468. [PubMed] [Google Scholar]
- 14.Boivin B, Vaniotis G, Allen BG, Hébert TE. G protein-coupled receptors in and on the cell nucleus: A new signaling paradigm? J Recept Signal Transduct Res. 2008;28(1-2):15–28. doi: 10.1080/10799890801941889. [DOI] [PubMed] [Google Scholar]
- 15.Gobeil F, et al. G-protein-coupled receptors signalling at the cell nucleus: An emerging paradigm. Can J Physiol Pharmacol. 2006;84(3-4):287–297. doi: 10.1139/y05-127. [DOI] [PubMed] [Google Scholar]
- 16.Verzijl D, Peters SL, Alewijnse AE. Sphingosine-1-phosphate receptors: Zooming in on ligand-induced intracellular trafficking and its functional implications. Mol Cells. 2010;29(2):99–104. doi: 10.1007/s10059-010-0041-z. [DOI] [PubMed] [Google Scholar]
- 17.Estrada R, et al. Ligand-induced nuclear translocation of S1P(1) receptors mediates Cyr61 and CTGF transcription in endothelial cells. Histochem Cell Biol. 2009;131(2):239–249. doi: 10.1007/s00418-008-0521-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favre N, et al. Chemokine receptor CCR2 undergoes transportin1-dependent nuclear translocation. Proteomics. 2008;8(21):4560–4576. doi: 10.1002/pmic.200800211. [DOI] [PubMed] [Google Scholar]
- 19.Don-Salu-Hewage AS, et al. Cysteine (C)-x-C receptor 4 undergoes transportin 1-dependent nuclear localization and remains functional at the nucleus of metastatic prostate cancer cells. PLoS ONE. 2013;8(2):e57194. doi: 10.1371/journal.pone.0057194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinsey CG, et al. Constitutive and ligand-induced nuclear localization of oxytocin receptor. J Cell Mol Med. 2007;11(1):96–110. doi: 10.1111/j.1582-4934.2007.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis*. J Biol Chem. 2001;276(22):19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 22.Seachrist JL, Ferguson SS. Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci. 2003;74(2-3):225–235. doi: 10.1016/j.lfs.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Conti F, Sertic S, Reversi A, Chini B. Intracellular trafficking of the human oxytocin receptor: Evidence of receptor recycling via a Rab4/Rab5 “short cycle.”. Am J Physiol Endocrinol Metab. 2009;296(3):E532–E542. doi: 10.1152/ajpendo.90590.2008. [DOI] [PubMed] [Google Scholar]
- 24.Pemberton LF, Paschal BM. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic. 2005;6(3):187–198. doi: 10.1111/j.1600-0854.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 25.Braem CV, et al. Identification of a karyopherin alpha 2 recognition site in PLAG1, which functions as a nuclear localization signal. J Biol Chem. 2002;277(22):19673–19678. doi: 10.1074/jbc.M112112200. [DOI] [PubMed] [Google Scholar]
- 26.Grotegut CA, et al. β-Arrestin mediates oxytocin receptor signaling, which regulates uterine contractility and cellular migration. Am J Physiol Endocrinol Metab. 2011;300(3):E468–E477. doi: 10.1152/ajpendo.00390.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamma R, et al. Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia. Proc Natl Acad Sci USA. 2013;110(46):18644–18649. doi: 10.1073/pnas.1318257110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima K, et al. Minireview: Novel aspects of M3 muscarinic receptor signaling in pancreatic β-cells. Mol Endocrinol. 2013;27(8):1208–1216. doi: 10.1210/me.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jong YJ, Kumar V, Kingston AE, Romano C, O’Malley KL. Functional metabotropic glutamate receptors on nuclei from brain and primary cultured striatal neurons. Role of transporters in delivering ligand. J Biol Chem. 2005;280(34):30469–30480. doi: 10.1074/jbc.M501775200. [DOI] [PubMed] [Google Scholar]
- 30.Re M, et al. The human gonadotropin releasing hormone type I receptor is a functional intracellular GPCR expressed on the nuclear membrane. PLoS ONE. 2010;5(7):e11489. doi: 10.1371/journal.pone.0011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaniotis G, et al. Nuclear β-adrenergic receptors modulate gene expression in adult rat heart. Cell Signal. 2011;23(1):89–98. doi: 10.1016/j.cellsig.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adebanjo OA, et al. Novel biochemical and functional insights into nuclear Ca(2+) transport through IP(3)Rs and RyRs in osteoblasts. Am J Physiol Renal Physiol. 2000;278(5):F784–F791. doi: 10.1152/ajprenal.2000.278.5.F784. [DOI] [PubMed] [Google Scholar]
- 33.Adebanjo OA, et al. A new function for CD38/ADP-ribosyl cyclase in nuclear Ca2+ homeostasis. Nat Cell Biol. 1999;1(7):409–414. doi: 10.1038/15640. [DOI] [PubMed] [Google Scholar]
- 34.Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995;80(3):439–444. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- 35.Jeong K, et al. Rab6-mediated retrograde transport regulates inner nuclear membrane targeting of caveolin-2 in response to insulin. Traffic. 2012;13(9):1218–1233. doi: 10.1111/j.1600-0854.2012.01378.x. [DOI] [PubMed] [Google Scholar]
- 36.Colucci S, Colaianni G, Mori G, Grano M, Zallone A. Human osteoclasts express oxytocin receptor. Biochem Biophys Res Commun. 2002;297(3):442–445. doi: 10.1016/s0006-291x(02)02009-0. [DOI] [PubMed] [Google Scholar]
- 37.Colaianni G, Sun L, Zaidi M, Zallone A. Oxytocin and bone. Am J Physiol Regul Integr Comp Physiol doi: 10.1152/ajpregu.00040.2014. , in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colaianni G, et al. The oxytocin-bone axis. J Neuroendocrinol. 2014;26(2):53–57. doi: 10.1111/jne.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.