Significance
Colorectal cancer is the third leading cause of cancer-related death in the United States, but treatment options for this disease are of limited effectiveness. Most human colorectal tumors begin with an inactivating mutation in the adenomatous polyposis coli (APC) gene. We demonstrate a mechanism by which nonsteroidal antiinflammatory drugs (NSAIDs) protect against colon cancer development by killing intestinal stem cells that have lost functional APC. NSAID treatment combines with APC loss-induced gene expression changes to selectively activate BID and induce apoptosis in these cells, while leaving normal cells unharmed. These results provide a rationale for developing more effective cancer prevention strategies and agents.
Keywords: colorectal cancer, APC, chemoprevention, BID, apoptosis
Abstract
Colorectal tumorigenesis is driven by genetic alterations in the adenomatous polyposis coli (APC) tumor suppressor pathway and effectively inhibited by nonsteroidal antiinflammatory drugs (NSAIDs). However, how NSAIDs prevent colorectal tumorigenesis has remained obscure. We found that the extrinsic apoptotic pathway and the BH3 interacting-domain death agonist (BID) are activated in adenomas from NSAID-treated patients. Loss of BID abolishes NSAID-mediated tumor suppression, survival benefit, and apoptosis in tumor-initiating stem cells in APCMin/+ mice. BID-mediated cross-talk between the extrinsic and intrinsic apoptotic pathways is responsible for selective killing of neoplastic cells by NSAIDs. We further demonstrate that NSAIDs induce death receptor signaling in both cancer and normal cells, but only activate BID in cells with APC deficiency and ensuing c-Myc activation. Our results suggest that NSAIDs suppress intestinal tumorigenesis through BID-mediated synthetic lethality triggered by death receptor signaling and gatekeeper mutations, and provide a rationale for developing more effective cancer prevention strategies and agents.
Colorectal cancer (CRC) is the third leading cause of cancer-related death in the United States (1). However, treatment options for advanced colorectal cancer, including radiation, cytotoxic, and targeted therapies, are of limited effectiveness. Colorectal tumorigenesis is driven by genetic alterations in the APC tumor suppressor pathway through aberrant Wnt signaling, leading to β-catenin accumulation and activation of oncogenes such as c-Myc and CCND1 (2). One of the most promising strategies for reducing the morbidity and mortality of CRC is to inhibit tumorigenesis by using natural products or pharmacologic agents, such as nonsteroidal antiinflammatory drugs (NSAIDs) (3). The chemopreventive activities of NSAIDs, such as aspirin and sulindac, have been demonstrated in epidemiological studies (4), clinical trials (5, 6), and animal models (7). However, the critical cellular activities and molecular targets of NSAIDs in chemoprevention have remained elusive.
It is suggested that the antitumor effects of NSAIDs require selective killing of neoplastic cells through apoptosis (8), a major turnover mechanism of intestinal epithelial cells (9). Apoptotic death is regulated by the death receptor (DR or extrinsic) and mitochondrial (intrinsic) pathways (10). The DR pathway involves activation of death receptors such as DR4 and DR5, recruitment of FADD and procaspase 8, and depletion of prosurvival proteins such as c-FLIP, resulting in activation of the recruited caspase 8 and other caspases (11). The mitochondrial pathway is regulated by the Bcl-2 family proteins (12) and characterized by mitochondrial dysfunction, release of cytochrome c, and activation of caspase 9 (10). These two pathways cross talk under certain conditions through caspase-8–mediated cleavage of BID, a BH3-only Bcl-2 family member (13). The activated and truncated BID (tBID) then engages the intrinsic pathway for efficient apoptosis induction (13). NSAIDs rely on the intrinsic pathway to kill CRC cells (14, 15). Nonetheless, it is still unclear whether apoptosis induction is essential for the antitumor activities of NSAIDs.
In this study, we found that NSAID treatment activates BID and the extrinsic apoptotic pathway in human intestinal adenomas. Loss of BID almost completely abolishes NSAID-mediated tumor suppression and killing of oncogenic intestinal stem cells in APC-deficient mice. BID is activated by a synthetic lethal interaction and mediates the effects of NSAIDs through cross-talk between the extrinsic and intrinsic pathways. Our results indicate that BID-dependent killing of tumor-initiating stem cells is critical for cancer prevention by NSAIDs.
Results
NSAIDs Activate Caspase 8 and BID in Human Colonic Adenomas.
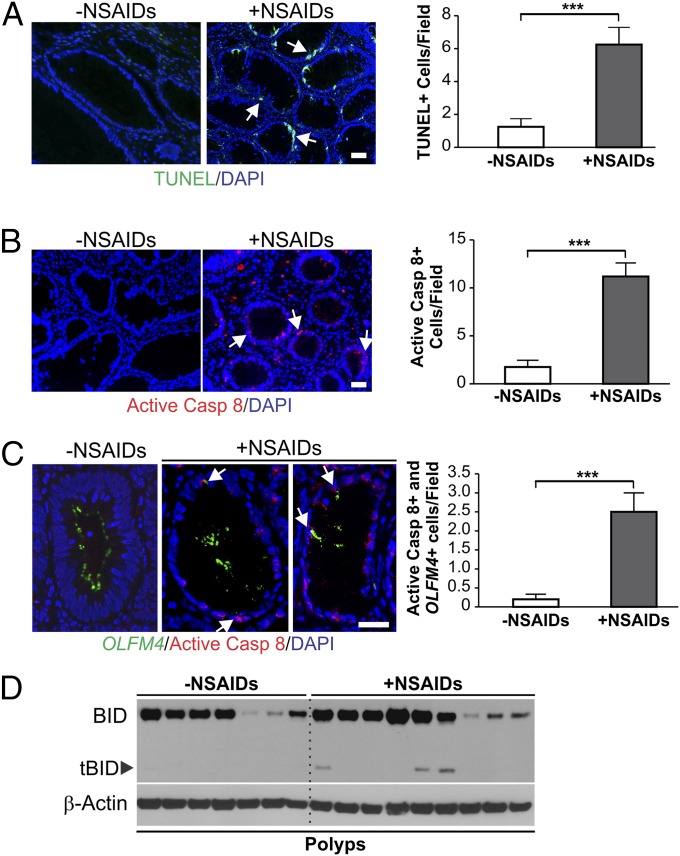
To determine the role of the extrinsic apoptotic pathway in NSAID-mediated tumor suppression, we used terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and active caspase 8 staining to analyze advanced colonic adenomas from patients taking aspirin or other NSAIDs and control samples from patients without NSAID use (16). The number of TUNEL-positive cells in the adenomas of the NSAID-treated patients was 5.0-fold higher compared with those in the control group (Fig. 1A). Active caspase 8 positive cells were markedly increased in the patients taking NSAIDs (Fig. 1B), and detected in 8 of 9 adenomas from NSAID users, but only in 1 of 7 from nonusers. Upon analyzing olfactomedin 4 (OLFM4) (Fig. 1C, Left), which marks human crypt base columnar cells (CBCs), representing active intestinal stem cells (ISCs) (17), we found active caspase 8/OLFM4 double-positive cells were significantly increased by more than 12-fold in the adenomas from NSAID takers compared with nonusers (Fig. 1C, Right). Most notably, BID cleavage was detected in several adenomas with high BID expression from NSAID users, but not in any adenomas from the patients not taking NSAIDs (Fig. 1D). These results suggest that NSAIDs kill oncogenic stem cells in human adenomas, and activation of the DR pathway and BID may contribute to this effect of NSAIDs.
Fig. 1.
NSAID treatment activates caspase 8 and BID in human advanced adenomas. (A–C) Advanced adenomas from nine patients taking NSAIDs and seven patients not taking NSAIDs were analyzed by TUNEL staining (green) (A), active caspase 8 (Casp 8) staining (red) (B), and OLFM4 (green) and active caspase 8 (red) double staining (C). (Left) Representative staining pictures. (Right) Mean numbers + SD of positive cells per field. (Scale bars: 25 µm.) ***P < 0.001. Arrows indicate example positive cells. (D) BID expression in the adenomas was analyzed by Western blotting using homogenized tissue lysates. Each lane represents an individual patient.
BID Is Required for Tumor Suppression by NSAIDs in APCMin/+ Mice.
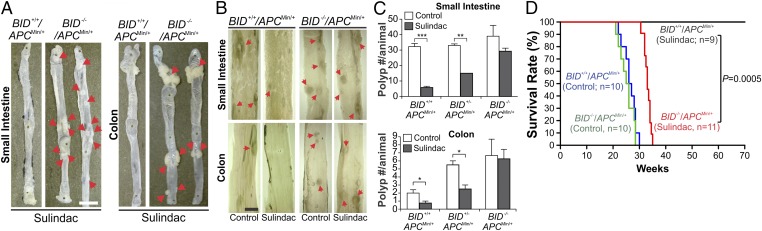
To determine whether BID is required for the chemopreventive effects of NSAIDs, we crossed APCMin/+ mice, a genetic model of intestinal adenomas (18), with BID KO mice (19) and generated age- and sex-matched cohorts of C57BL/6J APCMin/+ mice with different BID genotypes. These mice were then fed an AIN-93G diet containing 0 (control) or 200 ppm sulindac, and analyzed for intestinal polyp formation. Sulindac treatment suppressed small intestinal adenoma formation in BID+/+/APCMin/+ mice by more than 80% compared with the untreated mice (Fig. 2 A–C). Remarkably, this effect of sulindac was almost completely lost in BID−/−/APCMin/+ mice, in which the number of small intestinal polyps was only slightly reduced (Fig. 2C). Sulindac was also less effective in the small intestine of BID heterozygous APCMin/+ mice (Fig. 2C). Similar observations were made in the colon of APCMin/+ mice (Fig. 2 A–C). Indomethacin (10 ppm), also an NSAID with antitumor activity (15), was similarly BID-dependent in reducing small intestinal adenoma formation (Fig. S1). In untreated animals, loss of BID did not significantly affect the size and number of small intestinal polyps, but led to an increase in polyp number in the colon of APCMin/+ mice (Fig. 2C).
Fig. 2.
BID is required for tumor suppression by NSAIDs in APCMin/+ mice. Age- and sex-matched APCMin/+ mice with different BID genotypes were fed control or NSAID-containing AIN93G diet. (A) Representative images of small intestine and colon from BID+/+ and BID−/− APCMin/+ mice treated with sulindac (200 ppm) for 4 mo, with arrows indicating visible macroadenomas. (B) Representative images of small intestine and colon of control mice and mice treated with sulindac (200 ppm) for 2 mo, with arrows indicating microscopic lesions. (C) Mean numbers + SD of small intestinal and colonic adenomas (≥0.5 mm in diameter) in BID+/+, BID+/−, and BID−/− APCMin/+ mice treated ± sulindac as in B. *P < 0.05; **P < 0.01; ***P < 0.001 (n = 4 per group). (D) Kaplan–Meier survival curves of BID+/+ and BID−/− APCMin/+ mice treated ± sulindac (200 ppm) for 70 wk. BID+/+ vs. BID−/− APCMin/+ mice, P = 0.0005 (log-rank test). (Scale bars: A, 1 cm; B, 2 mm.)
We then compared the effects of prolonged sulindac treatment on the survival of BID+/+ and BID−/− APCMin/+ mice. In striking contrast to BID+/+/APCMin/+ mice (n = 9), which were alive after 70 wk of treatment, all of the BID−/−/APCMin/+ mice (n = 11) died before 35 wk, surviving only slightly longer than those on the control diet (∼30 wk) (Fig. 2D). The difference between the BID+/+ and BID−/− groups is highly significant (70 vs. 33 wk, P = 0.0005) (Fig. 2D). Together, these results demonstrate that BID plays an essential role in NSAID-mediated chemoprevention in APCMin/+ mice.
BID Is Required for NSAID-Induced Killing of Intestinal Stem Cells in APCMin/+ Mice.
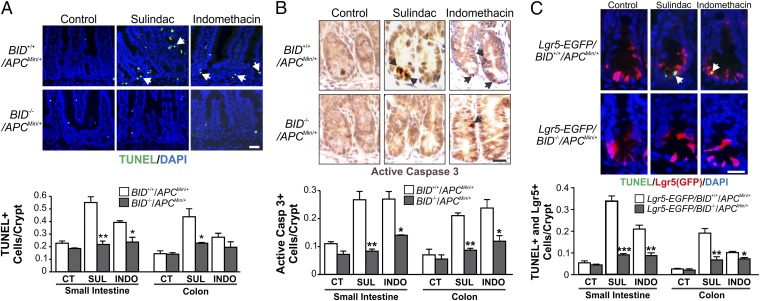
We investigated how BID mediates the chemopreventive effects of NSAIDs in APCMin/+ mice. We found that crypt cell apoptosis detected by TUNEL and active caspase 3 staining was significantly reduced in the small intestine and colon of sulindac- or indomethacin-treated BID−/−/APCMin/+ mice relative to BID+/+/APCMin/+ mice (Fig. 3 A and B and Fig. S2 A and B). In contrast, activation of caspase 8 by sulindac or indomethacin was not affected by the absence of BID (Fig. S2C). Sulindac was shown to preferentially kill ISCs in the crypt bottom of APCMin/+ mice (16), which can be detected by markers such as Lgr5 and OLFM4 (20). Analysis of Lgr5-EGFP–marked mice revealed that the killing effect of sulindac and indomethacin on ISCs indicated by TUNEL/EGFP double-positive staining was reduced in the small intestine and colon of BID−/−/APCMin/+ mice relative to BID+/+/APCMin/+ mice (Fig. 3C and Fig. S2D). Using OLFM4 RNA in situ hybridization to detect CBCs, we found the killing effect of sulindac and indomethacin on ISCs indicated by TUNEL/OLFM4 double-positive staining was also reduced in the small intestine of BID−/−/APCMin/+ mice (Fig. S2E). Inhibition of cyclooxygenase 2 (COX 2) by NSAIDs has been implicated as a mechanism of NSAID-mediated chemoprevention (21). Sulindac and indomethacin treatment reduced plasma prostaglandin E2 metabolites (PGEM) by ∼50% in BID+/+/APCMin/+, but these effects were intact in BID−/−/APCMin/+ mice (Fig. S2F), suggesting that BID functions downstream of COX 2 inhibition in suppressing tumorigenesis. Together, these results indicate that BID mediates the chemopreventive effects of NSAIDs through selective killing of APC-deficient ISCs.
Fig. 3.
BID deficiency abolishes NSAID-induced apoptosis in intestinal stem cells in APCMin/+ mice. (A and B) BID+/+ and BID−/− APC Min/+ mice were fed control (CT) or experimental AIN93G diet containing 200 ppm sulindac (SUL) or 10 ppm indomethacin (INDO) for 1 wk. Small intestinal and colonic sections were analyzed for apoptosis by TUNEL staining (green) (A) and active caspase 3 staining (brown) (B). (Upper) Representative staining pictures of small intestinal sections, with arrows indicating example cells with positive staining. (Lower) Mean + SD of positive signals in the small intestinal and colonic crypts. (C) Lgr5-EGFP–marked BID+/+ and BID−/− APCMin/+ mice were treated as in A and analyzed for intestinal stem cell apoptosis by TUNEL (green) and EGFP (Lgr5; red) double staining. (Upper) Representative staining pictures of small intestinal sections, with arrows indicating example double-positive cells. (Lower) Mean + SD of TUNEL/Lgr5 double-positive signals in the small intestinal and colonic crypts. (Scale bars: 25 µm.) *P < 0.05; **P < 0.01; ***P < 0.001 (n = 3 per group).
BID Mediates NSAID-Induced Apoptosis Through the Mitochondrial Pathway.
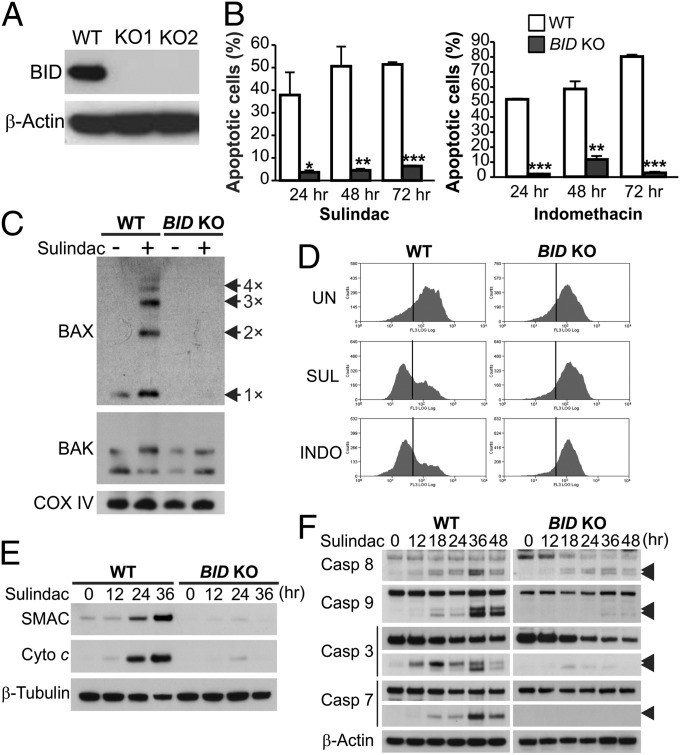
To further delineate the functional role of BID in NSAID-mediated tumor suppression, we analyzed HCT116 colon cancer cells, which contain a β-catenin–activating mutation and can recapitulate the anticancer and apoptotic effects of NSAIDs in mice (14–16). We generated BID knockout (BID KO) HCT116 cells by using homologous recombination (Fig. S3 and Fig. 4A) and compared WT and BID KO cells for their responses to NSAIDs and other anticancer agents. Strikingly, apoptosis induced by sulindac and indomethacin, as determined by nuclear fragmentation and annexin V staining, was almost completely blocked in BID KO cells (Fig. 4B and Fig. S4A). BID deficiency abrogated NSAID-induced Bax multimerization (Fig. 4C), mitochondrial membrane depolarization (Fig. 4D), cytosolic release of cytochrome c and SMAC (Fig. 4E), and activation of caspases 9, 7, and 3 (Fig. 4F). Reconstituting BID expression in BID KO cells restored sulindac-induced apoptosis (Fig. S4B). In contrast to wild-type (WT) cells, the long-term survival of BID KO cells, as analyzed by colony formation assay, did not decrease following NSAID treatment (Fig. S4C).
Fig. 4.
BID mediates NSAID-induced apoptosis in CRC cells through the mitochondrial pathway. (A) Western blot of BID in WT and two independent clones of BID knockout (BID KO) HCT116 cell lines. (B) Apoptosis at indicated time points in WT and BID KO HCT116 cells treated with 120 µM sulindac sulfide or 500 µM indomethacin was analyzed by counting cells with condensed and fragmented nuclei. Results were expressed as means + SD of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001. (C) BAX and BAK multimerization was analyzed by cross-linking the mitochondrial fractions isolated from cells treated as in B for 36 h, followed by SDS/PAGE under nonreducing conditions and Western blotting. Cox IV, a mitochondrial protein, was used as a control for loading and fractionation. (D) Mitochondrial membrane potential in cells untreated (UN) or treated with sulindac sulfide (SUL) or indomethacin (INDO) as in B for 48 h was analyzed by MitoTracker Red staining followed by flow cytometry. (E) Cytochrome c (Cyto c) and SMAC/DIABLO release were analyzed by Western blotting of cytosolic fractions isolated from cells treated with sulindac sulfide as in C. α-Tubulin, a cytosolic protein, was used as control for loading and fractionation. (F) Western blot of caspase (Casp) activation at the indicated time points in cells treated as in B. Arrowheads indicate caspase cleavage fragments.
NSAIDs also required BID to induce apoptosis in other CRC cell lines, including APC-mutant DLD1 and HT29 cells, and APC-WT RKO cells (Fig. S4D). Furthermore, BID-dependent apoptosis was induced by other NSAIDs, including sulindac sulfone, a sulindac derivative (22), and SC236, a COX 2-specific inhibitor structurally unrelated to sulindac and indomethacin (23) (Fig. S5 A–C). In contrast, BID deficiency did not affect apoptosis induced by staurosporine, camptothecin, or overexpression of the BH3-only protein PUMA, but attenuated apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (Fig. S5D). These results demonstrate a prominent and specific role of BID in mediating the anticancer and apoptotic effects of NSAIDs.
BID Is Activated by NSAIDs Through the Extrinsic Apoptotic Pathway.
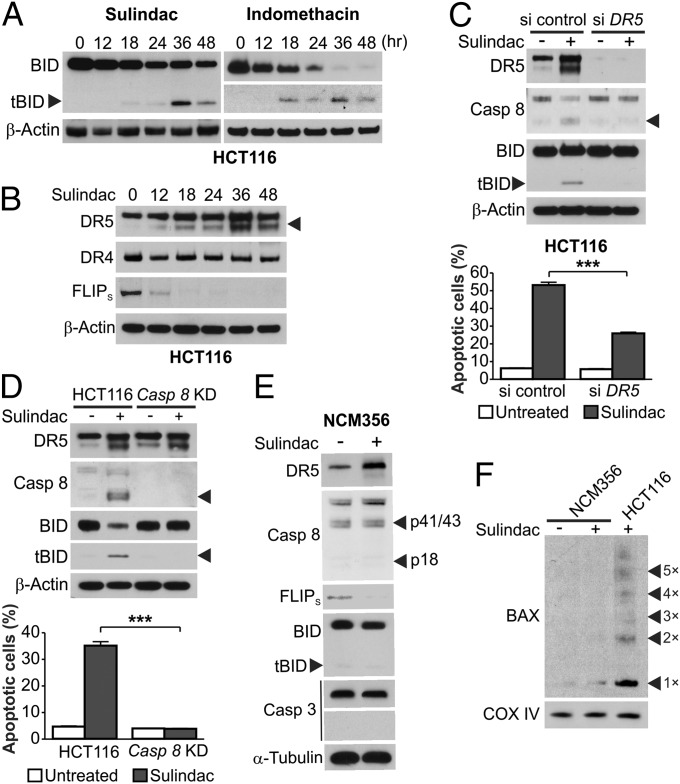
We determined how NSAIDs activate BID to engage the mitochondrial pathway to kill colon cancer cells. tBID was observed in HCT116 cells treated with different NSAIDs (Fig. 5A and Fig. S5 A and B) and could be detected as early as 18 h after treatment (Fig. 5A). The short form of DR5, but not DR4, was induced by NSAIDs in WT and BID KO HCT116 cells (Fig. 5B and Fig. S6 A and B). The induction of DR5 by NSAIDs was independent of p53 (Fig. S6C), which can activate DR5 following DNA damage (24), and observed in different CRC cells (Fig. S6D). The short form of c-FLIP (FLIPs), a negative regulator of caspase 8 activation, was markedly down-regulated before BID cleavage in NSAID-treated colon cancer cells (Fig. 5B and Fig. S6D). Similar to the finding in mice (Fig. S2C), caspase 8 activation was found in NSAID-treated BID KO cells (Fig. 4F), suggesting activation of BID through the extrinsic pathway. We therefore tested this possibility by perturbing the extrinsic pathway components. BID cleavage in sulindac-treated HCT116 cells was suppressed by transient knockdown of DR5 or stable knockdown of caspase 8 (Fig. 5 C and D). Sulindac-induced apoptosis was blocked by stable knockdown of caspase 8 (Fig. 5D) and partially inhibited by transient knockdown of DR5 or expression of dominant negative FADD (Fig. 5C and Fig. S6E), which is likely due to incomplete inhibition of DR5 expression and FADD activity. Together, these results demonstrate that BID is activated by NSAIDs through the extrinsic pathway and mediates apoptosis induction by engaging the intrinsic pathway to amplify the apoptotic signal.
Fig. 5.
BID is specifically activated by NSAIDs in cancer cells through the death receptor pathway. (A) Western blots of HCT116 cells treated with 120 µM sulindac sulfide or 500 µM indomethacin, with the arrowhead indicating tBID. (B) HCT116 cells were treated as in A, and analyzed for DR4, DR5, and FLIPs expression at indicated time points by Western blotting. (C) HCT116 cells were transfected with control scrambled or DR5 siRNA, and then treated with sulindac sulfide as in A. (Upper) Western blot analysis of indicated proteins at 36 h after treatment. (Lower) Apoptosis was analyzed by counting condensed/fragmented nuclei after nuclear staining at 24 h after treatment. (D) Parental and stable caspase 8 knockdown (Casp 8 KD) HCT116 cells were treated with sulindac sulfide as in A. (Upper) Western blot analysis of indicated proteins at 24 h after treatment. (Lower) Apoptosis was analyzed as in C at 24 h after treatment. (E) Indicated proteins were analyzed by Western blotting in NCM356 cells treated with sulindac sulfide as in A for 36 h. (F) BAX multimerization in NCM356 and HCT116 cells was analyzed as in Fig. 4C. Results in C and D were expressed as means + SD of three independent experiments. ***P < 0.001.
NSAID-Induced DR Signaling Triggers BID-Dependent Synthetic Lethality in APC-Deficient Cells with c-Myc Activation.
The anticancer effect of NSAIDs depends on selective killing of neoplastic cells (25). To determine this selectivity, we analyzed NCM356 normal intestinal epithelial cells (IECs), which have normal epithelial morphology and a functional APC pathway (26). NCM356 cells were resistant to NSAIDs and lacked BID cleavage, Bax multimerization, and caspases 8 and 3 activation, but remained sensitive to camptothecin and staurosporine (Fig. 5 E and F and Fig. S6F). Surprisingly, DR5 induction and FLIPs down-regulation were still observed in NCM356 cells following NSAID treatment (Fig. 5E). In both HCT116 and NCM356 cells, NSAID treatment similarly modulated the transcription and mRNA expression of the proximal DR pathway components, including DR5, c-FLIP, and the decoy receptors DcR1 and DcR3 (Fig. S7 A–D). Lack of apoptotic response even with DR pathway modulation was verified in another colon-derived and normal cell line, CRL-1831 (Fig. S7 E and F). Therefore, activation of the DR pathway by NSAIDs can occur in normal cells up to a point before caspase 8 and BID cleavage.
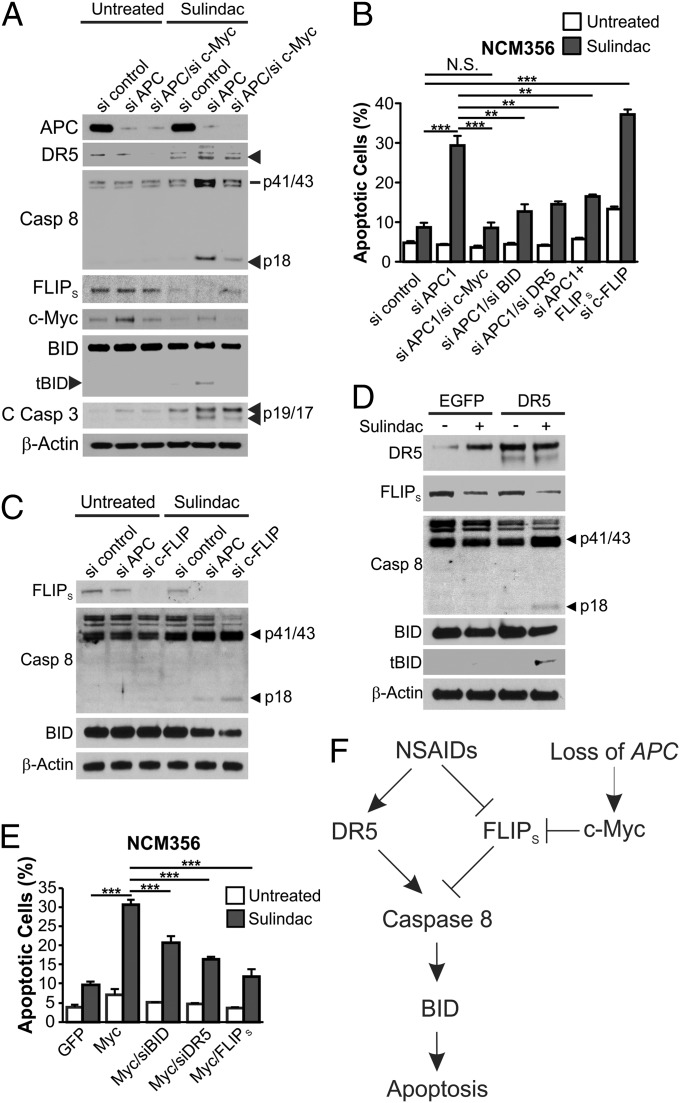
We then probed the selective activation of BID and caspase 8 by NSAIDs in neoplastic cells considering factors present in neoplastic cells but not normal cells. Loss of APC drives intestinal tumorigenesis by activating c-Myc (27, 28), which can trigger a synthetic lethal interaction in normal and tumor cells with high levels of DR5 (29). We therefore tested whether either APC loss or c-Myc activation sensitizes IECs to NSAIDs in an otherwise normal background. Knockdown of APC by siRNA, in conjunction with sulindac treatment, resulted in caspase 8 and BID cleavage, apoptosis induction, caspase 3 activation, loss of viability, and enhanced DR5 induction and FLIPs down-regulation in NCM356 cells (Fig. 6 A and B and Fig. S8 A and E). The killing effects of APC depletion require DR5, BID, and FLIPs depletion, as knockdown of DR5 or BID, or transfection of FLIPS suppressed apoptosis and restored cell viability in APC-depleted and NSAID-treated NCM356 cells (Fig. S8 B–E and Fig. 6B). Conversely, c-FLIP knockdown or DR5 transfection phenocopied APC depletion in inducing apoptosis, loss of cell viability, and caspase 8 and BID cleavage in NSAID-treated NCM356 cells (Fig. 6 C and D and Fig. S8E).
Fig. 6.
APC depletion or c-Myc induction triggers BID activation and BID-dependent apoptosis in NSAID-treated normal colonic epithelial cells. (A) NCM356 normal colonic epithelial cells were transfected with the indicated siRNA and then treated with 120 µM sulindac sulfide for 48 h. Indicated proteins were analyzed by Western blotting. C Casp 3, cleaved caspase 3. (B) NCM356 cells were transfected with indicated siRNA or siRNA/plasmid combinations, and then treated with 120 µM sulindac sulfide for 48 h. Apoptosis was measured by counting apoptotic nuclei after nuclear staining. (C and D) Western blotting of the indicated proteins in NCM356 cells transfected as indicated, and then treated with 120 µM sulindac sulfide for 36 h. (E) Apoptosis in NCM356 cells transfected as indicated and treated with 120 µM sulindac sulfide for 24 h was measured as in B. (F) A model of NSAID-mediated apoptosis in APC-deficient cells. Results in B and E were expressed as means + SD of three independent experiments. **P < 0.01; ***P < 0.001; N.S., not significant.
As expected, APC depletion led to c-Myc induction in NCM356 cells (Fig. 6A), and knockdown of c-Myc substantially decreased caspase 8 activation, and abolished BID cleavage, apoptosis, and loss of cell viability induced by APC deletion and sulindac treatment (Fig. 6 A and B and Fig. S8E). Transfection of c-Myc, but not a control vector, induced apoptosis in NSAID-treated NCM356 cells (Fig. 6E and Fig. S8 F and G). Consistent with the notion that c-Myc modulates the DR pathway (29), c-Myc transfection further down-regulated FLIPS protein, mRNA, and promoter activity and enhanced the expression of DR5, DR4, and DcR2 in sulindac-treated NCM356 cells (Figs. S8G and S9). The effects of c-Myc on apoptosis and cell viability also depended on BID and DR5 and were inhibited by FLIPS (Fig. 6E and Fig. S8F). These results demonstrate that APC deficiency and subsequent c-Myc activation interact with NSAID-induced DR signaling, leading to caspase 8 and BID cleavage and selective killing of APC-deficient IECs.
Discussion
Despite the well-defined precursor lesions and genetic changes in colorectal tumorigenesis (2), how NSAIDs inhibit this process has remained obscure. Our results demonstrate that activation of BID through the extrinsic pathway plays a vital role in NSAID-mediated chemoprevention. It is possible that the adenomas with high levels of BID and/or caspase 8 activity are less likely to recur, compared with those with relatively low levels. Long-term NSAID use is associated with a variety of side effects, such as gastrointestinal, renal, and cardiovascular toxicities (25). It is conceivable that the side effects of NSAIDs can be mitigated by selectively enhancing NSAID-induced apoptotic signaling, or by combining other agents with NSAIDs at lower and well-tolerated doses to achieve safer and more effective cancer prevention (30).
BID largely accounts for the antitumor and apoptotic effects of NSAIDs in APCMin/+ mice, a classical model that recapitulates the chemopreventive effects of NSAIDs in humans (7). The general role of BID and the extrinsic pathway in chemoprevention needs to be verified by using carcinogen tumor models and other genetically engineered mice. The increased polyp number in the colon, but not in the small intestine of BID-deficient APCMin/+ mice, suggests that a BID-mediated effect is more engaged in colonic epithelial cells and contributes to relatively lower tumor incidence in the colon of APCMin/+ mice. It may reflect differences in apoptosis and expression of other Bcl-2 family members in normal epithelial cells of small intestine and colon (31). ISCs with regenerative capability can sustain driver mutations and are likely to be the most relevant cell target of NSAIDs in chemoprevention. Tumor-initiating ISCs are likely to be more vulnerable to apoptosis relative to normal ISCs because of c-Myc activation by APC loss (32, 33), and compared with differentiated cells on account of intrinsic high levels of c-Myc in ISCs (34). In addition to their effects on colorectal tumorigenesis, NSAIDs have been shown to be useful in treating precancerous lesions of other tissues, and in adjuvant therapy for treating a variety of cancers (25). It is possible that these effects of NSAIDs are also mediated by BID through the DR pathway. Compared with other anticancer drugs, NSAIDs seem to be more reliant on BID to kill tumor cells, which is likely because BID is the major, if not the only, BH3-only Bcl-2 family protein activated by NSAIDs. In contrast, other drugs such as DNA-damaging agents modulate multiple BH3-only proteins, including BID, PUMA, Noxa, and BIM.
Suppression of intestinal tumorigenesis by NSAIDs seems to be mediated by the BID-dependent synthetic lethal interaction of DR signaling and aberrant Wnt signaling, which can be caused by inherited or sporadic APC mutations, activating β-catenin mutations, or other alterations (2). NSAIDs do not discriminate between cancer and normal cells in modulating the expression of DR5, c-FLIP, and other DR family members, which is insufficient for cell death. However, these changes in conjunction with activation of Wnt signaling and ensuing c-Myc induction are both sufficient and required to trigger caspase 8 and BID cleavage, leading to apoptosis induction in an otherwise normal background. Therefore, premalignant cells with APC loss and aberrant Wnt signaling are primed for apoptosis due to c-Myc–induced DR signaling, and the enhanced DR signaling by NSAIDs helps reach a critical threshold for caspase 8 activation (Fig. 6F). NSAIDs induce a number of apical signaling events (21), some of which, such as endoplasmic reticulum (ER) stress, can trigger DR signaling (35). Studying these signaling events may help identify the direct target of NSAIDs in chemoprevention, which should be an important area of future investigation. Exploiting synthetic lethal interactions has been recognized as a powerful targeting strategy (36) and may hold the key for developing effective preventive agents for other cancer types with defined gatekeeper or driver mutations.
Materials and Methods
Analysis of Human Colonic Polyps.
Frozen specimens from patients with sporadic colonic adenomas, including nine patients treated with NSAIDs and seven untreated patients, were acquired from the Health Sciences Tissue Bank of the University of Pittsburgh. Acquisition of the tissue samples was approved by the Institutional Review Board. Informed consent was received from all participating patients. TUNEL and the intestinal stem cell marker OLFM4 were analyzed by immunostaining of paraffin-embedded tissue as described (16). Active caspase 8 staining was performed as described in SI Materials and Methods.
Mice and Treatment.
The procedures for all animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. C57BL/6J BID−/− (BID KO) mice, which were described (19), were crossed with APCMin/+ mice (Jackson Laboratory) to generate APCMin/+ mice with different BID genotypes. Lgr5-EGFP–marked BID+/+ and BID−/− APCMin/+ mice were generated as described in SI Materials and Methods. For NSAID treatment, 4-wk-old mice were fed AIN-93G diets (Dyets) with or without 200 ppm sulindac (Sigma) or 10 ppm indomethacin (Sigma) for 1 wk for apoptosis analysis, or for 2 or 4 mo for tumor phenotype analysis. Following treatment and killing of mice, analysis of adenoma numbers and apoptosis in adenoma tissues was done as described in SI Materials and Methods. For survival analysis, BID+/+ and BID−/− APCMin/+mice were fed sulindac or control diet for up to 70 wk when all mice were killed.
Analysis of NSAID-Induced and BID-Mediated Apoptosis in Human CRC Cell Lines and NCM356 Cells.
Human CRC cell lines, including HCT116, HT29, RKO, DLD1 and their derivatives, and NCM356 cells (Incell) (26), were treated by NSAIDs and analyzed for apoptosis by nuclear staining, annexin V/propidium iodide staining, colony formation, mitochondrial membrane integrity, cytochrome c release, and Bax conformational change as described in SI Materials and Methods.
Statistical Analysis.
Statistical analyses were performed by using GraphPad Prism IV software. Differences were considered significant if the probability of the difference occurring by chance was less than 5 in 100 (P < 0.05).
Supplementary Material
Acknowledgments
We thank laboratory members, Dr. Wendie Cohick, and Dr. Thomas Kensler for critical reading. This work was supported by National Institute of Health Grants CA106348, CA121105, CA172136 (to L.Z.), CA129829, and U01DK085570 (to J.Y.) and American Cancer Society Grants RSG-07-156-01-CNE (to L.Z.) and RGS-10-124-01-CCE (to J.Y.). This project used the University of Pittsburgh Cancer Institute shared facilities that were supported in part by Award P30CA047904.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415178111/-/DCSupplemental.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10(8):789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 3.William WN, Jr, Heymach JV, Kim ES, Lippman SM. Molecular targets for cancer chemoprevention. Nat Rev Drug Discov. 2009;8(3):213–225. doi: 10.1038/nrd2663. [DOI] [PubMed] [Google Scholar]
- 4.Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. 2012. Aspirin and cancer risk: A quantitative review to 2011. Ann Oncol 23(6):1403–1415. [DOI] [PubMed]
- 5.Rothwell PM, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 6.Giardiello FM, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med. 2002;346(14):1054–1059. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corpet DE, Pierre F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur J Cancer. 2005;41(13):1911–1922. doi: 10.1016/j.ejca.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Sun SY, Hail N, Jr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst. 2004;96(9):662–672. doi: 10.1093/jnci/djh123. [DOI] [PubMed] [Google Scholar]
- 9.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: The importance of apoptosis. J Cell Sci. 1994;107(Pt 12):3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 10.Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 11.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7(12):1001–1012. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 12.Cory S, Adams JM. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 13.Yin XM. Bid, a BH3-only multi-functional molecule, is at the cross road of life and death. Gene. 2006;369:7–19. doi: 10.1016/j.gene.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 14.Kohli M, et al. SMAC/Diablo-dependent apoptosis induced by nonsteroidal antiinflammatory drugs (NSAIDs) in colon cancer cells. Proc Natl Acad Sci USA. 2004;101(48):16897–16902. doi: 10.1073/pnas.0403405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290(5493):989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 16.Qiu W, et al. Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proc Natl Acad Sci USA. 2010;107(46):20027–20032. doi: 10.1073/pnas.1010430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137(1):15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 18.Su LK, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256(5057):668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 19.Yin XM, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400(6747):886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1(1):11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 22.Piazza GA, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57(14):2909–2915. [PubMed] [Google Scholar]
- 23.Penning TD, et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: Identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib) J Med Chem. 1997;40(9):1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 24.Wu GS, et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet. 1997;17(2):141–143. doi: 10.1038/ng1097-141. [DOI] [PubMed] [Google Scholar]
- 25.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94(4):252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, et al. Chemoprevention of colorectal cancer by targeting APC-deficient cells for apoptosis. Nature. 2010;464(7291):1058–1061. doi: 10.1038/nature08871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He TC, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 28.Sansom OJ, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446(7136):676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, et al. Synthetic lethal targeting of MYC by activation of the DR5 death receptor pathway. Cancer Cell. 2004;5(5):501–512. doi: 10.1016/s1535-6108(04)00113-8. [DOI] [PubMed] [Google Scholar]
- 30.Meyskens FL, Jr, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: A randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1(1):32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merritt AJ, et al. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci. 1995;108(Pt 6):2261–2271. doi: 10.1242/jcs.108.6.2261. [DOI] [PubMed] [Google Scholar]
- 32.Barker N, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457(7229):608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 33.Powell AE, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149(1):146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Lu M, et al. Cell death. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345(6192):98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaelin WG., Jr Synthetic lethality: A framework for the development of wiser cancer therapeutics. Genome Med. 2009;1(10):99. doi: 10.1186/gm99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.