Significance
The main cause of death in autosomal-dominant polycystic kidney disease (ADPKD) patients is cardiac-related. However, the reasons why remain unclear. We show that mice lacking one copy of polycystin 2, a protein mutated in ADPKD, have altered calcium signaling and desensitized calcium-contraction coupling in cardiomyocytes. We also show that decreased polycystin 2 levels affect cardiac function by altering responses to adrenergic stimulus. We propose that altering polycystin levels in the heart directly contributes to remodeling of the heart in patients with ADPKD in the absence of renal failure or high blood pressure.
Keywords: calcium signaling, β-adrenergic receptor blocker, excitation contraction coupling
Abstract
Cardiac disorders are the main cause of mortality in autosomal-dominant polycystic kidney disease (ADPKD). However, how mutated polycystins predispose patients with ADPKD to cardiac pathologies before development of renal dysfunction is unknown. We investigate the effect of decreased levels of polycystin 2 (PC2), a calcium channel that interacts with the ryanodine receptor, on myocardial function. We hypothesize that heterozygous PC2 mice (Pkd2+/−) undergo cardiac remodeling as a result of changes in calcium handling, separate from renal complications. We found that Pkd2+/− cardiomyocytes have altered calcium handling, independent of desensitized calcium-contraction coupling. Paradoxically, in Pkd2+/− mice, protein kinase A (PKA) phosphorylation of phospholamban (PLB) was decreased, whereas PKA phosphorylation of troponin I was increased, explaining the decoupling between calcium signaling and contractility. In silico modeling supported this relationship. Echocardiography measurements showed that Pkd2+/− mice have increased left ventricular ejection fraction after stimulation with isoproterenol (ISO), a β-adrenergic receptor (βAR) agonist. Blockers of βAR-1 and βAR-2 inhibited the ISO response in Pkd2+/− mice, suggesting that the dephosphorylated state of PLB is primarily by βAR-2 signaling. Importantly, the Pkd2+/− mice were normotensive and had no evidence of renal cysts. Our results showed that decreased PC2 levels shifted the βAR pathway balance and changed expression of calcium handling proteins, which resulted in altered cardiac contractility. We propose that PC2 levels in the heart may directly contribute to cardiac remodeling in patients with ADPKD in the absence of renal dysfunction.
Cardiac-related deaths are the main cause of mortality in autosomal-dominant polycystic kidney disease (ADPKD) (1), a high-incidence genetic disorder affecting 1 in 800 in the general population, that manifests as renal cysts. Patients with ADPKD are born heterozygous, harboring one good copy and one bad copy of the gene, and cysts are believed to arise when a good copy of the genes are mutated in the kidney. In addition to renal cysts, more than 90% of patients exhibit left ventricular hypertrophy at death, and more than 70% have hypertension. However, how mutations in the polycystin genes (denoted pkd1 and pkd2 in humans and Pkd1 and Pkd2 in mice) initiate cardiac remodeling and cause cardiac dysfunction remains largely unexplored. The current theory suggests that cystic compression of the renal vasculature leads to an up-regulation of the renin–angiotensin–aldosterone system (RAAS), which increases vascular resistance, causing hypertension, and contributes to cardiac hypertrophy (2–7). The RAAS pathway is the subject of clinical trials in patients with ADPKD in the HALT study, and several studies have demonstrated the benefits of blood-pressure control by antihypertensive drugs on patients with ADPKD (8, 9). Although this theory explains the high prevalence of hypertension, it does not explain the evidence from several studies that demonstrates that a significant proportion of younger normotensive ADPKD carriers have no renal function deficit but have cardiac abnormalities (10–15). One possible means by which patients with ADPKD may have a cardiac phenotype in the absence of renal dysfunction is because the heterozygous nature of the disease is sufficient to render the function of polycystin in the heart ineffective.
Heart function is directly linked to the calcium-dependent contractile apparatus in cardiomyocytes (16), and mutations to the calcium handling proteins are associated with cardiac dysfunction (17). For example, mutations to the intracellular calcium release channel, ryanodine receptor 2 subtype (RyR2), or to the calcium binding protein calsequestrin, results in catecholaminergic polymorphic ventricular tachycardia (CPVT). These mutations promote spontaneous leak of calcium from the RyR2, leading to arrhythmogenesis. The protein product of pkd2, PC2, is also an intracellular calcium channel (18) that interacts and acts as a brake on RyR2 (19). We have recently demonstrated that zebrafish lacking PC2 have calcium alternans (13), and that mutations to PC2 in human patients with ADPKD (without renal dysfunction or hypertension) are associated with a 200-fold increased risk of idiopathic dilated cardiomyopathy compared with the general population (13).
Interestingly, previous reports suggest that mouse models of ADPKD do not have the same cardiovascular abnormalities observed in patients with ADPKD. Studies on heterozygous Pkd2 mice (Pkd2+/−) or mice with Pkd1 knocked out in smooth muscle (Pkd1sm−/−) have not reported cardiac deficits or changes in blood pressure (20–22), although increased aortic contractility was observed after phenylephrine challenge in Pkd2+/− mice, and altered vascular pressure sensing responses were observed in Pkd1sm−/− mice (20, 22, 23). Cardiac developmental defects have been observed in embryonic Pkd2−/− mice (24), but, as Pkd2−/− mice die by birth, it is impossible to properly characterize their cardiac function. Collectively, none of these studies provide a suitable explanation for the increased risk for cardiac dysfunction in patients with ADPKD.
In view of the association of RyR2 with PC2 and the incomplete characterization of the effect of PC2 in cardiac tissue, we investigated if adult Pkd2+/− mice have a cardiac phenotype independent of a renal phenotype. We hypothesized that calcium handling in cardiomyocytes would be impaired in Pkd2+/− mice. Here, we report alterations in calcium handling and contraction coupling in cardiomyocytes from Pkd2+/− mice. Intriguingly, we identified paradoxical changes in the phosphorylation status of phospholamban (PLB) and troponin I (TnI) that can be explained by activation of the β-adrenergic receptor (βAR)-2 pathway. Importantly, the Pkd2+/− mice did not have hypertension or renal cysts. These data suggest that Pkd2+/− mice have a cardiac phenotype whereby decreased PC2 levels are associated with changes that may mimic the remodeling that induces the cardiac phenotype seen in a subset of patients with ADPKD before onset of renal failure (13).
Results
Pkd2+/− Cardiomyocytes Have Altered Calcium Handling.
To examine if the loss of one allele of Pkd2 resulted in altered calcium homeostasis, isolated cardiomyocytes from 5-mo-old WT and Pkd2+/− male mice were characterized for contractility and calcium handling. The mRNA and protein for PC2 were decreased by ∼40% in left ventricular samples (Fig. S1). Relative diastolic calcium levels, determined by loading cardiomyocytes with Fura-2AM, were not significantly different between WT and Pkd2+/− cells (Fig. 1 A and B). However, Pkd2+/− cells had a 60% smaller response to 5 mM caffeine, which enables a bolus release of calcium from the sarcoplasmic reticulum via the RyR2, compared with WT cells (Fig. 1 C and D). This suggests that the calcium load in the sarcoplasmic reticulum of Pkd2+/− cells is lower than in WT cells, consistent with previous findings from neonate Pkd2−/− cardiomyocytes (19). We suggest that the discrepancy between release of calcium with pacing and with caffeine is caused not only by altered release from the RyR, but also by differential regulation of the uptake by the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pump.
Fig. 1.
Calcium handling in 5-mo-old WT and Pkd2+/− cardiomyocytes. (A) Representative cardiomyocytes loaded with Fura-2AM. (B) Diastolic calcium is unchanged between WT and Pkd2+/− cells. (C) Calcium transients elicited by the addition of 5 mmol/L caffeine to activate release from the RyR-controlled sarcoplasmic reticulum stores from WT (black trace) and Pkd2+/− cardiomyocytes (gray trace). (D) Quantification of area under the curve (AUC) after addition of 5 mmol/L caffeine. Data are the mean ± SEM from n = 9–10 cardiomyocytes, n = 3 animals (*P < 0.05). (E) Magnitude of calcium release (Ca2+ Rmag) is lower in WT (black) than in Pkd2+/− (gray) cardiomyocytes (n = 256 WT, n = 249 Pkd2+/−cells, n = 4 WT, n = 3 Pkd2+/− animals per group; ****P < 0.001). (F) Tau, the rate of calcium decay (TauCa2+), was faster in WT (open bar) than in Pkd2+/− (black bar) cardiomyocytes (n = 256 WT, n = 249 Pkd2+/−cells, n = 4 WT, n = 3 Pkd2+/− animals per group).
To determine the calcium response during electrical pacing, mechanically unloaded cardiomyocytes were field stimulated at 1 Hz. Calcium transients were recorded for ten beats and averaged (25). Under normal Tyrode conditions, Pkd2+/− cells had 39% higher magnitude of calcium release (Ca2+ Rmag, P < 0.001; Fig. 1E) compared with WT cells. Pkd2+/− cells had 9% slower decay constant for calcium reuptake (τCa; P < 0.001; Fig. 1F) compared with WT cells.
Protein and Posttranslational Changes in Pkd2+/− Mice.
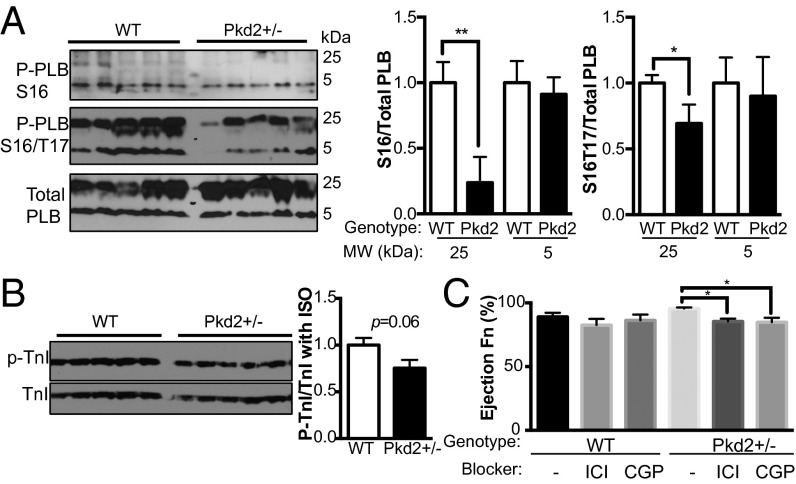
To determine a molecular mechanism for the changes in calcium handling, Western blot analyses of the left ventricle (LV) for calcium signaling proteins were conducted. RyR2 protein levels were 60% higher in Pkd2+/− mice (P < 0.05), even though mRNA expression levels were not significantly elevated (Fig. 2 A and B and Fig. S1). Strikingly, RyR2 protein expression levels were elevated only in the LV, but not the right ventricle (Fig. 2A and Fig. S2A). The elevation in RyR2 expression was consistent with a higher magnitude of calcium release in response to pacing (Fig. 1E). Different forces (e.g., sheer stress or pressure changes) experienced by the two ventricles may explain the difference in RyR2 expression. There was 40% higher protein expression of SERCA2A in Pkd2+/− mice compared with WT (P < 0.05; Fig. 2B). There was a 70% decrease in the phosphorylation status of PLB, a protein that inhibits SERCA, at Ser16 (phosphorylated by PKA) and Thr17 (phosphorylated by CaMKII) in Pkd2+/− mice compared with WT (P < 0.05; Fig. 2 A and B and Fig. S2B). The dephosphorylated state of PLB was consistent with the slower calcium reuptake (i.e., elevated τCa) seen in Pkd2+/− cells (Fig. 1F), as well as the higher peak of calcium release. The expression of βAR-1 and βAR-2, the G protein-coupled receptor kinases (GRKs), and downstream transcription factor CREB (cAMP response element-binding protein) were similar in WT and Pkd2+/− mice (Figs. S1 and S2).
Fig. 2.
Calcium handling proteins in WT and Pkd2+/− mice. (A) Protein expression of calcium-contractile proteins in WT and Pkd2+/− mice in 5-mo-old mice. Tissue taken from the LV (Left) and right ventricle (Right). Each lane is a separate animal. (B) Quantification of n = 5 WT and n = 4 Pkd2+/− mice normalized to tubulin (LV only; *P < 0.05).
Myofilaments in Pkd2+/− Cells Have Lower Calcium Sensitivity.
The time course of sarcomere shortening was collected simultaneously with calcium transients in electrically paced myocytes (25) (Fig. 3 A and B). Intriguingly, despite significant molecular and functional differences in calcium handling between WT and Pkd2+/− cells, there was no difference in the contractile properties of the cardiomyocytes with electrical stimulation. Analysis of the sarcomere length recordings demonstrated that peak sarcomere length shortening, time to peak shortening (TP), and time to 50% relaxation (RT50) were not significantly different (Fig. 3 A and B and Table S1). These data demonstrate that, although there was greater calcium release in Pkd2+/− cells, there was a similar degree of contractility in WT and Pkd2+/− cells. This is surprising because, based on normal physiology, the larger and longer calcium transients seen in Pkd2+/− mice (Fig. 1 E and F) would be expected to elicit corresponding increases in peak shortening and slower relaxation time of contractions.
Fig. 3.
Pkd2+/− cardiomyocytes have a paradoxical calcium-contractility relationship. (A) Peak sarcomere length shortening (peak SL shortening) in WT (open bar) and Pkd2+/− (black bar) cardiomyocytes is similar. (B) RT50 in WT (open bar) and Pkd2+/− (black bar) cardiomyocytes is similar. (C, Left) Representative traces of experimental cardiomyocyte dynamics for calcium transient traces (Top) from WT (black) and Pkd2+/− (gray) with their corresponding sarcomere length shortening traces (Bottom). (Middle) A computational model whereby the calcium transient for WT cardiomyocytes was used to adjust the shortening traces to match experimental data, then the Pkd2+/− calcium trace was entered and the resultant “expected” sarcomere shortening was recorded. Note that the simulated calcium transient does not match the experimental findings. (Right) A parameter that tunes the rate of calcium dissociation (i.e., TnI phosphorylation) was increased in the Pkd2+/− model, and the output was recorded to match the experimental data. (D) Phosphorylated TnI under baseline conditions in Pkd2+/− mice is higher than in WT mice. For Western blots, each lane represents a different animal (n = 5–6 animals per group) and values are normalized to the total amount of TnI. Tissue is from the LV. WT is represented by open bar, Pkd2+/− by black bar (**P < 0.01).
We used a computational model of myocyte shortening (26, 27) to explore the mechanisms that would explain the paradox of the cardiomyocyte calcium transient and cell shortening data. We first constructed idealized calcium transients (Fig. 3C, Left) that had peak concentrations and τCa values matching the mean values measured in WT and Pkd2+/− cells. The WT transient was then used as an input to the computational model to produce a simulated WT sarcomere shortening event. Model parameters were adjusted until simulated contraction properties (peak shortening and RT50) matched mean experimental values (Fig. 3C, Center, black traces). Then, the Pkd2+/− transient was used as input while keeping the same myofilament model parameters as determined for the WT case (“naïve” model). The predicted Pkd2+/− contraction (Fig. 3C, Center, gray trace) was much larger and longer than WT, contrary to experimental measurements. The obvious conclusion is that the properties of sarcomeric proteins cannot be identical in the two genotypes. We hypothesized that a decrease in myofilament calcium sensitivity in the Pkd2+/− cells would reconcile the model/experiment discrepancy. To test this, we repeated the Pkd2+/− simulation after increasing the dissociation rate of calcium from troponin C from 0.625 per second to 0.75 per second. This single parameter change caused the simulated Pkd2+/− shortening to match both peak contraction and RT50 values measured experimentally (Fig. 3C, Right).
The simulations suggested a desensitization of the myofilament to calcium in Pkd2+/− mice under baseline conditions, which is a known effect of TnI phosphorylation (28). Consistent with our model, we found that the phosphorylation status of TnI at the PKA phosphorylation site Ser22/23 in Pkd2+/− mice was 90% higher than in WT mice (Fig. 3D). This experimental observation begins to explain the decreased myofilament sensitivity to calcium in the Pkd2+/− mouse, and supports the findings from our model.
AKAP and Altered βAR Subtype Signaling in Pkd2+/− Mice.
The changes in the phosphorylation status of TnI are paradoxical to the phosphorylation status of PLB, as both sites are phosphorylated by PKA. In Pkd2+/− mice, the PKA site on PLB (Ser16) was less phosphorylated, but the PKA sites on TnI (Ser22/23) were more phosphorylated. Use of a PKA substrate-specific antibody demonstrated a clear difference in the phosphorylation of a number of proteins in Pkd2+/− mice compared with WT mice (Fig. S3), in agreement with the phosphorylation results from PLB and TnI. These data suggest the existence of microdomains of PKA in Pkd2+/− mice, potentially through increased interaction with A-kinase anchoring proteins (AKAPs). As the contractile apparatus protein TnT has been reported to have AKAP-like binding properties (29), we looked at the spatial localization of PKA phosphorylated proteins. From immunofluorescence studies, we suggest that TnI is associated with the contractile apparatus and that this association is greater in the Pkd2+/− tissue (Fig. 4A). This result suggests that TnI has a higher level of phosphorylation in Pkd2+/− mice through TnT, which can act as an AKAP to localize PKA close to the contractile apparatus. In contrast, PLB is excluded from many regions where TnI and PKA are localized (Fig. 4B).
Fig. 4.

PKA phosphorylated proteins localize with the contractile apparatus. (A) TnI (red) and the PKA phosphorylated substrate (green) colocalize. Note that there is a higher degree of colocalization in the Pkd2+/− cardiomyocytes compared with the WT, as quantified to the right. (B) PLB (red) is largely excluded from the contractile apparatus (TnI; green). Images are representative of three mice, and are taken from the LV (*P < 0.05).
Although the presence of an AKAP could promote a higher phosphorylation status of TnI, the lower level of PLB phosphorylation suggests the presence of a protein phosphatase that actively removes phosphorylation. Such a situation might arise from activation of βAR-2, which not only signals through Gs, but also the inhibitory Gi pathway, leading to downstream activation of protein phosphatase 1, a phosphatase known to dephosphorylate PLB. We therefore sought evidence that there were measurable differences in cardiac function after stimulation of the βAR signaling pathway in vivo.
Pkd2+/− Mice Have Increased Cardiac Contractility with Isoproterenol.
Function was measured by using M-mode echocardiography under baseline conditions and after injection with the βAR agonist isoproterenol (ISO; Fig. 5A). Pkd2+/− mice at 5 mo were compared with C57/Bl6 (i.e., WT) mice. In WT and Pkd2+/− mice, all baseline cardiac parameters including left ventricular ejection fraction (LVEF) were within the expected physiological ranges (Fig. 5B and Table S3), consistent with previously published data (20, 21). However, the inner ventricular septum (IVS) and the left ventricular posterior wall (LVPW) were both significantly thinner in the Pkd2+/− mice compared with WT, but the left ventricular internal diameter (LVID) was unchanged (Fig. S4A). The thinner measurements were also consistent with overall longer cell measurements taken from isolated cardiomyocytes (Fig. S4B).
Fig. 5.
Pkd2+/− mice have enhanced contractility after ISO stimulus. (A) Echocardiograms 3 min after ISO injection (0.1 mg/kg) demonstrate increased LV contractility in 5-mo-old Pkd2+/− mice. (B) No difference in LVEF at baseline between WT and Pkd2+/− mice. (C) LVEF in WT and Pkd2+/− mice after administration of 0.1 mg/kg ISO. Data are presented as mean ± SEM (n = 5–9 animals per group), and values are included in Tables S3 and S4). (D) Blood pressure measurements for 5-mo-old WT and Pkd2+/− mice (n = 5–9 animals per group). WT is represented by open bar, Pkd2+/− by black bar (*P < 0.05).
Acute administration of 0.1 mg/kg ISO increased cardiac contractility in WT and Pkd2+/− mice compared with baseline measurements (Fig. 5C and Fig. S5). However, the change in cardiac contractility with ISO treatment was greater in Pkd2+/− mice compared with WT controls (LVEF, P < 0.05; Fig. 5C). Pkd2+/− mice had smaller end systolic diameter and smaller end diastolic diameter upon ISO stimulation compared with WT (P < 0.05), which constitutes evidence of greater contractility forces in response to ISO (Fig. S5). The end systolic volume and end diastolic volumes were also lower in the Pkd2+/− mice compared with the WT mice after ISO application (Fig. S5). ISO administration induced an elevation in HR in WT and Pkd2+/− mice, with no statistical difference (Fig. S5 and Tables S3 and S4), indicating that the effect of ISO on the sinoatrial node is similar between genotypes.
Although leak of calcium via the RyR2 is associated with CPVT, and increased arrhythmogenic events are associated with βAR activation, no arrhythmias were observed at baseline in Pkd2+/− or WT mice. We found a similar incidence (∼10% of all animals) of 0.1 mg/kg ISO-induced arrhythmias in WT and Pkd2+/− mice. These arrhythmogenic events were not sustained, and there were no more than three observances per animal during the recording interval. All echocardiographic measurements for analysis were taken during periods of regular heart rhythm.
The possibility that the heightened sensitivity to ISO in Pkd2+/− mice was a result of factors outside the cardiac β-adrenergic signaling pathway was excluded by blood pressure and histological studies. Tail-cuff measurements of systemic blood pressure revealed no difference in the resting value for conscious 5-mo-old mice (∼130/90 mm Hg for both genotypes; Fig. 5D), consistent with previous studies (21). No pathological differences in Pkd2+/− were observed in the heart or kidneys (Fig. S6 A–C), suggesting that the remodeling in Pkd2 mice was not a result of renal compression by cyst formation (2).
As we had identified PLB and TnI phosphorylation as key differences between WT and Pkd2+/− mice under nonstimulated conditions, we reassessed these parameters in LV tissue 5 min after acute in vivo exposure to ISO. We found PLB was still less phosphorylated in Pkd2+/− mice compared with WT mice (Fig. 6A), although there was no longer a significant change in the phosphorylation status of TnI (Fig. 6B). These data are consistent with our interpretation that PLB is actively undergoing dephosphorylation, whereas TnI can still be phosphorylated in the Pkd2+/− mice. These findings also fit with the increase in cardiac contractility in the Pkd2+/− after ISO stimulation in the intact animal, as measured with echocardiography.
Fig. 6.
Effect of ISO on protein phosphorylation in Pkd2+/− mice and blockade by βAR blockers. (A) Injection of ISO does not induce the same level of phosphorylation of PLB at Ser16 or Ser16/Thr17 in Pkd2+/− mice as in WT mice. Each lane represents a different animal (n = 6 per genotype). (Right) Analysis of data at left. (B) ISO application induces phosphorylation of TnI in tissue from ISO-treated animals. (Right) Analysis of data at left. WT is represented by open bar, Pkd2+/− by black bar. (C) Effect of the β-blockers ICI 118,551 and CGP 20712 on WT and Pkd2+/− 5-mo-old mice 3 min after ISO application (n = 4–6 animals per group; *P < 0.05 and **P < 0.01).
βAR-Blockers Reveal β1 and β2 Signaling in Pkd2+/− Mice.
Although our mRNA and protein analysis did not point to differences in expression of the βAR proteins (Figs. S1 and S2), our functional studies suggested that recruitment of the βAR-1 and βAR-2 signaling pathways were required to explain our results. To determine if ISO treatment activated both βAR isoforms, and thus the Gs and the Gi downstream signaling pathways, we measured cardiac contractility through echocardiography in mice treated with βAR-blockers 30 min before ISO challenge. Five-month-old mice were treated with CGP 20712 (specific βAR-1 blocker) and ICI-118,551 (specific βAR-2 blocker) at dosages 10-fold higher than Ki, but below the concentration for nonspecific effects to other receptors (30, 31). There was no significant effect of the drugs on baseline ejection fractions in WT or Pkd2+/−. Three-way ANOVAs were performed for the factors: genotype, β-blocker, and presence of ISO. Using LVEF as the primary measured parameter, Pkd2+/− mice responded differently to β-blockers, comparing the 3-min time point against baseline measurements (interaction, F < 0.001, P = 0.034). At 3 min after ISO application, WT mice treated with either drug showed trends toward decreased LVEF, although not significant, although, collectively, over the 6-min time course, both CGP and ICI diminished LVEF. At 3 min after ISO application, Pkd2+/− mice had significantly decreased LVEF with β-blockers: CGP reduced LVEF (P < 0.05) and ICI reduced LVEF (P < 0.05; Fig. 6C). This result suggests that ISO administered to the Pkd2+/− mice activated the βAR-1 and βAR-2 pathways.
Taken together, these data suggest that Pkd2+/− mice have adaptive mechanisms that account for changes in calcium handling via a modified systolic response (increased RyR2 expression) as well as diastolic uptake response (increased SERCA2A expression), a paradoxical phosphorylation status of PLB and TnI that accounts for decoupling of the calcium-contractile apparatus, that can be explained by microsignaling domains and by activation of the βAR-2 signaling pathway.
Discussion
Cardiovascular complications are the most common cause of mortality in patients with ADPKD, and these dysfunctions can arise in patients without prior evidence of renal failure or hypertension. Despite the numerous ADPKD mouse models, we are aware of no study that has described cardiac deficiencies in adult mice. Here, we report a striking phenotype in Pkd2+/− mice: adult Pkd2+/− mice have decoupled calcium-contraction coupling, paradoxically caused by opposite phosphorylation states of PLB and TnI. These effects are exacerbated with βAR signaling stimulus and are suggestive of altered βAR signaling pathways. To our knowledge, the present study reveals the first cardiac phenotype in an adult ADPKD mouse model, and provides evidence by which cardiac remodeling may occur in the absence of renal dysfunction, elevated blood pressure, or cyst formation.
Although cardiac studies have been performed in other Pkd2 mouse models, no abnormalities were reported, and thus the Pkd2 mouse is thought to poorly mimic human ADPKD (21). We find here that Pkd2+/− mice have normal blood pressure and cardiac function. However, closer inspection revels that the Pkd2+/− mice have decoupled calcium contraction coupling and an enhanced response to ISO stimulus. We suggest that our findings represent an important and relevant model of cardiovascular dysfunction in ADPKD, as it reflects the loss of function from one allele of Pkd2, similar to patients with ADPKD. Thus, the whole-body Pkd2+/− mouse represents the nonhypertensive human ADPKD model with cardiac abnormalities (2, 4, 10–14). As the Pkd2+/− mouse does not have hypertension, this mouse model enables exploration of the mechanism underlying effects of Pkd2 deficiency in the heart before onset of hypertension and renal failure.
However, a caveat of the Pkd2+/− model we present here is that, although some patients with ADPKD display cardiac dysfunction before hypertension or renal cysts, we cannot overlook the possibility that endothelial dysfunction could occur in human patients with ADPKD before the development of cardiac dysfunction (3). Although the Pkd1sm−/− mouse does not have a hypertensive phenotype (22), we are aware of no existing studies that conclusively rule out a role for PC1 or PC2 in the endothelium. Therefore, future studies that specifically address the effect of Pkd1 and Pkd2 in the heart, with and without contribution from the renal or systemic vasculature, are needed to conclusively demonstrate the function of PC2 in the heart. These additional studies would provide further information into the importance of PC2 in human patients with ADPKD.
Changes in calcium handling in cardiomyocytes have been linked to a number of cardiac disorders (16, 32–35), including CPVT (36, 37). We have previously demonstrated that PC2 interacts with and can regulate the RyR2 by acting as a brake on calcium release, and that total loss of PC2 results in a slow but uncontrolled release of calcium through the RyR2 (19). We therefore hypothesized that decreased PC2 levels in mice may phenocopy the CPVT phenotype. However, decreased PC2 levels diminished sarcoplasmic reticulum calcium stores but there was no evidence of increased arrhythmic events in the Pkd2+/− mouse under baseline or ISO conditions.
Intriguingly, we observed changes in the duration and amplitude of the calcium signal in Pkd2+/− cells suggesting that calcium release and reuptake pathways are affected. In particular, PLB phosphorylation was decreased, which has been linked to dilated cardiomyopathy in humans (38) and associated with heart failure. However, the Pkd2+/− mouse did not recapitulate phenotypes observed in mice with mutated PLB or troponin (39, 40). Thus, the findings in the Pkd2+/− mouse may reflect a compensatory pathway that has enabled an adaptation to altered calcium homeostasis without altering contractility through TnI phosphorylation. PC2 has also been reported to interact with TnI, but the functional significance is not known (41). A functional link between PC2 and TnI may also contribute to the decoupling of the calcium myofilament.
As the Pkd2+/− mouse has a phenotype not previously described in other mouse models of cardiac calcium or contractile deregulation, our mouse model represents a signaling pathway whereby a cascade of changes results in altered βAR signaling. The βAR signaling pathway is important in cardiac development, aging, and failure (25, 42–45). Here, we demonstrate enhanced ionotropic responses to activation of the βAR pathway in Pkd2+/− mice, with no effect on basal cardiac function. Our finding that PLB is less phosphorylated in Pkd2+/− mice supports the idea that βAR-2 signaling pathways are activated. In contrast to βAR-1, which is coupled to Gs, βAR-2 is coupled to Gi and Gs pathways (46). One possible ramification of turning on the Gi pathway is activation of protein phosphatase 1, which can dephosphorylate PLB (47) (Fig. S7). Thus, activation of the βAR-2 pathway in Pkd2+/− mice may explain the reduction in phosphorylation of PLB. Interestingly, we observed that inhibition of the βAR-2 receptor significantly diminished the effects of ISO stimulus on cardiac function; this is surprising, as βAR-1 is the main isoform responsible for contractility (48). The idea that βAR-2 is contributing to the ISO response is consistent with studies demonstrating that subtle overexpression of βAR-2 enhanced cardiac function, although grossly high overexpression of βAR-2 resulted in cardiomyopathy (49–51).
Although we did not observe a change in βAR-1 or βAR-2 protein expression levels, we paradoxically found that the PKA site on PLB was less phosphorylated in Pkd2+/− mice, whereas the PKA site on TnI had elevated phosphorylation. These results suggest the existence of specific microsignaling domains within the Pkd2+/− cardiomyocytes that enables the selective phosphorylation of one target (TnI), while simultaneously allowing another target (i.e., PLB) to remain dephosphorylated. In support of this idea, AKAPs such as TnT may hold PKA in place on the contractile apparatus, thus preventing TnI from being dephosphorylated. The level of control could also be earlier, given that it has been demonstrated that the particular localization of βAR receptors, coupled with compartmentalization of the downstream signaling, particularly cAMP, can have significant ramifications in heart failure (52).
The effects of βAR blockers on Pkd2+/− mice demonstrate the potential ramifications for the treatment of cardiovascular pathologies in ADPKD. The current recommended treatment for hypertension in patients with ADPKD are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, rather than βAR-blockers, although the βAR-1 blocker metoprolol is recommended after angiotensin-converting enzyme inhibitors and ARBs in the PKD HALT trial (9). Indeed, our results are consistent with clinical findings in which noradrenaline, an endogenous ligand of βAR, was seen at significantly higher levels in patients with ADPKD (53). Our results suggest that βAR blockers that target βAR-1 and βAR-2 receptors may be more efficacious for patients with ADPKD.
Conclusion
Cardiopathologic conditions are the leading cause of mortality in patients with ADPKD, and, although the contribution from hypertension cannot be overlooked, there is evidence that the cardiac phenotype may precede the vascular phenotype. The Pkd2+/− mice have cardiac abnormalities independent of renal pathologies that mimic the cardiac disease progression in patients with ADPKD. We find that the Pkd2+/− mouse model represents a phenotype with a unique decoupling of the calcium-contraction interaction that is highlighted by a paradoxical phosphorylation status of PLB and TnI that leads to aberrant cardiac function. Therefore, these mice provide essential insight into the mechanisms of cardiac remodeling as well as a model for treatments to improve ADPKD patient outcomes.
Methods
Cardiomyocyte Cell Mechanics and Calcium Handling with Field Stimulus.
LV cardiomyocytes from 5-mo-old WT and Pkd2+/− male mice were isolated by Langendorff and loaded with Fura-2AM. Calcium transients and unloaded shortening contractions were measured under constant perfusion in 30 °C Tyrode solution. Cells were field-stimulated at 1 Hz.
Echocardiograms.
Anesthetized mice were continually monitored during echocardiogram recordings in M-mode with short axial measurements of the LV. After a baseline echocardiogram measurement, mice were given a single i.p. injection of ISO at 0.1 mg/kg. An extended description of the study methods is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Stefan Somlo (Yale University) for Pkd2+/− mice, Nikki Mikush for technical expertise with echocardiograms, Jacopo Ferruzi and Dr. Jay Humphrey (Yale University) for blood pressure equipment, Dr. Frank Giordano (Yale University) for the Langendorff apparatus, and Dr. Lawrence Young for constructive comments. This work was funded by NIH Grants P30 DK090744 (to B.E.E.), R01 DK61747 (to B.E.E.), and R01 DK087844 (to B.E.E.), American Heart Association Postdoctoral Fellowship R10682 (to I.Y.K.), and a Rudolph J. Anderson Postdoctoral Fellowship from Yale University (A.T.K.). Use of the Yale Cell Biology Confocal Core (NIH Grants P01 DK57751 and P30 DK34989) is acknowledged.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415933111/-/DCSupplemental.
References
- 1.Chapman AB, Stepniakowski K, Rahbari-Oskoui F. Hypertension in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17(2):153–163. doi: 10.1053/j.ackd.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ecder T, Schrier RW. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol. 2009;5(4):221–228. doi: 10.1038/nrneph.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrier RW. Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2009;20(9):1888–1893. doi: 10.1681/ASN.2008080882. [DOI] [PubMed] [Google Scholar]
- 4.Bardají A, et al. Cardiac involvement in autosomal-dominant polycystic kidney disease: a hypertensive heart disease. Clin Nephrol. 2001;56(3):211–220. [PubMed] [Google Scholar]
- 5.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 2008;74(9):1192–1196. doi: 10.1038/ki.2008.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeier M, Geberth S, Schmidt KG, Mandelbaum A, Ritz E. Elevated blood pressure profile and left ventricular mass in children and young adults with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993;3(8):1451–1457. doi: 10.1681/ASN.V381451. [DOI] [PubMed] [Google Scholar]
- 7.Ivy DD, et al. Cardiovascular abnormalities in children with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;5(12):2032–2036. doi: 10.1681/ASN.V5122032. [DOI] [PubMed] [Google Scholar]
- 8.Chapman AB. Approaches to testing new treatments in autosomal dominant polycystic kidney disease: insights from the CRISP and HALT-PKD studies. Clin J Am Soc Nephrol. 2008;3(4):1197–1204. doi: 10.2215/CJN.00060108. [DOI] [PubMed] [Google Scholar]
- 9.Chapman AB, et al. The HALT polycystic kidney disease trials: Design and implementation. Clin J Am Soc Nephrol. 2010;5(1):102–109. doi: 10.2215/CJN.04310709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saggar-Malik AK, et al. Left ventricular mass in normotensive subjects with autosomal dominant polycystic kidney disease. BMJ. 1994;309(6969):1617–1618. doi: 10.1136/bmj.309.6969.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman AB, et al. Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1997;8(8):1292–1297. doi: 10.1681/ASN.V881292. [DOI] [PubMed] [Google Scholar]
- 12.Bardají A, et al. Left ventricular mass and diastolic function in normotensive young adults with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1998;32(6):970–975. doi: 10.1016/s0272-6386(98)70071-x. [DOI] [PubMed] [Google Scholar]
- 13.Paavola J, et al. Polycystin-2 mutations lead to impaired calcium cycling in the heart and predispose to dilated cardiomyopathy. J Mol Cell Cardiol. 2013;58:199–208. doi: 10.1016/j.yjmcc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valero FA, et al. Ambulatory blood pressure and left ventricular mass in normotensive patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1999;10(5):1020–1026. doi: 10.1681/ASN.V1051020. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Vea A, et al. Exercise blood pressure, cardiac structure, and diastolic function in young normotensive patients with polycystic kidney disease: A prehypertensive state. Am J Kidney Dis. 2004;44(2):216–223. doi: 10.1053/j.ajkd.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 17.Venetucci L, Denegri M, Napolitano C, Priori SG. Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nat Rev Cardiol. 2012;9(10):561–575. doi: 10.1038/nrcardio.2012.93. [DOI] [PubMed] [Google Scholar]
- 18.Koulen P, et al. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4(3):191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 19.Anyatonwu GI, Estrada M, Tian X, Somlo S, Ehrlich BE. Regulation of ryanodine receptor-dependent calcium signaling by polycystin-2. Proc Natl Acad Sci USA. 2007;104(15):6454–6459. doi: 10.1073/pnas.0610324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian Q, et al. Pkd2+/- vascular smooth muscles develop exaggerated vasocontraction in response to phenylephrine stimulation. J Am Soc Nephrol. 2007;18(2):485–493. doi: 10.1681/ASN.2006050501. [DOI] [PubMed] [Google Scholar]
- 21.Stypmann J, et al. Cardiovascular characterization of Pkd2(+/LacZ) mice, an animal model for the autosomal dominant polycystic kidney disease type 2 (ADPKD2) Int J Cardiol. 2007;120(2):158–166. doi: 10.1016/j.ijcard.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Sharif-Naeini R, et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139(3):587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 23.Qian Q, et al. Pkd2 haploinsufficiency alters intracellular calcium regulation in vascular smooth muscle cells. Hum Mol Genet. 2003;12(15):1875–1880. doi: 10.1093/hmg/ddg190. [DOI] [PubMed] [Google Scholar]
- 24.Wu G, et al. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet. 2000;24(1):75–78. doi: 10.1038/71724. [DOI] [PubMed] [Google Scholar]
- 25.Campbell SG, Haynes P, Kelsey Snapp W, Nava KE, Campbell KS. Altered ventricular torsion and transmural patterns of myocyte relaxation precede heart failure in aging F344 rats. Am J Physiol Heart Circ Physiol. 2013;305(5):H676–H686. doi: 10.1152/ajpheart.00797.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell SG, Lionetti FV, Campbell KS, McCulloch AD. Coupling of adjacent tropomyosins enhances cross-bridge-mediated cooperative activation in a Markov model of the cardiac thin filament. Biophys J. 2010;98(10):2254–2264. doi: 10.1016/j.bpj.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheikh F, et al. Mouse and computational models link Mlc2v dephosphorylation to altered myosin kinetics in early cardiac disease. J Clin Invest. 2012;122(4):1209–1221. doi: 10.1172/JCI61134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ Res. 1995;76(6):1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]
- 29.Sumandea CA, et al. Cardiac troponin T, a sarcomeric AKAP, tethers protein kinase A at the myofilaments. J Biol Chem. 2011;286(1):530–541. doi: 10.1074/jbc.M110.148684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dooley DJ, Bittiger H, Reymann NC. CGP 20712 A: A useful tool for quantitating beta 1- and beta 2-adrenoceptors. Eur J Pharmacol. 1986;130(1-2):137–139. doi: 10.1016/0014-2999(86)90193-7. [DOI] [PubMed] [Google Scholar]
- 31.Bilski AJ, Halliday SE, Fitzgerald JD, Wale JL. The pharmacology of a beta 2-selective adrenoceptor antagonist (ICI 118,551) J Cardiovasc Pharmacol. 1983;5(3):430–437. doi: 10.1097/00005344-198305000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Bers DM. Cardiac sarcoplasmic reticulum calcium leak: Basis and roles in cardiac dysfunction. Annu Rev Physiol. 2014;76:107–127. doi: 10.1146/annurev-physiol-020911-153308. [DOI] [PubMed] [Google Scholar]
- 33.Marks AR. Calcium cycling proteins and heart failure: Mechanisms and therapeutics. J Clin Invest. 2013;123(1):46–52. doi: 10.1172/JCI62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drawnel FM, et al. Mutual antagonism between IP(3)RII and miRNA-133a regulates calcium signals and cardiac hypertrophy. J Cell Biol. 2012;199(5):783–798. doi: 10.1083/jcb.201111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harzheim D, et al. Increased InsP3Rs in the junctional sarcoplasmic reticulum augment Ca2+ transients and arrhythmias associated with cardiac hypertrophy. Proc Natl Acad Sci USA. 2009;106(27):11406–11411. doi: 10.1073/pnas.0905485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kushnir A, Marks AR. The ryanodine receptor in cardiac physiology and disease. Adv Pharmacol. 2010;59:1–30. doi: 10.1016/S1054-3589(10)59001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogrodnik J, Niggli E. Increased Ca(2+) leak and spatiotemporal coherence of Ca(2+) release in cardiomyocytes during beta-adrenergic stimulation. J Physiol. 2010;588(pt 1):225–242. doi: 10.1113/jphysiol.2009.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacLennan DH, Kranias EG. Phospholamban: A crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4(7):566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 39.Chu G, et al. A single site (Ser16) phosphorylation in phospholamban is sufficient in mediating its maximal cardiac responses to beta -agonists. J Biol Chem. 2000;275(49):38938–38943. doi: 10.1074/jbc.M004079200. [DOI] [PubMed] [Google Scholar]
- 40.Takimoto E, et al. Frequency- and afterload-dependent cardiac modulation in vivo by troponin I with constitutively active protein kinase A phosphorylation sites. Circ Res. 2004;94(4):496–504. doi: 10.1161/01.RES.0000117307.57798.F5. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Shen PY, Wu G, Chen XZ. Polycystin-2 interacts with troponin I, an angiogenesis inhibitor. Biochemistry. 2003;42(2):450–457. doi: 10.1021/bi0267792. [DOI] [PubMed] [Google Scholar]
- 42.Sucharov CC. Beta-adrenergic pathways in human heart failure. Expert Rev Cardiovasc Ther. 2007;5(1):119–124. doi: 10.1586/14779072.5.1.119. [DOI] [PubMed] [Google Scholar]
- 43.Tilley DG, Rockman HA. Role of beta-adrenergic receptor signaling and desensitization in heart failure: new concepts and prospects for treatment. Expert Rev Cardiovasc Ther. 2006;4(3):417–432. doi: 10.1586/14779072.4.3.417. [DOI] [PubMed] [Google Scholar]
- 44.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93(10):896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 45.Schutzer WE, Mader SL. Age-related changes in vascular adrenergic signaling: Clinical and mechanistic implications. Ageing Res Rev. 2003;2(2):169–190. doi: 10.1016/s1568-1637(02)00063-6. [DOI] [PubMed] [Google Scholar]
- 46.Xiao RP. Beta-adrenergic signaling in the heart: Dual coupling of the beta2-adrenergic receptor to G(s) and G(i) proteins. Sci STKE. 2001;2001(104):re15. doi: 10.1126/stke.2001.104.re15. [DOI] [PubMed] [Google Scholar]
- 47.Nicolaou P, Hajjar RJ, Kranias EG. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J Mol Cell Cardiol. 2009;47(3):365–371. doi: 10.1016/j.yjmcc.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999;51(4):651–690. [PubMed] [Google Scholar]
- 49.Liggett SB, et al. Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101(14):1707–1714. doi: 10.1161/01.cir.101.14.1707. [DOI] [PubMed] [Google Scholar]
- 50.Milano CA, et al. Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor. Science. 1994;264(5158):582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 51.Dorn GW, 2nd, Tepe NM, Lorenz JN, Koch WJ, Liggett SB. Low- and high-level transgenic expression of beta2-adrenergic receptors differentially affect cardiac hypertrophy and function in Galphaq-overexpressing mice. Proc Natl Acad Sci USA. 1999;96(11):6400–6405. doi: 10.1073/pnas.96.11.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nikolaev VO, et al. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327(5973):1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 53.Martinez-Vea A, et al. Left ventricular hypertrophy in hypertensive patients with autosomal dominant polycystic kidney disease: influence of blood pressure and humoral and neurohormonal factors. Am J Nephrol. 2000;20(3):193–200. doi: 10.1159/000013583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.