Significance
Decisions concerning the future are often informed by past experiences. However, in a complex world, we frequently have to make choices for prospective scenarios that we haven’t yet encountered. The present study demonstrates a critical role for the ventromedial prefrontal cortex in simulating what it may feel like to experience such future events. We show that this region contributes to integrating knowledge related to the elements that constitute the episode (e.g., the episode’s location and protagonists). Its activation then indicates the episode’s emergent or overall anticipated affective quality. By this process, the ventromedial prefrontal cortex fundamentally supports our ability to predict possible future affective states, a mechanism that can be flexibly used to augment future oriented decisions.
Keywords: episodic future thinking, episodic memory, functional MRI, ventromedial prefrontal cortex, subjective value
Abstract
Although the future often seems intangible, we can make it more concrete by imagining prospective events. Here, using functional MRI, we demonstrate a mechanism by which the ventromedial prefrontal cortex supports such episodic simulations, and thereby contributes to affective foresight: This region supports processes that (i) integrate knowledge related to the elements that constitute an episode and (ii) represent the episode’s emergent affective quality. The ventromedial prefrontal cortex achieves such integration via interactions with distributed cortical regions that process the individual elements. Its activation then signals the affective quality of the ensuing episode, which goes beyond the combined affective quality of its constituting elements. The integrative process further augments long-term retention of the episode, making it available at later time points. This mechanism thus renders the future tangible, providing a basis for farsighted behavior.
The mental simulation of possible future episodes provides great adaptive value: it supports planning (1), problem solving (2), and carrying out prospective intentions (3) (for a review, see ref. 4). A particular advantage of episodic simulations is their capacity to convey the affective qualities that a future event might hold (5). This mental experience, in turn, can induce motivational incentives for farsighted decisions (6, 7). Moreover, by encoding imagined scenarios into long-term memory, we can use our prior simulations at later time points so that they can substitute for real experiences (8, 9).
This study examines a key role of ventromedial prefrontal cortex (vmPFC) in mediating simulations of future affective episodes and, thereby, in contributing to affective foresight. The vmPFC is part of a network that is consistently engaged during the construction of potential future scenarios and during the recollection of past events (10, 11). It is thus part of a system that has been proposed to provide episodic details and the constructive processes to recombine these details for the simulation of possible scenarios (12).
The vmPFC exhibits bidirectional anatomical connections with other nodes of this simulation network, such as the hippocampal formation, and also with several structures involved in the processing of affective information (13). Parts of the vmPFC feature a higher spine density and number of dendritic spines per cell than comparable cortical areas (14, 15), making it especially suitable for the integration of inputs (16). Here, we hypothesize that the vmPFC supports processes that integrate knowledge related to the elements that constitute a possible future episode to simulate the episode’s emergent or overall affective quality.
This hypothesis is based on two lines of evidence that associate vmPFC functioning both with mnemonic processes and the computation of subjective value. On the one hand, this region contributes to the superior memory for episodes whose elements entail preexperimental associations (17, 18). The vmPFC seems to support this mnemonic benefit of prior knowledge via interactions with posterior cortical regions that are likely involved in the representation of the individual elements (19, 20).
If the vmPFC augments new memories by supporting integration of prior knowledge, it may support a similar function during episodic future simulations. Specifically, the vmPFC should particularly enhance simulations that can draw on richer knowledge about the episode’s elements. Recent observations are consistent with this account: the vmPFC is more strongly activated when people imagine episodes in familiar rather than unfamiliar contexts (21), when they simulate episodes that are personally relevant rather than those that are not personally relevant (22, 23), and when they think about themselves and similar others (24). The vmPFC may thus support flexible episodic simulations by merging prior knowledge about diverse elements of a possible future episode.
On the other hand, there is also considerable evidence for a contribution of the vmPFC to the computation of emotional and subjective value (25–29). Activation in the medial PFC and adjacent anterior cingulate cortex is greater during the simulation of positive rather than negative episodes (30, 31), and it is coupled with the anticipated reward magnitude of imagined experiences (6, 7). The vmPFC may support such value representations by acting as a hub that links information about the simulated episodic details with associated affective responses (15, 32). Based on these lines of research, we hypothesize that this region is critical for the simulation and evaluation of possible future experiences. Specifically, we suggest that the vmPFC supports processes that integrate arbitrary combinations of knowledge structures to simulate the emergent affective quality that a possible future episode may hold.
To test this hypothesis, we designed a procedure that examines blood-oxygen level-dependent signal changes during episodic simulation as a function of both (i) the degree of knowledge about the constituting elements, and (ii) the anticipated affective quality of the event (Fig. S1). Before the functional MRI (fMRI) session, participants named 200 people and 200 places they personally knew, and rated these according to their familiarity and pleasantness. In the scanner, they were then presented with arbitrary person/place pairings and imagined interacting with the given person in a location-specific manner. In addition, participants also completed a functional localizer task during which they imagined known people and places in isolation. Outside the scanner, participants were later cued with one element of each pairing to recall the other, before they indicated the affective value of each episode by rating the associated anticipated pleasantness. We also assessed whether a simulated episode could have been based on previous experiences with the respective person/place combination or was likely to be completely novel.
This procedure allowed us to assess four critical predictions. First, if the vmPFC supports episodic simulations to the degree that they can draw on rich knowledge structures (i.e., extensive associations), this region should be more strongly engaged to the extent that the episode’s elements (i.e., the person and place) are more familiar. Second, if the vmPFC is involved in integrating knowledge structures about the episode’s constituting elements, this should be reflected in its connectivity pattern with cortical regions preferentially involved in processing either places or people. Specifically, there is evidence that the parahippocampal cortex (PHC) supports the imagination of scenes, whereas the dorsal medial PFC (dmPFC) is more strongly recruited during the imagination of scenarios that include familiar people (33, 34). Thus, the vmPFC should exhibit stronger coupling with the PHC during the simulation of more familiar places, whereas its coupling with the dmPFC should vary as a function of the familiarity with the person. Third, if this familiarity-dependent connectivity pattern reflects the integration of prior knowledge that also facilitates memory formation (18), it should be more pronounced for those individuals who subsequently exhibit a greater mnemonic benefit of prior knowledge than those who exhibit a lesser mnemonic benefit. Finally, if the vmPFC supports integration of knowledge structures to process the affective quality of a potential future episode, activation in the same part of this region should scale with the episode’s anticipated pleasantness. Specifically, if the activation reflects the emergent, or overall, affective quality, this should be the case even when controlling for the affective quality of the episode’s elements. Moreover, if this mechanism can be flexibly used to process the value of any arbitrary situation, this should also be the case when examining only those episodes that are likely to be completely novel.
Together, these functional properties would provide a basis for ascribing a key role to the vmPFC in mediating adaptive benefits of episodic simulations. By supporting the anticipation and retention of future episodes and associated affective states, this region could augment future-oriented decisions, even for situations that we have yet to encounter for the first time.
Results
A Greater vmPFC Engagement During the Simulation of More Familiar Elements.
Imagining location-specific interactions (compared with a control task; see Methods) was associated with activation of the typical simulation network, comprising structures such as the medial temporal lobes (including the hippocampus), lateral temporal cortex, and posterior cingulate (4) (Table S1). Critically, these simulations also engaged the vmPFC.
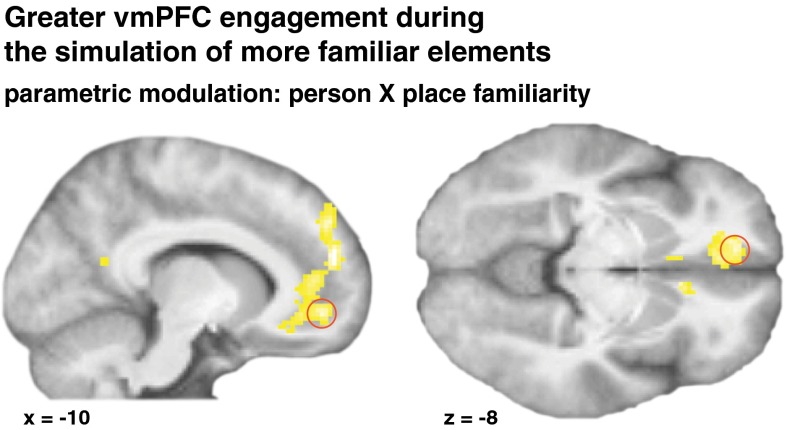
We hypothesized that this region supports episodic simulations to the degree that it can facilitate knowledge of the episode’s elements. If so, its activation should vary from trial to trial as a function of the combined familiarity of person and place. To test this prediction, we examined the effects of a parametric modulator that coded for the product of the two familiarity ratings. This analysis reveals those brain regions that show greater activation when simulations can be based on the integration of richer knowledge structures. As predicted, it yielded a cluster in vmPFC [peaking at x, y, z: −10, 48, −8; zmax = 5.15; P < 0.05, familywise error rate (FWE)-corrected] along with more dorsal mPFC subregions, and small clusters in the anterior cingulate, left prefrontal and parietal cortices (Fig. 1 and Table S2). Thus, activation in a part of the vmPFC indeed varied with the combined familiarity of the person and place, consistent with the hypothesis that this region mediates the integration of knowledge structures in service of episodic simulation.
Fig. 1.
Activation in the vmPFC was greater during the imagination of combinations of more familiar people and places, consistent with a role of this region in facilitating knowledge structures for the simulation of possible episodes. The red circle indicates the ROI for subsequent analyses. The statistical map is displayed at P < 1 × 10−5 uncorrected, and at least 20 voxels.
To further scrutinize the functional properties of this vmPFC region, the following analyses focus on a region-of-interest (ROI) centered on the observed peak (i.e., a sphere with a radius of 6 mm). These results are complemented by exploratory whole-brain analyses in SI Methods. We first further focus on the integrative mechanism by which the vmPFC supports integration of knowledge structures, before we examine whether this region may use the integrated information to process the anticipated affective quality of the simulated episode.
A Familiarity-Dependent Coupling Between More Content-Specific Cortical Regions and the vmPFC.
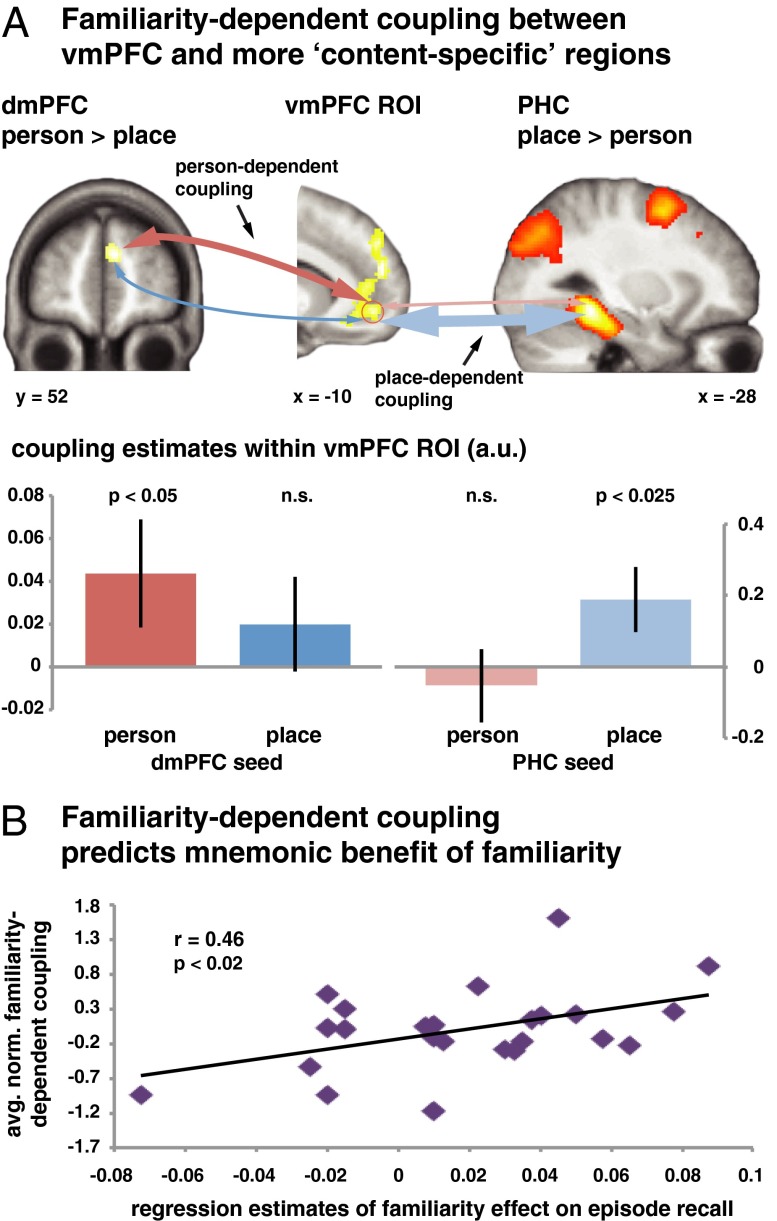
The observed pattern of activation within the vmPFC is consistent with the region’s putative role in integrating knowledge of an episode’s elements. To support such an integrative function, we further hypothesized that the vmPFC would need to interact with distributed regions involved in processing these individual components. These interactions should be stronger in a case of greater knowledge about the respective elements. Activation in those distributed regions should thus more strongly influence activation in the vmPFC to the extent that the vmPFC is likely to integrate richer knowledge structures.
To test this hypothesis, we first identified cortical regions that were preferentially involved in the simulation of either people or places. Consistent with prior observations (33, 34), the functional localizer revealed a stronger engagement of the PHC during the imagination of familiar places (x, y, z: −28, −40, −10, zmax > 8) and of the dmPFC during the imagination of known people (x, y, z: 8, 52, 24, zmax = 5.11; both P < 0.05, whole-brain FWE-corrected) (Table S3). We then assessed changes in coupling between those content-preferring regions and the vmPFC using psychophysiological interaction (PPI) analyses (35).
We estimated two general linear models (GLM) that were based on the time series of activation from either the dmPFC or PHC. Each model included two PPI regressors that were created by convolving the respective seed activation with either the familiarity ratings of the person or of the place. (Note that these ratings were uncorrelated across trials, as described in Methods). We then examined the resulting coupling parameters within the vmPFC ROI. Positive contrast estimates for a given PPI regressor (e.g., dmPFC × person familiarity) indicate a stronger coupling between the seed region (e.g., dmPFC) and vmPFC during the simulation of episodes including more familiar elements (e.g., more familiar people). If the vmPFC integrates existing information processed by the two seed regions, its connectivity with the dmPFC should vary as a function of the person-familiarity, and its connectivity with the PHC should vary as a function of the place-familiarity.
To test this prediction, we conducted a repeated-measures ANOVA on the coupling parameters from the vmPFC ROI with the factors “seed” (dmPFC, PHC) and “element” (person, place) (Fig. 2A and Tables S4 and S5). This analysis yielded a significant interaction [F(1, 23) = 4.44, P = 0.046], reflecting the expected stronger dmPFC coupling for the simulation of more familiar people [t(23) = 1.74, P = 0.048 one-tailed], and the stronger PHC coupling for the simulation of more familiar places only [t(23) = 2.07, P < 0.025, one-tailed]. However, the difference in coupling parameters for places versus people was only significant for the PHC seed [t(23) = −1.78, P = 0.045, one-tailed].
Fig. 2.
Familiarity-dependent integration of distributed knowledge. (A) Psychophysiological interaction analyses examined the connectivity pattern between the vmPFC and regions identified by a localizer to be more preferentially involved in the simulation of either people (dmPFC) or places (PHC). Coupling with the dmPFC varied as a function of the person-familiarity, and coupling with the PHC as a function of the place-familiarity, consistent with a role of the vmPFC in integrating knowledge about the elements of a simulated episode. (B) Regression analyses demonstrated that participants were more successful at recalling simulated episodes that were comprised of more familiar elements. The parameter estimates of these analyses correlated positively with the strength of the (average normalized) familiarity-dependent connectivity, suggesting that the mnemonic benefit was mediated by the integrative function supported by the vmPFC. Data are shown as mean ± SEM; P values are one-tailed. Statistical maps are displayed at P < 1 × 10−5, uncorrected, and at least 20 voxels; n.s., not significant.
Thus, the vmPFC indeed exhibited greater coupling with regions preferentially engaged during the simulation of people or places with the degree that the respective elements were more familiar. This familiarity-dependent connectivity pattern supports the hypothesis that the vmPFC acts as an integrative hub in the service of simulating possible future episodes.
A Stronger Familiarity-Dependent Coupling Predicts a Greater Mnemonic Benefit of Familiarity.
The previous section provided evidence that the vmPFC integrates knowledge structures in the service of episodic simulations. We next test the hypothesis that this integrative process also augments the retention of simulated episodes, similar to the mnemonic benefit for material that is consistent with prior knowledge (17, 18). This retention benefit, in turn, would make the simulations more accessible in later situations for predictions about future affairs (8). To examine this hypothesis, we first tested whether participants indeed recalled more details of simulated episodes that included more familiar elements. We then assessed whether such a putative mnemonic benefit may be mediated by the involvement of the vmPFC in integrating knowledge.
To test the impact of prior knowledge on episodic memory, we performed a quartile split, for each individual, of the person/place pairings based on the combined familiarity ratings (i.e., the product of the person and place familiarity). We then calculated their recall rates separately for each quartile, and conducted subject-specific regression analyses, indicating the increase in recall accuracy with increasing combined familiarity across the four bins. Across participants, the parameter estimates of the regression slopes were significantly positive, consistent with the expected mnemonic benefit of familiarity [t(23) = 2.41, P = 0.024].
We then examined the hypothesis that this benefit is a consequence of the integrative function supported by the vmPFC. Individuals who showed a stronger influence of familiarity on subsequent recall should have exhibited stronger familiarity-dependent coupling during the preceding simulations. To assess the coupling strength across the two seeds, we separately normalized the parameters reflecting the place-familiarity–dependent coupling with the PHC and the person-familiarity dependent coupling with the dmPFC. These normalized values were averaged to yield an overall measure of familiarity-dependent coupling with the vmPFC that is not biased by the numerically larger connectivity parameters for the PHC seed. Critically, as predicted, these connectivity values correlated positively with the behavioral parameter estimates [r(22) = 0.46, P = 0.012, one-tailed] [the corresponding Spearman correlation was also significant: ρ(22) = 0.37, P = 0.038, one-tailed] (Fig. 2B). Thus, individuals who showed a greater benefit of familiarity on subsequent recall had exhibited a stronger familiarity-dependent integration fostered by the vmPFC during the preceding simulations. This observation supports the hypothesis that the facilitation of existing knowledge, mediated by the vmPFC, strengthens memory for the simulations. The enhanced long-term retention makes the episodes available for later planning and decision-making (4, 8, 9).
A vmPFC Representation of the Emergent Affective Quality of Future Episodes.
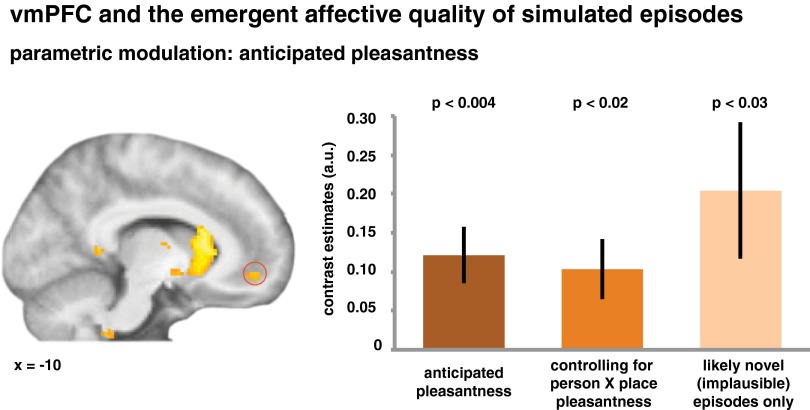
We hypothesized that the vmPFC support processes that integrate knowledge about the constituting elements of a simulated episode to process the affective state that one might experience in that event. If so, the subregion that supports the integration of knowledge structures should also be involved in representing the affective quality of the future episode. We accordingly expected activation in the same part of the vmPFC to also vary as a function of the anticipated affective state. We tested this account by examining the effect of a parametric modulator that coded for the pleasantness assigned to each episode (Table S6). As predicted, this effect was significant in the vmPFC ROI [t(23) = 3.38, P = 0.003] (Fig. 3), demonstrating that activation in this region was stronger during the simulation of more positive future scenarios. This finding suggests that the subregion supporting the integration of knowledge in the service of episodic simulation is also involved in the representation of the episode’s affective quality. Moreover, the effect remained significant when controlling for the combined familiarity of the episode’s elements by including this variable as a first parametric modulator [t(23) = 2.33, P = 0.029], indicating that it did not just reflect possible shared variance between these measures (36).
Fig. 3.
vmPFC activation coded for the emergent affective quality of the simulated future episode. Activation in this region was modulated by the anticipated pleasantness of the future episode. This was also the case when controlling for the combined pleasantness of the episodes’ constituting elements, and when analyzing only those episodes that are likely to be completely novel (because the co-occurrence of the respective person and place is deemed implausible; for example, school teacher in college dorm room). Data are shown as mean ± SEM. The statistical map is displayed at P < 1 × 10−3, uncorrected, and at least 20 voxels.
Critically, the effect of anticipated pleasantness was also present when we controlled for the combined pleasantness of the episode’s elements (i.e., the product of the person and place pleasantness) [t(23) = 2.69, P = 0.013]. Therefore, the vmPFC activation did not merely reflect the affective quality of those elements but coded for the emergent, or overall, affective quality of the future episodes. Intriguingly, we further observed the effect when only focusing on those episodes that were likely to be completely novel (i.e., when the given person/place combination was judged to be implausible, and thus not compatible with one’s prior experiences; e.g., school teacher in college dorm) [t(23) = 2.33, P = 0.029]. The vmPFC thus exhibits functional properties consistent with the computation of possible emotional experiences that novel future episodes may hold. By supporting such computations, this region could mediate our ability to predict future emotional states, even in situations that we have yet to encounter for the first time.
Discussion
Decisions concerning the future are often informed by past experiences. However, we are also frequently confronted with possible scenarios that we have not yet encountered. In those situations, the capacity of episodic simulation conveys a particular adaptive benefit because it allows for the imagination of any possible event that can be constructed based on recombined details of the past (12, 37, 38). The mental experience can then shape future-oriented decisions (6, 39, 40).
The present data demonstrate a key role for the vmPFC in mediating this process. This region was more strongly recruited when the constituting elements of the simulation were more familiar. The activation profile is thus consistent with the hypothesis that the vmPFC contributes to integrating knowledge structures in the service of constructing episodes. This account is further supported by the pattern of effective connectivity with the dmPFC and PHC. A functional localizer task revealed activation of these regions during the imagination of known people versus places, suggesting that they preferentially process these two elements of the simulation (33, 34). Critically, the connectivity between the two regions and the vmPFC was stronger to the degree that the respective elements were more familiar. The interactions of the vmPFC with the dmPFC and PHC thus indicate that the vmPFC may support processes that integrate knowledge structures represented in distributed cortical areas.
How does the vmPFC achieve such integration? On the one hand, this region may coordinate the activation of other cortical modules that are involved in processing the required information. This idea is consistent with general frameworks of prefrontal functioning (e.g., ref. 41), and more specifically with the pattern of spontaneous confabulation associated with lesions to this region (42, 43). Confabulations have been argued to arise from an impairment in selecting and monitoring schematic knowledge that is stored in other, distributed cortical areas (44–47; see also ref. 48). Such a putative control function may support episodic simulations via the selection of arbitrary combinations of knowledge structures.
On the other hand, for the vmPFC to coordinate the activation of distributed knowledge, its neurons may need to code for a summary representation. Indeed, neuroimaging evidence suggests that this region represents abstract summaries of frequent events (e.g., going out for dinner) (49), and there is also evidence for the emergence of novel neuronal representations within the vmPFC (50). Using fMRI repetition suppression, the latter study observed changes in the activation profile of the vmPFC during the simulation of novel foods. When people initially imagined a novel food comprised of two familiar components, this simulation coactivated the components’ representations within the vmPFC. This effect diminished over time, suggesting that simulations led to experience-dependent plasticity that created a novel neuronal representation coding for the novel food. The representation of the food was thus no longer dependent on the representations of its individual components. The observed plasticity may accordingly support the merging of individual elements into a common knowledge structure, an interpretation that is consistent with other recent evidence: For example, activation in this region has been shown to increase as participants learn to use patterns of object features to guide decisions (51). The region also becomes more strongly engaged when partly overlapping sets of items have successfully been integrated into a common representation, as indicated by a transitive inference test (52).
Accordingly, if the vmPFC supports the merging of knowledge structures during the simulation of novel episodes, this process could contribute to the long-term retention of the simulations. In the current study, those simulated episodes that were better remembered were comprised of more familiar elements. Individuals who exhibited the greatest mnemonic benefit of familiarity had previously shown the strongest familiarity-dependent coupling between the vmPFC and the more content-specialized regions. These data suggest that the integrative process supported by the vmPFC enhanced memory for the simulated episodes, a process that may more generally augment long-term memory (17, 18). The vmPFC would thus also contribute to the construction of “memory for the future” (8), as it would make the simulations accessible in later situations when they can aid decisions.
Critically, we further hypothesized that the vmPFC would support integration of knowledge structures to compute the affective state that one may experience in the simulated episode. Indeed, we observed that the same vmPFC region associated with the integration of knowledge also represented the anticipated pleasantness of the situation, suggesting that overlapping neuronal populations support both functions (36). The latter function is consistent with the region’s proposed role in the computation of value (e.g., refs. 25–28). The vmPFC has been suggested to compute the overall value of an item by integrating the values of the item’s attributes, a process that seems to be achieved via interactions with posterior regions involved in representing the individual attributes (53). A similar mechanism may also have been instantiated by the familiarity-dependent coupling in the current experiment.
However, we observed that activation in the vmPFC was modulated by the anticipated pleasantness, even when we controlled for the pleasantness of the episode’s constituting elements. The vmPFC thus coded for the emergent affective quality of the prospective events, suggesting that its integrative process goes beyond the summation of the elements’ absolute values. Although the overlap of activation associated with integration and valuation suggests that overlapping neuronal populations support both of these processes, more work will be necessary to pinpoint their interrelations and temporal dynamics (see, for example, ref. 36). Nonetheless, given that the activation in the vmPFC also reflected the pleasantness of episodes that were likely completely novel (because the place/person combinations were deemed implausible), this region seems to support a powerful adaptive function: it mediates the affective state that we may experience in a range of familiar and novel situations.
Such a mental glimpse of the affective future can help overcome the intangibility of prospective scenarios (cf. 5, 54). For example, people are prone to forfeit a larger reward that they would receive after a delay in favor of a smaller but immediate reward (54). This tendency to discount the future is attenuated when they first imagine the consumption of the delayed reward (6, 7), and thus mentally experience the emotional impact that the delayed reward would hold. Recent evidence suggested that the mPFC supports this effect by representing the magnitude of the imagined reward, thereby conveying motivational incentives for farsighted choices (6; see ref. 55 for a related discussion). The current study elucidates the mechanism by which this region could contribute to the construction of such affective forecasts.
To conclude, the vmPFC supports processes that draw on the past to simulate possible happenings. It thus augments episodic simulations with the richness of past experiences (56). Although prospection is not always accurate (57), and exaggerated or distorted future thinking may, in some cases, be detrimental to our well-being (58, 59), the vmPFC offers a flexible mechanism that enables us to experience the merits and pitfalls of possible future episodes, thereby aiding farsighted decisions.
Methods
Participants.
Twenty-seven right-handed volunteers participated, who all reported no history of psychiatric or neurological disorder and gave written, informed consent as approved by the Harvard University Institutional Review Board. Two participants were excluded because of excessive movements and one for not complying with task instructions. We thus analyzed data from 24 participants (15 males; mean age: 21.5 y, range: 18–29 y).
Tasks and Procedure.
The study was comprised of a preparation session and a simulation session (Fig. S1). In the preparation session, participants first named 200 people and 200 places they were personally familiar with (SI Methods). Participants then rated (i) how familiar they were with the given person or place (1: unfamiliar; 9: very familiar), indicating the degree of knowledge about the item, and (ii) how pleasant the respective person or place was (1: unpleasant; 9: very pleasant).
We then selected the 80 most-familiar and 80 least-familiar people and places. These were pseudorandomly combined to create 160 person/place pairings, ensuring that their familiarity ratings were uncorrelated (mean r = 0.03, SD = 0.03) and maximizing the variance of their combined familiarity (i.e., the product of their familiarity ratings) across trials. Four additional pairings served for practice.
Participants returned for the simulation session (median delay: 7 d; range: 5–8 d), which entailed four phases: (i) the critical simulation phase, (ii) a functional localizer phase, (iii) a simulation recall phase, and (iv) a final rating phase. The simulation phase was comprised of the simulation and a control task, which alternated pseudorandomly from trial to trial. Each trial started with a task cue for 2 s (Fig. S1). In the simulation task, we then presented a person/place pairing for 7.5 s (e.g., wife/restaurant), and participants imagined interacting with the person in a location-specific manner (e.g., trying the wife’s appetizer) as vividly as possible. Participants were carefully instructed to always imagine a novel episode. Participants then rated the vividness of their imagination with their right hand (1: not vivid; 5: very vivid), within a maximum of 2.5 s. The screen then went blank for the remainder of the 2.5 s plus a jittered interstimulus interval (≥1 s, mean ± SD: 3 ± 3.45 s), optimized to maximize the design efficiency using optseq2 (surfer.nmr.mgh.harvard.edu/optseq). The sentence task (described in SI Methods) merely served to assess whether episodic simulation yielded activation in the same regions previously identified in comparison with similar control tasks (10). After practicing the tasks, participants completed five runs of 32 simulation and 6 sentence trials.
Participants then performed a localizer task to identify regions within the dmPFC and PHC that we expected to be preferentially involved in simulating people or places (33, 34). Participants therefore pseudorandomly alternated between imagining either just a person or a place in isolation (SI Methods).
Outside the scanner, we assessed memory for the simulations. Participants were shown the place or person of a given episode, and attempted to recall the respective other element. Finally, participants provided two ratings for each person/place pairing. They first indicated the plausibility of meeting the person at the location (1: implausible; 9: very plausible). We reasoned that implausible combinations (e.g., school teacher/college dorm) would likely require the construction of completely novel episodes. On average, 61.5 (SD: 18.3) of the combinations were deemed implausible (i.e., received a rating of 1), suggesting that a substantial proportion (38%) of the simulations could not have been based on past experiences. Participants then indicated the anticipated affective quality of the episode by rating how pleasant it would be to meet the person at the location (1: unpleasant; 9: very pleasant).
fMRI Data Acquisition and Preprocessing.
Using a 3T Siemens TIM Trio MRI scanner with a 32-channel head coil, we acquired T2*-weighted echoplanar images (TR: 2.5 s; TE: 30 ms; flip angle: 90°; FOV: 216 × 216 mm; 3 × 3 × 3 mm3 voxels; interslice gap: 0.3 mm; −30° tilted from the AC-PC plane; 39 slices obtained in descending order; 232 volumes for each of the five runs of the simulation phase, 231 volumes for the localizer task, including 4 dummy volumes each). MPRAGE structural images were acquired with a multiecho scan (TR: 2,530 ms; TEs: 1.6, 3.5, 5.36, 7.22 ms; flip angle: 7°; 1-mm3 isotropic voxels; interslice gap: 0.5 mm; 176 slices in interleaved order).
Data were analyzed using SPM8 (www.fil.ion.ucl.ac.uk/spm). The functional images were realigned, corrected for slice acquisition times, and coregistered with the structural image. This was spatially normalized and the resulting parameters served to normalize the functional images into 2 × 2 × 2-mm3 cubic voxels by fourth-degree B-spine interpolation (using the Montreal Neurological Institute reference brain). The images were then smoothed by an isotropic 8-mm full-width half-maximum Gaussian kernel.
fMRI Analyses.
Regional activation.
fMRI data were analyzed with a series of GLMs, each of which decomposed the blood-oxygen level-dependent time series separately for each run. In addition to the respective model-specific regressors, all GLMs included regressors representing the mean over scans and residual movement artifacts. Analyses of regional activation focused on parametric modulations. These GLMs included a regressor that coded for the 7.5-s periods of the simulation task, a regressor coding for the respective parametric modulation, and a regressor coding for the 7.5-s periods of the sentence task. Only trials were modeled that had received a rating response within the 2.5 s.
To analyze the emergent pleasantness of the episodes, we controlled for the combined pleasantness of the constituting elements by first entering a parametric regressor coding for this covariate and then the regressor of interest. We also analyzed the pleasantness while controlling for effects of combined familiarity in this way. To restrict the pleasantness analysis to likely completely novel episodes, the regressors for the simulation task and the parametric effect of pleasantness only coded for trials of implausible person/place combinations. This GLM included a further regressor for trials with plausible pairings.
Each task regressor was convolved with the canonical hemodynamic response function. We applied a 1/128 Hz high-pass filter to model and data before estimating the model parameters from the least-square fit.
Effective connectivity analyses.
We tested for familiarity-dependent coupling of the dmPFC and PHC using PPI analyses (35). We estimated two GLMs that were based on the activation from either the dmPFC or the PHC region identified by the functional localizer (SI Methods). The first eigenvariate of the respective region, adjusted for effects of interest, constituted the physiological variable. Two psychological variables were defined as the mean-centered familiarity ratings of either the people or the places. Two PPI regressors were then created by convolving the regional activation with either the people or places variable. The respective GLM then included the physiological, the two psychological, and the two resulting PPI regressors.
Supplementary Material
Acknowledgments
We thank N. Rungratsameetaweemana for help in data collection, and B. Staresina for comments on an earlier draft. This work was supported by National Institute of Mental Health Grant R01MH60941 (to D.L.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419274111/-/DCSupplemental.
References
- 1.Taylor SE, Pham LB, Rivkin ID, Armor DA. Harnessing the imagination: Mental simulation, self-regulation, and coping. Am Psychol. 1998;53(4):429–439. doi: 10.1037//0003-066x.53.4.429. [DOI] [PubMed] [Google Scholar]
- 2.Sheldon S, McAndrews MP, Moscovitch M. Episodic memory processes mediated by the medial temporal lobes contribute to open-ended problem solving. Neuropsychologia. 2011;49(9):2439–2447. doi: 10.1016/j.neuropsychologia.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Brewer GA, Marsh RL. On the role of episodic future simulation in encoding of prospective memories. Cogn Neurosci. 2010;1(2):81–88. doi: 10.1080/17588920903373960. [DOI] [PubMed] [Google Scholar]
- 4.Schacter DL. Adaptive constructive processes and the future of memory. Am Psychol. 2012;67(8):603–613. doi: 10.1037/a0029869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer P. Evolutionary economics of mental time travel? Trends Cogn Sci. 2008;12(6):219–224. doi: 10.1016/j.tics.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. J Neurosci. 2011;31(18):6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66(1):138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Ingvar DH. “Memory of the future”: An essay on the temporal organization of conscious awareness. Hum Neurobiol. 1985;4(3):127–136. [PubMed] [Google Scholar]
- 9.Szpunar KK, Addis DR, McLelland VC, Schacter DL. Memories of the future: New insights into the adaptive value of episodic memory. Front Behav Neurosci. 2013;7:47. doi: 10.3389/fnbeh.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11(7):299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Schacter DL, et al. The future of memory: Remembering, imagining, and the brain. Neuron. 2012;76(4):677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76(6):1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs B, et al. Regional dendritic and spine variation in human cerebral cortex: A quantitative Golgi study. Cereb Cortex. 2001;11(6):558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- 15.Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: A comparative study of area 10. Am J Phys Anthropol. 2001;114(3):224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Ramnani N, Owen AM. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5(3):184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 17.Tse D, et al. Schema-dependent gene activation and memory encoding in neocortex. Science. 2011;333(6044):891–895. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- 18.van Kesteren MT, Ruiter DJ, Fernández G, Henson RN. How schema and novelty augment memory formation. Trends Neurosci. 2012;35(4):211–219. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 19.van Dongen EV, Takashima A, Barth M, Fernández G. Functional connectivity during light sleep is correlated with memory performance for face-location associations. Neuroimage. 2011;57(1):262–270. doi: 10.1016/j.neuroimage.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 20.van Kesteren MT, et al. Differential roles for medial prefrontal and medial temporal cortices in schema-dependent encoding: From congruent to incongruent. Neuropsychologia. 2013;51(12):2352–2359. doi: 10.1016/j.neuropsychologia.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Szpunar KK, Chan JCK, McDermott KB. Contextual processing in episodic future thought. Cereb Cortex. 2009;19(7):1539–1548. doi: 10.1093/cercor/bhn191. [DOI] [PubMed] [Google Scholar]
- 22.Abraham A, Schubotz RI, von Cramon DY. Thinking about the future versus the past in personal and non-personal contexts. Brain Res. 2008;1233:106–119. doi: 10.1016/j.brainres.2008.07.084. [DOI] [PubMed] [Google Scholar]
- 23.D’Argembeau A, et al. The neural basis of personal goal processing when envisioning future events. J Cogn Neurosci. 2010;22(8):1701–1713. doi: 10.1162/jocn.2009.21314. [DOI] [PubMed] [Google Scholar]
- 24.Benoit RG, Gilbert SJ, Volle E, Burgess PW. When I think about me and simulate you: Medial rostral prefrontal cortex and self-referential processes. Neuroimage. 2010;50(3):1340–1349. doi: 10.1016/j.neuroimage.2009.12.091. [DOI] [PubMed] [Google Scholar]
- 25.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litt A, Plassmann H, Shiv B, Rangel A. Dissociating valuation and saliency signals during decision-making. Cereb Cortex. 2011;21(1):95–102. doi: 10.1093/cercor/bhq065. [DOI] [PubMed] [Google Scholar]
- 27.Peters J, Büchel C. Neural representations of subjective reward value. Behav Brain Res. 2010;213(2):135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 28.Winecoff A, et al. Ventromedial prefrontal cortex encodes emotional value. J Neurosci. 2013;33(27):11032–11039. doi: 10.1523/JNEUROSCI.4317-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tusche A, Smallwood J, Bernhardt BC, Singer T. Classifying the wandering mind: Revealing the affective content of thoughts during task-free rest periods. Neuroimage. 2014;97:107–116. doi: 10.1016/j.neuroimage.2014.03.076. [DOI] [PubMed] [Google Scholar]
- 30.D’Argembeau A, Xue G, Lu ZL, Van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. Neuroimage. 2008;40(1):398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450(7166):102–105. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- 32.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassabis D, et al. Imagine all the people: How the brain creates and uses personality models to predict behavior. Cereb Cortex. 2014;24(8):1979–1987. doi: 10.1093/cercor/bht042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szpunar KK, St. Jacques PL, Robbins CA, Wig GS, Schacter DL. Repetition-related reductions in neural activity reveal component processes of mental simulation. Soc Cogn Affect Neurosci. 2014;9(5):712–722. doi: 10.1093/scan/nst035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 36.Shenhav A, Feldman Barrett L, Bar M. Affective value and associative processing share a cortical substrate. Cogn Affect Behav Neurosci. 2013;13(1):46–59. doi: 10.3758/s13415-012-0128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullally SL, Maguire EA. Memory, imagination, and predicting the future: A common brain mechanism? Neuroscientist. 2014;20(3):220–234. doi: 10.1177/1073858413495091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behav Brain Sci. 2007;30(3):299–313. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- 39.Schacter DL, Benoit RG, De Brigard F, Szpunar KK. Episodic future thinking and episodic counterfactual thinking: Intersections between memory and decisions. Neurobiol Learn Mem. 2013 doi: 10.1016/j.nlm.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smallwood J, Andrews-Hanna J. Not all minds that wander are lost: The importance of a balanced perspective on the mind-wandering state. Front Psychol. 2013;4:441. doi: 10.3389/fpsyg.2013.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 42.Gilboa A, Moscovitch M. The cognitive neuroscience of confabulation: A review and a model. In: Baddeley AD, Kopelman MD, Wilson BA, editors. Handbook of Memory Disorders. 2nd Ed. John Wiley & Sons; London: 2002. pp. 315–342. [Google Scholar]
- 43.Turner MS, Cipolotti L, Yousry TA, Shallice T. Confabulation: Damage to a specific inferior medial prefrontal system. Cortex. 2008;44(6):637–648. doi: 10.1016/j.cortex.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Burgess PW, Shallice T. Confabulation and the control of recollection. Memory. 1996;4(4):359–411. doi: 10.1080/096582196388906. [DOI] [PubMed] [Google Scholar]
- 45.Burgess PW, McNeil JE. Content-specific confabulation. Cortex. 1999;35(2):163–182. doi: 10.1016/s0010-9452(08)70792-5. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh VE, Gilboa A. What is a memory schema? A historical perspective on current neuroscience literature. Neuropsychologia. 2014;53:104–114. doi: 10.1016/j.neuropsychologia.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh VE, Moscovitch M, Melo Colella B, Gilboa A. Schema representation in patients with ventromedial PFC lesions. J Neurosci. 2014;34(36):12057–12070. doi: 10.1523/JNEUROSCI.0740-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warren D, Jones SH, Duff MC, Tranel D. False recall is reduced by damage to the ventromedial prefrontal cortex: Implications for understanding the neural correlates of schematic memory. J Neurosci. 2014;34(22):7677–7682. doi: 10.1523/JNEUROSCI.0119-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krueger F, Barbey AK, Grafman J. The medial prefrontal cortex mediates social event knowledge. Trends Cogn Sci. 2009;13(3):103–109. doi: 10.1016/j.tics.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Barron HC, Dolan RJ, Behrens TE. Online evaluation of novel choices by simultaneous representation of multiple memories. Nat Neurosci. 2013;16(10):1492–1498. doi: 10.1038/nn.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumaran D, Summerfield JJ, Hassabis D, Maguire EA. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009;63(6):889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75(1):168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim SL, O’Doherty JP, Rangel A. Stimulus value signals in ventromedial PFC reflect the integration of attribute value signals computed in fusiform gyrus and posterior superior temporal gyrus. J Neurosci. 2013;33(20):8729–8741. doi: 10.1523/JNEUROSCI.4809-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rick S, Loewenstein G. Intangibility in intertemporal choice. Philos Trans R Soc Lond, B. 2008;363(1511):3813–3824. doi: 10.1098/rstb.2008.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernhardt BS, et al. Medial prefrontal and anterior cingulate cortical thickness predicts shared individual differences in self-generated thought and temporal discounting. Neuroimage. 2014;90:290–297. doi: 10.1016/j.neuroimage.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 56.Robin J, Moscovitch M. The effects of spatial contextual familiarity on remembered scenes, episodic memories, and imagined future events. J Exp Psychol Learn Mem Cogn. 2014;40(2):459–475. doi: 10.1037/a0034886. [DOI] [PubMed] [Google Scholar]
- 57.Gilbert DT, Wilson T. Prospection: Experiencing the future. Science. 2007;317(5843):1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- 58.Holmes EA, et al. Mood stability versus mood instability in bipolar disorder: A possible role for emotional mental imagery. Behav Res Ther. 2011;49(10):707–713. doi: 10.1016/j.brat.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacLeod AK, Byrne A. Anxiety, depression, and the anticipation of future positive and negative experiences. J Abnorm Psychol. 1996;105(2):286–289. doi: 10.1037//0021-843x.105.2.286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.