Significance
Anopheles gambiae females are the principal vectors of malaria, a disease that kills more than 600,000 people every year. Current control methods using insecticides to kill mosquitoes are threatened by the spread of resistance in natural populations. A promising alternative control strategy is based on interfering with mosquito reproduction to reduce the number of malaria-transmitting females. Here we show that a male hormone transferred to the female during sex induces large changes in female behavior. These changes, defined as the postmating switch, include a physical incapacity for fertilization by additional males and the ability to lay mature eggs. Tampering with the function of this hormone generates unprecedented opportunities to reduce the reproductive success of Anopheles mosquitoes and impact malaria transmission.
Keywords: ecdysone, hormone, reproduction, malaria, mosquito
Abstract
Female insects generally mate multiple times during their lives. A notable exception is the female malaria mosquito Anopheles gambiae, which after sex loses her susceptibility to further copulation. Sex in this species also renders females competent to lay eggs developed after blood feeding. Despite intense research efforts, the identity of the molecular triggers that cause the postmating switch in females, inducing a permanent refractoriness to further mating and triggering egg-laying, remains elusive. Here we show that the male-transferred steroid hormone 20-hydroxyecdysone (20E) is a key regulator of monandry and oviposition in An. gambiae. When sexual transfer of 20E is impaired by partial inactivation of the hormone and inhibition of its biosynthesis in males, oviposition and refractoriness to further mating in the female are strongly reduced. Conversely, mimicking sexual delivery by injecting 20E into virgin females switches them to an artificial mated status, triggering egg-laying and reducing susceptibility to copulation. Sexual transfer of 20E appears to incapacitate females physically from receiving seminal fluids by a second male. Comparative analysis of microarray data from females after mating and after 20E treatment indicates that 20E-regulated molecular pathways likely are implicated in the postmating switch, including cytoskeleton and musculature-associated genes that may render the atrium impenetrable to additional mates. By revealing signals and pathways shaping key processes in the An. gambiae reproductive biology, our data offer new opportunities for the control of natural populations of malaria vectors.
Sex profoundly affects the reproductive physiology of Anopheles gambiae mosquitoes, the major vector of human malaria. After copulation females of this species experience a permanent loss of receptivity to further copulation, so that they rely on sperm from a single male for a lifetime production of offspring (1). Sex also increases the number of eggs developed after a blood meal and induces laying of mature eggs (2). Parallel to these modifications in postcopulatory behavior, the female reproductive tract undergoes significant morphological changes, possibly reflecting a switch from an insemination to an egg-laying requirement in these tissues (3). The evolutionary forces behind monandry in An. gambiae are largely uncertain; however, this reproductive strategy may have evolved because of the costs associated with mating in swarms, including use of precious energy resources and elevated risk of predation (4).
What are the possible triggers of the postmating switch that renders females refractory to copulation and allows them to lay eggs? During mating, An. gambiae males package seminal fluid produced in the male accessory glands (MAGs) into a coagulated mating plug that is transferred to the female atrium (uterus), where it is digested in 1–2 d (5–7). Conversely, sperm are permanently stored by the female in a storage organ named the spermatheca, where they must be maintained as viable during multiple reproductive cycles to fertilize eggs developed after blood feeding. The relative roles of MAG secretions and sperm transfer in inducing female postmating behavior in insects have been the subject of numerous studies. In Drosophila melanogaster, a species in which females experience a temporary loss of mating receptivity after copulation, a short 36-amino acid sex peptide (SP) synthesized in the MAGs has a major role in the regulation of the postmating switch (8). Once transferred to the female, SP interacts with the sex peptide receptor, a G-protein–coupled receptor expressed in the reproductive tract and central nervous system of females, to stimulate egg-laying and induce a temporary refractoriness to further mating (9). The refractory period in fruit fly females is extended up to 1 wk beyond the first day postcopulation by the slow release of SP bound to sperm tails (10). The An. gambiae postmating switch, on the other hand, does not seem to depend on sperm transfer, because females mated to spermless males are capable of laying eggs and do not mate again (11). A number of methodologies have been used to elucidate the triggers of egg-laying and monandry in these mosquitoes (12–15); although these studies yield contrasting results, they suggest that the MAG secretions that compose the mating plug have a prominent role in regulating these responses.
Intriguing and rare among insects, the An. gambiae mating plug contains the steroid hormone 20-hydroxyecdysone (20E) (16, 17) in addition to proteins and peptides (6, 7). 20E is the major ecdysteroid in insects and regulates molting during juvenile stages (18). This hormone has been found to affect multiple aspects of adult insect physiology: It controls lifespan (19), learning (20), stress-induced responses (21), sleep regulation, and social interactions (22). Moreover 20E acts as a sexual hormone, influencing male sexual behavior in Drosophila (23). We recently have determined that in An. gambiae the interaction between sexually transferred 20E and the female atrial protein Mating-Induced Stimulator of Oogenesis (MISO) increases female fecundity by stimulating a series of events that lead to a greater number of eggs produced after blood feeding (17). In adult mosquitoes 20E is also produced by the female after every blood meal to stimulate vitellogenesis and egg development (16, 17, 24). In An. gambiae male-produced 20E is delivered specifically to the female atrium during mating, whereas the synthesis of this hormone after blood feeding occurs in ovaries and, likely, in fat body and reaches peak levels much lower than those transferred by males (16, 17).
Here we demonstrate that sexually transferred 20E is a key molecular trigger of the postmating switch in An. gambiae females. We show that this hormone induces a great transcriptional response in two major female reproductive organs, the atrium and the spermatheca. The cascades of events triggered by 20E in these tissues have profound effects on female physiology, leading to a loss of receptivity to further insemination and triggering egg-laying after blood feeding. Moreover, we identify the molecular pathways possibly implicated in these postmating responses. Our findings unveil the multiple roles of this steroid hormone in mosquito reproductive biology and open possibilities for reducing the reproductive success of the most important malaria vector.
Results
Mating Induces a Large 20E-Mediated Transcriptional Response in the Female Lower Reproductive Tract.
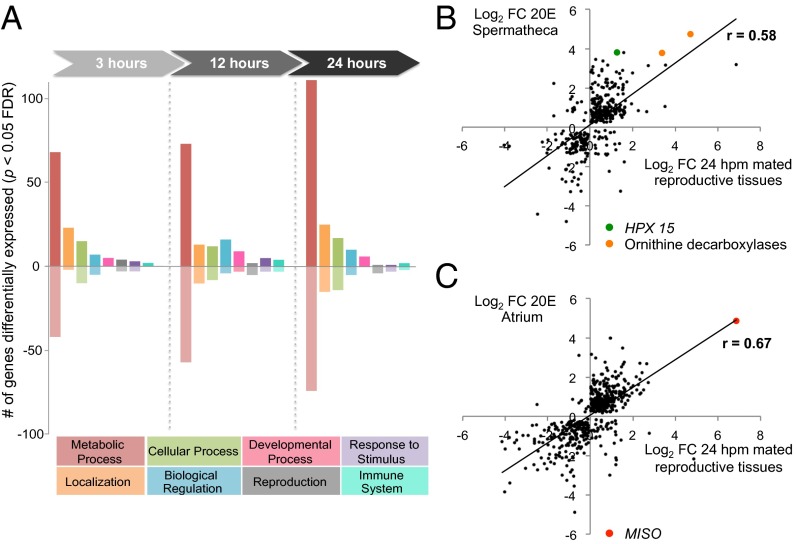
To gain better insight into the molecular link between the postmating transcriptional and morphological changes and the behavioral and physiological shift induced by copulation (3, 17, 25), we initially performed an in-depth transcriptional analysis of the female lower reproductive tract (LRT), comprising the atrium and the spermatheca. To identify the molecular pathways specifically regulated in these female reproductive tissues, we analyzed three time points (3, 12, and 24 h post mating; hpm) that would capture transcriptional changes occurring within a broad time window after copulation. These analyses identified 708 genes induced and 412 genes repressed by mating across the different time points [P < 0.05; false-discovery rate (FDR) corrected] (Dataset S1), unveiling a greater induction of transcription than of repression and a much larger transcriptional response than previously observed in the whole-female analysis, in which only 141 mating-regulated genes were identified (3). Metabolic processes represented the great majority of assignable Gene Ontology (GO) terms in the dataset (Fig. 1A). Up-regulated metabolic genes included factors associated with proteolysis, amino acid biosynthesis, and energy production, suggestive of high-energy demand and protein cycling post mating. Down-regulated genes included cytochrome P450s and carboxy/metallopeptidases that may indicate a shift of resources away from general metabolic activities toward more reproduction-associated processes. The response to mating increased with time, and the highest number of differentially expressed genes (41% of all gene changes) was recorded at 24 hpm. This finding suggests an accumulation of the mating signal during the first day postcopulation, possibly extending into later time points not analyzed here, in contrast to D. melanogaster, in which transcriptional changes were largest 1–6 hpm (26, 27).
Fig. 1.
Mating induces large transcriptional changes in the female LRT. (A) Significant gene changes (P < 0.05 FDR corrected) at each time point (3, 12, and 24 hpm) are represented by their GO biological process (BP) categories. Genes not assignable to GO BP terms (3 hpm, n = 216; 12 hpm, n = 254; 24 hpm, n = 318) are omitted. (B and C) Correlation between mating-induced transcriptional changes at 24 hpm and 20E treatment in the spermatheca (B) and atrium (C). Log2-transformed fold-change data from unique array identifiers showing significant differences (P < 0.05 mating, P < 0.01 20E treatment) are correlated. The Pearson correlation coefficient (r) is indicated and is statistically significant in both cases (P < 0.0001, two-tailed test). The mating and 20E coinduction of ODCs, peroxidase HPX15 [previously associated with fertility in the spermatheca (25)], and MISO (17) is highlighted on the graphs.
What factors could cause such a powerful and prolonged transcriptional response in the female reproductive tract? During mating males transfer large amounts of 20E, a strong regulator of gene expression (28), that is released gradually from the mating plug into the female reproductive tract over the first day after copulation (17). We observed that a considerable number of genes (vitellogenin, prophenoloxidases, proteasome subunits, V-type ATPases, cytochrome P450s, MISO, the heme peroxidase HPX15) identified in our microarrays analysis generally are regulated by 20E in Anopheles and other insects (17, 25, 29–32). Therefore we explored the full extent of a possible 20E-mediated regulation of postmating transcriptional activity by comparing the transcriptional profile of the female reproductive tract observed at 24 hpm and the transcriptional response induced in the atrium and spermatheca at the same time point after injection of 20E into the thorax of virgin females. [This injection delivers to these reproductive tissues 20E levels comparable to those detected after mating (17).] Remarkably, 73% (459/628) of the genes regulated at 24 hpm responded to 20E injections in one or both tissues, and highly significant correlations were found between the datasets (P < 0.0001) (Fig. 1 B and C). Although 20E was injected rather than transferred during copulation, the striking overlap between the responses in female reproductive tissues induced by mating and 20E injection suggests that sexual transfer of this steroid hormone has a major role in the postmating regulation of gene expression. However, at 24 hpm, 169 genes were induced by mating but not by hormone injection, suggesting that other male factors might contribute to the mating-induced transcriptional response in female reproductive organs. It is important to note that the changes in gene expression induced by 20E injections were analyzed at a single time point, not at the three time points examined after mating, and that injections of a large amount of 20E in the thorax may not fully reproduce the slow release of 20E from the plug occurring after mating.
Sexual Transfer of 20E Induces a Physical Refractoriness to Mating.
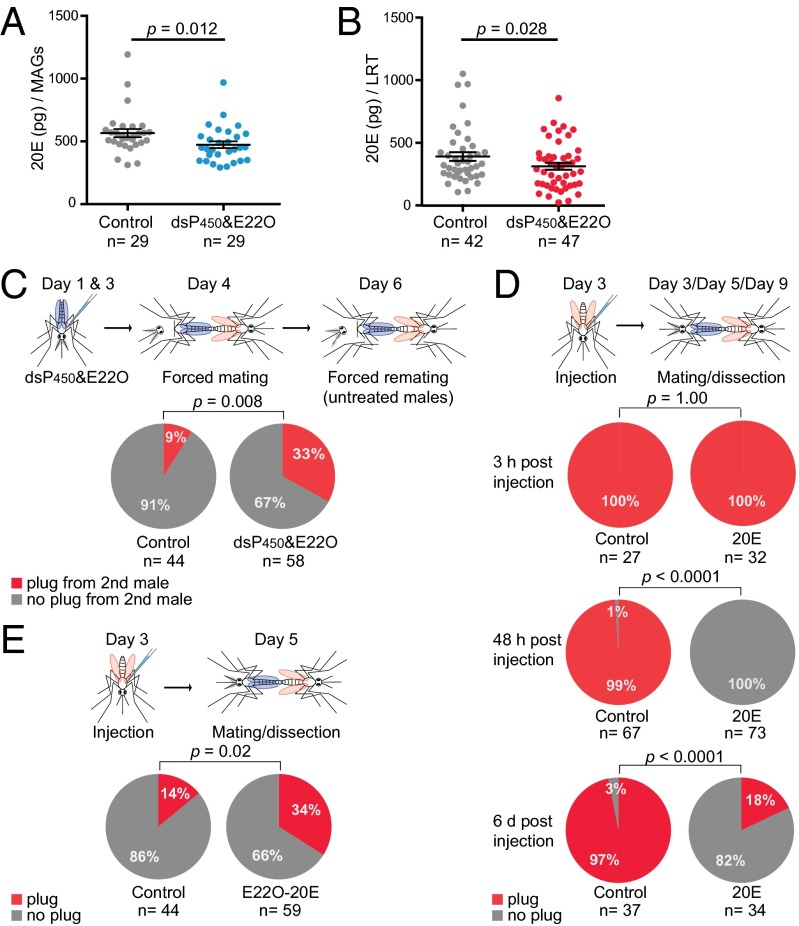
The substantial 20E signature on the transcriptional response to mating indicated that this steroid hormone might be a significant factor in regulating female postmating biology. To determine whether 20E transfer induces loss of susceptibility to further insemination, we generated males impaired in their ability to produce full amounts of 20E. The 20E-impaired males were generated by combining two different methods: We injected young males with RNAi to silence two cytochrome P450 enzymes (Cyp315a1 and Cyp314a1) that catalyze the final steps in the 20E biosynthesis cascade (Fig. S1A) (33), and 2 d later we partially inactivated this steroid hormone by injecting the same males with extracts from the fungus Nomuraea rileyi, which contains an ecdysteroid 22-oxidase (E22O) that oxidizes the 20E hydroxyl group in position 22, inducing loss of function (34). Enzyme immunoassay (EIA) and ultra performance liquid chromatography-mass spectrometry (UPLC-MS) analysis confirmed a 20E reduction of 16–21% (P = 0.012) in MAGs from impaired males as compared with controls (Fig. 2A and Fig. S1B). Injected males then were forced-mated to virgin females, and a reduced transfer of 20E (20% decrease, P = 0.028) was recorded via EIA in mating plugs collected from females shortly after mating (Fig. 2B). Because 20E regulates male sexual behavior in other insects (23), the use of forced mating allowed us to exclude possible confounding effects caused by reduced male mating competitiveness: In this protocol the female is anesthetized before insemination and therefore cannot select a mating partner or avoid copulation (35). Two days after this initial copulation, females again were force-mated, but this time to males with normal 20E levels, and the atrium was analyzed for the presence of a mating plug, indicative of successful copulation (6). Control females (initially mated to control males injected with dsGFP and BSA) showed a low remating frequency (9%) despite being exposed to a second forceful mating event. Conversely, refractoriness was released in females initially mated to 20E-impaired males, and 33% of females in this group showed evidence of insemination by a second male (Fig. 2C), in agreement with the reduced levels of sexually transferred 20E in the first forced mating (Fig. 2B). These results suggest that male-transferred 20E reduces the female’s receptivity to further copulation and that females that receive full titers of 20E become physically incapacitated to reinsemination, because in our experimental settings possible behavioral components were removed by using forced copulations.
Fig. 2.
Sexual transfer of 20E triggers a long-lasting loss of receptivity in females. (A) 20E levels in MAGs dissected from males injected with dsCyp314a1, dsCyp315a1, and E22O (dsP450&E22O) are significantly lower than the levels in MAGs dissected from control males (Control) (Mann–Whitney u test = 259, P = 0.012). (B) Females mated to males injected with dsCyp314a1 and with dsCyp315a1 and E22O (dsP450&E22O) receive significantly lower levels of 20E than control females (Control) (unpaired t test using log(Y)-transformed data, t = 2.235, df = 87, P = 0.028). In both A and B data are shown with the mean (horizontal bar) ± SEM (error bars). (C) Females mated with males doubly injected with dsCyp314a1 and dsCyp315a1 and with E22O receive a mating plug by a second male at higher frequency than females from the control group (doubly injected with dsGFP and BSA; Fisher’s exact test, P = 0.008). (D) The great majority of 20E-injected females are not inseminated when captured in copula 48 hpi as compared with controls (Control) (Fisher’s exact test, P < 0.0001), whereas the mating plug was present in females captured 3 hpi (Fisher’s exact test, P = 1.000). Females still fail to become inseminated at day 6 post 20E injection (Fisher’s exact test P < 0.0001). (E) 20E oxidation by E22O (E22O-20E) rescues the female’s ability to be inseminated by males (Fisher’s exact test, P = 0.02). The number (n) of females analyzed is indicated in A–E.
Following natural copulations, loss of mating receptivity occurs gradually over time, with full refractoriness observed 1–2 d after mating and persisting for the female’s lifetime (6, 36). In agreement with the results obtained using 20E-impaired males, 20E injections into the thorax of virgin females mimicked the mating effects, inducing a striking and long-lasting refractoriness to copulation in a time- and dose-dependent manner (Fig. 2D and Fig. S2). Successful insemination was observed in 100% of females captured in copula at 3 h post injection (hpi), but no 20E-injected females were inseminated when captured at 48 hpi, as compared with 99% of controls. Partial inactivation of 20E, achieved by prior incubation of the hormone with the oxidase E22O, abolished the inhibition of insemination in 34% of females, demonstrating that the hormone has a specific role in reducing the ability of females to become inseminated (Fig. 2E). Lack of insemination was persistent and also was observed 6 d postinjection, when only 18% of 20E-treated females were inseminated as compared with 97% of controls (Fig. 2D). Interestingly, a number of females that were not inseminated had a mating plug deposited on their last abdominal segment, reinforcing the notion that the female’s atrium becomes physically incapable of receiving the plug.
The Release of 20E from the Mating Plug Induces Oviposition.
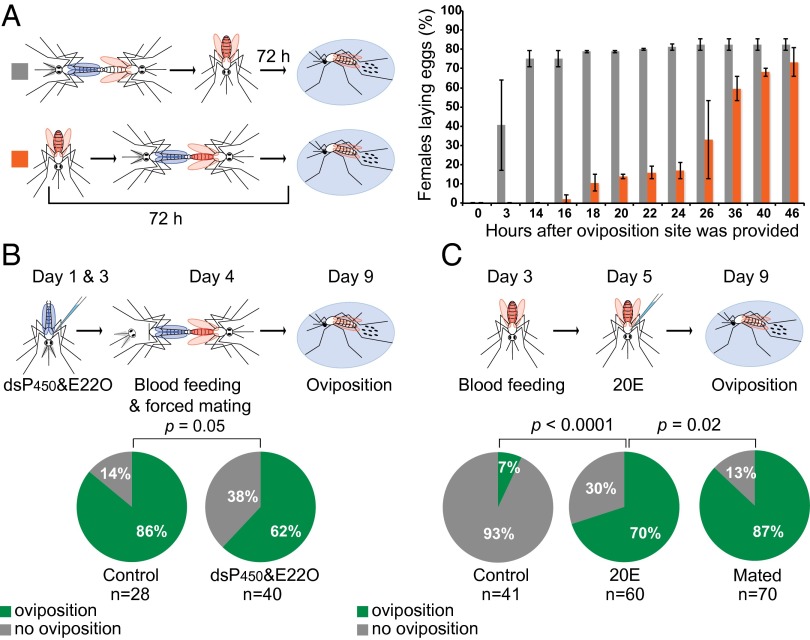
We next determined a possible role of 20E in another crucial mating-dependent reproductive process: oviposition. In An. gambiae this process is tightly regulated and, with a few exceptions, is triggered only in mated blood-fed females after egg development is completed (48–72 h after blood feeding). We initially determined how long after mating blood-fed females start laying their eggs. To this end, experimental females were blood fed as virgins and then were captured in copula 72 h post blood feeding and were placed immediately in oviposition cups to allow egg-laying. Control females instead were first mated and then were blood fed and were placed in oviposition cups at the same time after blood feeding (72 h) as experimental females (Fig. 3A). Despite having fully developed eggs, experimental females initiated oviposition only 16–18 h after mating and completed this process over the course of 2 d, whereas control females started and terminated egg-laying in a considerably shorter time (Fig. 3A). These results suggest that, similar to the loss of mating receptivity, oviposition is regulated by mating signals accumulating over time.
Fig. 3.
20E transferred during mating induces oviposition. (A) A time course of oviposition after mating. Control mosquitoes were blood fed immediately after mating (gray, n = 80), but in the experimental group mating occurred when oogenesis was terminated (orange, n = 94). Data are presented as the mean of two separate replicates with SE. Experimental females took considerably longer than controls to start and complete egg-laying (comparison of oviposition curve functions with Gehan–Breslow–Wilcoxon test: χ2 = 116.7; df = 1; P < 0.0001). The median time to initiate oviposition was 14 h in controls and 36 h in experimental females. (B) Males injected with dsP450s and E22O fail to induce oviposition in a larger number of females than did control males injected with dsGFP and BSA (Fisher’s exact test, P = 0.05). (C) Egg-laying is induced in blood-fed virgins injected with 20E but not in controls injected with 10% ethanol 48 h after blood feeding (Fisher’s exact test, P < 0.0001), although not to the same levels induced by mating (Mated, Fisher’s exact test P = 0.02).
We then tested whether egg-laying is dependent on sexual transfer of 20E. A significant proportion (38%) of females mated to 20E-impaired males injected with dsCyp315a1, dsCyp314a1, and E22O failed to oviposit after blood feeding, compared with 14% of controls (Fig. 3B), a finding that is consistent with the reduced 20E transfer achieved (Fig. 2B). Moreover, 20E injections in the thorax of blood-fed virgin females stimulated laying of (sterile) eggs in 70% of virgins, compared with very low oviposition levels (7%) in controls injected with a 10% ethanol solution (Fig. 3C), and the effects were dose-dependent, as seen in the mating refractoriness experiments (Fig. S2). Taken together, these data show that the transfer of 20E is required for the induction of egg-laying.
Comparative Analysis of Microarray Data Reveals Molecular Pathways Regulating the Postmating Switch.
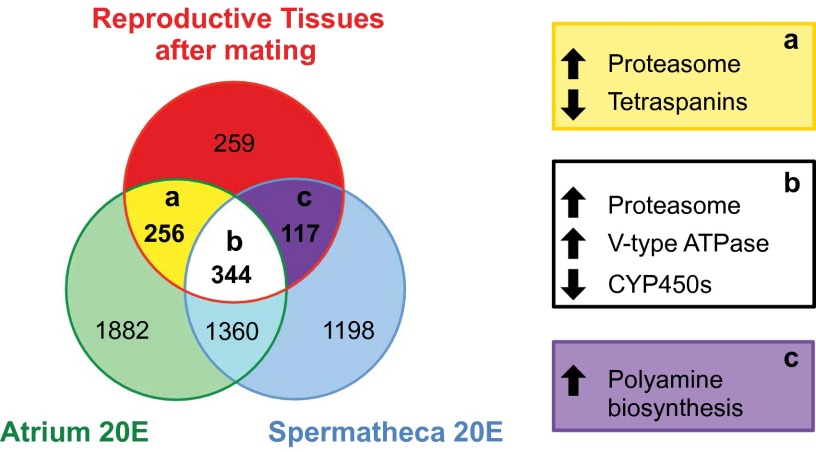
To identify possible molecular pathways underlying the postmating effects induced by 20E, we performed a more detailed comparison of the reproductive genes regulated in the atrium and the spermatheca by both mating and 20E injections. In total, 256 genes were regulated exclusively in the atrium by both 20E treatment and mating at all time points (Dataset S2). Among up-regulated transcripts (n = 137) were a considerable number of genes associated with tissue structure, including the cytoskeleton genes Actin5C, Actincytosk, and β-tubulin I and an adducin-like protein important for cytoskeleton network assembly (37). Changes in the atrial musculature were indicated through induction of Mlp84B, which functions in muscle differentiation (38), and two myosins. Functional enrichment analysis (Table S1) identified the proteasome pathway as significantly up-regulated, whereas down-regulated genes (n = 119) were overrepresented by membrane-spanning tetraspanins, with potential roles in intracellular signaling, cellular motility, and hemocyte cell–cell interactions (Fig. 4, section a) (39, 40). The proteasome was also highly enriched in the group of genes up-regulated by mating and 20E treatment in both the atrium and spermatheca (344 genes; Dataset S2), including the proteasome maturation factor POMP and proteins from the core and regulatory particles (Fig. 4, section b). In this group vacuolar-type ATPases were also overrepresented (9 of the 13 subunits; P < 0.05) and likely function to acidify intracellular lysosomes as well as to facilitate epithelial transport. Six insect-specific cytochrome P450 monooxygenases (CYP6 and CYP9 members) generally linked to xenobiotic and insecticide detoxification (41) were enriched instead in the down-regulated group (n =133). Interestingly, these enzymes are also down-regulated 24 h after blood feeding (42), another time of high 20E titers. Overall, the induction of cytoskeleton/musculature-associated genes supports the hypothesis that 20E may trigger remodeling of the atrium, possibly rendering this tissue impenetrable to additional mates.
Fig. 4.
Gene-expression overlap between mating and 20E treatment in the female LRT. Unique and common significantly differentially expressed genes from three microarray experiments investigating mating-induced changes in the female LRT 3, 12, and 24 hpm (P < 0.05 FDR corrected) and 20E-induced changes in two LRT tissues (atrium and spermatheca) (P < 0.001 FDR). Values in overlap sections a–c represent the number of common genes with the same directional fold-change (Dataset S2). Terms representing significantly enriched clusters (P < 0.05) are shown in boxes a–c. The number of genes in each section is indicated.
Finally, we analyzed the 117 genes regulated by both mating and 20E treatment specifically in the spermatheca (Fig. 4, section c and Dataset S2), a tissue that has been implicated in controlling egg-laying in mosquitoes and fruit flies (36, 43). Interestingly, a significant enrichment was found only for the up-regulation of genes involved in the biosynthesis of polyamines, including two ornithine decarboxylases (ODCs) that catalyze the first committing step in polyamine synthesis, and an antizyme inhibitor that binds ODC antizymes preventing ODC degradation (44). Because the function of polyamines has been associated with various reproductive processes, (45) including oviposition (46), this finding may suggest a possible role for these enzymes in regulating the 20E-mediated release of mature eggs from the ovaries.
Discussion
Our results reveal the male molecular trigger and the female molecular pathways that switch An. gambiae females to a mated status. The discovery that male-transferred 20E is a key regulator of female reproductive biology in An. gambiae adds to the myriad of effects that this hormone exerts on insect adult physiology (2, 3, 17–23). Interestingly, the steady decline of 20E levels in the atrium during the first day after copulation (17) is reminiscent of the process of mating plug digestion, which is completed in a similar time frame (5–7, 16). Our hypothesis is that the incorporation of 20E in the mating plug and its gradual release when the plug is deposited into the female atrium are necessary for the correct function of this hormone, and these requirements could explain past controversial evidence on the role of MAG secretions obtained using different approaches (12–15). Sexual delivery of 20E specifically into the female atrium seems crucial to induce postmating processes, because these processes are not triggered when virgin females produce smaller peaks of 20E in other tissues after blood feeding (16, 17, 24). Although there is an underlying risk that synthesis of 20E after a blood meal potentially could induce refractoriness to mating in blood-fed virgins, males may have evolved mechanisms to exploit the natural female responsiveness to 20E to induce an irreversible chain of signaling events in tissues (the atrium and spermatheca) where 20E normally is not synthesized after blood feeding. Our data, however, do not exclude the possibility that other male factors, possibly including MAG peptides and proteins homologous to the Drosophila SP, may cooperate with 20E in shaping the female postmating switch. Indeed, when we used 20E-impaired males, we prevented induction of oviposition and mating refractoriness in only a proportion of females (less than 40%). However, this result may also be due to the observed incomplete 20E depletion (Fig. 2 A and B), which, in fact, is comparable to the level of phenotypes recorded in females. Furthermore 20E’s pivotal role was supported by the striking induction of these female postmating responses after 20E injections in virgins.
Loss of sexual receptivity after copulation in natural mating events may be the consequence of different components: It may be caused by a change in sexual behavior, with mated females failing to rejoin the mating swarms following a first copulation, and/or by the physical inability of the female to become inseminated by a second male. Importantly, in the remating experiments using 20E-impaired males (Fig. 2C), we completely excluded the behavioral component by forcefully mating females to a second male. Only 9% of females mated to control males received a plug from a second male, whereas this percentage increased to 33% in females mated to 20E-impaired males. These results suggest that transfer of 20E during mating must induce physical modifications of reproductive tissues that prevent females from becoming reinseminated. This hypothesis is in agreement with studies linking ecdysteroids to dramatic and long-lasting modifications in cell morphology (47, 48) and with our previous findings showing that the atrium undergoes large morphological changes during the first few hours after mating (3). In accordance with these observations, our comparative analysis of genes regulated by both mating and 20E in the atrium identified a number of cytoskeleton- (Actin 5C, β-tubulin, adducin) and muscle-associated (myosins, Mlp84B) genes that may be involved in remodeling of this tissue. Atrial remodeling may also promote future oviposition; dramatic ultrastructural changes recorded in the oviduct of Drosophila females after mating ultimately induce the opening of the upper oviduct lumen, allowing egg release from the ovaries (49). Another plausible mechanism for the effects of 20E in egg-laying emerges from our analyses. ODCs, enzymes catalyzing polyamine production, were highly up-regulated in the spermatheca by both mating and 20E treatment. Interestingly, ODCs are hormonally controlled in insects (50), and both ODCs and polyamine synthesis have been linked to egg development and egg-laying in some species in which inhibition of ODC activity resulted in impaired vitellogenesis (51) and a delay in oviposition (46). 20E released from the mating plug therefore may induce egg-laying via the stimulation of polyamine synthesis by the sperm storage organ, which in turn may trigger yet unknown downstream cascades. However, it also is possible that 20E may act directly or indirectly on other tissues (including the ovaries and those of the nervous system) to promote oviposition.
Finally, by identifying the mechanisms regulating postmating biology in Anopheles mosquitoes, our study generates opportunities for malaria control. Compounds mimicking the action of sexually transferred 20E could be developed to tamper with egg-laying or to switch virgin females to a mated status, preventing further insemination. The use of these sterilizing compounds alone or in combination with insecticides would help reduce the size of natural mosquito populations, ultimately contributing to the control of this devastating infectious disease.
Materials and Methods
For mating microarray experiments, 4-d-old mated females were dissected at 3, 12, and 24 hpm in parallel with aged-matched virgins. Reproductive tissues were collected from four biological replicates. For 20E treatment microarrays, 3-d-old virgin females were injected with a 20E solution (138 nL of a 38-mM solution, equivalent to 2.5 μg per mosquito) or 10% ethanol as control. The atrium and spermatheca from four biological replicates were dissected separately 24 hpi. RNA extraction, microarray labeling, and analysis methods are detailed in SI Materials and Methods. For the remating assays, 20E-impaired males were generated by dsRNA against Cyp314a1 and Cyp315a1 (or dsGFP as control) followed by injection with E22O extracts or with BSA as control. Reduction in 20E levels in both MAGs and mated LRTs was measured using an acetylcholinesterase (AChE) Competitive EIA (Cayman Chemical). The impaired males were used in forced-mating experiments with virgin females, and after 2 d the mated females were forced-mated again with untreated males. Occurrence of reinsemination was established by analyzing the presence of a mating plug in the atrium. For mating assays after 20E injection in females, virgin females injected with 20E [as previously, in an amount that delivers 20E levels in the LRT comparable to those detected after mating (17)] or with 10% ethanol (control) were introduced into a cage containing males at 3 h, 48 h, or 6 d postinjection. The mated females were caught in copula, and their insemination status was determined by the presence of a mating plug in the atrium. For oviposition assays, oviposition cups were provided to blood-fed females and the number of females that laid eggs was recorded. For timing of oviposition experiments, females were blood fed before or after mating. Detailed experimental protocols are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Agricultural Research Section Culture Collection of the Northern Region Research Laboratory (NRRL) for kindly donating the Nomuraea rileyi spores (NRRL no. 13755) and members of the F.C. laboratory for helpful suggestions and careful reading of the manuscript. This study was sponsored by European Research Council 7th Research Framework Programme Project ‘Anorep’ Starting Grant 260897, by Biotechnology and Biological Sciences Research Council Grant BB/I002898/1, and by National Institutes of Health Grant 1R01AI104956-01A1 (to F.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray data reported in this paper have been deposited in the ArrayExpress database, www.ebi.ac.uk/arrayexpress (accession nos. E-MTAB-3022 and E-MTAB-3023).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410488111/-/DCSupplemental.
References
- 1.Tripet F, Touré YT, Dolo G, Lanzaro GC. Frequency of multiple inseminations in field-collected Anopheles gambiae females revealed by DNA analysis of transferred sperm. Am J Trop Med Hyg. 2003;68(1):1–5. [PubMed] [Google Scholar]
- 2.Klowden MJ, Russell RC. Mating affects egg maturation in Anopheles gambiae Giles (Diptera: Culicidae) J Vector Ecol. 2004;29(1):135–139. [PubMed] [Google Scholar]
- 3.Rogers DW, et al. Molecular and cellular components of the mating machinery in Anopheles gambiae females. Proc Natl Acad Sci USA. 2008;105(49):19390–19395. doi: 10.1073/pnas.0809723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuval B, Bouskila A. Temporal dynamics of mating and predation in mosquito swarms. Oecol. 1993;95(1):65–69. doi: 10.1007/BF00649508. [DOI] [PubMed] [Google Scholar]
- 5.Giglioli MEC, Mason GF. The mating plug of anopheline mosquitoes. Proc R Ent Soc Lond. 1966;41(7-9):123–129. [Google Scholar]
- 6.Rogers DW, et al. Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol. 2009;7(12):e1000272. doi: 10.1371/journal.pbio.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldini F, Gabrieli P, Rogers DW, Catteruccia F. Function and composition of male accessory gland secretions in Anopheles gambiae: A comparison with other insect vectors of infectious diseases. Pathog Glob Health. 2012;106(2):82–93. doi: 10.1179/2047773212Y.0000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubli E, Bopp D. Sexual behavior: How Sex Peptide flips the postmating switch of female flies. Curr Biol. 2012;22(13):R520–R522. doi: 10.1016/j.cub.2012.04.058. [DOI] [PubMed] [Google Scholar]
- 9.Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451(7174):33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- 10.Peng J, et al. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol. 2005;15(3):207–213. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 11.Thailayil J, Magnusson K, Godfray HC, Crisanti A, Catteruccia F. Spermless males elicit large-scale female responses to mating in the malaria mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2011;108(33):13677–13681. doi: 10.1073/pnas.1104738108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryan JH. Results of consecutive matings of female Anopheles gambiae species B with fertile and sterile males. Nature. 1968;218(5140):489. doi: 10.1038/218489a0. [DOI] [PubMed] [Google Scholar]
- 13.Klowden MJ. Sexual receptivity in Anopheles gambiae mosquitoes: Absence of control by male accessory gland substances. J Insect Physiol. 2001;47(7):661–666. doi: 10.1016/s0022-1910(00)00127-x. [DOI] [PubMed] [Google Scholar]
- 14.Shutt B, Stables L, Aboagye-Antwi F, Moran J, Tripet F. Male accessory gland proteins induce female monogamy in anopheline mosquitoes. Med Vet Entomol. 2010;24(1):91–94. doi: 10.1111/j.1365-2915.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- 15.Dottorini T, Persampieri T, Palladino P, Spaccapelo R, Crisanti A. Silencing of the Hsf gene, the transcriptional regulator of A. gambiae male accessory glands, inhibits the formation of the mating plug in mated females and disrupts their monogamous behaviour. Pathog Glob Health. 2012;106(7):405–412. doi: 10.1179/2047773212Y.0000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pondeville E, Maria A, Jacques JC, Bourgouin C, Dauphin-Villemant C. Anopheles gambiae males produce and transfer the vitellogenic steroid hormone 20-hydroxyecdysone to females during mating. Proc Natl Acad Sci USA. 2008;105(50):19631–19636. doi: 10.1073/pnas.0809264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldini F, et al. The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biol. 2013;11(10):e1001695. doi: 10.1371/journal.pbio.1001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamanaka N, Rewitz KF, O’Connor MB. Ecdysone control of developmental transitions: Lessons from Drosophila research. Annu Rev Entomol. 2013;58:497–516. doi: 10.1146/annurev-ento-120811-153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tricoire H, et al. The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mech Ageing Dev. 2009;130(8):547–552. doi: 10.1016/j.mad.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Geddes LH, McQuillan HJ, Aiken A, Vergoz V, Mercer AR. Steroid hormone (20-hydroxyecdysone) modulates the acquisition of aversive olfactory memories in pollen forager honeybees. Learn Mem. 2013;20(8):399–409. doi: 10.1101/lm.030825.113. [DOI] [PubMed] [Google Scholar]
- 21.Hirashima A, Rauschenbach IYu, Sukhanova MJh Ecdysteroids in stress responsive and nonresponsive Drosophila virilis lines under stress conditions. Biosci Biotechnol Biochem. 2000;64(12):2657–2662. doi: 10.1271/bbb.64.2657. [DOI] [PubMed] [Google Scholar]
- 22.Ishimoto H, Kitamoto T. Beyond molting—roles of the steroid molting hormone ecdysone in regulation of memory and sleep in adult Drosophila. Fly (Austin) 2011;5(3):215–220. doi: 10.4161/fly.5.3.15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganter GK, et al. Increased male-male courtship in ecdysone receptor deficient adult flies. Behav Genet. 2007;37(3):507–512. doi: 10.1007/s10519-006-9140-1. [DOI] [PubMed] [Google Scholar]
- 24.Li C, et al. Conserved molecular mechanism for the stage specificity of the mosquito vitellogenic response to ecdysone. Dev Biol. 2000;224(1):96–110. doi: 10.1006/dbio.2000.9792. [DOI] [PubMed] [Google Scholar]
- 25.Shaw WR, et al. Mating activates the heme peroxidase HPX15 in the sperm storage organ to ensure fertility in Anopheles gambiae. Proc Natl Acad Sci USA. 2014;111(16):5854–5859. doi: 10.1073/pnas.1401715111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mack PD, Kapelnikov A, Heifetz Y, Bender M. Mating-responsive genes in reproductive tissues of female Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103(27):10358–10363. doi: 10.1073/pnas.0604046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGraw LA, Clark AG, Wolfner MF. Post-mating gene expression profiles of female Drosophila melanogaster in response to time and to four male accessory gland proteins. Genetics. 2008;179(3):1395–1408. doi: 10.1534/genetics.108.086934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonsalves SE, Neal SJ, Kehoe AS, Westwood JT. Genome-wide examination of the transcriptional response to ecdysteroids 20-hydroxyecdysone and ponasterone A in Drosophila melanogaster. BMC Genomics. 2011;12:475. doi: 10.1186/1471-2164-12-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bownes M, Blair M, Kozma R, Dempster M. 20-hydroxyecdysone stimulates tissue-specific yolk-protein gene transcription in both male and female Drosophila. J Embryol Exp Morphol. 1983;78:249–268. [PubMed] [Google Scholar]
- 30.Ahmed A, et al. Genomic structure and ecdysone regulation of the prophenoloxidase 1 gene in the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA. 1999;96(26):14795–14800. doi: 10.1073/pnas.96.26.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reineke S, Wieczorek H, Merzendorfer H. Expression of Manduca sexta V-ATPase genes mvB, mvG and mvd is regulated by ecdysteroids. J Exp Biol. 2002;205(Pt 8):1059–1067. doi: 10.1242/jeb.205.8.1059. [DOI] [PubMed] [Google Scholar]
- 32.Davies L, et al. Expression and down-regulation of cytochrome P450 genes of the CYP4 family by ecdysteroid agonists in Spodoptera littoralis and Drosophila melanogaster. Insect Biochem Mol Biol. 2006;36(10):801–807. doi: 10.1016/j.ibmb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochem Soc Trans. 2006;34(Pt 6):1256–1260. doi: 10.1042/BST0341256. [DOI] [PubMed] [Google Scholar]
- 34.Kamimura M, et al. Fungal ecdysteroid-22-oxidase, a new tool for manipulating ecdysteroid signaling and insect development. J Biol Chem. 2012;287(20):16488–16498. doi: 10.1074/jbc.M112.341180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benedict MQ, Rafferty CS. Unassisted isolated-pair mating of Anopheles gambiae (Diptera: Culicidae) mosquitoes. J Med Entomol. 2002;39(6):942–944. doi: 10.1603/0022-2585-39.6.942. [DOI] [PubMed] [Google Scholar]
- 36.Klowden MJ. Switchover to the mated state by spermathecal activation in female Anopheles gambiae mosquitoes. J Insect Physiol. 2006;52(7):679–684. doi: 10.1016/j.jinsphys.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Zaccai M, Lipshitz HD. Role of Adducin-like (hu-li tai shao) mRNA and protein localization in regulating cytoskeletal structure and function during Drosophila Oogenesis and early embryogenesis. Dev Genet. 1996;19(3):249–257. doi: 10.1002/(SICI)1520-6408(1996)19:3<249::AID-DVG8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Clark KA, Bland JM, Beckerle MC. The Drosophila muscle LIM protein, Mlp84B, cooperates with D-titin to maintain muscle structural integrity. J Cell Sci. 2007;120(Pt 12):2066–2077. doi: 10.1242/jcs.000695. [DOI] [PubMed] [Google Scholar]
- 39.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6(10):801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 40.Zhuang S, Kelo L, Nardi JB, Kanost MR. An integrin-tetraspanin interaction required for cellular innate immune responses of an insect, Manduca sexta. J Biol Chem. 2007;282(31):22563–22572. doi: 10.1074/jbc.M700341200. [DOI] [PubMed] [Google Scholar]
- 41.David JP, Ismail HM, Chandor-Proust A, Paine MJ. Role of cytochrome P450s in insecticide resistance: Impact on the control of mosquito-borne diseases and use of insecticides on Earth. Philos Trans R Soc Lond B Biol Sci. 2013;368(1612):20120429. doi: 10.1098/rstb.2012.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marinotti O, et al. Genome-wide analysis of gene expression in adult Anopheles gambiae. Insect Mol Biol. 2006;15(1):1–12. doi: 10.1111/j.1365-2583.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 43.Schnakenberg SL, Siegal ML, Bloch Qazi MC. Oh, the places they’ll go: Female sperm storage and sperm precedence in Drosophila melanogaster. Spermatogenesis. 2012;2(3):224–235. doi: 10.4161/spmg.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pegg AE. Regulation of ornithine decarboxylase. J Biol Chem. 2006;281(21):14529–14532. doi: 10.1074/jbc.R500031200. [DOI] [PubMed] [Google Scholar]
- 45.Lefèvre PL, Palin MF, Murphy BD. Polyamines on the reproductive landscape. Endocr Rev. 2011;32(5):694–712. doi: 10.1210/er.2011-0012. [DOI] [PubMed] [Google Scholar]
- 46.Cayre M, et al. Inhibition of polyamine biosynthesis alters oviposition behavior in female crickets. Behav Neurosci. 1996;110(5):1117–1125. [PubMed] [Google Scholar]
- 47.Baehrecke EH. Steroid regulation of programmed cell death during Drosophila development. Cell Death Differ. 2000;7(11):1057–1062. doi: 10.1038/sj.cdd.4400753. [DOI] [PubMed] [Google Scholar]
- 48.Thummel CS. Steroid-triggered death by autophagy. BioEssays. 2001;23(8):677–682. doi: 10.1002/bies.1096. [DOI] [PubMed] [Google Scholar]
- 49.Kapelnikov A, Rivlin PK, Hoy RR, Heifetz Y. Tissue remodeling: A mating-induced differentiation program for the Drosophila oviduct. BMC Dev Biol. 2008;8:114. doi: 10.1186/1471-213X-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wyatt GR, Rothaus K, Lawler D, Herbst EJ. Ornithine decarboxylase and polyamines in silkmoth pupal tissues: Effects of ecdysone and injury. Biochim Biophys Acta. 1973;304(2):482–494. doi: 10.1016/0304-4165(73)90268-7. [DOI] [PubMed] [Google Scholar]
- 51.Kogan PH, Hagedorn HH. Polyamines, and effects from reducing their synthesis during egg development in the yellow fever mosquito, Aedes aegypti. J Insect Physiol. 2000;46(7):1079–1095. doi: 10.1016/s0022-1910(99)00084-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.