Significance
Helminths and allergens stimulate type 2 immune responses by largely unknown mechanisms. Proteolytic activity is a common feature of many helminths and allergens and can promote activation of the immune system. Signaling pathways activated by these proteases remain poorly characterized and are the focus of this study. Using basophils as model type 2 immune cells, we identified roles for the immunoreceptor tyrosine-based activation motif (ITAM)-containing adaptor Fc receptor γ-chain and calcium signaling in protease-stimulated basophil activation. We suggest models to explain how protease sensing, ITAM signaling, and nuclear factor of activated T cells pathways contribute to produce allergic type 2 responses. Elucidation of these signaling pathways and ultimately the identity of protease allergen sensors will be important for the development of pharmacologic strategies to target the initiation of allergic responses.
Keywords: allergen, basophil, signaling
Abstract
Allergic diseases represent a significant burden in industrialized countries, but why and how the immune system responds to allergens remain largely unknown. Because many clinically significant allergens have proteolytic activity, and many helminths express proteases that are necessary for their life cycles, host mechanisms likely have evolved to detect the proteolytic activity of helminth proteases, which may be incidentally activated by protease allergens. A cysteine protease, papain, is a prototypic protease allergen that can directly activate basophils and mast cells, leading to the production of cytokines, including IL-4, characteristic of the type 2 immune response. The mechanism of papain’s immunogenic activity remains unknown. Here we have characterized the cellular response activated by papain in basophils. We find that papain-induced IL-4 production requires calcium flux and activation of PI3K and nuclear factor of activated T cells. Interestingly, papain-induced IL-4 production was dependent on the immunoreceptor tyrosine-based activation motif (ITAM) adaptor protein Fc receptor γ-chain, even though the canonical ITAM signaling was not activated by papain. Collectively, these data characterize the downstream signaling pathway activated by a protease allergen in basophils.
Allergic diseases affect ∼25% of the population of industrialized countries, and their prevalence is rising (1). The properties that make a particular agent allergenic are not well understood, especially considering the wide variety of agents, ranging from helminths and venoms to small-protein allergens, which can induce ostensibly similar type 2 immune responses. Although pattern recognition receptors, such as Toll-like receptors (TLRs), are involved in the recognition of some allergens (2–5), an alternative recognition strategy may be based on the detection of the allergens’ biochemical activities. A similar strategy likely is used to detect enzymatic activities of proteins secreted by multicellular parasites. Secreted proteases are necessary for helminth reproduction and life cycles in their hosts (6), and it has been hypothesized that specific innate immune mechanisms have evolved to detect their proteolytic activity (7). Interestingly, many allergens lack unique structural features that might allow recognition by the immune system, but, like helminths, allergens can exhibit proteolytic activity (8), and it has been hypothesized that they incidentally activate the same pathways activated by helminth proteases and elicit similar type 2 responses (7, 9). In any case, the resulting type 2 immune response and tissue repair may be beneficial in protecting against protease-inflicted tissue damage (10).
Basophils play multiple roles in type 2 immunity and allergy (11–13). Basophils also have been shown to be activated by various cysteine proteases, including papain, bromelain (9), and Der p 1, an allergen produced by the house dust mite Dermatophagoides pteronyssinus (14). Treating in vitro-derived bone marrow basophils (BMBs) with papain leads to the induction of cytokines involved in the regulation of allergic immune responses, including the type 2 cytokines IL-4 and IL-13 (9). At present, basophils and mast cells are the only cell types we have found to respond specifically to the proteolytic activity of papain by producing type 2 cytokines.
Here we have examined signaling pathways activated by papain in basophils. We found that papain-induced IL-4 production requires calcium flux and is sensitive to inhibitors of the calcium release-activated calcium (CRAC) channels. The basophil response is mediated by PI3K and nuclear factor of activated T cells (NFAT). Moreover, papain-induced cytokine production in basophils requires the expression of an intact immunoreceptor tyrosine-based activation motif (ITAM)-containing adaptor, Fc receptor γ (FcRγ)-chain, but does not require its binding to the Fc ε receptor I (FcεRI) α-chain. Although the specific function of this adaptor has yet to be determined, it likely plays a role as a transducer of tonic permissive signals or noncanonical ITAM signals emanating from a heterologous receptor in basophils and is required for setting up an allergen-responsive state.
Results
The Proteolytic Activity of Papain Directly Stimulates Basophil IL-4 Production.
The proteolytic activity of papain is necessary for basophil activation, because chemically inactivated and heat-inactivated papain does not induce basophil IL-4 production (9). There are several possible mechanisms by which basophils may be activated by papain (Fig. S1A). One possibility is that papain enzymatically cleaves a basophil cell-surface receptor to release a proteolytic fragment, which in turn serves as a ligand for a different surface receptor. Alternatively, a cleavage event may activate the cleaved receptor itself directly, either on the cell surface, as in the protease-activated receptor (PAR) family of G protein-coupled receptors (GPCRs), or within a cytosolic or endosomal compartment. Conversely, papain cleavage of a receptor may result in the inactivation of the receptor and, potentially, in the disruption of an inhibitory signal. Papain also could cleave and activate a precursor protein present in the serum. However, it is unlikely that serum-derived proteins are involved in the activation of basophils, because basophils produce similar levels of IL-4 in response to papain in serum-free conditions and in serum-replete conditions (9).
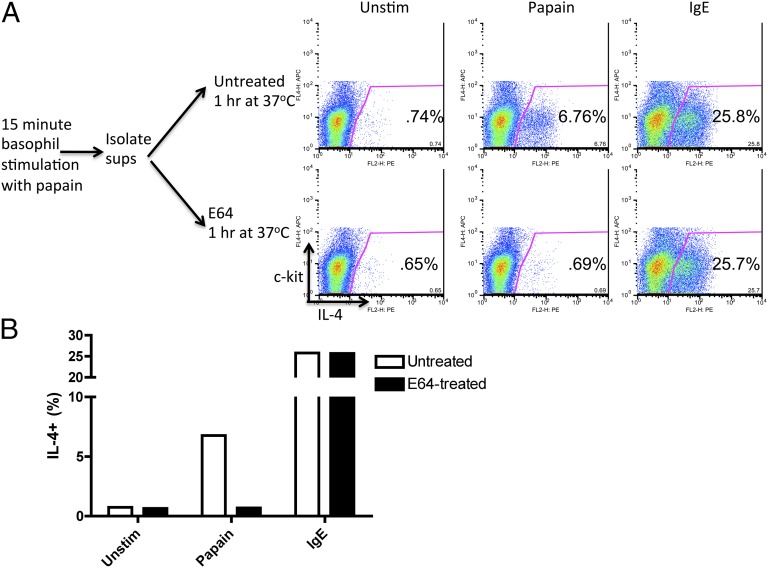
To investigate whether a soluble proteolytic fragment cleaved by papain is involved in basophil activation, mouse BMBs were left unstimulated or were stimulated with papain for 15 min, at which point the supernatants of the samples, which contained cleaved soluble proteins and papain itself, were isolated. The supernatants were treated with E-64, an irreversible cysteine protease inhibitor that was used to inactivate the remaining papain activity, or were left untreated. Fresh, unstimulated basophils were resuspended in test and control supernatants, and intracellular IL-4 production was analyzed subsequently. To confirm that the presence of E-64 did not affect basophil responsiveness in general, basophils also were stimulated subsequently by IgE cross-linking after incubation in the presence of E-64–treated or untreated supernatants. The E-64–treated supernatants from papain-stimulated basophils did not induce IL-4 production in fresh responder basophils, whereas the untreated, active papain-containing supernatants induced IL-4 production (Fig. 1). These data suggest that the proteolytic activity is necessary to activate intrinsic basophil receptors directly and not that a cleaved product from the basophil surface elicits activation of neighboring cells. In addition, the production of IL-4 by basophils was intact in TLR4-deficient basophils, suggesting that papain stimulation of basophils does not involve TLR4 signaling (Fig. S1B) and ruling out a possible contribution of LPS contamination to papain activity.
Fig. 1.
The proteolytic activity of papain directly stimulates basophil IL-4 production. (A) Intracellular IL-4 produced by BALB/c BMBs. Cells were stimulated with papain for 15 min or were left unstimulated; their cell supernatants were left untreated or were treated with E-64 for 1 h; fresh basophils were resuspended in supernatants for 7 h and stained. Unstimulated cell supernatants were used for Unstim and IgE samples. IgE samples were prestimulated with IgE for 30 min before anti-IgE stimulation. Gated populations are ckit-IL-4+, representing IL-4+ basophils. (B) Graphical display of data. Data are representative of at least three similar experiments.
Induction of Cytokines in Basophils Is Dependent on Intracellular and Extracellular Calcium, Calcium Flux Through CRAC Channels, and the Activity of NFAT.
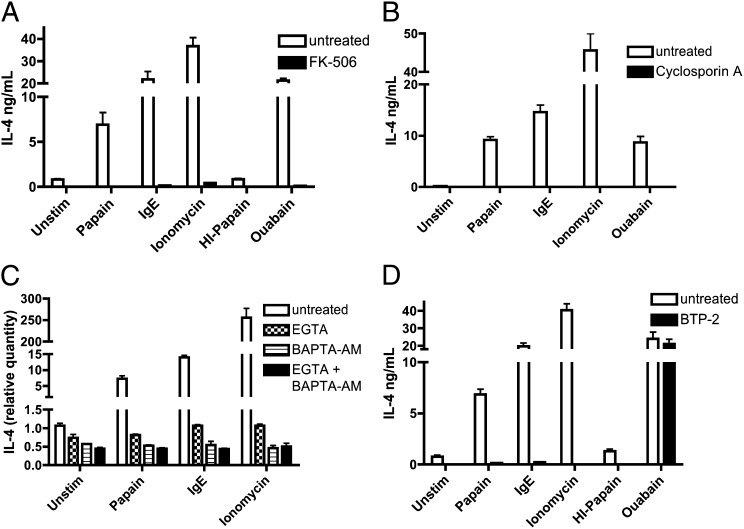
NFAT is a key transcription factor downstream of many activating signals in hematopoietic cells and specifically is activated downstream of signaling through the FcεRI (15). The calcium-dependent NFATs (NFAT1–4) are activated upon dephosphorylation by the serine/threonine phosphatase calcineurin (16). We tested the requirement of NFAT activity for basophil responses to papain by treating cells with calcineurin inhibitors. Stimuli included papain, IgE cross-linking, and ionomycin, a calcium ionophore, as well as the cardiac glycoside, ouabain. Ouabain has been shown previously to increase mast cell cytosolic calcium levels and histamine release (17), although the specific mechanism of its action in our studies is unclear. Papain-induced IL-4 production in BMBs was blocked by pretreatment with the calcineurin inhibitors cyclosporin A and FK-506, and IL-4 production in response to all other basophil stimuli tested also was blocked completely, suggesting a critical requirement for NFAT activity in basophil IL-4 production (Fig. 2 A and B).
Fig. 2.
IL-4 production in basophils is dependent on intracellular and extracellular calcium, calcium flux through the CRAC channel, and the activity of NFAT. (A and B) IL-4 secretion by BALB/c BMBs prestimulated with FK-506 (A) or cyclosporin A (B) and subsequently stimulated as indicated (anti-IgE samples were prestimulated with IgE for 30 min). (C) Relative IL-4 induction by BMBs prestimulated with inhibitors (BAPTA-AM for 35 min or EGTA for 25 min) and then stimulated as indicated for 45 min. (D) IL-4 secretion by BMBs prestimulated with BTP-2 for 30 min and subsequently stimulated as indicated for 6 h. Data are representative of at least three similar experiments. Error bars show SEM.
Activation of NFAT1-4 requires a sustained increase in intracellular calcium from intracellular flux and extracellular calcium entry through CRAC channels (18). Pretreatment of basophils with the extracellular calcium chelator EGTA or the intracellular calcium chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM abrogated papain-induced IL-4 production (Fig. 2C). Cells were equally viable in the presence or absence of chemical inhibitors, as indicated by visual inspection and equal expression of unrelated (e.g., housekeeping) genes in similar experimental samples. CRAC channels are store-operated calcium channels activated upon the depletion of endoplasmic reticulum (ER) calcium stores, which play an important role in calcium-dependent processes in the immune system, including FcεR signaling (19). Prestimulation with the CRAC channel inhibitor N-[4-[3,5-bis(trifluoromethyl)-1H-pyrazol-1-yl]phenyl]-4-methyl-1,2,3-thiadiazole-5-carboxamide (BTP-2) prevented basophils from responding to papain, IgE cross-linking, and ionomycin treatment but did not have an effect on ouabain stimulation (Fig. 2D). Collectively, these results suggest that CRAC channels and calcium flux play an important role in papain signaling in basophils.
Papain Activates ERK and Protein Kinase B Pathways.
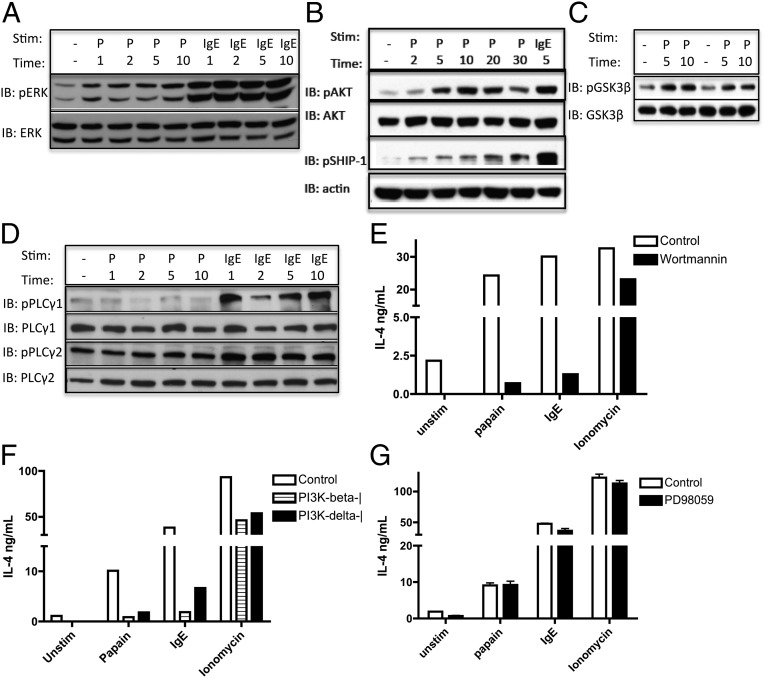
We next tested whether papain stimulation induces other signaling pathways commonly activated in basophils by other known stimuli, including the FcεRI. Papain induced ERK activation of BMBs at early time points (Fig. 3A). In addition, protein kinase B (AKT) and glycogen synthase kinase 3 (GSK3-β) were phosphorylated at early time points after papain stimulation (Fig. 3 B and C), suggesting a role for the AKT-inducing PI3K pathway downstream of the papain sensor. AKT potentially may act by phosphorylating and inactivating GSK3-β, which typically phosphorylates NFAT and induces its nuclear export and the termination of NFAT-driven transcription. Normally, the duration of PI3K-dependent signaling and downstream transcription is dictated by negative feedback by regulatory phosphatases, such as Src homology 2-containing inositol phosphatase (SHIP). SHIP already has been shown to regulate basophil IL-4 production in some settings, because SHIP−/− basophils secrete higher levels of IL-4 in response to IL-3 or FcεRI cross-linking (20). The role of SHIP in allergen sensing has not been tested to date. We found that SHIP-1 is phosphorylated in basophils at later time points after stimulation by papain, suggesting activation of this PI3K negative feedback pathway downstream of the allergen sensor as well (Fig. 3B). The activation of the above-described pathways was not caused by LPS contamination, because the signaling persisted in TLR4-deficient basophils. The response also was dependent on the proteolytic activity of papain, because heat-inactivated papain could not induce signaling in TLR4-deficient basophils (Fig. S2).
Fig. 3.
Papain stimulates ERK and AKT pathway activation, whereas an intact PI3K pathway is required for IL-4 production. (A–D) Immunoblot analysis of pERK/ERK (A), pAKT/AKT and pSHIP-1/actin (B), pGSK3β/GSK3β (C), and pPLCγ1/PLCγ1 and pPLCγ2/PLCγ2 (D) was prepared from BALB/c BMBs stimulated with papain or IgE cross-linking for the times (in minutes) noted. Protein lysates were sonicated before nuclei were removed in D. P, papain. IB, immunoblot. (E–G) IL-4 production by BMBs prestimulated for 30 min with general PI3K inhibitor Wortmannin (E), PI3K-isoform–specific inhibitors (PI3K p110β inhibitor TGX-221 and PI3K p110δ inhibitor IC87114) (F), or the MEK inhibitor PD98059 (G) and subsequently stimulated as indicated for 6 h. Data are representative of at least three similar experiments.
The best-characterized mechanism of NFAT activation in immune cells is by the activation of phospholipase Cγ (PLCγ) by the spleen tyrosine kinase (Syk) downstream of ITAM-containing receptors, such as antigen receptors or the FcεRI (21). There are many isoforms of PLC, and we tested several of them for a role in papain sensing. Surprisingly, unlike in IgE cross-linking, we found that PLCγ1 and PLCγ2 are not phosphorylated in response to papain, although PLCγ2 is highly phosphorylated at baseline in unstimulated BMBs (Fig. 3D). Furthermore, using double-knockout mice, we have ruled out a role for signaling through PLCβ2 and PLCβ3, the PLC isoforms commonly used by GPCR signaling pathways (see Fig. S5D). Exclusion of these pathways suggests that cysteine protease allergens activate NFAT through a currently uncharacterized pathway in basophils that perhaps, in part, is distinct from that used by FcεRI. Consistently, we found no change in JNK or p38 mitogen-activated protein kinase (p38) phosphorylation or IκBα degradation, suggesting that the papain-induced response most likely is independent of JNK, p38, and NFκB.
Papain Stimulation of Basophil IL-4 Production Requires PI3K, but Not ERK, Activity.
We next tested whether signaling through these pathways is required for the transcriptional responses elicited by papain. Stimulation of basophils in the presence of the PI3K inhibitor Wortmannin or the mitogen-activated protein kinase kinase (MEK) inhibitor PD98059 revealed that PI3K, but not ERK, signaling is required for IL-4 production in basophils in response to papain (Fig. 3 E and G). Incubation with the PI3K-β or PI3K-δ isoform-specific inhibitors suggested a potential role for each of these isoforms in the response to papain, because both were able to block papain-induced IL-4 production as well as partially block the response to IgE cross-linking (Fig. 3F). The lack of a requirement for ERK signaling in basophils is in contrast to the requirement for ERK in mouse embryonic fibroblasts and may be cell type specific (22). Furthermore, our data do not exclude the possibility that some other aspects of allergen responses in basophils (e.g., induction of other cytokines) might rely on an intact ERK pathway.
Papain-Induced Basophil Activation in Vitro Is Dependent on the FcεRI γ-Chain.
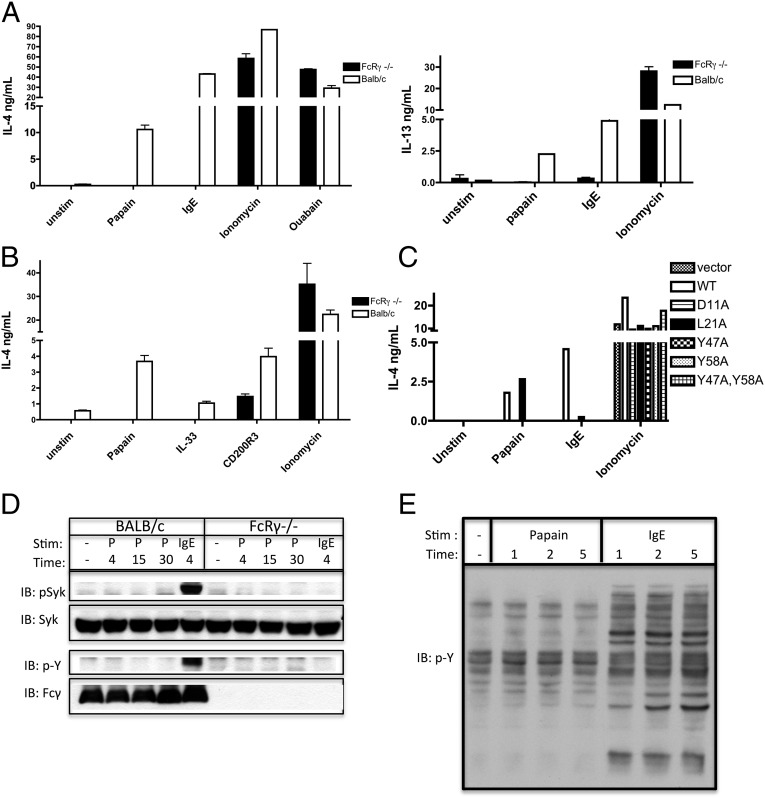
A common mechanism of calcium signaling induction in leukocytes is through the ITAM-containing adaptors. In basophils, two such adaptors exist: FcRγ and DNAX activation protein of 12 kDa (DAP12). They typically are used by classical immunoreceptors, such as the FcεRI, in basophils. However, it is becoming clear that in many cell types other types of surface receptors somehow couple to ITAM signaling pathways upon activation to elicit specific subsets of their functions. This phenomenon is seen particularly in signaling pathways that require calcium fluxes if a receptor does not have the intrinsic ability to elicit such flux on its own (23). It also has been shown that IL-3 stimulation of basophils leads to IL-4 production in a manner that is dependent on the ITAM-containing adaptor FcRγ and is independent of its binding to its classical client immunoreceptor, FcεRI α-chain (24). Unexpectedly, we found that papain-induced IL-4 and IL-13 production is FcRγ dependent (Fig. 4A). To evaluate whether this requirement for FcRγ expression was restricted to papain stimulation, we tested other stimuli known to activate basophils, including IL-33 (25) and an antibody to CD200R3 (26), in FcRγ-deficient basophils. Although the response to anti-CD200R3 antibody was relatively intact (presumably because of compensation by a related adaptor Dap12), the response to IL-33 required FcRγ expression (Fig. 4B). These data indicate that the FcRγ-chain is a required adaptor for papain-induced basophil activation.
Fig. 4.
FcRγ-deficient basophils do not respond to papain or IL-33 stimulation. Stimulation by papain requires the expression of FcRγ with an intact ITAM domain. (A and B) IL-4 and IL-13 production by BALB/c and FcRγ-deficient BMBs stimulated as noted. (C) IL-4 production by FcRγ-deficient basophils transduced with constructs as indicated and enriched for transduced cells by FACsorting based on the expression of hCD2. Transduced cells were stimulated as noted for 6 h. (D) Immunoblot analysis of pSyk/Syk and phosphotyrosine (p-Y)/FcRγ prepared from BALB/c and FcRγ−/− BMBs stimulated with papain and IgE cross-linking. Antiphosphotyrosine was used to identify phosphorylation of the FcRγ chain. (E) Immunoblot analysis of phosphotyrosine prepared from BALB/c BMBs stimulated with papain and IgE cross-linking. Data are representative of at least three similar experiments. Error bars show SEM.
Role of FcRγ in the Papain Response.
It is possible that papain uses the FcRγ-chains that are part of the signaling complexes for the FcεRI or the IL-3 receptor (IL-3R), or, alternatively, that papain signals through FcRγ bound to a different receptor playing a yet undetermined role. To test these possibilities, we made several FLAG-tagged mutants of FcRγ and retrovirally transduced them into BMBs deficient in FcRγ, as modeled after experiments done by Hida et al. (24) and as we have described previously (27). Mutants were designed to disrupt (i) signaling through ITAM domain tyrosines (single or double Y→A mutants within the ITAM motif), (ii) interaction with the FcεRI α-chain (L21A transmembrane region mutation), or (iii) interaction with the FcεRI α-chain and IL-3R βc (D11A transmembrane region mutation), in addition to other receptors that might require this charged residue for interactions (Fig. 4C). This design allowed us to test whether papain sensing requires FcRγ-chain signaling through an ITAM-mediated mechanism and, more specifically, whether papain sensing requires association of the FcRγ-chain with either the FcεRI complex or the IL-3R βc complex. After retroviral transduction of these mutants into FcRγ-deficient basophils and enrichment for transduced cells, we stimulated them with papain, IgE, and ionomycin. In each sample, we also monitored surface expression of the FcεRI α-chain (Fig. S3). As expected, the response to ionomycin was unaffected by mutation of FcRγ, because it acts independently of FcRγ expression. IgE cross-linking could not activate basophils transduced with the ITAM mutant or D11A mutant but was recovered, as expected, in the cells transduced with the wild-type construct and was much diminished in the cells transduced with the L21A mutant. Interestingly, the response to papain was enhanced in the cells that were transduced with the mutant that disrupts interaction with the FcεRI α-chain (L21A) but was lost in cells transduced with the mutant that disrupts interaction with both FcεRI α-chain and IL-3R βc and presumably other transmembrane receptors (D11A). This enhanced signaling in response to papain with the L21A mutant is similar to that seen in response to IL-3, perhaps suggesting that when the FcεRI α-chain is not expressed a greater proportion of the pool of FcRγ is free to bind other receptors, including the IL-3R βc (24). The response to papain also required an intact ITAM domain, suggesting that papain does require activation of the ITAM signaling pathway downstream of FcRγ, but, in contrast to IgE, does not require the interaction of FcRγ with the FcεRI complex. Furthermore, papain sensing required an intact D11 transmembrane residue of FcRγ, suggesting either a requirement for an intact IL-3R signaling complex or signaling through another surface receptor that uses this same residue for association with FcRγ.
Two scenarios describe how the putative papain sensor could engage an ITAM pathway for basophil stimulation: The sensor either could activate the ITAM pathway directly by phosphorylating ITAM tyrosine residues or could rely on the activation of the pathway by another surface receptor, which in this case would serve as a permissive signal to papain stimulation. In testing the former possibility, we found that although IgE cross-linking led to strong phosphorylation of FcRγ and Syk, papain stimulation did not have an effect on their phosphorylation (Fig. 4D). These data suggest that papain may not activate FcRγ ITAM phosphorylation directly, and the lack of phosphorylation of Syk is consistent with the lack of phosphorylation of PLCγ. Furthermore, unlike IgE cross-linking, papain does not elicit significant tyrosine phosphorylation in the total cell lysate (Fig. 4E). Interestingly, the FcRγ ITAM domain still seems to be required for basophil activation, suggesting a role for a permissive signal created by another ITAM-coupled surface receptor or, alternatively, suggesting uncharacterized functions of the ITAM module downstream of nonclassical ITAM-dependent receptors.
IL-3 Enhances the Basophil Response to Papain.
IL-3 has been shown to play many roles in basophil functioning, because it can lead to direct basophil cytokine production (24), play a key role in basophil expansion and survival (28, 29), and also can enhance the stimulatory activity of many basophil activators in vitro (30, 31). Because IL-3 is used as a growth factor in our in vitro basophil cultures and because of its published role in signaling through the FcRγ-chain (24), we wanted to determine if IL-3 is required for responsiveness to papain. A requirement for IL-3 might explain the dependency on FcRγ expression. We determined that although many basophil stimuli have moderately enhanced signaling in the presence of IL-3, depriving cells of IL-3 before stimulation had the greatest effect on papain’s ability to stimulate cytokine production (Fig. 5A). We tested whether papain might cleave the IL-3R, thereby leading to ligand-independent activity of the receptor and activation of IL-4 production. We found that prestimulation of basophils with papain did not prevent IL-3 from signaling through the receptor to phosphorylate Stat5, nor did papain stimulation itself lead to Stat5 phosphorylation (Fig. S4A), suggesting that papain does not cleave the IL-3R itself. To verify that papain does not cleave the FcεRI α-chain, we treated basophils with papain and found that papain treatment did not prevent the binding of IgE to the basophil cell surface (Fig. S4B).
Fig. 5.
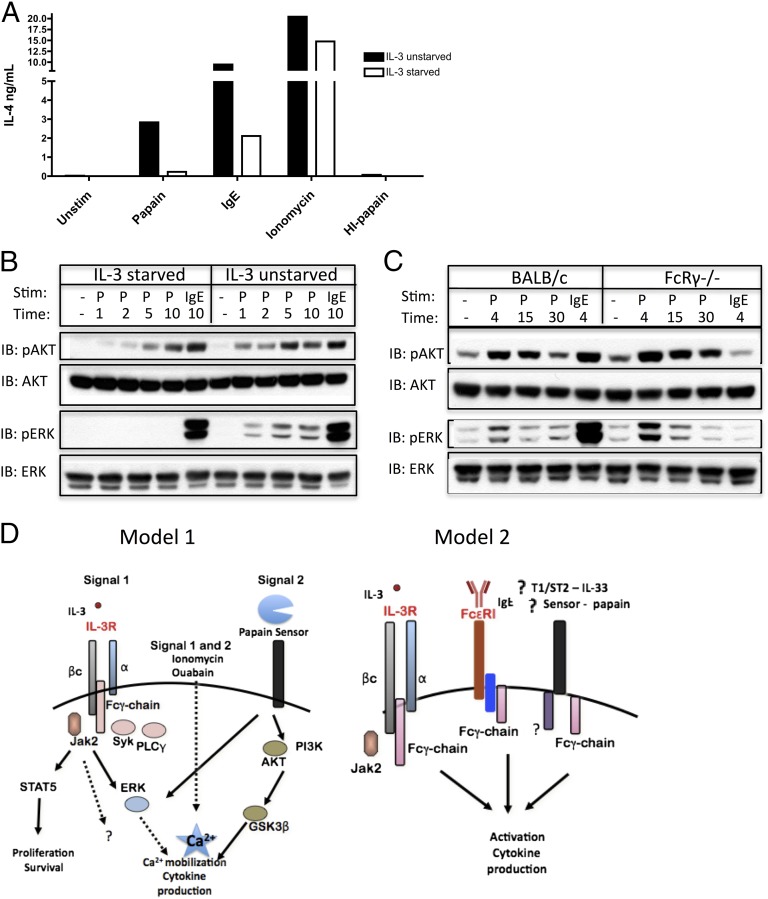
Papain stimulation of IL-4 production by BMBs is IL-3 dependent. Neither IL-3 starvation nor FcRγ deficiency prevents papain from activating the AKT pathway. (A) IL-4 production from BALB/c BMBs either starved of IL-3 or incubated in IL-3–replete medium for 4 h and subsequently stimulated as indicated for 6 h. (B) Immunoblot analysis of pAKT/AKT or pERK/ERK prepared from BMBs that were either starved of IL-3 or unstarved for 4 h and then subsequently stimulated as indicated. (C) BALB/c and FcRγ-deficient BMBs were stimulated as indicated and immunoblotted as in B. (D) Possible models that explain the requirement for FcRγ and IL-3R expression for papain signaling. Data are representative of at least three similar experiments.
Having determined that ERK and AKT are activated downstream of papain stimulation, we wanted to investigate if IL-3–deprived basophils could be stimulated by papain to activate ERK and AKT and whether IL-3–starved basophils would phenocopy the signaling profile of FcRγ-deficient basophils, given that both conditions blocked IL-4 production by papain. Phosphorylation of AKT was intact in IL-3–starved basophils, but papain was unable to phosphorylate ERK in the same cultures, suggesting that IL-3 signaling is required for activation of the ERK pathway by papain (Fig. 5B). Surprisingly, phosphorylation of both AKT and ERK was intact in FcRγ-deficient basophils, suggesting that papain activates more than one signaling pathway in basophils required for IL-4 production (Fig. 5C).
Papain-Induced Basophil Activation Is Independent of Several Common Signaling Pathways.
The studies described above have given insight into the types of pathways activated in response to papain and have suggested the type of receptor triggered by papain cleavage. It is likely that the sensing receptor is not basophil-specific but also senses this protease activity on other cell types. We have considered various potential mechanisms of basophil activation by papain and have tested a number of candidate receptors and signaling pathway components. We evaluated those and other candidates’ contribution to the response by using mice deficient in key signaling mediators (Fig. S5A). Aside from FcRγ, none of these gene deficiencies prevented basophil production of IL-4 in response to papain stimulation in vitro. A role for PAR domain-containing GPCRs, and specifically PAR2 (F2RL1), in sensing exogenous proteases has been discussed in the literature. Interestingly, our data suggest that papain stimulation of basophils is sensitive to pertussis toxin (Fig. S5B), but responsiveness to papain is intact in Gαi2−/− mice, F2RL1−/− mice, and in mice deficient in key signaling mediators downstream of the Gαi-containing GPCRs, PLCβ2,3, and PI3Kγ (Fig. S5D). We addressed the possibility that papain might contribute to ectodomain shedding of a receptor similar to Notch, which is processed by extracellular metalloprotease cleavage and subsequent intracellular cleavage by γ-secretase (32), but prestimulation with γ-secretase inhibitors had little effect on papain-induced IL-4 production (Fig. S5C). It also is possible that papain has a more generalized effect on membrane integrity, similar to the stimuli that activate the caspase-1 inflammasome. Although possible, this mechanism did not seem to operate in vitro, because we found that basophil activation by papain was intact in cells deficient in caspase-1 or an adaptor, Apoptosis-associated speck-like protein containing CARD (Asc) (Fig. S5E). The specific sensing mechanisms of papain-induced cellular activation have yet to be determined.
Discussion
This study provides several important insights into how protease allergens are sensed by innate immune cells. We found that a basophil intrinsic receptor responds to cleavage by papain by producing a key type 2 cytokine, IL-4. Although not shown for all experiments, other type 2 cytokines, such as IL-13, were produced under the same conditions as IL-4. Furthermore, production of these cytokines requires calcium flux, presumably through CRAC channels, and activation of the NFAT family of transcription factors. The role of CRAC channels in mast cell activation and allergic responses has generated much interest, and CRAC channels have been suggested as a drug target for allergy (33). To our knowledge, however, this is one of the first findings of a role for CRAC channels in basophils. The mechanism by which cytokines are induced involves activation of PI3K and phosphorylation of AKT and GSK3-β, which are known to perpetuate NFAT signaling in the nucleus (34). Interestingly, activation of cytokine production requires expression of the FcRγ-chain, including intact expression of its ITAM domain. Papain would not be expected to signal through an Fc receptor, but the FcRγ-chain has been shown to play signaling roles in several non-Fc receptors (35, 36). Surprisingly, FcRγ is not phosphorylated upon stimulation with papain, nor are its downstream mediators Syk and PLCγ. Considering that some papain-induced responses (e.g., IL-4 production), but not all (e.g., AKT and ERK phosphorylation), are impaired in FcRγ-deficient mice, it is likely that the FcRγ-chain is not the only adaptor required for papain sensing in basophils. Consistently, our studies found intact T-cell IL-4 production and basophil lymph node migration upon papain immunization of FcRγ-deficient mice in vivo (Fig. S6), suggesting the existence of additional activation mechanisms in vivo. It is likely other ITAM adaptors can compensate for FcRγ deficiency in vivo.
The data presented here suggest that multiple signals are required to lead to papain-induced type 2 cytokine production: one that induces activation of the PI3K pathway, and another that leads to an increase in cytosolic calcium levels. These signals can be illustrated in two different models (Fig. 5D). In model 1, signal 1, the calcium-inducing signal, is supplied by traditional ITAM signaling through the FcRγ-chain, potentially through signaling of IL-3 through the IL-3R or a yet undetermined FcRγ-coupled receptor. Signal 2 presumably is provided upon cleavage of a putative papain sensor, which is required for GSK3β phosphorylation and increased NFAT activity. Together, signal 1 and 2 are needed for activation of cytokine production. The stimuli that were found to be FcRγ independent, such as ionomycin and α-CD200R3, likely are able to generate significant calcium flux on their own, ionomycin through ER calcium release (37), and α-CD200R3 through DAP12 signaling (26), and therefore do not need the FcRγ ITAM adaptor for the generation of signal 1. In model 2, FcRγ exists as an intrinsic part of a signaling sensor for papain, as found for other stimulatory receptors of basophils, including IL-3, IgE, and perhaps IL-33. We believe that FcRγ signaling is required for calcium mobilization and activation of NFAT in response to papain, because activation of the PI3K pathway is still intact in FcRγ-deficient basophils.
Although the mechanistic details of FcRγ signaling in this setting are unknown, possible calcium-inducing signals include activation of a phospholipase C isoform (38), the sphingosine kinase pathway (39), or direct activation of an ion channel, such as a TRP channel (40). Given that Syk is not activated in our studies, it also will be interesting to determine what signaling adaptors are required for this response. It is possible that tonic signals through the FcRγ-chain are required to make cells permissive to stimulation. A similar concept is illustrated by a requirement for antigen-independent tonic signals through the B-cell receptor for B-cell development (41–43). It has been suggested that isotype-switched antibodies and FcRγ expression play a role in supporting Heligmosomoides polygyrus-induced expansion of basophils (44). This and other studies suggest that expression of FcRγ plays an instrumental role in basophil responses (24), and here we suggest an additional role for FcRγ that is independent of antibody binding.
Many receptors that require calcium signals for the induction of transcription factors such as NFAT do not have a clear mechanism for activating these signals on their own and may require ITAM-dependent costimulatory signals. For example, signaling of RANKL and M-CSF requires the expression of DAP12 or FcRγ for appropriate osteoclastogenesis (45). In general, these receptors may bind directly to ITAM-containing adaptors, or they may couple to ITAM-containing receptors on demand (i.e., upon activation) to accomplish the required calcium signaling indirectly and help achieve a specific response (23).
In conclusion, this study describes signaling pathways activated in response to a cysteine protease allergen. This knowledge may aid in the identification of the protease allergen sensor. More importantly, it may help in designing strategies to modulate these signaling pathways in vivo, so as to prevent their activation and to slow disease processes, because many of the current medical interventions target the effects of allergic reactions on the tissue and not the initiation of the response.
Materials and Methods
Mice.
Mice were bred at the Yale Animal Resources Center at Yale University in specific pathogen-free conditions, and all experiments were done in accordance with approved guidelines, regulations, and protocols as determined by the Institutional Animal Care and Use Committee at Yale University. BALB/c and C57BL/6 mice were purchased from the National Cancer Institute or Jackson Laboratories. TLR4Lps-d, Gata1−/−, Ltb4r1−/−, F2rl1−/−, and CD44−/− mice were purchased from Jackson Laboratories. FcRγ−/− BALB/c mice were obtained from Taconic Laboratories. DAP12−/− bone marrow was kindly provided by Lewis Lanier (University of California, San Francisco); PLCβ2,3−/− and PI3Kγ−/− bone marrow by Dan Wu (Yale University, New Haven, CT); β-arrestin 1−/− and β-arrestin 2−/− bone marrow by Robert Lefkowitz (Duke University, Durham, NC); Trpa1−/− and Trpv1−/− mice by Sven-Eric Jordt (Yale University, New Haven, CT); Gαi2−/− bone marrow by JianPing He (National Institutes of Health, Bethesda); CD1d−/− mice from Peter Cresswell (Yale University, New Haven, CT); and MyD88−/− BALB/c mice by Mark Shlomchik (Yale University, New Haven, CT). Asc−/− (46), Caspase-1−/− (47), 4get mice (IL-4–GFP reporter mice) (48), and MyD88/Trif−/−, MYD88−/−, and TLR2/4−/− mice were bred in our colony and have been described previously.
Reagents and Antibodies.
Papain, E64, ionomycin, cyclosporin A, BAPTA-AM, BTP2, Wortmannin, PD98059, IC87114, and pertussis toxin were purchased from Calbiochem. FK-506 and TGX-221 were purchased from Cayman Chemical Co. IL-3 was purchased from Peprotech, and recombinant mIL-33, mIL-13, and mIL-4 were purchased from R&D Systems. Ouabain, o-phenylenediamine dihydrochloride, complete protease inhibitor tablets, ovalbumin, EGTA, and L-685,458 were purchased from Sigma. The antibody to mCD200R3, clone Ba103, was purchased from Hycult Biotech. GolgiPlug and BD Perm/Wash were from BD Biosciences. The following reagents are from the indicated sources: DX5 beads (Miltenyi Biotec), Ack lysing buffer (Lonza), FCS (Benchmark), Lipofectamine 2000 (Invitrogen), RNA-Bee (Tel-TesT), SMART MMLV RT reagents (Clontech), and SYBR Green QPCR mix (Quanta). Antibodies to FcεRI γ-chain and phosphotyrosine (Clone 4G10) were from Upstate Cell Signaling. Antibodies to pERK, ERK, pAKT, AKT, pPLCγ1, PLCγ1, pPLCγ2, PLCγ2, pSyk, pSTAT5, GSK3β, and pGSK3β were purchased from Cell Signaling. The antibody to β-actin was from Sigma, and Syk was from Santa Cruz. Dx5-PE, Ckit-APC, Dx5-APC, FcεRI-bio, hCD2-PE, CD44-FITC, CD4-APC, Streptavidin–PE-Cy7, Streptavidin-HRP, anti–mIL-4 (clone: 11B11), biotinylated anti–mIL-4 (clone: BVD6-24G2), anti–mIL-13, biotinylated anti–mIL-13, and anti-CD16/32 were from eBioscience, and IL-4-PE, IgE-bio, Streptavidin-PerCP, purified mouse IgE, κ isotype, and anti-mIgE (clone R35-72) were from BD Pharmingen.
Enzyme Inactivation.
Papain was heat inactivated by heating at 100 °C for 1 h. For experiments involving E-64–mediated inactivation of papain in cell supernatants, BMBs were left unstimulated or were stimulated with 100 μg/mL papain for 15 min. Supernatants were left untreated or were treated with 430 μM E-64 for 1 h at 37 °C. Fresh basophils were resuspended in supernatants and incubated for 7 h. Supernatants from basophils that had not been treated with papain were used for IgE stimulations, as a control for E64 toxicity. After fresh basophils were resuspended in these supernatants, they were prestimulated with IgE for 30 min before anti-IgE stimulation. Intracellular IL-4 was detected by flow cytometry.
Bone Marrow-Derived Basophil Cultures.
Mixed mast cell and basophil cultures were derived by incubating total bone marrow cells that had undergone red blood cell lysis with Ack lysing buffer in the presence of RPMI supplemented with 30 ng/mL mIL-3 and 10% FCS as described (9). Briefly, cells were plated at 5 × 106 cells/mL and subsequently were replated on days 3 and 7 at 1 × 106 cells/mL. Cultures were used on day 10–11. DX5+ cells were enriched from these cultures by AutoMACS positive selection.
Basophil Stimulations and Inhibitor Studies.
Macsorted DX5+ BMBs were prestimulated with all inhibitors for 30 min (2 μg/mL Cyclosporin A, 100 nM FK-506, 20 μM BAPTA-AM, 2 mM EGTA, 1 μM BTP-2, 50 nM Wortmannin, 20 μM PD98059, 10 μM PI3K p110β inhibitor TGX-221, 10 μM PI3K p110δ inhibitor IC87114), except for pertussis toxin (100 ng/mL), which was prestimulated overnight, L-685,458 (5 μM), which was prestimulated for 24 h, and EGTA, which was prestimulated for 25 min. DX5+ enriched basophils were resuspended at 2 × 106 cells per mL and stimulated with papain (100 μg/mL), ionomycin (500 ng/mL), heat-inactivated papain (100 μg/mL), ouabain (500 μM), IL-33 (20 ng/mL), and anti-CD200R3 (10 μg/mL). For IgE stimulations, cells were prestimulated with purified mouse IgE, κ isotype (5 μg/mL) for 30 min followed by incubation with purified rat anti-mouse IgE (5 μg/mL).
ELISA.
Supernatants from stimulated Macsorted DX5+ BMBs were added to Nunc Maxisorp 96-well plates that had been coated with purified anti-mouse IL-4 antibody (2 μg/mL) or IL-13 antibody (4 μg/mL) and subsequently were blocked with 1% BSA. Recombinant IL-4 or IL-13 was used as a standard. IL-4 was detected with biotinylated anti-mouse IL-4 (0.5 μg/mL) and IL-13 with biotinylated anti-mouse IL-13 (0.5 μg/mL). Antibody binding was detected with streptavidin-HRP and developed with o-phenylenediamine dihydrochloride.
Flow Cytometry.
BMBs were incubated with anti-CD16/32 to block nonspecific Fc-antibody binding before staining with various directly conjugated or biotinylated surface antibodies. Streptavidin-conjugated fluorophores were used for secondary antibody staining of biotinylated antibodies. For detection of intracellular cytokines, cells were stimulated for a total of 6–7 h with the addition of GolgiPlug 2 h after stimulation. Cells were stained with antibodies to extracellular markers and subsequently were fixed with 4% paraformaldehyde. Fixed cells were permeabilized with BD Perm/Wash before intracellular staining. Cells were analyzed on a FACSCalibur cytometer (BD Biosciences) and analyzed with FloJo software (Treestar).
DNA Construction and Retroviral Transduction.
The wild-type and mutant constructs of FcRγ were designed similarly to those published previously (24, 49) and as we have described in a recent publication (27). The wild-type FcRγ sequence was isolated using basophil cDNA for the PCR. First the product was cloned into the Hind3/EcoR1 sites of the pFLAG–CMV-1 expression vector to introduce an N-terminal FLAG tag into the FcRγ protein. From this construct, transmembrane and ITAM-domain mutants (D11A, L21A, Y47A, Y58A, and Y47,58A) were generated by PCR-based mutagenesis. All products then were subcloned into the modified pMSCV.hCD2 retroviral vector, MIGR2, (kindly provided by Daniel Stetson, University of Washington, Seattle). All retroviral vectors were transduced into FcRγ-deficient bone marrow-derived basophil cultures. Briefly, constructs were transiently transfected along with pCL-Eco for packaging into 293T cells with Lipofectamine 2000 (Invitrogen). After 24 h, 293T medium was exchanged for target basophil medium, and cells were moved to 32 °C for another 24 h to package the virus. Virus-containing supernatants were collected, treated with Lipofectamine (4 μL Lipofectamine/mL supernatant) for 10 min at room temperature, and used to infect day 6 bone marrow-derived basophil cultures. To do so, basophils were resuspended in retrovirus-containing supernatants, plated in six-well plates, and spinfected at 1,000 × g for 90 min (32 °C). The process was repeated the following day with fresh viral supernatants from the original packaging cells. After the second spinfection, the day 7 basophils were plated at 1 × 106 cells/mL in basophil medium. Day 10–11 mixed cell cultures were stained with DX5-APC, FcεRI-bio, and hCD2-PE and were FACSorted to enrich for transduced hCD2+ DX5+ basophils on a Beckman Coulter MoFlo sorter (BD Biosciences) at 30 psi.
RNA Extraction and Quantitative PCR.
Macsorted DX5+ BMBs were plated at 2 × 106 cells/mL. Basophils were stimulated as indicated in respective figures, and total RNA was extracted with RNA-Bee. Total RNA was reverse transcribed with an oligo (dT) primer. cDNA was analyzed by quantitative PCR (qPCR) amplification using SYBR Green qPCR Master Mix on an MX300P qPCR System (Stratagene) with analysis by comparative quantification using MXPro software. Primers were designed to amplify mRNA-specific sequences, and analysis of the melt-curve confirmed the amplification of single products. Unstimulated samples were used as calibrators, and samples were normalized to β-actin or HPRT expression.
Immunoblots.
Macsorted DX5+ BMBs were stimulated as indicated in the respective figures for the indicated time points. Cells were lysed in cold buffer consisting of 20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, 2× complete protease inhibitors, 17.5 mM β-glycerophosphate, 20 mM NaF, 1 mM sodium orthovanadate, and 500 μM E64. Nuclei were spun down, and supernatants were boiled in SDS protein sample buffer before being run on 4–12% gradient SDS/PAGE gels (Novex) and transferred to PVDF membranes (Millipore Corporation). Secondary antibodies were HRP-conjugated goat anti-rabbit or anti-mouse (Jackson ImmunoResearch). Antibodies were detected with ECL reagent (Amersham/GE Healthcare).
Draining Lymph Node Basophil and T-Cell Detection.
FcRγ-heterozygous 4get and FcRγ-deficient 4get mice were immunized s.c. in each hind footpad with 50 μg papain or ovalbumin. Popliteal lymph nodes were isolated and stained 3–4 d after immunization. CD4-PE was used to detect GFP+ T cells; DX5-APC was used to detect GFP+ basophils.
Supplementary Material
Acknowledgments
We thank N. Palm, E. Kopp, and the other members of the R.M. laboratory for advice and useful discussions; L. Lanier for providing DAP12−/− bone marrow; D. Wu for providing PLCβ2,3−/− and PI3Kγ−/− bone marrow; R. Lefkowitz for providing β-arrestin 1−/− and β-arrestin 2−/− bone marrow; J. He for providing Gαi2−/− bone marrow; S. Jordt for providing Trpa1−/− and Trpv1−/− mice; P. Cresswell for providing CD1d−/− mice; and M. Shlomchik for providing MyD88−/− BALB/c mice. This study was supported by the Howard Hughes Medical Institute and by NIH Grants AI08977 and AI046688. R.K.R. was supported by the Yale Medical Scientist Training Program.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418959111/-/DCSupplemental.
References
- 1.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454(7203):445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt M, et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol. 2010;11(9):814–819. doi: 10.1038/ni.1919. [DOI] [PubMed] [Google Scholar]
- 3.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008;181(6):4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenbarth SC, et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196(12):1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trompette A, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457(7229):585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly S, Dalton JP, Loukas A. Proteases in helminth- and allergen- induced inflammatory responses. Chem Immunol Allergy. 2006;90:45–64. doi: 10.1159/000088880. [DOI] [PubMed] [Google Scholar]
- 7.Finkelman FD, Urban JF., Jr Cytokines: Making the right choice. Parasitol Today. 1992;8(9):311–314. doi: 10.1016/0169-4758(92)90105-b. [DOI] [PubMed] [Google Scholar]
- 8.Stewart GA, Thompson PJ. The biochemistry of common aeroallergens. Clin Exp Allergy. 1996;26(9):1020–1044. [PubMed] [Google Scholar]
- 9.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9(3):310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen JE, Wynn TA. Evolution of Th2 immunity: A rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7(5):e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karasuyama H, Mukai K, Obata K, Tsujimura Y, Wada T. Nonredundant roles of basophils in immunity. Annu Rev Immunol. 2011;29:45–69. doi: 10.1146/annurev-immunol-031210-101257. [DOI] [PubMed] [Google Scholar]
- 12.Siracusa MC, Kim BS, Spergel JM, Artis D. Basophils and allergic inflammation. J Allergy Clin Immunol. 2013;132(4):789–801, quiz 788. doi: 10.1016/j.jaci.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motomura Y, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40(5):758–771. doi: 10.1016/j.immuni.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Phillips C, Coward WR, Pritchard DI, Hewitt CR. Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J Leukoc Biol. 2003;73(1):165–171. doi: 10.1189/jlb.0702356. [DOI] [PubMed] [Google Scholar]
- 15.Law M, et al. Structural requirements for the inhibition of calcium mobilization and mast cell activation by the pyrazole derivative BTP2. Int J Biochem Cell Biol. 2011;43(8):1228–1239. doi: 10.1016/j.biocel.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Rao A, Hogan PG. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011;21(2):91–103. doi: 10.1016/j.tcb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lago J, Alfonso A, Vieytes MR, Botana LM. Ouabain-induced enhancement of rat mast cells response. Modulation by protein phosphorylation and intracellular pH. Cell Signal. 2001;13(7):515–524. doi: 10.1016/s0898-6568(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 18.Crabtree GR, Olson EN. NFAT signaling: Choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 19.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuroda E, et al. SHIP represses Th2 skewing by inhibiting IL-4 production from basophils. J Immunol. 2011;186(1):323–332. doi: 10.4049/jimmunol.1002778. [DOI] [PubMed] [Google Scholar]
- 21.Vig M, Kinet JP. Calcium signaling in immune cells. Nat Immunol. 2009;10(1):21–27. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu HS, Angkasekwinai P, Chang SH, Chung Y, Dong C. Protease allergens induce the expression of IL-25 via Erk and p38 MAPK pathway. J Korean Med Sci. 2010;25(6):829–834. doi: 10.3346/jkms.2010.25.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bezbradica JS, Medzhitov R. Role of ITAM signaling module in signal integration. Curr Opin Immunol. 2012;24(1):58–66. doi: 10.1016/j.coi.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Hida S, et al. Fc receptor gamma-chain, a constitutive component of the IL-3 receptor, is required for IL-3-induced IL-4 production in basophils. Nat Immunol. 2009;10(2):214–222. doi: 10.1038/ni.1686. [DOI] [PubMed] [Google Scholar]
- 25.Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leukoc Biol. 2009;86(4):769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima T, et al. Mast cells and basophils are selectively activated in vitro and in vivo through CD200R3 in an IgE-independent manner. J Immunol. 2007;179(10):7093–7100. doi: 10.4049/jimmunol.179.10.7093. [DOI] [PubMed] [Google Scholar]
- 27.Bezbradica JS, Rosenstein RK, DeMarco RA, Brodsky I, Medzhitov R. A role for the ITAM signaling module in specifying cytokine-receptor functions. Nat Immunol. 2014;15(4):333–342. doi: 10.1038/ni.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Didichenko SA, Spiegl N, Brunner T, Dahinden CA. IL-3 induces a Pim1-dependent antiapoptotic pathway in primary human basophils. Blood. 2008;112(10):3949–3958. doi: 10.1182/blood-2008-04-149419. [DOI] [PubMed] [Google Scholar]
- 29.Shen T, et al. T cell-derived IL-3 plays key role in parasite infection-induced basophil production but is dispensable for in vivo basophil survival. Int Immunol. 2008;20(9):1201–1209. doi: 10.1093/intimm/dxn077. [DOI] [PubMed] [Google Scholar]
- 30.Lyngholm JM, Nielsen HV, Holm M, Schiøtz PO, Johnsen AH. Calreticulin is an interleukin-3-sensitive calcium-binding protein in human basophil leukocytes. Allergy. 2001;56(1):21–28. doi: 10.1034/j.1398-9995.2001.00115.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimoto T, et al. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci USA. 1999;96(24):13962–13966. doi: 10.1073/pnas.96.24.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29(5):258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Capite JL, Bates GJ, Parekh AB. Mast cell CRAC channel as a novel therapeutic target in allergy. Curr Opin Allergy Clin Immunol. 2011;11(1):33–38. doi: 10.1097/ACI.0b013e32834232b0. [DOI] [PubMed] [Google Scholar]
- 34.Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3) Trends Immunol. 2010;31(1):24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mócsai A, et al. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7(12):1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zarbock A, et al. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. J Exp Med. 2008;205(10):2339–2347. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 38.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6(3):218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 39.Olivera A, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26(3):287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaffer AL, Schlissel MS. A truncated heavy chain protein relieves the requirement for surrogate light chains in early B cell development. J Immunol. 1997;159(3):1265–1275. [PubMed] [Google Scholar]
- 42.Wienands J, Larbolette O, Reth M. Evidence for a preformed transducer complex organized by the B cell antigen receptor. Proc Natl Acad Sci USA. 1996;93(15):7865–7870. doi: 10.1073/pnas.93.15.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lam KP, Kühn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 44.Herbst T, et al. Antibodies and IL-3 support helminth-induced basophil expansion. Proc Natl Acad Sci USA. 2012;109(37):14954–14959. doi: 10.1073/pnas.1117584109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koga T, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428(6984):758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 46.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24(3):317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Sutterwala FS, et al. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204(13):3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15(2):303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 49.Sakurai D, et al. Fc epsilon RI gamma-ITAM is differentially required for mast cell function in vivo. J Immunol. 2004;172(4):2374–2381. doi: 10.4049/jimmunol.172.4.2374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.