Significance
Traditional immunizations involve the controlled introduction of attenuated bacteria or viruses, allowing for generation of immunity prior to exposure to the dangerous native pathogen. In contrast, subunit immunization utilizes only pieces of the pathogen combined with a separate immune stimulatory agent (adjuvant). Although subunit immunizations do generate effective neutralizing antibodies, they do not generate robust T-cell responses. T cells provide therapeutic benefit by directly inducing cell lysis and shaping the immune response through soluble proteins (cytokines) critical for intervening in cancer and viral infection. Here, we demonstrate that subunit vaccines are uniquely and unexpectedly dependent on the cytokine IL-27 for making strong T-cell responses.
Abstract
An elusive goal of cellular immune vaccines is the generation of large numbers of antigen-specific T cells in response to subunit immunization. A broad spectrum of cytokines and cell-surface costimulatory molecules are known to shape the programming, magnitude, and repertoire of T cells responding to vaccination. We show here that the majority of innate immune receptor agonist-based vaccine adjuvants unexpectedly depend on IL-27 for eliciting CD4+ and CD8+ T-cell responses. This is in sharp contrast to infectious challenge, which generates T-cell responses that are IL-27–independent. Mixed bone marrow chimera experiments demonstrate that IL-27 dependency is T cell-intrinsic, requiring T-cell expression of IL-27Rα. Further, we show that IL-27 dependency not only dictates the magnitude of vaccine-elicited T-cell responses but also is critical for the programming and persistence of high-affinity T cells to subunit immunization. Collectively, our data highlight the unexpected central importance of IL-27 in the generation of robust, high-affinity cellular immune responses to subunit immunization.
The efficacy of vaccination exploits the highly specific adaptive arm of the immune response. To date, the objective of most clinical-use vaccines has been the generation of high titers of antigen-specific neutralizing antibodies. Initially antibody production was achieved through direct exposure to attenuated pathogens. However, a host of issues (manufacturing, stability, toxicity, and virulence) limit the use of these types of vaccines. An alternative strategy constructs vaccines using only strategic portions of pathogens combined with innate immune agonists. These subunit vaccines are more stable, versatile, and safe relative to traditional attenuated pathogen vaccines. Combined, these platforms have saved countless lives in a little over 200 years of practiced vaccinology. Despite this success, vaccination has been unable to consistently achieve medically meaningful responses against most solid tumors and several persistent viral infections (i.e., HIV and hepatitis C). Interestingly, the major correlate for sterilizing immunity to both viral and tumor challenge is not antigen-specific antibody titer but rather the number of antigen-specific T cells generated, known as cellular immunity (1). Unfortunately, T-cell responses to subunit immunization typically require multiple boosts to achieve even detectable antigen-specific T-cell numbers, which often have little clinical impact. As such, identifying the factors that dictate the magnitude of antigen-specific T cells in response to immunization is of paramount importance.
Classically, robust CD4+ and CD8+ antigen-specific T-cell responses are dependent upon multiple inputs derived from various kinds of receptors on the T-cell surface (2–5). Particular cytokine receptors, such as the type I interferon receptor and IL-12R, execute targeted up-regulation of key transcription factors necessary for supporting T-cell expansion and the initiation of both T-cell effector and memory-fate programs (6, 7). Encounters that produce longstanding cellular immunity induce a balanced cytokine milieu, using both stimulatory (STAT1) and suppressive (STAT3) signaling pathways. IL-27 is a member of the IL-12 family of cytokines and, via its signaling through both STAT1 and STAT3 (8–12), contributes to a spectrum of T-cell functions and phenotypes. Although in vitro studies demonstrate a role for IL-27 in CD4 Th1 differentiation, IL-27 deficiency in vivo also leads to severe inflammatory immunopathology in parasite/pathogen infection models as well as in vaccination-induced autoimmunity (13–16). Additionally, IL-27 displays different effects on CD4+ and CD8+ T-cell responses, enhancing tumor-specific CD8+ T-cell responses (17–19) while also inducing IL-10-producing CD4+ T cells (13, 20, 21) and Tregs (22).
We report here an unexpected and central requirement for T cell-intrinsic IL-27 signaling in the generation of maximal T-cell responses to subunit vaccination. Besides dictating the overall magnitude of the T-cell response, IL-27 was also required for the survival of high-affinity antigen-specific cells. In the absence of IL-27, the pool of memory T cells was of lower affinity, was of reduced effector function, and was less protective on a per-cell basis against infectious challenge. Importantly, these observations are unique to subunit immunization because the T-cell responses to infectious challenge remain intact in an IL-27Rα–deficient environment. Furthermore, the influence of IL-27 on CD8 T-cell expansion, affinity, function, and memory programming was mediated via a STAT1/3-dependent mechanism. Collectively, these observations point to a unique and previously unappreciated role for IL-27R signaling on T cells in response to subunit vaccination.
Results
Vaccine Adjuvant-Elicited Cellular Immunity Is Dependent on IL-27Rα Signaling in T Cells.
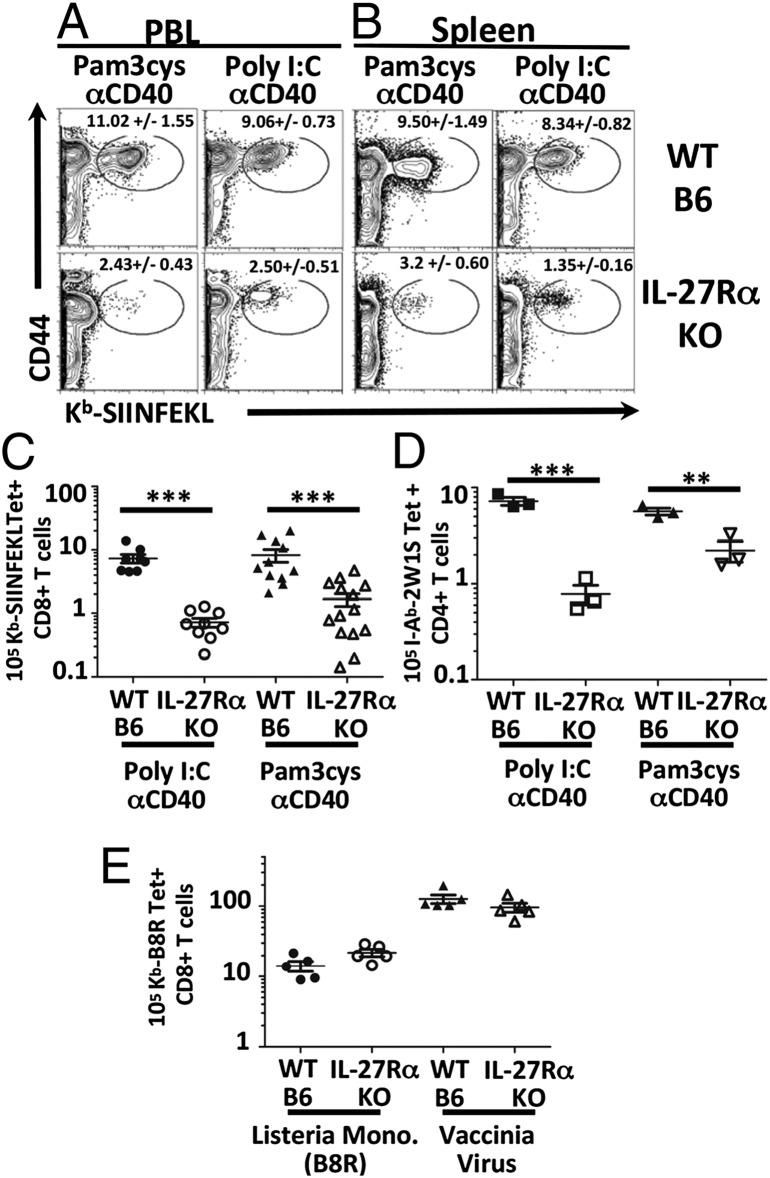
Previously we reported on large, durable antigen-specific CD4+ and CD8+ T-cell responses generated through the combined used of Toll-like receptor (TLR) and CD40 agonists (combined TLR/CD40 vaccination) (23, 24). In contrast to other forms of vaccination/immunization, T-cell intrinsic stimulation from classical Signal 3 cytokines such as type I IFN or IL-12 were not required for T-cell expansion, polarization, or memory generation (25, 26). Literature searches for other cytokines capable of dramatically influencing T-cell polarization/differentiation drew attention to the IL-12 family member IL-27 (11, 27, 28). To investigate the potential role of IL-27 signaling in response to our subunit vaccination (TLR/CD40), IL-27RαKO and wild-type (WT) mice were immunized with antigen in the context of either Poly I:C/αCD40 or Pam3cys/αCD40, and the magnitude of the antigen-specific T-cell responses was monitored by tetramer staining at the peak of the response (day 7). Surprisingly, we found a significant role for IL-27 in mediating the maximal T-cell response to this vaccination. The CD8+ T-cell response in the IL-27RαKO mice was substantially reduced (∼5- to 10-fold) in both the peripheral blood (Fig. 1A) and spleen (Fig. 1B) to either vaccination, compared with WT hosts. This reduction was apparent based on the assessment of the percentage and total numbers of antigen-specific CD8 + T cells (Fig. 1C). This IL-27 dependency was not solely a feature of the CD8+ response, being also observed in the CD4+ T-cell response to the 2W1S peptide antigen (29) (Fig. 1D). Thus, both CD8+ and CD4+ T-cell responses to combined TLR/CD40 vaccination are dependent upon IL-27.
Fig. 1.
Combined agonist TLR/CD40 vaccination-induced T-cell responses are IL-27Rα–dependent. IL-27RαKO and WT mice were immunized with Pam3Cys/αCD40 or Poly I:C/αCD40, and ova. Seven days postimmunization (dpi), T-cell responses in the peripheral blood lymphocytes (PBL) (A) and spleens (B) were determined by tetramer stain as described in SI Materials and Methods. All data gated on B220−CD3+CD8+ cells. (C) Number of splenic antigen-specific T cells. Data shown are representative of at least five independent experiments. (D) IL-27RαKO and WT mice immunized as in A except with I-Ab binding peptide 2W1S (39) and stained I-Ab/2W1S tetramer on CD3+CD4+B220− gated events. Data shown are representative of two independent experiments. (E) Numbers of CD8+ B8R-specific T cells in WT B6 and IL-27RαKO mice challenged with Lm-B8R, or with vaccinia virus (Vv) and stained with Kb-B8R tetramer. Data shown are representative of three independent experiments.
These data were in apparent contrast to previous reports where IL-27 deficiency in vivo more often led to an elevation in the magnitude of T-cell response (13, 15, 22, 30). However, these published data monitored T-cell responses during infectious challenge, raising the possibility that the IL-27 dependence might be unique to the cellular response elicited by subunit vaccination. We therefore next examined the CD8+ T-cell responses in WT and IL-27RαKO mice to primary challenge with either Listeria monocytogenes or vaccinia virus. Consistent with previous reports, there was no defect in the CD8+ T-cell response to either of these pathogens in IL-27RαKO mice (Fig. 1E). These data therefore demonstrate a significant, but selective, IL-27 dependency of the CD4+ and CD8+ T-cell responses elicited after combined TLR/CD40 vaccination but not infectious challenge.
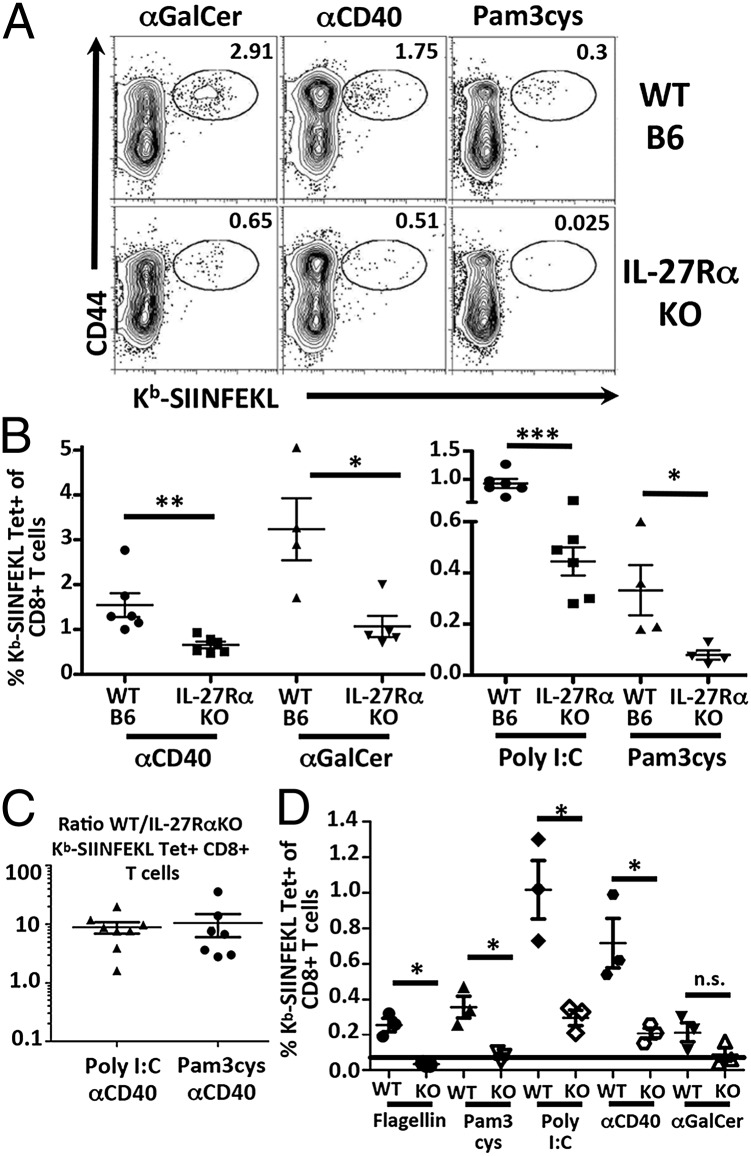
Modern subunit vaccines may be composed of one or multiple agonists for innate receptor pathways. As the response to both Pam3/αCD40 and Poly I:C/αCD40 were IL-27–dependent, we questioned whether this dependency was applicable only to combined adjuvants using αCD40 or whether a broad range of innate receptor agonists shared this trait. Therefore, WT and IL-27RαKO mice were immunized with antigen in the context of single adjuvants, and the T-cell responses were analyzed by tetramer staining of cells in the blood and spleen as before. Surprisingly, all single adjuvant-treated IL-27RαKO mice showed a reduced percentage of antigen-specific T cells (Fig. 2 A and B). IL-27 dependency applied to more robust single adjuvants, able to produce 1–3% antigen-specific T cells in the WT host (αCD40, αGalCer), as well as to adjuvants that produce overall weaker (<1%) cellular responses (Poly I:C, Pam3Cys). These data therefore demonstrate a previously unappreciated central and broad requirement for IL-27 in multiple vaccine adjuvant-elicited cellular immune responses.
Fig. 2.
IL-27Rα dependency of subunit vaccine-elicited T-cell responses is T cell-intrinsic. (A and B) WT and IL-27RαKO mice immunized with ova in conjunction with indicated innate receptor stimulus. Seven dpi, spleens were harvested and stained with Kb-SIINFEKL tetramer as in Fig. 1. Data show are representative of three independent experiments for each adjuvant. (B) B6-Ly5.2 (CD45.1) were irradiated and reconstituted with bone marrow from IL-27RαKO (CD45.2) and B6 (CD45.1) donors as described in SI Materials and Methods. Twelve weeks after reconstitution, mice were immunized with Pam3cys/αCD40 or Poly I:C/αCD40 (C), or with the indicated single adjuvants (D). Seven dpi, spleens were harvested and stained with Kb-SIINFEKL tetramer and CD45.1. (C) Representative of four independent experiments. (D) Representative of two independent experiments. Black line denotes limit of detection for tetramer.
Potent immune modulation by IL-27 has been observed within both T cells and dendritic cells (DCs) (31). It was therefore feasible that our observed IL-27 dependency of the T-cell response to vaccine adjuvants could be due to a requirement for IL-27 stimulation of T cells, DCs, or a combination of different cell subtypes (32). To understand which cell type required IL-27R signaling for the cellular response to vaccination, we generated IL-27RαKO:WT mixed bone marrow chimeras (BMCs). These hosts have both WT and IL-27RαKO DCs and T cells, allowing the assessment of the T-cell dependency of IL-27 signaling in an environment that has competent, WT antigen-presenting cells. Vaccination of these chimeric mice with either dual agonist vaccine (Fig. 2C) or single adjuvants (Fig. 2D) recapitulated the impairments in the CD8+ T-cell compartment observed in the IL-27RαKO mice regardless of the innate receptor agonist used. Although these data do not eliminate the possibility that IL-27 signaling may also be important for some aspects of DC activation/maturation, the disparity between WT and knockout (KO) CD8+ T-cell responses within the same host demonstrates that IL-27 dependency of the vaccine-elicited T-cell response is T cell-intrinsic.
IL-27 Signaling Shapes the Affinity Distribution of T cells.
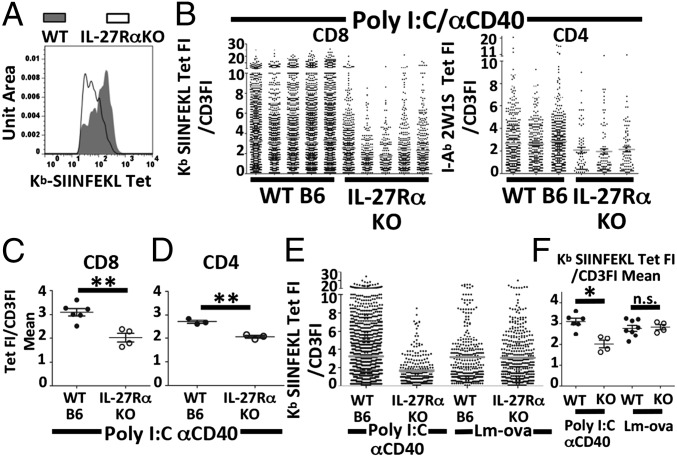
In addition to the overall numbers of tetramer-positive cells being dramatically reduced, we observed that the residual pool of tetramer-staining T cells in the IL-27Rα–deficient host had a lower level of tetramer fluorescence intensity (FI) compared with the WT cells (Fig. 3A). However, because tetramer staining varies directly as a function of TCR levels, this lower tetramer staining could be the result of lower TCR expression in the IL-27RαKO host. We therefore adopted two methodologies for normalizing our data for relative quantities of surface TCR complex. A commonly used method restricts analysis of tetramer fluorescence to narrow ranges of CD3 expression (Fig. S1). After controlling for TCR levels in this fashion, statistically significant differences between WT and IL-27RαKO tetramer mean fluorescence intensity (MFI) remained (Fig. S2), indicating a broad difference in affinity between WT and IL-27Rα deficient cells.
Fig. 3.
High-affinity T cells responding to vaccination are dependent on IL-27 and STAT3. Indicated mice were immunized, and T-cell responses were analyzed as described in Fig. 1. (A) Tetramer fluorescence intensity histogram for tetramer-positive cells by gating on B220−CD3+CD8+CD44Hi Kb SIINFEKL tetramer plus cells for either WT (gray filled) or IL-27RαKO (white open). (B) Output scatter plot of tetramer FI/CD3FI for each tetramer-positive event as described in Results. (C) Display of the mean tetramer FI/CD3FI for each mouse. (D) CD4 T cells immunized as described in Fig. 1 with 2W1S peptide. Statistical significance determine by t test. Data shown for A–F are representative of three independent experiments. (E and F) WT or IL-27RαKO immunized with Poly I:C/αCD40 or 2,000 cfu of Lm-ova.
This form of analysis is limited however, taking into account only the events within each CD3 gate. We therefore confirmed our results by correcting for CD3 levels on a per-cell basis. We defined an output parameter that divided the tetramer FI for each event by the CD3 FI for that event (tet FI/CD3 FI). This parameter allowed for the inclusion of a data point for every detectable antigen-specific T cell. Plotting these ratios for each tetramer-positive cell for each mouse confirmed a paucity of high-affinity (high tetramer/CD3 ratio) tetramer-positive cells in both the CD4+ and CD8+ T-cell pools from IL-27RαKO mice in response to combined TLR/CD40 immunization (Fig. 3B). To concisely represent and statistically validate these observations, the average TetFI/CD3FI for each mouse was determined. These average ratio values were then compared between cohorts by t test. This unbiased method of analysis confirmed significant differences in the affinity distribution of the responding CD8+ and CD4+ T cells in WT and IL-27RαKO hosts (P value < 0.01) (Fig. 3 C and D). This conclusion was further supported through results obtained by more traditional tetramer disassociation assays (Fig. S3 A–D). The reduced affinity of responding T cells in the IL-27Rα–deficient host was not due to an altered naive repertoire because Lm-ova–challenged IL-27RαKO mice produced T cells with a broad distribution of tetramer/CD3 ratios (Fig. 3E). The overall average affinities of IL-27RαKO and WT T cells in Lm-ova–challenged mice were statistically indistinguishable from one another and from WT mice immunized with Poly I:C/αCD40 (Fig. 3F). Thus, IL-27 signaling influences the generation of high-affinity antigen-specific CD4+ and CD8+ T cells, but only in response to subunit vaccination.
IL-27 Alters Programming and Effector Function of T Cells in Response to Subunit Immunization.
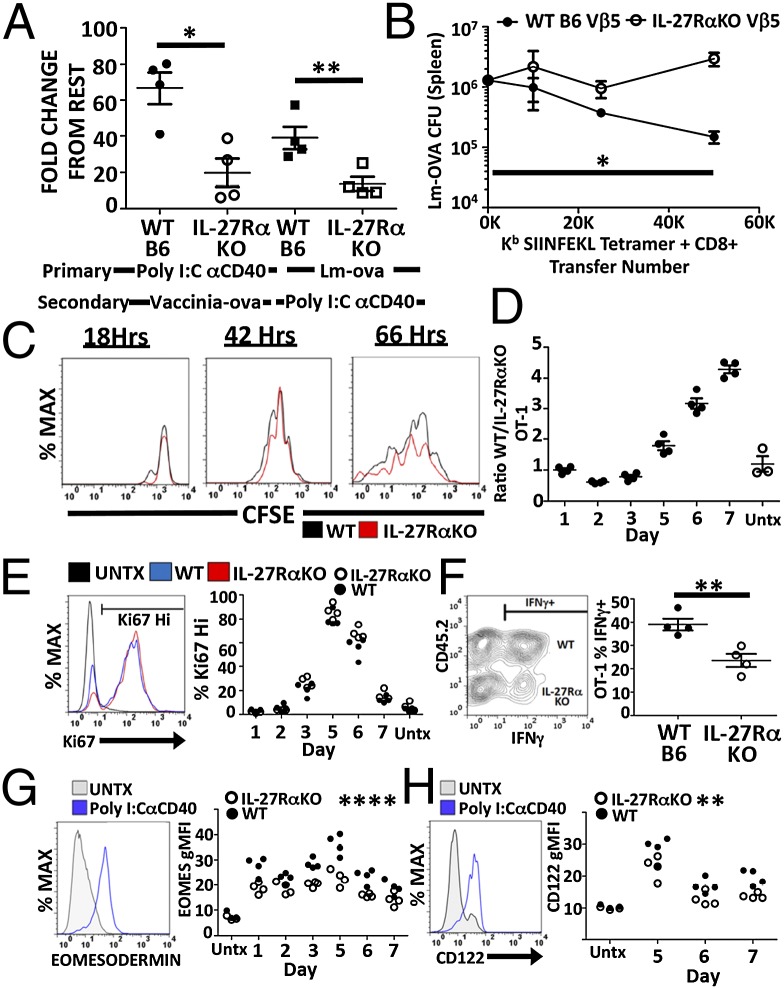
The larger goal of vaccination is the generation of long-lasting, protective immune memory. Our data thus far have characterized defects in the primary T-cell numbers in response to subunit vaccination in the absence of IL-27 signaling. We therefore examined immune memory formation and function in the IL-27RαKO/WT mixed BMCs. IL-27RαKO/WT BMCs were given a primary immunization with Poly I:C/αCD40 (IL-27–dependent) or Lm-ova (IL-27–independent). Fifty days later, the mice were boosted with either Vv-ova or Poly I:C/αCD40, and the secondary T-cell response was observed 5 d later. Two observations are noteworthy. First, despite having similar resting memory populations (Fig. S4), IL-27Rα–deficient T cells from Poly I:C/αCD40-primed mice showed defective secondary expansion to Vv-ova challenge, compared with their WT counterparts (Fig. 4A). Thus, in the absence of IL-27 signaling, primary subunit vaccination results in a deficit in memory programming. Second, the secondary response of IL-27Rα–deficient T cells was also reduced in mice primed with Lm-ova and boosted with Poly I:C/αCD40 (Fig. 4A). These data indicate that even pathogen-elicited memory T cells also have an acute requirement for IL-27 signaling in response to subunit vaccination. Importantly, IL-27RαKO T cells in these chimeras respond comparable to WT cells if primed with Lm-ova and boosted with VV-ova (Fig. S5), once again reinforcing the unique role of IL-27 in subunit vaccination compared with infectious challenge.
Fig. 4.
IL-27 shapes T-cell memory, function, and survival. (A) Memory expansion assay of WT and IL-27RαKO mixed BMCs were generated as described in Fig. 2 and 12 wk after reconstitution were immunized as indicated. Fifty dpi, mice were boosted with either Vv-ova or Poly I:C/αCD40/ova. Fold expansion was calculated as fold increase of the percentage of tetramer-positive cells from rest to peak of secondary response (day 5). (B) Lm-ova cfu in mice transferred with indicated numbers of WT or IL-27RαKO antigen-specific memory T cells as described in SI Materials and Methods. (C–H) WT (CD45.1/2) and IL-27RαKO (CD45.1) OT-1s were cotransferred into WT B6 (CD45.2) hosts and immunized with Poly I:C/αCD40/ova. Mice were killed at the time points indicated, and OT-1s were compared for (C) CFSE dilution days 1–3, (D) ratio between WT/IL-27RαKO, (E) proportion of cycling cells by % Ki67, (F) IFNγ production by intracellular cytokine staining 7dpi, (G) eomesodermin by intracellular transcription factor antibody staining, and (H) CD122 expression by surface stain and flow cytometry. A–H are representative data from single experiments repeated at least twice with an n greater than or equal to 3 for each group. Statistics determined within an experiment comparing WT and IL-27RαKO groups. For C–H, single time point significance was determine using paired Student t test whereas time course significance was determined by two-way ANOVA.
To demonstrate the impact of all of these factors in aggregate, we compared the protective capacity of antigen-specific WT and IL-27RαKO T cells in response to an infectious challenge. Equal numbers of either WT or IL-27RαKO antigen-specific memory T cells (generated by Poly I:C/CD40 vaccination) were transferred into congenically disparate naive WT B6 mice and subsequently challenged with a lethal dose of Lm-ova. Five days later, mice were killed, and the bacterial load in the spleens was determined as a measure of the protective capacity of the transferred cells (Fig. 4B). Mice receiving IL-27RαKO T cells showed elevated bacterial load over a broad range of transferred T cells, indicating that IL-27Rα–deficient T cells are less protective on a per-cell basis relative to WT cells. Collectively, we conclude from these data that vaccine-induced protective T-cell memory is compromised in the absence of IL-27.
IL-27 signaling after subunit vaccination could conceivably be influencing T-cell proliferation, survival, differentiation, or some combination of all of the above. To detect which of these factors was influencing the abundance of antigen-specific T cells, we transferred equal numbers of congenically marked WT (CD45.1/2) and IL-27RαKO (CD45.1/1) ova-specific TCR-transgenic T cells (OT-1) into B6 recipients (CD45.2/2) and subsequently immunized them with Poly I:C/αCD40/ova. The response of the transferred T cells was then monitored each day to determine the functional consequences of IL-27 deficiency on early events in the T-cell response. By transferring carboxyfluorescein succinimidyl ester (CFSE)-labeled OT-1s, we were able to observe that IL-27RαKO T cells had no deficit in antigen recognition or cellular division because they display similar numbers of divisions as WT OT-1s throughout the first 2–3 d after immunization (Fig. 4C). However, by monitoring the ratio of WT/IL-27RαKO OT-1s, we observed a striking increase in the ratio of WT to IL-27RαKO OT-1s between the peak of the OT-1 response at day 5 and growing through day 7 (Fig. 4D). Thus, either the survival of IL-27RαKO T cells was compromised past day 3 or their division was reduced compared with WT. Intracellular staining for the cell cycle-regulated protein Ki67 showed no differences at any time between WT and IL-27RαKO OT-1s in the proportion of cells undergoing division (Fig. 4E). We therefore concluded that it was likely that survival of IL-27Rα–deficient T cells is compromised at late times after subunit vaccination.
We next examined the role of IL-27 in shaping the effector functions and transcriptional profile in response to the subunit immunization. Intracellular cytokine staining of the cotransferred WT/IL-27RαKO OT-1 T cells 7 d after vaccination revealed a deficiency in IFNγ production by IL-27RαKO T cells (Fig. 4F). No deficits in granzyme A or granzyme B were noted, and all of these observations were consistent with previous reports of IL-27Rα deficiency in other systems (33, 34). Although alterations in expression levels of a variety of transcription factors [IRF4, Tbet, Gata3, c-MAF, Blimp-1, Bcl6, and eomesodermin (Eomes)] were easily observed between naive and vaccine-experienced T cells, the only transcription factor that showed a difference between WT and IL-27RαKO OT-1s in expression profile was Eomes. IL-27RαKO OT-1 T cells had an early and persistent decrease in Eomes expression (Fig. 4G), consistent with previous reports demonstrating the ability of IL-27 to amplify Eomes expression via STAT3 (28, 35). We confirmed the functional significance of this reduced Eomes expression by observing a subsequent reduction in the expression of CD122 (Fig. 4H), a protein known to be regulated by Eomes and to support the proliferation and cell survival of T cells via IL-2/15 signaling (36, 37).
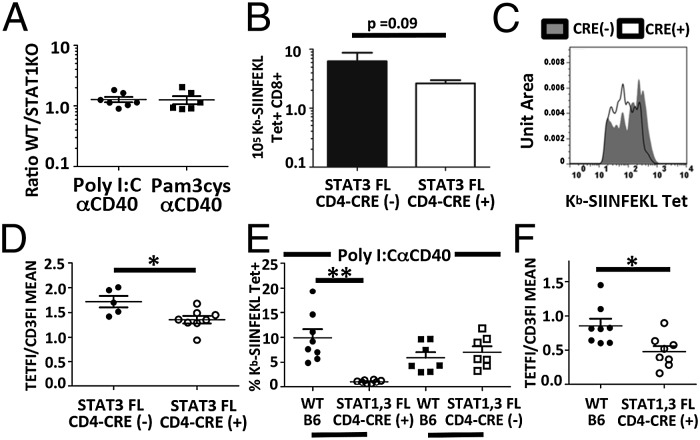
The functional IL-27R, composed of the unique IL-27Rα and shared gp130 subunits, allows signaling through both STAT1- and STAT3-dependent signaling pathways. To address whether either of these signaling pathways was responsible for the unique defects we have observed in IL-27RαKO mice, we immunized mice with T cells deficient in STAT1 or STAT3. Due to the known effects of STAT1 deficiency within dendritic cells on antigen presentation and T-cell costimulation (38, 39), we generated STAT1KO/WT mixed bone marrow chimeras and examined the magnitude and phenotype of STAT1KO T cells compared with WT T cells. In the chimera, we observed no differences in the magnitude (Fig. 5A) or in the affinity distribution between STAT1KO and WT T cells in response to either Pam3/αCD40 or Poly I:C/αCD40 immunization. Similarly, we found in experiments using conditional deletion of STAT3 within the T-cell compartment (STAT3 fl × CD4 cre) (40), the magnitude of the T-cell response to subunit vaccination was largely unaffected (Fig. 5B). Interestingly, however, the responding CD8+ T-cell pool did show a skewed affinity distribution similar to that observed in IL-27RαKO T cells (Fig. 5 C and D), indicating the importance of STAT3 in mediating IL-27–mediated expansion/survival of high-affinity T cells.
Fig. 5.
STAT1 and STAT3 deficiency in T cells recapitulates IL-27RαKO deficiency. (A) STAT1KO:WT BMCs were immunized as indicated, and the relative percentage of tetramer plus cells was determined 7 dpi. (B–D) Seven dpi, STAT3 fl mice ± CD4-Cre immunized with Poly I:C/αCD40/ova were analyzed for (B) number of tetramer plus cells. (C) The tetramer staining histograms of tetramer plus cells from STAT3 fl plus cre (white) and minus cre mice (filled) 7 dpi overlayed. (D) Ratio of tetramer FI/CD3 FI was determined as described in Fig. 3 and Results. (E and F) STAT1fl × STAT3 fl ± CD4 Cre:WT BMCs were immunized with Poly I:C/αCD40 and examined 7 dpi in blood for (E) the percentage of tetramer plus CD8+ T cells and (F) TetFI/CD3FI. Data displayed are from single experiments (A–F). A–D were repeated two or more times with n greater than 3 for each experiment. E and F were powered to determine n required for determination of statistical significance. Significance was determined by unpaired (A–D) and paired (E and F) Student t tests.
To address the possibility that STAT1 and STAT3 share redundant functions downstream of IL-27R signaling, we generated STAT1 fl × STAT3 fl × CD4 CRE (+/−) mice. Due to concerns with a more general STAT1 defect in the STAT1 fl mice (41), we again generated congenic mixed BMCs using WT bone marrow mixed with either Cre-sufficient or Cre-deficient STAT1 fl × STAT3 fl mice. As before, BMCs were immunized with Poly I:C/αCD40, and the magnitude and affinity distribution of the responding CD8+ T cells were analyzed. In contrast to the loss of either STAT1 or -3 alone, T cells deficient in both STAT1 and -3 showed a reduction in the magnitude of the T-cell response similar to that observed in the IL-27RαKO T cells (Fig. 5E). This reduction was not observed for cre-STAT1/3 fl/fl T cells (Fig. 5E), indicating once again that STAT1/3 dependency was T cell-intrinsic. Similar to both IL-27RαKO and STAT3 KO T cells, the affinity distribution of the CD8 T-cell response in the STAT1/3 double deficient T cells showed a loss of high-affinity responders (Fig. 5F). Taken together, our data reveal both unique and redundant roles for STAT1 and STAT3 in IL-27–mediated survival and affinity distribution for T cells responding to subunit vaccination.
Discussion
Our data identify a previously unobserved, highly selective, nonredundant role for IL-27 in instigating the survival of subunit vaccine-elicited cellular immune responses, contrasting with the role for IL-27 in pathogen-elicited cellular immunity. Consistent with previously published literature (33, 34, 36), IL-27 has effects on T-cell effector function (IFNγ) and transcriptional profile through the augmentation of expression of eomesodermin. In light of the highly pleiotropic nature of IL-27 signaling in both inflammation and immune regulation, we uncovered a surprisingly consistent and dramatic effect of IL-27 on the magnitude of T-cell responses across a spectrum of vaccine adjuvant-elicited cellular responses. Our data join only a few existing publications (9, 19, 42) directly demonstrating in vivo a direct positive role of IL-27R signaling in nonregulatory T-cell phenotypes. Many examples exist in the literature with indirect evidence of a positive role for IL-27 in enhancing CD8 T-cell responses, particularly in cancer therapy (17, 18), but rarely is the direct effect of IL-27 on T cells examined.
More recently, another report (9) also demonstrated an in vivo, T cell-intrinsic requirement for IL-27R signaling in the preservation of activated effector CD4 T-cell numbers undergoing homeostatic expansion. In this report, IL-27 mediated the survival of activated T cells through up-regulation of cFLIP, which counteracted FAS-mediated caspase 8 activation. Thus far, we have observed that none of the classical instigators (Fas; caspase-1, -3, or -8; and Bim) or inhibitors (Bcl2 and BclxL) of caspase-mediated cell death appear to be involved in IL-27–mediated T-cell survival after subunit vaccination. In fact, observed decreases in active caspase-8, -3, and -1 in IL-27RαKO OT-1 T cells suggested less cell death by extrinsic apoptosis, intrinsic apoptosis, and pyroptotic pathways, respectively.
Although these observations leave open the question as to how IL-27 mediates T-cell survival in the setting of subunit vaccination, our observation of decreased Eomes/CD122 in IL-27RαKO T cells may be consistent with a role for these molecules in survival and metabolic regulation of proliferating T cells. During rapid growth, such as that experienced by T cells after antigenic stimulation, T cells switch their energy metabolism from favoring oxidative phosphorylation (TCA cycle) to aerobic glycolysis (43), a process that can be enhanced by IL-15 signaling through CD122 (44). Cells that are stuck favoring the TCA cycle and unable to meet increased energy demands are more likely to die from necroptosis (45). Necroptosis is another form of programmed cell death often mediated by the protein kinases RIPK1 and RIPK3 (46, 47) and enhanced by the presence of reactive oxygen species. Importantly, necroptotic pathways have been shown to be in direct opposition to apoptotic and pyroptotic cell death. This pathway is consistent with our preliminary caspase observations in IL-27Rα–deficient T cells responding to subunit vaccination. Whatever cell death pathway is involved, our data indicate that its prevention requires STAT1/3 signaling.
Our use of both IL27RαKO and STAT1/3 KOs in mixed bone marrow chimeras not only confirms the T-cell intrinsic dependency of IL-27 and STAT1/3 but also demonstrates the exclusive dependency of the vaccine-elicited response on IL-27. A host of STAT1/3-signaling cytokines are induced during the course of vaccination, some of which (IL-21, IL-10) are downstream of IL-27 (13, 21, 48, 49). In the absence of the mixed BMC data, it could be argued that IL-27 is simply the initial cytokine in a cascade of STAT1/3-dependent cytokines. Indeed, Braciale and co-workers demonstrated that, in the response to influenza, IL-27 acts directly on CD4 T cells to induce their production of IL-10, which ultimately affects the magnitude of the primary response and the differentiated state of the memory cells (21). However, because WT-derived T cells in the mixed chimeras did not provide any rescuing effect to IL-27Rα–deficient T cells, this argues against any causal role for paracrine cytokine factors. Consistent with this, abrogation of IL-6 (50) or IL-10 (Fig. S6), both STAT3 cytokines, had no impact on the vaccine-elicited T-cell response. Thus, not only is the dependency of IL-27 subunit-elicited cellular immunity inverse from what is observed in primary T-cell responses to pathogen, but the pathways downstream of IL-27 are not conserved between pathogen exposure and subunit immunization.
Perhaps even more surprising is the central importance of IL-27 signaling via STAT3 for the response of high-affinity T cells. STAT3 signaling often participates in resolving inflammation and suppressing immune responses and was recently shown to be critical for generation of memory CD8 T cells, ultimately through induction of SOCS3 and suppression of Tbet (51). These and other studies indicate that unabated proinflammatory signals lead to terminal-effector differentiation and immune exhaustion. Seen in this context, STAT3 signals might facilitate the survival/transition of high-affinity T cells into long-lived memory T cells, as has been demonstrated in both mouse and human (16, 19, 51–53). In our system, IL-27, signaling through STAT3, would serve as the immunological restraint, critically dampening the higher perceived signal within the higher-affinity T-cell clones.
Finally, it is interesting to note that, in the absence of IL-27Rα, responses to the combined TLR/CD40 adjuvant platform more closely resemble the magnitude of responses of WT mice to single adjuvants. Consistent with this, use of recombinant IL-27 increases antigen-specific T-cell responses above use of αCD40 alone (Fig. S7). However, these responses are well short of the 50- to 100-fold increases reported previously from combined TLR/CD40. This failure to recapitulate the magnitude of the T-cell response to Poly I:C/αCD40 indicates that IL-27 cannot completely replicate the complex inflammatory environment instigated by the TLR agonist. We and others previously published on an important role for CD70–CD27 interactions in the T-cell response to combined TLR/CD40 immunization (24, 25, 54) as well as to infectious challenge/vaccination (55–58). Because IL-27 and CD27 separately are required for maximal T-cell responses after subunit vaccination, neither alone is therefore sufficient for maximal T-cell expansion. All available data suggest that it is the synchronized delivery of signals through these two signaling pathways that is at the heart of the combined TLR/CD40 subunit vaccine adjuvant potency. Thus, we propose that future efforts at novel vaccine adjuvant discovery would do well to monitor the kinetics and magnitude of DC expression of IL-27, as well as the ligand for CD27, CD70.
Materials and Methods
Specific methods concerning mice, reagents, immunizations, flow-cytometric methodology, infectious challenge and immunologic protection assay, production of mixed bone marrow chimeras, adoptive T-cell transfers, and statistical analysis are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Catherine Haluszczak for generation of PE- and BV421-conjugated Kb tetramers and covalently loaded Kb-SIINFEKL tetramers. N.D.P. is supported by National Research Service Award Training Grant T32-AI007405. This work was funded by National Institute of Allergy and Infectious Diseases Grant R01AI066121 (to R.M.K.)
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407393111/-/DCSupplemental.
References
- 1.Budhu S, et al. CD8+ T cell concentration determines their efficiency in killing cognate antigen-expressing syngeneic mammalian cells in vitro and in mouse tissues. J Exp Med. 2010;207(1):223–235. doi: 10.1084/jem.20091279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett SR, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393(6684):478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 3.Curtsinger JM, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162(6):3256–3262. [PubMed] [Google Scholar]
- 4.DeBenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28- T lymphocytes by 4-1BB ligand. J Immunol. 1997;158(2):551–559. [PubMed] [Google Scholar]
- 5.Linsley PS, et al. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal P, et al. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol. 2009;183(3):1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182(5):2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamiya S, et al. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol. 2004;173(6):3871–3877. doi: 10.4049/jimmunol.173.6.3871. [DOI] [PubMed] [Google Scholar]
- 9.Kim G, Shinnakasu R, Saris CJM, Cheroutre H, Kronenberg M. A novel role for IL-27 in mediating the survival of activated mouse CD4 T lymphocytes. J Immunol. 2013;190(4):1510–1518. doi: 10.4049/jimmunol.1201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 2003;100(25):15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owaki T, et al. A role for IL-27 in early regulation of Th1 differentiation. J Immunol. 2005;175(4):2191–2200. doi: 10.4049/jimmunol.175.4.2191. [DOI] [PubMed] [Google Scholar]
- 12.Owaki T, et al. STAT3 is indispensable to IL-27-mediated cell proliferation but not to IL-27-induced Th1 differentiation and suppression of proinflammatory cytokine production. J Immunol. 2008;180(5):2903–2911. doi: 10.4049/jimmunol.180.5.2903. [DOI] [PubMed] [Google Scholar]
- 13.Anderson CF, Stumhofer JS, Hunter CA, Sacks D. IL-27 regulates IL-10 and IL-17 from CD4+ cells in nonhealing Leishmania major infection. J Immunol. 2009;183(7):4619–4627. doi: 10.4049/jimmunol.0804024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong WP, et al. IL-27p28 inhibits central nervous system autoimmunity by concurrently antagonizing Th1 and Th17 responses. J Autoimmun. 2014;50:12–22. doi: 10.1016/j.jaut.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villarino A, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19(5):645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 16.Villegas-Mendez A, et al. IL-27 receptor signalling restricts the formation of pathogenic, terminally differentiated Th1 cells during malaria infection by repressing IL-12 dependent signals. PLoS Pathog. 2013;9(4):e1003293–e1003293. doi: 10.1371/journal.ppat.1003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiyo M, et al. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer. 2005;115(3):437–442. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- 18.Hisada M, et al. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64(3):1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, et al. IL-27 enhances the survival of tumor antigen-specific CD8+ T cells and programs them into IL-10-producing, memory precursor-like effector cells. Eur J Immunol. 2013;43(2):468–479. doi: 10.1002/eji.201242930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8(12):1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Dodd H, Moser EK, Sharma R, Braciale TJ. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nat Immunol. 2011;12(4):327–334. doi: 10.1038/ni.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall AOH, et al. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37(3):511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahonen CL, et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199(6):775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J Immunol. 2007;178(3):1564–1572. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]
- 25.Kurche JS, Haluszczak C, McWilliams JA, Sanchez PJ, Kedl RM. Type I IFN-dependent T cell activation is mediated by IFN-dependent dendritic cell OX40 ligand expression and is independent of T cell IFNR expression. J Immunol. 2012;188(2):585–593. doi: 10.4049/jimmunol.1102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez PJ, Kedl RM. An alternative signal 3: CD8⁺ T cell memory independent of IL-12 and type I IFN is dependent on CD27/OX40 signaling. Vaccine. 2012;30(6):1154–1161. doi: 10.1016/j.vaccine.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morishima N, et al. A pivotal role for interleukin-27 in CD8+ T cell functions and generation of cytotoxic T lymphocytes. J Biomed Biotechnol. 2010;2010:605483. doi: 10.1155/2010/605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morishima N, et al. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175(3):1686–1693. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- 29.Rees W, et al. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad Sci USA. 1999;96(17):9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosas LE, et al. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to leishmania donovani infection but develop severe liver immunopathology. Am J Pathol. 2006;168(1):158–169. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J Immunol. 2007;179(10):6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 32.Robinson CM, Nau GJ. Interleukin-12 and interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J Infect Dis. 2008;198(3):359–366. doi: 10.1086/589774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Q, et al. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407(6806):916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 34.Mayer KD, et al. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J Immunol. 2008;180(2):693–697. doi: 10.4049/jimmunol.180.2.693. [DOI] [PubMed] [Google Scholar]
- 35.Kidder BL, Yang J, Palmer S. Stat3 and c-Myc genome-wide promoter occupancy in embryonic stem cells. PLoS ONE. 2008;3(12):e3932. doi: 10.1371/journal.pone.0003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6(12):1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 37.Marks-Konczalik J, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci USA. 2000;97(21):11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson SH, Yu C-R, Mahdi RM, Ebong S, Egwuagu CE. Dendritic cell maturation requires STAT1 and is under feedback regulation by suppressors of cytokine signaling. J Immunol. 2004;172(4):2307–2315. doi: 10.4049/jimmunol.172.4.2307. [DOI] [PubMed] [Google Scholar]
- 39.Pilz A, et al. Dendritic cells require STAT-1 phosphorylated at its transactivating domain for the induction of peptide-specific CTL. J Immunol. 2009;183(4):2286–2293. doi: 10.4049/jimmunol.0901383. [DOI] [PubMed] [Google Scholar]
- 40.Takeda K, et al. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161(9):4652–4660. [PubMed] [Google Scholar]
- 41.Klover PJ, et al. Loss of STAT1 from mouse mammary epithelium results in an increased Neu-induced tumor burden. Neoplasia. 2010;12(11):899–905. doi: 10.1593/neo.10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harker JA, Dolgoter A, Zuniga EI. Cell-intrinsic IL-27 and gp130 cytokine receptor signaling regulates virus-specific CD4⁺ T cell responses and viral control during chronic infection. Immunity. 2013;39(3):548–559. doi: 10.1016/j.immuni.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Windt GJW, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36(1):68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinn L, Anderson BG, Conner J, Pistilli E, Wolden-Hanson T. Overexpression of interleukin-15 in mice promotes resistance to diet-induced obesity, increased insulin sensitivity, and markers of oxidative skeletal muscle metabolism. Int J Infereron Cytokine Mediator Res. 2011;3:29–42. doi: 10.2147/IJICMR.S19007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He MX, He YW. A role for c-FLIP(L) in the regulation of apoptosis, autophagy, and necroptosis in T lymphocytes. Cell Death Differ. 2013;20(2):188–197. doi: 10.1038/cdd.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunai ZA, et al. Staurosporine induces necroptotic cell death under caspase-compromised conditions in U937 cells. PLoS ONE. 2012;7(7):e41945. doi: 10.1371/journal.pone.0041945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han J, Zhong C-Q, Zhang D-W. Programmed necrosis: Backup to and competitor with apoptosis in the immune system. Nat Immunol. 2011;12(12):1143–1149. doi: 10.1038/ni.2159. [DOI] [PubMed] [Google Scholar]
- 48.Batten M, et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J Exp Med. 2010;207(13):2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pot C, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183(2):797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamburini BA, Kedl RM, Bellgrau D. IL-6-inducing whole yeast-based immunotherapy directly controls IL-12-dependent CD8 T-cell responses. J Immunother. 2012;35(1):14–22. doi: 10.1097/CJI.0b013e3182356888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35(5):792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darrah PA, et al. IL-10 production differentially influences the magnitude, quality, and protective capacity of Th1 responses depending on the vaccine platform. J Exp Med. 2010;207(7):1421–1433. doi: 10.1084/jem.20092532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novy P, Huang X, Leonard WJ, Yang Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J Immunol. 2011;186(5):2729–2738. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McWilliams JA, Sanchez PJ, Haluszczak C, Gapin L, Kedl RM. Multiple innate signaling pathways cooperate with CD40 to induce potent, CD70-dependent cellular immunity. Vaccine. 2010;28(6):1468–1476. doi: 10.1016/j.vaccine.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong H, et al. CD27 stimulation promotes the frequency of IL-7 receptor-expressing memory precursors and prevents IL-12-mediated loss of CD8(+) T cell memory in the absence of CD4(+) T cell help. J Immunol. 2012;188(8):3829–3838. doi: 10.4049/jimmunol.1103329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hendriks J, et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1(5):433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 57.Roberts DJ, et al. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J Immunother. 2010;33(8):769–779. doi: 10.1097/CJI.0b013e3181ee238f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Gisbergen KP, et al. The costimulatory molecule CD27 maintains clonally diverse CD8(+) T cell responses of low antigen affinity to protect against viral variants. Immunity. 2011;35(1):97–108. doi: 10.1016/j.immuni.2011.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.