Significance
Defects in mitochondrial substrate selection, mediated by inhibition of the pyruvate dehydrogenase complex (PDH), have been proposed to be a major contributor to lipid-induced muscle insulin resistance. To examine this hypothesis, we assessed insulin action in a genetic mouse model of constitutive PDH activation. Surprisingly, we found that preferential glucose oxidation in skeletal muscle in this mouse was accompanied by muscle insulin resistance. Muscle insulin resistance could be attributed to increased glucose oxidation at the expense of reduced fatty acid oxidation, leading to increased intramyocellular lipid accumulation and diacylglycerol-PKC-θ–mediated reductions in proximal insulin signaling. These findings have important clinical implications for novel antidiabetic therapies currently in development that activate PDH and enhance glucose oxidation in muscle.
Keywords: insulin action, diacylglycerol, protein kinase C theta, nuclear magnetic resonance, liquid chromatography mass spectrometry
Abstract
The pyruvate dehydrogenase complex (PDH) has been hypothesized to link lipid exposure to skeletal muscle insulin resistance through a glucose-fatty acid cycle in which increased fatty acid oxidation increases acetyl-CoA concentrations, thereby inactivating PDH and decreasing glucose oxidation. However, whether fatty acids induce insulin resistance by decreasing PDH flux remains unknown. To genetically examine this hypothesis we assessed relative rates of pyruvate dehydrogenase flux/mitochondrial oxidative flux and insulin-stimulated rates of muscle glucose metabolism in awake mice lacking pyruvate dehydrogenase kinase 2 and 4 [double knockout (DKO)], which results in constitutively activated PDH. Surprisingly, increased glucose oxidation in DKO muscle was accompanied by reduced insulin-stimulated muscle glucose uptake. Preferential myocellular glucose utilization in DKO mice decreased fatty acid oxidation, resulting in increased reesterification of acyl-CoAs into diacylglycerol and triacylglycerol, with subsequent activation of PKC-θ and inhibition of insulin signaling in muscle. In contrast, other putative mediators of muscle insulin resistance, including muscle acylcarnitines, ceramides, reactive oxygen species production, and oxidative stress markers, were not increased. These findings demonstrate that modulation of oxidative substrate selection to increase muscle glucose utilization surprisingly results in muscle insulin resistance, offering genetic evidence against the glucose-fatty acid cycle hypothesis of muscle insulin resistance.
Lipid-induced muscle insulin resistance plays a major role in the pathogenesis of type 2 diabetes (T2D), but the cellular mechanisms remain unknown (1, 2). More than 50 y ago Randle et al. (3) postulated the glucose-fatty acid cycle to explain the impairment of insulin-stimulated glucose disposal by fatty acids in muscle. In this model, fat oxidation increases mitochondrial acetyl-CoA/CoA and NADH/NAD+ ratios. Acetyl-CoA and NADH allosterically inhibit pyruvate dehydrogenase complex (PDH), the mitochondrial enzyme that links glycolysis to the TCA cycle by converting pyruvate to acetyl-CoA. Additionally, fatty acid-derived acetyl-CoA produces citrate, which inhibits phosphofructokinase. This in turn increases glucose-6-phosphate (G6P), a potent allosteric inhibitor of hexokinase. By these mechanisms, increased fatty acid oxidation was hypothesized to reduce glycolytic flux and prevent further muscle glucose uptake. However, in vivo studies of human skeletal muscle metabolism have challenged the Randle hypothesis. Five hours of a lipid infusion, combined with heparin to activate lipoprotein lipase, raised plasma fatty acids and induced muscle insulin resistance in healthy individuals, yet intramyocellular G6P and glucose concentrations were reduced compared with control glycerol infusion studies, implicating defects in insulin-stimulated glucose transport activity (4, 5). An alternative hypothesis to explain the muscle insulin resistance associated with lipid exposure posits that accumulation of bioactive lipid intermediates initiates signaling cascades that impair insulin action. Lipid species implicated include diacylglycerols (DAGs) (6–10), ceramides (11, 12), and long-chain acyl-CoAs (13). DAG activation of PKC-θ in skeletal muscle has been shown to impair canonical insulin signaling at the level of insulin receptor substrate-1 (IRS-1) tyrosine phosphorylation through increased IRS-1 serine phosphorylation at the 1101 position (2, 6, 7, 14).
More recently, incomplete fat oxidation and subsequent accumulation of mitochondrially derived acylcarnitines has been proposed to contribute to lipid-induced muscle insulin resistance (15–17). According to this model, insulin resistance stems from increased fat oxidation, leading to increased conversion of acyl-CoA to medium- and long-chain acylcarnitines, which may mediate insulin resistance via unknown mechanisms. In contrast, short-chain acylcarnitines have been suggested to promote metabolic flexibility. The shortest acylcarnitine, acetylcarnitine, is synthesized from acetyl-CoA and carnitine by carnitine acetyltransferase (CrAT), a mitochondrial matrix enzyme, and is responsible for buffering the mitochondrial acetyl-CoA pool and mitigating acetyl-CoA inhibition of PDH (18). Consistent with the notion that CrAT regulates substrate selection by modulating PDH flux, mice with muscle-specific deletion of CrAT exhibited reduced PDH activity during the fed-to-fasted transition, resulting in glucose intolerance and metabolic inflexibility, a term coined by Kelley and Mandarino (19) to explain the impairment in the ability to adjust fuel oxidation to fuel availability.
Although these studies emphasize the importance of PDH in the promotion of metabolic inflexibility, the role of PDH and mitochondrial oxidative substrate selection in the regulation of basal and insulin-stimulated muscle glucose metabolism has not been directly assessed in vivo. To examine this question, we sought to determine whether modulation of oxidative substrate selection in a genetic mouse model with constitutively active PDH activity would affect insulin sensitivity in skeletal muscle.
Results
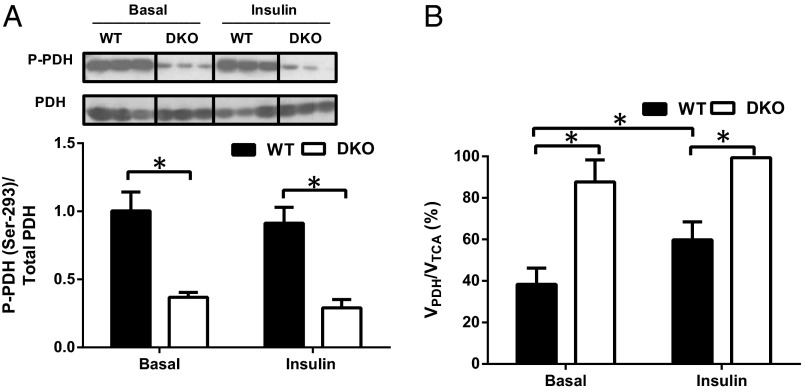
Reduced PDH Phosphorylation Promotes Increased in Vivo VPDH/VTCA in the Muscle of Double-Knockout Mice.
PDH activity is regulated by four pyruvate dehydrogenase kinases (PDK1–4), which phosphorylate and inhibit PDH. Because PDK2 and PDK4 are the most highly expressed isoforms in skeletal muscle (20, 21), we hypothesized that mice with global deletion of PDK2 and PDK4 (double knockout, DKO) would preferentially oxidize glucose in muscle. Consistent with this hypothesis, muscle PDH was highly dephosphorylated in both basal and insulin-stimulated conditions in DKO mice (Fig. 1A). To assess PDH activity in vivo, we measured PDH flux (VPDH) relative to TCA cycle flux (VTCA) in skeletal muscle by proton-observed carbon-edited (POCE) magnetic resonance spectroscopy (22, 23). This ratio represents the relative contribution of glucose oxidation to TCA cycle flux. As anticipated, on the basis of the highly dephosphorylated PDH state, VPDH/VTCA was increased by ∼fourfold in the skeletal muscle of fasted DKO mice (Fig. 1B). Basal VPDH/VTCA was 88% ± 12% in DKO mice, suggesting near-maximal utilization of glucose for oxidation. Upon insulin stimulation, VPDH/VTCA increased by ∼twofold in skeletal muscle of WT mice, reflecting a substrate switch toward increased muscle glucose oxidation (Fig. 1B). However, insulin had no effect on VPDH/VTCA in skeletal muscle of DKO mice, indicating that glucose oxidation was maximal in both basal and insulin-stimulated conditions. An impaired capacity to increase muscle glucose oxidation during the insulin stimulated state in the DKO mice reflects metabolic inflexibility.
Fig. 1.
Decreased phosphorylation of the PDH complex subunit E1α increases PDH flux in the skeletal muscle of DKO mice. (A) Representative immunoblots of the Ser293-phosphorylated form of the PDH complex E1α from WT and PDK2/PDK4 DKO mice. Histograms constructed from the data obtained from Western blot analysis. (B) Relative PDH flux (VPDH/VTCA) in the skeletal muscle of WT and DKO mice after overnight fasting (basal) and at the end of the hyperinsulinemic-euglycemic clamp (insulin). Data are mean ± SEM. n = 8 mice per group.
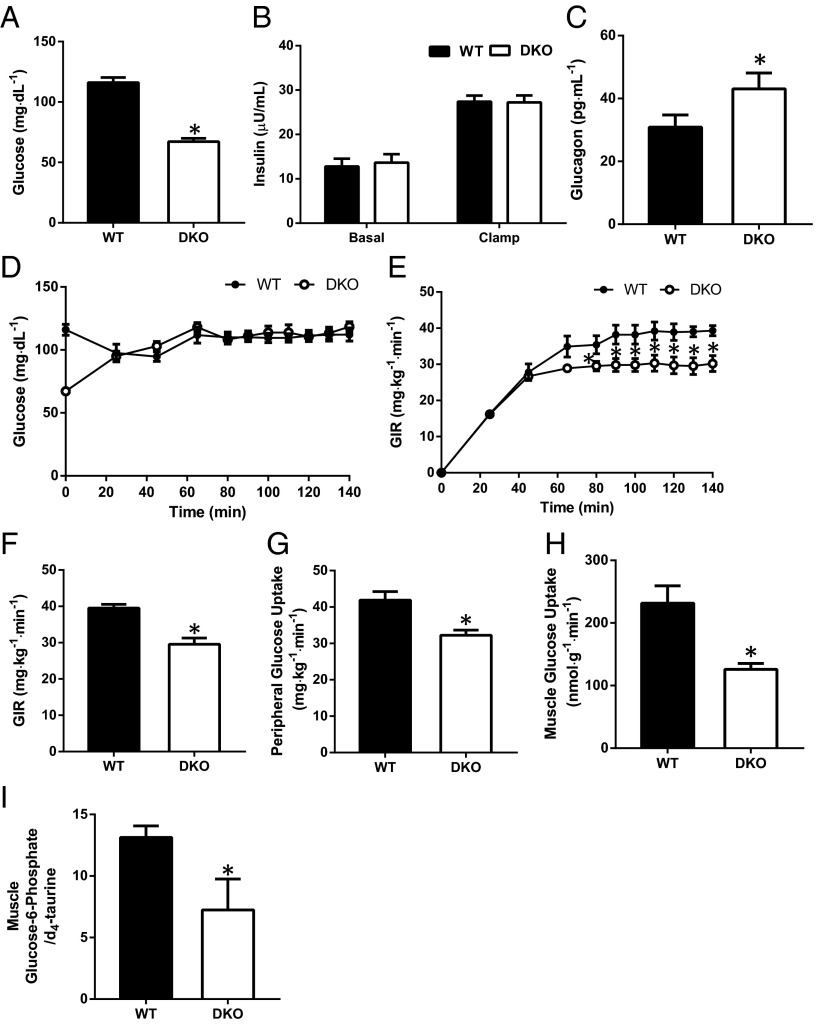
DKO Mice Are Insulin Resistant Owing to Impaired Insulin-Stimulated Muscle Glucose Uptake.
To further examine whether the increased muscle glucose oxidation of DKO mice affected whole-body glucose metabolism and muscle insulin sensitivity, we performed hyperinsulinemic-euglycemic clamp studies combined with radiolabeled glucose to assess basal and insulin-stimulated rates of whole-body glucose turnover. Overnight-fasted DKO mice had reduced fasting plasma glucose concentrations (Fig. 2A), with no differences in plasma insulin concentrations in the basal state or during the clamp (Fig. 2B) and a modest increase in plasma glucagon concentrations (Fig. 2C) compared with WT mice. Surprisingly, despite striking increases in skeletal muscle glucose oxidation, DKO mice manifested impaired insulin-stimulated whole-body glucose metabolism, as reflected by a lower glucose infusion rate required to maintain euglycemia during the hyperinsulinemic-euglycemic clamp (Fig. 2 D–F). This impaired whole-body response to insulin in the DKO mice could mostly be attributed to ∼25% reduction in insulin-stimulated peripheral glucose uptake (Fig. 2G), which in turn could be attributed to a 50% reduction in insulin-stimulated muscle glucose uptake (Fig. 2H). Myocellular G6P levels were reduced in DKO mice in the basal state (Fig. 2I). Taken together, these data indicate that PDK2/PDK4 deficient mice are insulin resistant owing to impaired insulin-stimulated muscle glucose uptake.
Fig. 2.
Glucose uptake is reduced in the skeletal muscle of DKO mice. (A–C) Fasting (basal) plasma glucose (A), insulin in the basal state and at the end of the clamp (B), and basal glucagon (C). (D and E) Plasma glucose levels (D) and glucose infusion rates (E) required to maintain euglycemia during the hyperinsulinemic-euglycemic clamp. (F) Mean glucose infusion rate required to maintain euglycemia during the steady-state period (final 40 min) of the clamp. (G) Insulin-stimulated peripheral glucose disposal during the hyperinsulinemic-euglycemic clamp studies. (H) Insulin-stimulated glucose uptake in the quadriceps. Data are mean ± SEM. n = 8–10 mice per group. (I) G6P levels in the gastrocnemius of overnight fasted WT and DKO mice. Data are mean ± SEM. n = 4 mice per group.
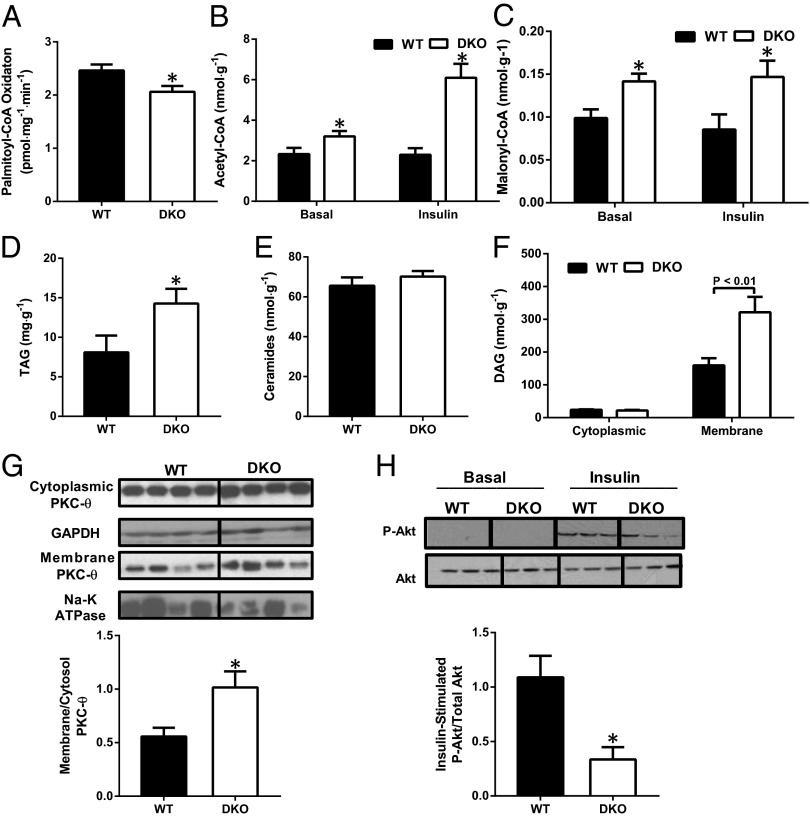
Reduced Rate of Fatty Acid Oxidation Attributes to DAG Activation of PKC-θ with Subsequent Inhibition of Insulin Signaling in Muscle of DKO Mice.
To examine this apparent paradox, we next examined whether the enhanced muscle glucose oxidation of DKO mice affected fatty acid catabolism owing to the reciprocal relationship between fat oxidation and glucose oxidation. As expected, rates of fatty acid oxidation were 15–20% lower in isolated skeletal muscle from DKO mice (Fig. 3A). To gain further insight into the effects of PDK deficiency on fatty acid oxidation, we measured concentrations of key allosteric regulators of fatty acid oxidation. We hypothesized that some of the excess acetyl-CoA produced by PDH activation would be converted to malonyl-CoA, an allosteric inhibitor of carnitine palmitoyltransferase 1 (CPT1, the rate-controlling enzyme of mitochondrial fatty acid oxidation). Indeed, muscle from DKO mice had increased concentrations of acetyl-CoA (Fig. 3B) and malonyl-CoA (Fig. 3C) in both overnight-fasted and insulin-stimulated conditions. To address whether reduced fatty acid oxidation might be explained by changes in the expression of fatty acid oxidation enzymes, we measured mRNA levels of key fatty acid oxidative enzymes and found no differences in CPT1, acetyl-CoA carboxylase 2, peroxisome-proliferator-activated receptor α, long-chain acyl-CoA dehydrogenase, and medium-chain acyl-CoA dehydrogenase between the two groups (Fig. S1A). These findings suggest that allosteric inhibition by malonyl-CoA of mitochondrial fatty acid uptake is the mechanism responsible for reduced fatty acid oxidation in the skeletal muscle of DKO mice. Next, we investigated whether the reduced fat oxidation in the muscle of DKO mice caused intramyocellular lipid accumulation. Intramuscular triacylglycerol (TAG) and membrane DAG content were increased by approximately twofold in DKO mice, whereas ceramide content was unchanged (Fig. 3 D–F). However, mRNA levels of key enzymes regulating lipid synthesis were similar in the muscle of WT and DKO mice (Fig. S1B). Because DAG has been well documented to cause muscle insulin resistance through activation of PKC-θ, we measured membrane translocation of PKC-θ and found it to be increased in DKO mice (Fig. 3G). Further, insulin-stimulated Akt Ser473 phosphorylation was reduced in muscle from DKO mice (Fig. 3H). Together, these data suggest that the reduced insulin-stimulated glucose uptake of DKO mice may be attributed at least in part to DAG activation of PKC-θ, leading to inhibition of insulin signaling (6–10).
Fig. 3.
Reduction in fatty acid oxidation leads to muscle insulin resistance in PDK-deficient mice. (A) Rate of fatty acid oxidation after addition of palmitoyl-CoA assessed in permeabilized skeletal muscle fibers taken from the quadriceps of overnight fasted WT and DKO mice. (B and C) Concentrations of acetyl-CoA (B) and malonyl-CoA (C) in the quadriceps of overnight fasted (basal) and at the end of the hyperinsulinemic-euglycemic clamp (insulin) WT and DKO mice. (D–F) Quadriceps TAG (D), ceramide (E), and cytoplasmic and membrane DAG (F) of 6-h fasted WT and DKO mice. (G) Western blot analysis and densitometry of PKC-θ in the cytosol and membrane fraction in the muscle of WT and DKO mice. (H) Representative immunoblot densitometry of phosphorylated Akt2 (Ser473) in the skeletal muscle of WT and DKO mice in the basal state or 20 min after i.p. insulin injection (0.75 U/kg). Data are mean ± SEM. n = 4–6 mice per group.
Energy Expenditure and Intramyocellular Glycogen Content Is Unchanged in DKO Mice.
To examine whether increased TAG levels could be attributed to alterations in whole-body energy expenditure, we performed metabolic cage studies in chow-fed WT and DKO mice. Interestingly, we observed no differences in whole-body oxygen consumption, carbon dioxide production, respiratory quotient, energy expenditure, food intake, or activity (Fig. S2 A–F) in the dark or light cycle. Additionally, accumulation of TAG in the muscle was not a result of greater glycogen stores, as reflected by similar glycogen content in the quadriceps of WT and DKO mice (Fig. S3A).
High-Fat Diet Feeding Abrogated Insulin-Stimulated Increases in PDH Flux in Both Wild-Type and DKO Mice.
Next, we examined the effect of enhanced PDH activation on diet-induced insulin resistance by feeding WT and DKO mice a high-fat diet (HFD) for 4 wk. We found that HFD feeding abrogated insulin-induced increases in PDH flux in muscle of both WT and DKO mice, although DKO mice maintained higher PDH flux in both conditions (Fig. S4A). As with chow-fed mice, HFD-fed DKO mice displayed similar lower fasting plasma glucose concentrations, whereas insulin concentrations remained unchanged compared with WT mice (Fig. S4 B and C). We next performed hyperinsulinemic-euglycemic clamp studies to assess the effect of PDK2/4 deficiency on HFD-induced insulin resistance. High-fat feeding induced similar degrees of peripheral insulin resistance in both the WT and DKO mice, as reflected by similar reductions in the glucose infusion rate required to maintain plasma glucose concentrations during the hyperinsulinemic-euglycemic clamp and similar reductions in insulin-stimulated peripheral and muscle glucose uptake in both WT and DKO mice (Fig. S4 D–F). We next assessed intramuscular lipid content in high-fat-fed WT and DKO mice and found that high-fat feeding induced an accumulation of intramuscular TAG and DAG in both groups of mice (Fig. S5 A and B). Furthermore, membrane translocation of PKC-θ in the muscle was not different among high-fat DKO and WT mice but was increased compared with WT chow-fed mice (Fig. S5C). Taken together these data demonstrate that the muscle insulin resistance observed in the chow-fed DKO mice was not further exacerbated by high-fat feeding.
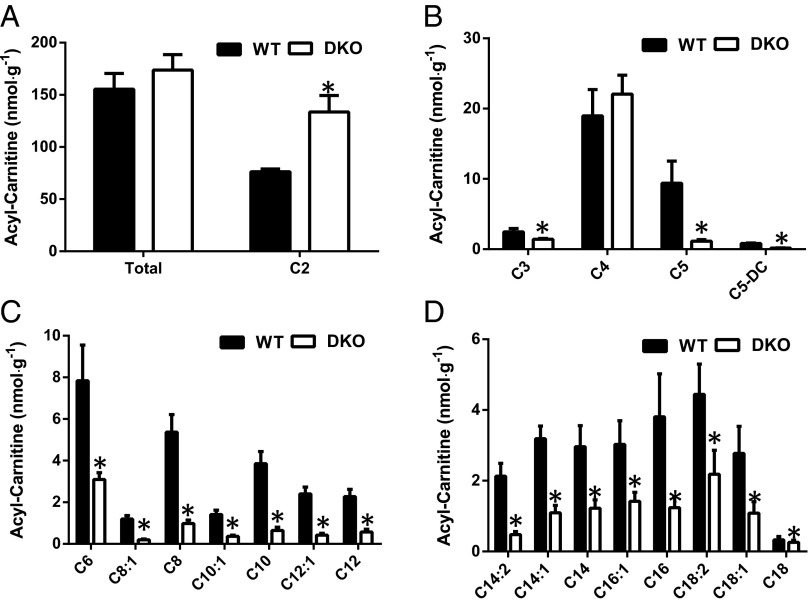
Intramuscular Acylcarnitine Profile Is Altered in DKO Mice.
Because accumulation of acylcarnitines resulting from incomplete fat oxidation has been associated with muscle insulin resistance in rodent models of obesity and diabetes (15–17, 24), we next measured muscle acylcarnitines in WT and DKO mice. Whereas total acylcarnitine levels (Fig. 4A) in muscle were similar between WT and DKO mice, short-, medium-, and long-chain species were all significantly decreased in the quadriceps of DKO mice (Fig. 4 B–D). As observed in chow-fed mice, DKO mice fed an HFD for 8 wk displayed significant decreases in short-, medium-, and long-chain acylcarnitines, but increases in total and C2-acylcarnitines (Fig. S6 A–D). Previous studies have reported that intramuscular accumulation of acylcarnitines, particularly long-chain species, contributes to insulin resistance (17, 24). However, the present data, which show that muscle insulin resistance is accompanied by decreases in both the medium- and long-chain acylcarnitine species, do not support this hypothesis.
Fig. 4.
Acylcarnitine profiling in the skeletal muscle of DKO mice. (A–D) Total acylcarnitine and acetylcarnitine (A), short-chain acylcarnitines (B), medium-chain acylcarnitine (C),and long-chain acylcarnitine (D) levels in the quadriceps taken from overnight fasted WT and DKO mice (n = 6–8 mice per group) were measured by mass spectroscopy.
Acetyl-carnitine is of particular interest because it has been hypothesized to control the fate of acetyl-CoA during substrate switching and promote increased insulin sensitivity (18). Accumulation of acetyl-carnitine has been proposed to buffer the mitochondrial acetyl-CoA pool and abrogate acetyl-CoA inhibition of PDH, resulting in metabolic flexibility and improved glucose tolerance. Contrary to this hypothesis we found that acetyl-carnitine (C2) levels were increased by more than twofold in the insulin-resistant muscle of DKO mice (Fig. 4A). Despite the decreased muscle acylcarnitine levels, plasma acylcarnitines were similar in DKO mice compared with WT mice except for short-chain acylcarnitines, which were decreased (Fig. S7 A–D), suggesting that the plasma acylcarnitine profile does not reflect the profile of skeletal muscle acylcarnitines and cannot be used as a surrogate marker of muscle acylcarnitines.
Reactive Oxygen Species Production Rates Are Similar Between Wild-Type and DKO Mice.
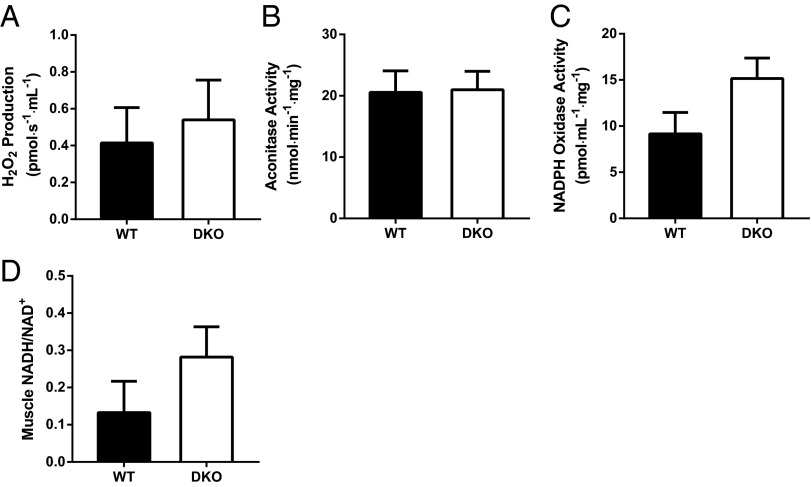
Because the generation of reactive oxygen species (ROS) via PDH and 2-oxoglutarate dehydrogenase has been strongly associated with muscle insulin resistance (25–29), we measured myocellular ROS in three separate assays. We found no differences in ROS production (Fig. 5A), oxidative damage as assessed by aconitase activity (Fig. 5B), or NADPH oxidase activity (Fig. 5C) in the muscle of WT and DKO mice, providing evidence against a role of ROS in the muscle insulin resistance of this model. Additionally, redox balance was not altered in the muscle of DKO mice, as reflected by similar NADH to NAD+ ratios in WT and DKO mice (Fig. 5D).
Fig. 5.
Oxidative stress and redox state in the skeletal muscle of DKO mice. (A) Reactive oxygen species production measured in isolated mitochondria, (B) aconitase activity, (C) NADPH oxidase activity, and (D) NADH/NAD+ in the gastrocnemius taken from overnight fasted WT and DKO mice (n = 6–8 mice per group).
Discussion
Activation of PDH, through inhibition of PDKs, has been proposed to be a potentially important therapeutic target for the treatment of T2D (30–33). Interest in targeting the inhibition of PDKs was based on several studies that showed that PDK2 and PDK4 were responsible for inactivation of PDH in T2D (34), genetic animal models of T2D (35), animals fed HFDs (36), and humans consuming HFDs (37). Previous studies also explored this hypothesis by feeding PDK4 knockout mice and WT mice an HFD enriched with unsaturated fatty acids (38) and saturated fatty acids (38, 39). On both diets, PDK4 KO mice had lower plasma glucose levels and improved glucose tolerance, despite induction of hyperinsulinemia after 8 mo of high-fat feeding (39). These studies proposed that PDH activation through PDK inhibition would reduce available substrate for glucose production, resulting in lower blood glucose levels and improved glucose tolerance. However, this study and previous studies have not evaluated the effect of knocking down PDKs on in vivo rates of PDH flux or the effect of PDH activation on insulin responsiveness in skeletal muscle. Importantly, these studies did not assess the impact of constitutively increased PDH flux on insulin action in skeletal muscle in vivo.
In this study, we applied POCE NMR spectroscopy in combination with [1-13C] glucose and [1-14C]-2-deoxyglucose tracers during hyperinsulinemic-eugylycemic clamp studies to address these questions in awake mice lacking both PDK2 and PDK4. We show that knocking out PDK2 and PDK4 results in constitutively active PDH activity and increased muscle glucose oxidation. Furthermore, we show in vivo that insulin stimulates the shifts of substrate for oxidative metabolism from fatty acids to glucose in the skeletal muscle of WT mice, as reflected by increased VPDH/VTCA. These findings are consistent with previous studies in the perfused working heart, in which insulin did not cause a significant increase in glucose utilization at high workload when glucose was the sole substrate (40).
Surprisingly, DKO mice manifested profound muscle insulin resistance despite marked increases in muscle glucose oxidation. The muscle insulin resistance in the DKO mice could be attributed to increased DAG content, from increased acetyl-CoA production secondary to increased PDH activity, leading to activation of PKC-θ and inhibition of insulin signaling. Although previous studies have implicated increased ceramide content (12), ROS (25–27), and long-chain acylcarnitines (17, 24) in causing insulin resistance, we found no relationship between these factors and muscle insulin resistance in DKO mice. Furthermore, medium-chain and long-chain chain acylcarnitines were decreased in the skeletal muscle of DKO mice, despite reports implicating increased long-chain acylcarnitines in promoting insulin resistance. Finally, it has also been proposed that acetylcarnitines buffer the mitochondrial acetyl-CoA pool and abrogate acetyl-CoA inhibition of PDH, resulting in increased metabolic flexibility and improved glucose tolerance (18). Contrary to this hypothesis, we found that DKO mice had severe muscle insulin resistance despite a twofold increase in muscle acetylcarnitine content.
Taken together these studies demonstrate that modulation of oxidative substrate selection to increase muscle glucose utilization surprisingly results in muscle insulin resistance, offering genetic evidence against the glucose-fatty acid cycle hypothesis of muscle insulin resistance. Furthermore, these findings have important clinical implications for novel antidiabetic therapies that are currently being developed to activate PDH and enhance glucose oxidation in skeletal muscle.
Methods
SI Methods provides a detailed description of the methods used. The methods describe animal, basal, and insulin-stimulated glucose turnover studies, tissue determination of VPDH/VTCA, fatty acid oxidation rates, oxidative stress and redox state in the skeletal muscle, metabolite profiling, muscle glycogen content, basal metabolism and exercise capacity, RT-PCR analysis, immunoblotting, cell fractionation, and statistical analysis.
Supplementary Material
Acknowledgments
We thank Robert A. Harris for providing the PDK2/PDK4 DKO mice, Mario Kahn and Blas A. Guigni for their expert technical assistance, Dr. Renata L. S. Goncalves for help with the ROS assay, and Oroboros Instruments for the loan of an Oxygraph-2k. This work was supported by National Institutes of Health Grants R01 DK-40936, R01 DK-49230, U24 DK-059635, P30 DK-45735, P30 DK-034989, and K01 DK-099402, a mentor-based award from the American Diabetes Association (Merck 1-14-Merck-10), and the Novo Nordisk Foundation Center for Basic Metabolic Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419104111/-/DCSupplemental.
References
- 1.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 2.Samuel VT, Shulman GI. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 4.Dresner A, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103(2):253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roden M, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97(12):2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin ME, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48(6):1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 7.Yu C, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277(52):50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 8.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51(7):2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 9.Samuel VT, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279(31):32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 10.Samuel VT, et al. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117(3):739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ussher JR, et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010;59(10):2453–2464. doi: 10.2337/db09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holland WL, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5(3):167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Thompson AL, Cooney GJ. Acyl-CoA inhibition of hexokinase in rat and human skeletal muscle is a potential mechanism of lipid-induced insulin resistance. Diabetes. 2000;49(11):1761–1765. doi: 10.2337/diabetes.49.11.1761. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, et al. Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101) J Biol Chem. 2004;279(44):45304–45307. doi: 10.1074/jbc.C400186200. [DOI] [PubMed] [Google Scholar]
- 15.An J, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10(3):268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- 16.Koves TR, et al. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol. 2005;288(5):C1074–C1082. doi: 10.1152/ajpcell.00391.2004. [DOI] [PubMed] [Google Scholar]
- 17.Koves TR, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Muoio DM, et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012;15(5):764–777. doi: 10.1016/j.cmet.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: A reexamination. Diabetes. 2000;49(5):677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 20.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329(Pt 1):191–196. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu P, et al. Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. Biochem J. 1998;329(Pt 1):197–201. doi: 10.1042/bj3290197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shulman GI, Rossetti L, Rothman DL, Blair JB, Smith D. Quantitative analysis of glycogen repletion by nuclear magnetic resonance spectroscopy in the conscious rat. J Clin Invest. 1987;80(2):387–393. doi: 10.1172/JCI113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alves TC, et al. Regulation of hepatic fat and glucose oxidation in rats with lipid-induced hepatic insulin resistance. Hepatology. 2011;53(4):1175–1181. doi: 10.1002/hep.24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seiler SE, et al. Obesity and lipid stress inhibit carnitine acetyltransferase activity. J Lipid Res. 2014;55(4):635–644. doi: 10.1194/jlr.M043448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tretter L, Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J Neurosci. 2004;24(36):7771–7778. doi: 10.1523/JNEUROSCI.1842-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinlan CL, et al. The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I. J Biol Chem. 2014;289(12):8312–8325. doi: 10.1074/jbc.M113.545301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher-Wellman KH, et al. Mitochondrial glutathione depletion reveals a novel role for the pyruvate dehydrogenase complex as a key H2O2-emitting source under conditions of nutrient overload. Free Radic Biol Med. 2013;65:1201–1208. doi: 10.1016/j.freeradbiomed.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson EJ, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119(3):573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoehn KL, et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA. 2009;106(42):17787–17792. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugden MC, Holness MJ. Therapeutic potential of the mammalian pyruvate dehydrogenase kinases in the prevention of hyperglycaemia. Curr Drug Targets Immune Endocr Metabol Disord. 2002;2(2):151–165. [PubMed] [Google Scholar]
- 31.Majer M, Popov KM, Harris RA, Bogardus C, Prochazka M. Insulin downregulates pyruvate dehydrogenase kinase (PDK) mRNA: Potential mechanism contributing to increased lipid oxidation in insulin-resistant subjects. Mol Genet Metab. 1998;65(2):181–186. doi: 10.1006/mgme.1998.2748. [DOI] [PubMed] [Google Scholar]
- 32.Mayers RM, et al. AZD7545, a novel inhibitor of pyruvate dehydrogenase kinase 2 (PDHK2), activates pyruvate dehydrogenase in vivo and improves blood glucose control in obese (fa/fa) Zucker rats. Biochem Soc Trans. 2003;31(Pt 6):1165–1167. doi: 10.1042/bst0311165. [DOI] [PubMed] [Google Scholar]
- 33.Mayers RM, Leighton B, Kilgour E. PDH kinase inhibitors: A novel therapy for Type II diabetes? Biochem Soc Trans. 2005;33(Pt 2):367–370. doi: 10.1042/BST0330367. [DOI] [PubMed] [Google Scholar]
- 34.Rosa G, et al. Reduced PDK4 expression associates with increased insulin sensitivity in postobese patients. Obes Res. 2003;11(2):176–182. doi: 10.1038/oby.2003.28. [DOI] [PubMed] [Google Scholar]
- 35.Bajotto G, et al. Downregulation of the skeletal muscle pyruvate dehydrogenase complex in the Otsuka Long-Evans Tokushima Fatty rat both before and after the onset of diabetes mellitus. Life Sci. 2004;75(17):2117–2130. doi: 10.1016/j.lfs.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Holness MJ, Kraus A, Harris RA, Sugden MC. Targeted upregulation of pyruvate dehydrogenase kinase (PDK)-4 in slow-twitch skeletal muscle underlies the stable modification of the regulatory characteristics of PDK induced by high-fat feeding. Diabetes. 2000;49(5):775–781. doi: 10.2337/diabetes.49.5.775. [DOI] [PubMed] [Google Scholar]
- 37.Chokkalingam K, et al. High-fat/low-carbohydrate diet reduces insulin-stimulated carbohydrate oxidation but stimulates nonoxidative glucose disposal in humans: An important role for skeletal muscle pyruvate dehydrogenase kinase 4. J Clin Endocrinol Metab. 2007;92(1):284–292. doi: 10.1210/jc.2006-1592. [DOI] [PubMed] [Google Scholar]
- 38.Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295(1):E46–E54. doi: 10.1152/ajpendo.00536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwang B, Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) deficiency attenuates the long-term negative effects of a high-saturated fat diet. Biochem J. 2009;423(2):243–252. doi: 10.1042/BJ20090390. [DOI] [PubMed] [Google Scholar]
- 40.Taegtmeyer H, Hems R, Krebs HA. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J. 1980;186(3):701–711. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.