Significance
Androgen is essential for the masculinization of external genitalia such as the organ size and the male-type urethra in mammals. However, the genes downstream of androgen, which are responsible for these masculinization processes, have not been identified. Here, we show v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog B (Mafb) as an essential masculinization gene for embryonic urethral formation. Mafb expression is prominent in developing male external genitalia, driving masculinization of embryonic urethral formation in an androgen-dependent manner. External genitalia of Mafb KO males exhibit urethral defects, giving insight into human hypospadias. The current findings indicate that Mafb is a crucial mediator of urethral masculinization and is a possible new candidate gene for hypospadias derived from embryonic abnormalities.
Keywords: Mafb, masculinization, urethra, hypospadias, androgen receptor
Abstract
Masculinization of external genitalia is an essential process in the formation of the male reproductive system. Prominent characteristics of this masculinization are the organ size and the sexual differentiation of the urethra. Although androgen is a pivotal inducer of the masculinization, the regulatory mechanism under the control of androgen is still unknown. Here, we address this longstanding question about how androgen induces masculinization of the embryonic external genitalia through the identification of the v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog B (Mafb) gene. Mafb is expressed prominently in the mesenchyme of male genital tubercle (GT), the anlage of external genitalia. MAFB expression is rarely detected in the mesenchyme of female GTs. However, exposure to exogenous androgen induces its mesenchymal expression in female GTs. Furthermore, MAFB expression is prominently down-regulated in male GTs of androgen receptor (Ar) KO mice, indicating that AR signaling is necessary for its expression. It is revealed that Mafb KO male GTs exhibit defective embryonic urethral formation, giving insight into the common human congenital anomaly hypospadias. However, the size of Mafb KO male GTs is similar with that of wild-type males. Moreover, androgen treatment fails to induce urethral masculinization of the GTs in Mafb KO mice. The current results provide evidence that Mafb is an androgen-inducible, sexually dimorphic regulator of embryonic urethral masculinization.
External genitalia exhibit marked sexual dimorphism in mammals. The organ size of external genitalia is longer in male mice than female mice. Androgen-dependent control of organ size is widely accepted as a masculinization parameter. In addition to androgen-dependent size control, mesenchymal differentiation is an essential process of masculinization to form sexually dimorphic structures such as corporal tissues, the penile bone, and the urethra. Sexual differentiation of corporal tissues and penile bones occurs after birth (1, 2). In contrast, urethral formation shows sexual dimorphism during embryogenesis (Fig. 1) (3). Androgen actions are central to masculinization of external genitalia (4, 5). However, the mechanisms mediating androgen-dependent masculinization processes are largely unknown. Failure of exposure to androgen causes lack of masculinization of male genital tubercles (GTs) (3, 6). Therefore, proper timing of embryonic exposure to androgen is essential for inducing GT masculinization. Androgen actions are mediated through a specific receptor, the androgen receptor (AR). Recent genetic studies on a conditional allele of Ar suggest that mesenchymal androgen actions are essential for GT masculinization (3).
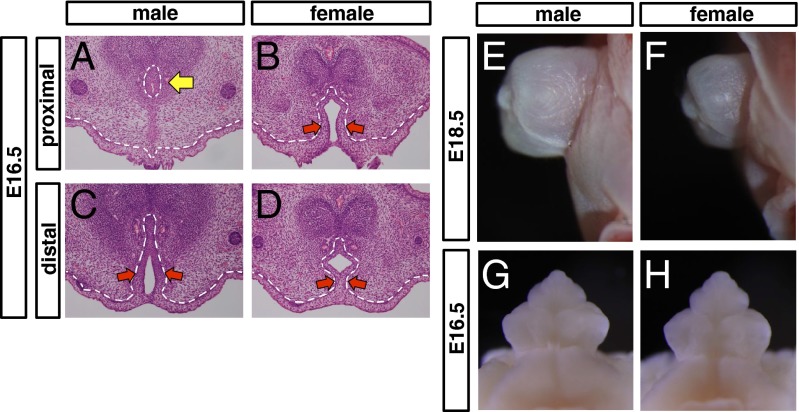
Fig. 1.
Sexually dimorphic structures of mouse embryonic external genitalia. (A–D) Histological analysis of the embryonic external genitalia at E16.5 by H&E staining. In male embryos, the male-type urethra (tubular urethra) is formed in the proximal region (yellow arrow in A). The urethral plate (red arrows in C) remains in the distal region of the GT, indicating that the male-type urethra is formed from the proximal to the distal. In female embryos, the urethral plate remains from the proximal to the distal (red arrows in B and D). (E–H) Appearance of the embryonic external genitalia at E18.5 (E and F) and E16.5 (G and H). Red arrows in B, C, and D indicate a urethral plate. Dashed lines in A–D, epithelial–mesenchymal border.
Embryonic masculinization processes of male GTs are divided into androgen-dependent organ size control and androgen-dependent mesenchymal differentiation to form the urethra. Researchers have focused on the identification of masculinization genes under the control of androgen actions for many years. However, such genes have not been identified. Recently, growth factor signals have been suggested to regulate the masculinization of the size of GTs (3, 7). However, the modulation of such growth factor signals does not induce the urethral masculinization (3, 7). These observations indicate that distinct regulators may induce embryonic urethral masculinization under androgen actions. Thus, it is still unclear how such unique morphogenetic processes are mediated by androgen-driven regulators.
The external genitalia are the most common sites of congenital abnormalities in humans (8–10). Of those, hypospadias is the frequent abnormality in which the urethral opening is ectopically located on the ventral (lower) side of the penis (8, 10–12). The male urethra connects the bladder to the penis and transports urine and semen outside of the body. In males, the urethra is incorporated into the glans to form the tubular urethra (Fig. 1). The urethral plate is an early, transitory developmental structure that develops into the tubular urethra in males. Fusion of the urethral plate is an essential process for tubular urethral formation (13). Various environmental substances are speculated to modulate developmentally essential hormonal pathways. Maternal exposure to estrogenic compounds may increase the risk of hypospadias because these compounds may interfere with the effects of fetal androgen (14–16). These studies indicate that hypospadias may be caused by disruption of the androgen exposure required for embryonic urethral masculinization. The prevalence of hypospadias is reported as increasing, but its pathogenic mechanism remains unknown.
Recently, we identified several candidate masculinization genes showing the sexually dimorphic expression during GT development by microarray analyses (17). Of note, Mafb (v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog B) was expressed prominently in the male GT mesenchyme during urethral formation. Mafb is a member of the large Maf family, which encodes leucine-zipper transcription factors (18). Mafb contributes to cell differentiation and organogenesis in a variety of cell types and tissues, including macrophages, the pancreas, and the kidney (19). The current study suggests that Mafb is required for androgen-dependent embryonic urethral masculinization. Mafb KO male embryos exhibited defective urethra with phenotypes similar to those of human hypospadias. Moreover, exposure to androgen in the absence of Mafb failed to masculinize the urethra. Of note, the organ size of mutant male GTs was similar to that of wild-type males, despite the presence of severe urethral defects. These results indicate that the embryonic masculinization processes that regulate organ size are independent of Mafb functions. Taken together, current results indicate that Mafb is an essential masculinization gene for embryonic urethral formation under androgen actions. Our results provide new insights into the molecular mechanisms of masculinization of the embryonic external genitalia and into the etiology of the human congenital anomaly hypospadias.
Results
The Urethra Is a Sexually Dimorphic Structure of Embryonic External Genitalia.
It is generally accepted that organ size of external genitalia is longer in males than in females. In addition to such size differences, the urethral structure differs prominently between males and females. To identify the onset of masculinization of the embryonic external genitalia, we reanalyzed the developing GT. In male embryos, the urethra is incorporated into the glans that formed the tubular urethra (hereafter designated as male-type urethra) (13). In contrast, the female urethra, which is not incorporated into the clitoris, is located in the ventral GT (13). The urethral plate, an early developmental epithelium essential for urethral formation, fused at the midline of the GT from the proximal region of male GT at E16.5. Subsequently, the male-type urethra formed in the glans (Fig. 1A, yellow arrow). The urethral plate remained in the distal region of the GT, indicating that the male-type urethra started to form in the proximal region at E16.5 and continued to form distally thereafter (Fig. 1C, red arrows). Unlike male-type urethral formation, masculinization of the GT organ size was not prominent at E16.5, indicating that the timing of induction was different for each masculinization process: GT organ size and male-type urethral formation (Fig. 1 E–H). Thus, the tubular urethral formation in the male GT is a representative process of its embryonic masculinization.
Mesenchymal AR Signaling Is Essential for Embryonic Urethral Masculinization.
AR is expressed in the GT mesenchyme and in the urethral epithelium (3). Genetic analyses using the Gli1CreERT2 Cre-expressing strain indicate that mesenchymal AR signaling may be essential for embryonic urethral masculinization. However, Cre activity in the Gli1CreERT2 Cre-expressing strain is detected broadly in the GT mesenchyme and in the epithelia (3). To identify the critical mesenchymal region for embryonic urethral masculinization, we reexamined the AR expression during the GT development by immunohistochemistry. AR was prominently expressed in the GT mesenchyme adjacent to the urethral plate (Fig. 2A, square). These results prompted us to investigate whether such a mesenchymal region was involved in embryonic urethral masculinization. To examine this possibility, we used a conditional Ar null allele (Arlox/flox) by using the Sall1CreERT2 Cre-expressing strain (20). Cre activity in such a strain was detected in the mesenchyme adjacent to the urethral plate, including the AR-expressing region (Fig. 2B). Arflox/–;Sall1CreERT2 (hereafter designated as Ar cKO) mice exhibited a defective male-type urethra that was open at the ventral side of the GT, indicating the failure of embryonic urethral masculinization (Fig. 2D). In contrast, the male-type urethra was formed in wild-type male embryos (Fig. 2C, yellow arrow). These results indicate that AR signaling in the mesenchyme adjacent to the urethral plate is essential for embryonic urethral masculinization.
Fig. 2.
Mesenchymal AR signaling is essential for embryonic masculinization of the urethra. (A) Expression of AR in male GT mesenchyme adjacent to the urethral plate (square) at E16.5. (B) Detection of Cre-expressing cells in the developing GT of Sall1CreERT2 male embryos at E15.5 using the R26R-LacZ indicator line. (C and D) Histological analysis of the male GT of wild-type (WT) and Arflox/–;Sall1CreERT2 (Ar cKO) at E16.5. The yellow arrow in C indicates the male-type urethra.
Sexually Dimorphic Expression of Mafb During Male-Type Urethral Formation.
Recently, we identified several candidate masculinization genes showing sexually dimorphic expression during GT development (17). Intriguingly, MAFB was expressed prominently in male GT mesenchyme adjacent to the urethral plate at E16.5 (Fig. 3A, square). To determine the expression profile of Mafb during GT development, we used a Mafb–GFP knock-in mouse line (hereafter designated as MafbGFP/+) as a reporter for its expression (21). Male-type urethral formation occurred from the proximal to distal midventral region of male GTs (Fig. 1). Of note, Mafb was prominently expressed in the male GTs during urethral formation (Fig. 3 B, D, F, H, J, and L). Such sexually dimorphic expression of Mafb was observed at E14.5 before any morphological indications of male-type urethral formation (Fig. 3 B, red arrow, and H, yellow arrows). In contrast, Mafb did not show such an expression pattern in the female GTs (Fig. 3 C, E, G, I, K, and M). MAFB expression was detected in both male and female GTs earlier than E12.5 (Fig. S1). These results indicate that Mafb is a developmental gene associated with GT masculinization. Hence, we investigated whether Mafb played a role as a downstream gene of AR signaling during the embryonic GT masculinization.
Fig. 3.
Sexually dimorphic expression of Mafb during GT development. (A) Image of anti-MAFB pAb staining (brown) that represents MAFB expression in male GT mesenchyme adjacent to the urethral plate (square) at E16.5. (B–G) Appearance of the developing GT of MafbGFP/+ male mice (B, D, and F) and MafbGFP/+ female mice (C, E, and G). GFP fluorescence indicates the Mafb-expressing region (red arrow in B, D, and F). (H–M) Expression pattern of Mafb in the developing GT of male (H, J, and L) and female (I, K, and M) embryos. Its expression was detected by GFP immunostaining. Yellow arrows in H, GFP-positive GT mesenchymal cells. Insets in H–M, higher magnification of the GT mesenchyme adjacent to the urethral plate.
Requirement of AR Signaling for Mafb Expression.
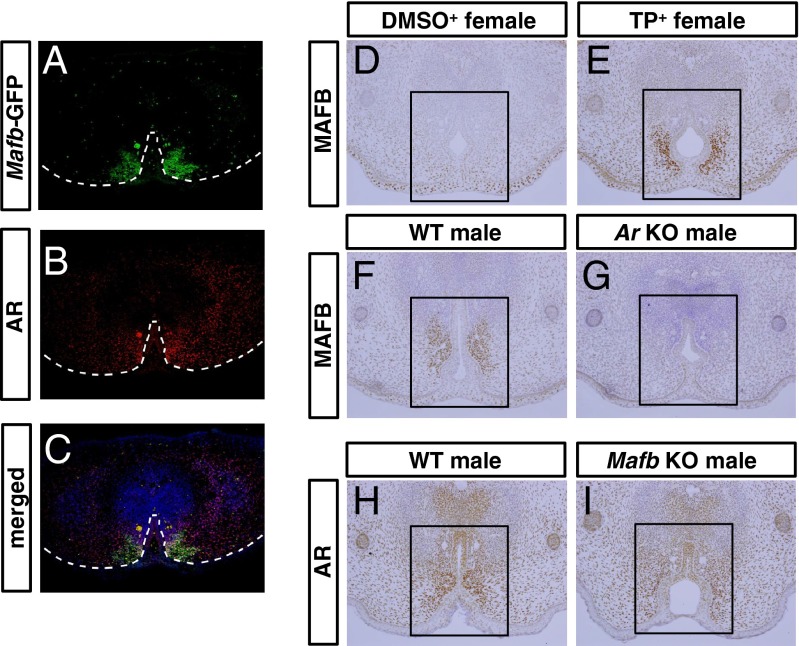
The Mafb-expressing region in the GT mesenchyme adjacent to the urethral plate was overlapped with the region of prominent AR expression (Fig. 4 A–C). We therefore investigated whether androgen signals were required for Mafb expression in such mesenchyme. The female embryos exposed to androgen between E15.5 and E17.5 exhibit urethral masculinization (3). To determine whether androgen was required for the mesenchymal Mafb expression, we administrated androgen, testosterone propionate (TP), to pregnant mice. Expression of MAFB was induced in female GT mesenchyme after 24 h of androgen treatment (Fig. 4E, square). Such induction of MAFB expression was evident within 6 h of androgen treatment (Fig. S2). To further investigate the androgen dependence of Mafb expression, we analyzed MAFB expression in Ar KO male GTs. MAFB expression was much lower in the GT mesenchyme of Ar KO males than that of wild-type males (Fig. 4G). In contrast, nuclear localization of AR was maintained in the GT mesenchyme of Mafb KO males, indicating that AR signaling was still activated in the mesenchyme of Mafb KO males (Fig. 4I). These results indicate that AR signaling is required for Mafb expression.
Fig. 4.
AR signaling is required for the expression of MAFB. (A and B) Expression patterns of MAFB (green) and AR (red) in the male GT at E16.5. (C) Merged image of expression of MAFB and AR at E16.5. (D and E) MAFB expression of DMSO-treated female GT as the control and TP-treated female GT. (F and G) MAFB expression in the GTs of wild-type males and Ar KO males at E16.5. (H and I) AR expression in the GTs of wild-type males and Mafb KO males at E16.5. Square in D–I, mesenchyme adjacent to the urethral plate. Dashed lines in A–C, epithelial–mesenchymal border.
Mafb Is Essential for Male-Type Urethral Formation.
To investigate the role of Mafb in GT development, we examined Mafb KO embryos (21). Notably, Mafb KO male GTs exhibited hypospadias-like phenotypes in which the prepuce failed to fuse along the ventral midline (Fig. 5B, red arrow). Furthermore, histological analysis revealed that Mafb KO male GTs exhibited defective urethral formation (Fig. 5 C–E). The urethra was incorporated into the glans in wild-type males (Fig. 5C, yellow arrow). However, the male-type urethra did not form, with abnormal urethral opening at the ventral side of the glans in Mafb KO male GTs (Fig. 5D). The phenotype of Mafb KO female GTs was not obviously different from that of wild-type females. Mafb is expressed in steroidogenic Leydig cells (22). Testosterone (T) is locally converted into the dihydrotestosterone (DHT) by 5α-reductase type 2 in the GT mesenchyme (23, 24). To confirm the production of androgens, we measured the level of testicular T and the local amount of DHT in the GT. For each level of T and DHT, steroid levels in mutant males were not significantly different from those in wild-type males (Table 1). The perineal region is another sexually dimorphic region and is sensitive to androgen actions (25). The phenotype of perineal regions did not prominently differ between mutant and wild-type males (bracket in Fig. 5 A and B). Notably, the organ size of GT was similar between mutant and wild-type males, despite severe urethral defects in the mutants (Fig. 5 F–H). These results indicate that Mafb is essential for embryonic urethral masculinization.
Fig. 5.
Mafb is essential for masculinization of the urethra but not for masculinization of the GT organ size. (A and B) Appearance of wild-type and Mafb KO male GTs at E18.5. (C–E) H&E staining of wild-type male, Mafb KO male, and wild-type female GTs at E19.0. (F–H) Appearance of wild-type male, Mafb KO male, and wild-type female GTs at E18.5. Bracket in A and B, perineal region. Red arrow in B, defective urethral formation in Mafb KO male GTs. Yellow arrow in C, tubular urethra. Dashed lines in C–E, epithelial–mesenchymal border.
Table 1.
Measurement of T and DHT in the testes and GTs
| Androgen | WT ♂ | KO ♂ |
| T (testis) | 887.9 (n = 10) | 780.1 (n = 13) |
| DHT (genital tubercle) | 6.3 (n = 10) | 5.5 (n = 17) (ng/g) |
Similar level of T and DHT in the testes and GTs of Mafb KO compared with those of wild-type males.
Mafb Is Essential for Embryonic Urethral Masculinization Induced by Androgen Actions.
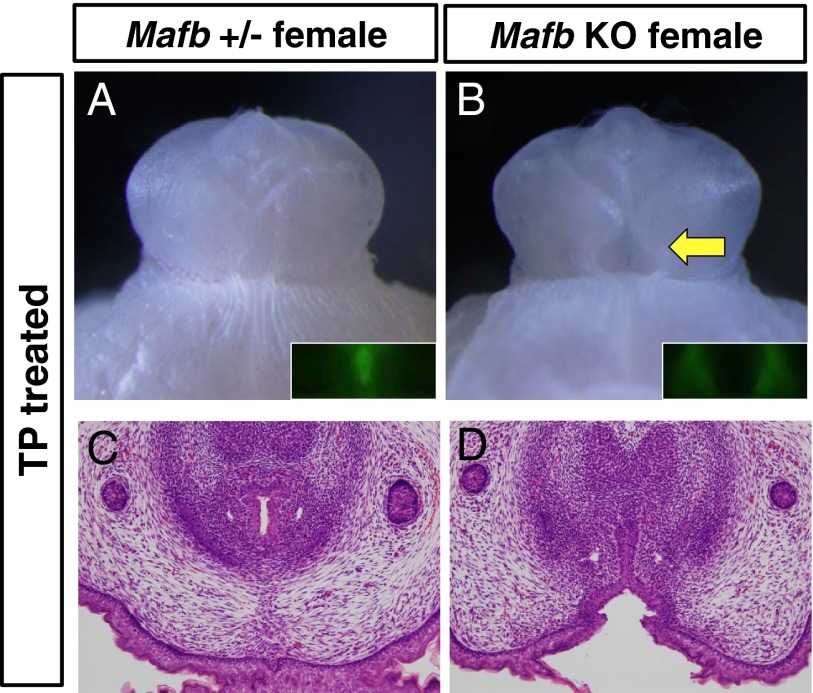
Our data indicate that Mafb is a masculinization gene important for androgen-dependent male-type urethral formation. Given such circumstances, Mafb KO female GTs were not expected to be masculinized under excessive androgen exposure. To test this hypothesis, we analyzed whether GT masculinization occurred in androgen-treated Mafb KO female mice. Control female GTs exposed to exogenous androgen showed the male-type urethra (tubular urethra), indicating the induction of urethral masculinization (Fig. 6 A and C). Although Mafb–GFP expression was induced in Mafb KO female GTs exposed to exogenous androgen (Fig. 6B, Inset), a male-type urethra did not form in these females (Fig. 6 B, yellow arrow, and D). On the other hand, the size of the GT in mutant females exposed to exogenous androgen was larger than that in DMSO-treated control females, indicating that masculinization of the GT size was induced in the mutants (Fig. S3). These results suggest that Mafb is a pivotal masculinizing gene responsible for embryonic urethral masculinization under androgen actions.
Fig. 6.
Mafb is essential for the urethral masculinization induced by androgen. (A and B) Appearance of TP-treated control (MafbGFP/+) female and Mafb KO (MafbGFP/GFP) female GTs at E18.5. Inset in A and B, detection of Mafb-expressing cells by using the Mafb–GFP knock-in mouse line. (C and D) Histological analysis of control (MafbGFP/+) female and TP-treated Mafb KO GTs at E18.5, respectively. Yellow arrow in B, defective urethral formation.
Discussion
Sexual differentiation, such as embryonic masculinization of external genitalia, is one of the essential processes in male reproductive organ formation (26). Androgens drive masculinization of external genitalia. Despite many efforts to understand the mechanisms mediating this masculinization, the identity of such masculinization genes remained unknown. Here, we identified Mafb as an essential gene for embryonic urethral masculinization. Prominent characteristics of GT masculinization include the enlarged organ size and the male-type urethral formation. Fusion of the urethral plate along the midline of the GT is essential for male-type urethral formation. Mafb was expressed prominently in the mesenchyme adjacent to the urethral plate in male GTs. Additionally, Mafb KO male GTs failed to induce embryonic urethral masculinization. During GT development, AR is expressed broadly in the mesenchyme of the male GT. We observed that Ar conditional knockout (cKO) (Arflox/–;Sall1CreERT2) male mice without AR functions in the mesenchyme adjacent to the urethral plate showed the defective urethral formation. These results indicate that AR signaling in such mesenchyme is essential for regulating the embryonic urethral masculinization. Furthermore, the male-dominant Mafb expression in such mesenchymal regions was dramatically down-regulated in Ar KO male GTs relative to wild-type males. These results indicate that Mafb acts downstream of androgen signaling during the embryonic urethral masculinization.
Several works have identified the critical time window for masculinization of external genitalia (3, 6, 27). Exposure to a proper amount of androgen within this period is essential for the induction of its masculinization. Although failure of such androgen exposure induces the defective masculinization, the degree of anomalies depends on the timing of androgen exposure. In rat models, blocking androgen actions in late gestation induces the defective penile growth, but does not induce hypospadias (6, 28). Mafb exhibited sexually dimorphic expression during this critical time window. Mafb KO male GTs exhibited defects of embryonic urethral masculinization. In contrast, GT organ size was not affected in the mutants. These results indicate that Mafb is essential for embryonic masculinization of the urethra, but not for masculinization of the GT organ size. In clinical medicine, administration of androgen to a human patient with micropenis and hypospadias is reportedly effective for restoring penile growth, but not for alleviating the hypospadias phenotype. Although the expression of Mafb was induced by treatment of exogenous androgen, such induction was not observed before E14.5 (e.g., E12.5). These results indicate that inducing the expression of the masculinization genes for the urethral formation is tightly regulated during the masculinization processes.
Hypospadias is a common congenital anomaly (8–10). Although the incidence of hypospadias is increasing, its etiology remains unknown. Several genes are suggested as the candidate genes for the urethral formation based on analyses of knockout mice (10, 29–33). However, many of these KO mice exhibit severe urethral defects in early embryonic stages before sexual differentiation. Notably, Mafb KO mice show prominent urethral defects specifically during male-type urethral formation in late-stage embryos. Thus, Mafb KO mouse is the new mouse model showing such human hypospadias-like phenotypes. Furthermore, androgen treatment failed to induce embryonic urethral masculinization in Mafb KO mice embryos. Our findings indicate that Mafb is a pivotal mediator of androgen actions during embryonic urethral masculinization.
Another characteristic of the masculinization of external genitalia is the difference in the GT organ size. Regulation of cell proliferation by growth factors has been suggested as an essential process for the GT development (30, 34–36). Gene-driven GT outgrowth regulated by several growth factors has been proposed as essential for androgen-independent early embryonic GT growth. Genetic analyses based on several KO mouse models indicate that the failure of such early growth leads to abnormal size of the GT in either sex (31, 37, 38). In addition to such early organ size control, several growth factor signals can modulate embryonic masculinization of the size of the GT (3, 7). However, such growth factor-mediated modulation is not sufficient to induce full masculinization of the GT, because gain-of-function mutants for an individual growth factor gene do not exhibit urethral masculinization (3, 7). Thus, masculinization of the GT may require definitive tissue differentiation processes as well as its growth regulation. Current results indicate that each masculinization process, including GT organ size control and tubular urethral formation, is regulated independently by androgen actions. The coordinated regulation of these processes may be essential for the proper masculinization of the embryonic GT.
Materials and Methods
Collections of Mutant Mouse Embryos and Hormone Treatments.
Each mouse strain used in this study—MafbGFP/+, Ar KO (Ar knockout), Arflox/flox conditional mutant alleles, Sall1CreERT2, and R26R-LacZ indicator—was described previously (20, 21, 39–41). The genetic background of this mice line was C57BL/6J. For embryonic sampling, each pregnant female was killed between E12.5 and E19.0, and the embryos were examined. Each protocol involving animals was approved by the animal study committee of Wakayama Medical University. The tamoxifen (TM)-inducible Cre recombinase system removes the floxed sequence from the target genome (42). TM (Sigma) was dissolved in sesame oil at 100 mg/kg of body weight to treat the pregnant mice via oral gavage at E11.5. Under these conditions, no overt teratologic effects are observed in the external genitalia (43). For androgen treatment, each pregnant CD1 mouse (Charles River Laboratories) was i.p. injected with TP (Sigma) or DMSO (Nacalai Tesque, Inc.) dissolved in sesame oil at 100 mg/kg of body weight. To induce masculinization of the female GT via exogenous androgen, each pregnant female MafbGFP/+ mouse was injected daily via i.p. injection with TP dissolved in sesame oil at 100 mg/kg body weight from day 13.5–17.5 of gestation.
Histology, Immunohistochemistry, and X-Gal Staining.
Embryonic specimens were fixed overnight in 4% (wt/vol) paraformaldehyde (PFA)/PBS, dehydrated in methanol, and embedded in paraffin. Serial sections (6-μm thick) were prepared for hematoxylin/eosin (H&E) staining and immunohistochemistry. Rabbit anti-MAFB pAb (polyclonal antibody) (1:1,500, Bethyl Laboratories, IHC-00351), anti-AR pAb (1:300, Santa Cruz Biotechnology, sc-816), and anti-GFP pAb (1:200, abcam, ab6556) were used in this study. Immunostaining was visualized via 3,3′-diaminodbenzidine staining (Wako Pure Chemical Industries) or via fluorescent staining (an Alexa 488-conjugated IgG or an Alexa 546-conjugated IgG) (Molecular Probes). Nuclei were counterstained with Hoechst (Nacalai Tesque, Inc.). X-gal staining for the detection of Sall1-expressing cells was performed as previously described (43).
Hormone Measurement.
For measurement of T and DHT, we used pooled testes and GTs from wild-type or MafB KO male mice. T and DHT concentrations were determined as described previously (44) with some modifications (ASKA Pharma Medical. Co., Ltd.). Briefly, testes or GTs were homogenized in distilled water. After T-13C3 and DHT-13C3, as internal standard, were added to the homogenized tissue, the steroids were extracted with ethyl acetate. Subsequently, the extracts were purified using Oasis MAX cartridge (Waters Co.). After derivatization to picolinoyl ester forms, the derivatized steroids were purified using an InertSep SI cartridge (GL Sciences Inc.). The concentrations of T and DHT were determined with LC-MS/MS (liquid chromatography-tandem mass spectrometry).
Supplementary Material
Acknowledgments
We thank Drs. D. Duboule, A. A. Thomson, P. Chambon, R. R. Behringer, D. W. Russell, M. Mahendroo, N. Shiraki, Y. Ogino, S. Miyagawa, T. Matsumoto, S. Kato, L. Liqing, D. Matsumaru, A. Murashima, and H. Sasamoto for their invaluable support and M. Matsumoto, S. Fujikawa, M. Eto, S. Miyaji, E. Chun, and T. Iba for their assistance. This work was supported by Grant-in-Aid for Scientific Research on Innovative Areas: Molecular Mechanisms for Establishment of Sex Differences (22132006), the National Institutes of Health Grant R01ES016597, and Grant-in-Aid for Scientific Research (24590235).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413273111/-/DCSupplemental.
References
- 1.Schlomer BJ, et al. Sexual differentiation in the male and female mouse from days 0 to 21: A detailed and novel morphometric description. J Urol. 2013;190(4) Suppl:1610–1617. doi: 10.1016/j.juro.2013.02.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez E, Jr, et al. New insights on the morphology of adult mouse penis. Biol Reprod. 2011;85(6):1216–1221. doi: 10.1095/biolreprod.111.091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyagawa S, et al. Genetic interactions of the androgen and Wnt/beta-catenin pathways for the masculinization of external genitalia. Mol Endocrinol. 2009;23(6):871–880. doi: 10.1210/me.2008-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiort O. Clinical and molecular aspects of androgen insensitivity. Endocr Dev. 2013;24:33–40. doi: 10.1159/000342499. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed SF, et al. Phenotypic features, androgen receptor binding, and mutational analysis in 278 clinical cases reported as androgen insensitivity syndrome. J Clin Endocrinol Metab. 2000;85(2):658–665. doi: 10.1210/jcem.85.2.6337. [DOI] [PubMed] [Google Scholar]
- 6.Welsh M, et al. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008;118(4):1479–1490. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyagawa S, et al. The role of sonic hedgehog-Gli2 pathway in the masculinization of external genitalia. Endocrinology. 2011;152(7):2894–2903. doi: 10.1210/en.2011-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed SF, et al. Prevalence of hypospadias and other genital anomalies among singleton births, 1988-1997, in Scotland. Arch Dis Child Fetal Neonatal Ed. 2004;89(2):F149–F151. doi: 10.1136/adc.2002.024034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiort O. The differential role of androgens in early human sex development. BMC Med. 2013;11:152. doi: 10.1186/1741-7015-11-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaschko SD, Cunha GR, Baskin LS. Molecular mechanisms of external genitalia development. Differentiation. 2012;84(3):261–268. doi: 10.1016/j.diff.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation. 2003;71(8):445–460. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
- 12.van der Zanden LF, et al. Aetiology of hypospadias: A systematic review of genes and environment. Hum Reprod Update. 2012;18(3):260–283. doi: 10.1093/humupd/dms002. [DOI] [PubMed] [Google Scholar]
- 13.Baskin LS, et al. Urethral seam formation and hypospadias. Cell Tissue Res. 2001;305(3):379–387. doi: 10.1007/s004410000345. [DOI] [PubMed] [Google Scholar]
- 14.Kim KS, et al. Induction of hypospadias in a murine model by maternal exposure to synthetic estrogens. Environ Res. 2004;94(3):267–275. doi: 10.1016/S0013-9351(03)00085-9. [DOI] [PubMed] [Google Scholar]
- 15.Baskin LS, Liu W, Bastacky J, Yucel S. Anatomical studies of the mouse genital tubercle. Adv Exp Med Biol. 2004;545:103–121. doi: 10.1007/978-1-4419-8995-6_7. [DOI] [PubMed] [Google Scholar]
- 16.Carmichael SL, et al. National Birth Defects Prevention Study Hypospadias and maternal intake of phytoestrogens. Am J Epidemiol. 2013;178(3):434–440. doi: 10.1093/aje/kws591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishida H, et al. Gene expression analyses on embryonic external genitalia: Identification of regulatory genes possibly involved in masculinization processes. Congenit Anom (Kyoto) 2008;48(2):63–67. doi: 10.1111/j.1741-4520.2008.00180.x. [DOI] [PubMed] [Google Scholar]
- 18.Kataoka K, Fujiwara KT, Noda M, Nishizawa M. MafB, a new Maf family transcription activator that can associate with Maf and Fos but not with Jun. Mol Cell Biol. 1994;14(11):7581–7591. doi: 10.1128/mcb.14.11.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eychène A, Rocques N, Pouponnot C. A new MAFia in cancer. Nat Rev Cancer. 2008;8(9):683–693. doi: 10.1038/nrc2460. [DOI] [PubMed] [Google Scholar]
- 20.Inoue S, Inoue M, Fujimura S, Nishinakamura R. A mouse line expressing Sall1-driven inducible Cre recombinase in the kidney mesenchyme. Genesis. 2010;48(3):207–212. doi: 10.1002/dvg.20603. [DOI] [PubMed] [Google Scholar]
- 21.Moriguchi T, et al. MafB is essential for renal development and F4/80 expression in macrophages. Mol Cell Biol. 2006;26(15):5715–5727. doi: 10.1128/MCB.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeFalco T, Takahashi S, Capel B. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol. 2011;352(1):14–26. doi: 10.1016/j.ydbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian H, Russell DW. Expression and regulation of steroid 5 alpha-reductase in the genital tubercle of the fetal rat. Dev Dyn. 1997;209(1):117–126. doi: 10.1002/(SICI)1097-0177(199705)209:1<117::AID-AJA11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 24.Kim KS, et al. Expression of the androgen receptor and 5 alpha-reductase type 2 in the developing human fetal penis and urethra. Cell Tissue Res. 2002;307(2):145–153. doi: 10.1007/s004410100464. [DOI] [PubMed] [Google Scholar]
- 25.Ipulan LA, et al. Nonmyocytic androgen receptor regulates the sexually dimorphic development of the embryonic bulbocavernosus muscle. Endocrinology. 2014;155(7):2467–2479. doi: 10.1210/en.2014-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilhelm D, Koopman P. The makings of maleness: Towards an integrated view of male sexual development. Nat Rev Genet. 2006;7(8):620–631. doi: 10.1038/nrg1903. [DOI] [PubMed] [Google Scholar]
- 27.Leihy MW, Shaw G, Wilson JD, Renfree MB. Development of the penile urethra in the tammar wallaby. Sex Dev. 2011;5(5):241–249. doi: 10.1159/000334053. [DOI] [PubMed] [Google Scholar]
- 28.Husmann DA. Micropenis: An animal model and its human correlates. Adv Exp Med Biol. 2002;511:41–54; discussion 54-56. doi: 10.1007/978-1-4615-0621-8_4. [DOI] [PubMed] [Google Scholar]
- 29.Yamada G, et al. Molecular genetic cascades for external genitalia formation: An emerging organogenesis program. Dev Dyn. 2006;235(7):1738–1752. doi: 10.1002/dvdy.20807. [DOI] [PubMed] [Google Scholar]
- 30.Petiot A, Perriton CL, Dickson C, Cohn MJ. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development. 2005;132(10):2441–2450. doi: 10.1242/dev.01778. [DOI] [PubMed] [Google Scholar]
- 31.Lin C, Yin Y, Long F, Ma L. Tissue-specific requirements of beta-catenin in external genitalia development. Development. 2008;135(16):2815–2825. doi: 10.1242/dev.020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalfa N, Sultan C, Baskin LS. Hypospadias: Etiology and current research. Urol Clin North Am. 2010;37(2):159–166. doi: 10.1016/j.ucl.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Dravis C, et al. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271(2):272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Miyagawa S, et al. Dosage-dependent hedgehog signals integrated with Wnt/beta-catenin signaling regulate external genitalia formation as an appendicular program. Development. 2009;136(23):3969–3978. doi: 10.1242/dev.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin C, et al. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development. 2009;136(23):3959–3967. doi: 10.1242/dev.039768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haraguchi R, et al. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128(21):4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- 37.Kondo T, Zákány J, Innis JW, Duboule D. Of fingers, toes and penises. Nature. 1997;390(6655):29. doi: 10.1038/36234. [DOI] [PubMed] [Google Scholar]
- 38.Cohn MJ. Development of the external genitalia: Conserved and divergent mechanisms of appendage patterning. Dev Dyn. 2011;240(5):1108–1115. doi: 10.1002/dvdy.22631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato T, et al. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci USA. 2004;101(6):1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21(1):70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 41.Murashima A, et al. Essential roles of androgen signaling in Wolffian duct stabilization and epididymal cell differentiation. Endocrinology. 2011;152(4):1640–1651. doi: 10.1210/en.2010-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237(3):752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 43.Haraguchi R, et al. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development. 2007;134(3):525–533. doi: 10.1242/dev.02736. [DOI] [PubMed] [Google Scholar]
- 44.Arai S, et al. Effect of castration monotherapy on the levels of adrenal androgens in cancerous prostatic tissues. Steroids. 2011;76(3):301–308. doi: 10.1016/j.steroids.2010.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.