Abstract
Alkaline phosphatase (ALP) flare phenomenon documented as scintigraphic flare phenomenon due to elevated serum ALP levels produced by osteoblasts reflects an osteoblastic reaction in response to the effective therapy of non-small cell lung cancer (NSCLC). Here, we report a case of ALP flare following gefitinib treatment for NSCLC. We also retrospectively analyzed the prevalence of ALP flare in lung cancer patients treated via epidermal growth factor receptor-tyrosine kinase inhibitor in our hospital. Recognition of this phenomenon is important for physicians treating NSCLC patients to avoid discontinuation of a potentially beneficial treatment because of misdiagnosis for refractory multiple bone metastasis or adverse effect.
Keywords: Alkaline phosphatase, Flare phenomenon, Non-small cell lung cancer, Epidermal growth factor receptor-tyrosine kinase inhibitor
Introduction
Flare phenomenon on bone scintigraphy was first documented in 1970s [1] and is defined based on an increase in the number of lesions or intensity observed in a bone scan and the subsequent decreased uptake of radiotracers in these lesions on repeated examinations. Flare phenomenon reflects a favorable response to treatment, which has been well documented in prostate cancer and breast cancer patients undergoing chemotherapy or hormone therapy [2–4]. This phenomenon is typically observed approximately 3 weeks to 3 months following therapy [2,5,6]. Alkaline phosphatase (ALP) flare phenomenon has also been documented as a scintigraphic flare phenomenon, as elevated serum ALP levels produced by osteoblasts reflect an osteoblastic reaction [2,4,7]. Both of these flare phenomena are thought to occur in response to effective therapy of non-small cell lung cancer (NSCLC).
Although reports have been filed in recent years on flare phenomenon following anti-cancer chemotherapy in lung cancer [5,8–12], little information is available regarding the incidence of ALP flare during anti-cancer chemotherapy, particularly among patients treated with epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI).
Here, we report a case of ALP flare following gefitinib treatment for NSCLC and the results of retrospective analysis of the prevalence of ALP flare in lung cancer patients treated with EGFR-TKI in our hospital.
Case
A 62-year-old man presenting with adenocarcinoma, multiple lung nodules, hilar lymphadenopathy, and pleural dissemination was admitted to our hospital (Fig. 1). Gefitinib was administered following four cycles of cisplatin and pemetrexed. Serum alkaline phosphatase (ALP) levels increased to 1.85 times admission levels following 2 weeks of gefitinib therapy. Although the patient's bone scan revealed multiple hot spots, he denied pain (Fig. 2). We considered these phenomena to be due to flare phenomenon rather than treatment failure, and gefitinib was therefore continued. Approximately one month after gefitinib treatment, serum ALP levels returned to pre-treatment values, and 2-[18F]fluoro-2-deoxy-d-glucose/positron-emission tomography (FDG/PET) indicated no uptake of radiotracer in the bone (Fig. 3). The patient received gefitinib for 119 days, and then erlotinib was initiated 258 days later. However, the patient's disease progressed, and he refused to remain on chemotherapy. The patient died of cancer 635 days after diagnosis.
Fig. 1.
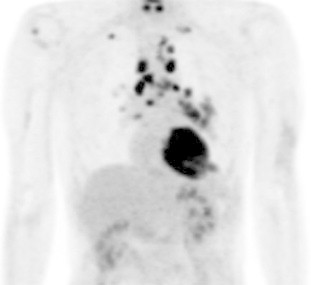
2-[18F]fluoro-2-deoxy-d-glucose/positron-emission tomography (FDG/PET) performed before any treatment revealed increased uptake of radiotracer to the lesion located in left lower lobe and multiple mediastinum and hilar lymph nodes not to any bone area.
Fig. 2.
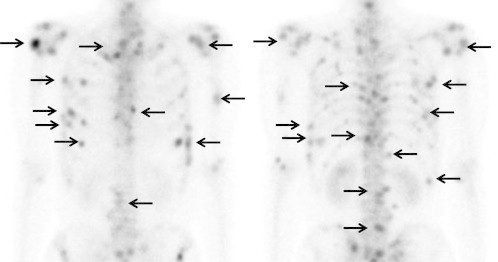
Bone scintigraphy performed 14 days after the initiation of gefitinib treatment revealed increased uptake of radiotracer in multiple bone lesions.
Fig. 3.
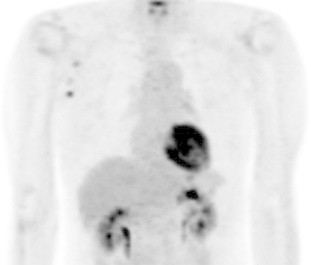
FDG/PET performed 58 days after the start of gefitinib treatment revealed no uptake of radiotracer to the tumor, bone, and lymph nodes. The clinician evaluated the patient as partial response (PR) and continued gefitinib therapy.
To analyze the prevalence of ALP flare phenomenon, we retrospectively evaluated 224 NSCLC patients who received EGFR-TKI treatment in the form of gefitinib (n = 126) or erlotinib (n = 98) between April 2006 and September 2012 in our institution. Table 1 shows the list of patients with transient elevation of ALP levels following EGFR-TKI treatment. We extracted those who experienced transient elevation within 3 months of EGFR-TKI treatment and examined their phenomena with regard to “ALP flare”.
Table 1.
Characteristics of patients who were observed ALP flare during EGFR-TKI treatment.
| No. | Age | Sex | EGFR mutation | Bone metastasis | Baseline ALP | Peak ALP (day) | Baseline peak ratio | Peakout ALP (day) | Response | Total medication (day) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | F | 19 deletions | + | 783 | 1006 (8) | 1.28 | 319 (115) | PR | 185 |
| 2 | 60 | M | 21 L858R | + | 534 | 731 (9) | 1.36 | 316 (32) | PR | 174 |
| 3 | 68 | M | 21 L858R | unknown | 385 | 554 (28) | 1.43 | 293 (126) | PR | 196 |
| 4 | 74 | F | – | + | 362 | 538 (16) | 1.48 | 309 (44) | PR | 314 |
| 5 | 81 | F | 21 L858R | + | 291 | 548 (12) | 1.88 | 301 (40) | PR | 339 |
| 6 | 63 | M | 21 L858R | unknown | 530 | 1121 (18) | 2.11 | 298 (66) | PR | 119 |
| 7 | 60 | M | 21 L858R | + | 1324 | 3236 (15) | 2.44 | 1065 (55) | PR | 56< |
| 8 | 73 | M | 18 G719X | + | 842 | 1009 (7) | 1.2 | 350 (91) | PR | 182 |
| 9 | 73 | F | 19 deletions | – | 289 | 401 (101) | 1.39 | 321 (136) | PR | 986< |
| 10 | 80 | M | 19 deletions | + | 239 | 429 (15) | 1.79 | 238 (35) | PR | 255< |
| 11 | 71 | M | 19 deletions | + | 1012 | 1854 (21) | 1.83 | 318 (197) | PR | 226 |
No.1–7: gefitinib group, No.8–11: erlotinib group.
Eleven (5%) patients were extracted as having possible ALP flare. All patients had adenocarcinoma, but only 10 had a mutation in the EGFR gene. In all cases, EGFR-TKI treatment was evaluated as partial response (PR), and 8 patients had bone metastasis defined by bone scintigraphy or FDG/PET. In the gefitinib-treated group, 7 patients (5.6%) exhibited ALP flare, and the median time to ALP peak was 16 days (range 8–28) after the initiation of gefitinib treatment. Baseline ALP median was 783 (range 291–1324), and peak ALP median was 731 (range 568–3536). In the erlotinib-treated group, four patients (4%) exhibited ALP flare, and the median time to ALP peak was 18 days (range 7–101) following the initiation of erlotinib treatment. Median baseline ALP was 586 (range 239–1012), and median peak ALP 719 (range 401–1854). Serum ALP values returned to normal range at a median of 114 days (range 35–197) (Table 1).
Discussion
We reported a case of ALP flare following gefitinib treatment for NSCLC and retrospectively evaluated the prevalence of ALP flare in lung cancer patients treated with EGFR-TKI in our hospital. The incidence of flare was approximately 5% and occurred within almost a month of EGFR-TKI therapy. These findings suggest that, in certain cases, effective treatment of NSCLC may still be continued even on recognition of flare phenomenon.
Determining whether the adverse event was ALP flare from new bone metastasis (evaluated as disease progression [PD]) or due to liver dysfunction was difficult. Bone scan flare is characterized by an increase in uptake of radiotracer as a result of the stimulation of osteoblastic activity during the repair process. A lytic lesion that has been overlooked during a bone scan before therapy might also present a new site of increased uptake and, therefore, be misinterpreted as indicating possible PD. Bone ALP is secreted by osteoblasts depending on the osteoblastic reaction, with serum ALP often being elevated due to ALP flare during bone scan flare [7]. Itotani et al. (2012) reported 10 patients with liver dysfunction during gefitinib treatment, which was detected at a median of 40 days after initiation of gefitinib [13]. Yoshimoto et al. also reported 5 cases of liver dysfunction detected between 28 and 56 days after initiation of gefitinib treatment, 2 of which had ALP elevation in association with transaminase elevation [14]. Given that this interval resembles that of flare phenomenon, it is difficult to distinguish ALP flare from liver dysfunction as an adverse effect. In our case, both elevated serum ALP and uptake of radiotracer during bone scan were observed. However, the patient denied experiencing bone pain, and the mass detected via chest X-ray was decreased in size compared to before treatment. Other liver function tests were also normal. We therefore continued gefitinib treatment, and after approximately one month, serum ALP values returned to pre-treatment levels, and FDG/PET did indicated no radiotracer uptake in the bone. These findings suggest that physicians should evaluate not only the bone scan but also clinical information, other serum liver function tests and other imaging modalities, when we meet elevated serum ALP following EGFR-TKI treatment. If an increase in ALP is observed during EGFR-TKI treatment, particularly within 3 months and even in Grade 3 case (Common Terminology Criteria for Adverse Events: CTCAE v4.0), but is not accompanied by elevated transaminase, physicians might not have to discontinue EGFR-TKI treatment.
We retrospectively evaluated the prevalence of ALP flare in lung cancer patients treated with EGFR-TKI in our hospital. The incidence of flare was approximately 5% and occurred within a month of initiating EGFR-TKI therapy. Chao et al. (2009) observed that bone scan flare phenomenon was present in 21.2% of NSCLC patients treated with gefitinib and was detected an average of 40 days after initiation of gefitinib treatment [5]. Colemann et al. (1988) examined the correlation between responses to hormonal treatment and alkaline phosphatase bone isozyme (ALP-BI) levels in 53 breast cancer patients [4]. According to their report, 1 month after hormonal treatment, 16 patients achieved a partial response (PR), and 15 (28%) experienced an increase in ALP-BI.
All patients who received EGFR-TKI treatment in our cases did not have confirmed bone metastasis before beginning of treatment. The incidence of ALP flare may increase if there are limited cases of confirmed bone metastasis before treatment. Confirmation of bone metastasis, however, might not be practical, as bone scans are performed relatively infrequently. Although this was a retrospective study, the result of an ALP peak being observed within one month, but one case and ALP peak-out being observed from 1 to 6 months fit the criteria of bone scan flare phenomenon.
Conclusion
We reported one case of ALP flare following gefitinib treatment for NSCLC. Given the difficulty in distinguishing ALP flare from new bone metastasis or liver dysfunction as an adverse event, recognition of these phenomena is important for physicians treating NSCLC patients to avoid discontinuation of a potentially beneficial treatment. The incidence of ALP flare was approximately 5% in NSCLC treated by EGFR-TKI and occurred within 1 month of EGFR-TKI therapy.
References
- 1.Gillespie P.J., Alexander J.L., Edelstyn G.A. Changes in 87mSr concentractions in skeletal metastases in patients responding to cyclical combination chemotherapy for advanced breast cancer. J Nucl Med. 1975;16(3):191–193. [PubMed] [Google Scholar]
- 2.Coleman R.E., Mashiter G., Whitaker K.B., Moss D.W., Rubens R.D., Fogelman I. Bone scan flare predicts successful systemic therapy for bone metastases. J Nucl Med. 1988;29:1354–1359. [PubMed] [Google Scholar]
- 3.Aizawa T., Tochimoto M., Ito T., Tsujino S., Akiyama A., Namiki K. Flare response on bone scintigraphy in metastatic prostate cancer. Nihon Hinyokika Gakkai Zasshi. 1994;85(5):815–818. doi: 10.5980/jpnjurol1989.85.815. [DOI] [PubMed] [Google Scholar]
- 4.Coleman R.E., Whitaker K.B., Moss D.W., Mashiter G., Fogelman I., Rubens R.D. Biochemical prediction of response of bone metastases to treatment. Br J Cancer. 1988;58:205–210. doi: 10.1038/bjc.1988.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao H.S., Chang C.P., Chiu C.H., Chu L.S., Chen Y.M., Tsai C.M. Bone scan flare phenomenon in non-small-cell lung cancer patients treated with gefitinib. Clin Nucl Med. 2009;34:346–349. doi: 10.1097/RLU.0b013e3181a344df. [DOI] [PubMed] [Google Scholar]
- 6.Janicek M.J., Hayes D.F., Kaplan W.D. Healing flare in skeletal metastases from breast cancer. Radiology. 1994;192(1):201–204. doi: 10.1148/radiology.192.1.8208938. [DOI] [PubMed] [Google Scholar]
- 7.Even-Sapir E. Imaging of malignant bone involvement by morphologic, scintigraphic, and hybrid modalities. J Nucl Med. 2005;46(8):1356–1367. [PubMed] [Google Scholar]
- 8.Krupitskaya Y., Eslamy H.K., Nguyen D.D., Kumar A., Wakelee H.A. Osteoblastic bone flare on F18-FDG PET in non-small cell lung cancer (NSCLC) patients receiving bevacizumab in addition to standard chemotherapy. J Thorac Oncol. 2009;4(3):429–431. doi: 10.1097/JTO.0b013e3181989e12. [DOI] [PubMed] [Google Scholar]
- 9.Lemieux J., Guimond J., Laberge F., St-Pierre C., Cormier Y. The bone scan flare phenomenon in non-small-cell lung cancer. Clin Nucl Med. 2002;27(7):486–489,. doi: 10.1097/00003072-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Lind J.S., Postmus P.E., Smit E.F. Osteoblastic bone lesions developing during treatment with erlotinib indicate major response in patients with non-small cell lung cancer: a brief report. J Thorac Oncol. 2010;5(4):554–557. doi: 10.1097/JTO.0b013e3181d3e47e. [DOI] [PubMed] [Google Scholar]
- 11.Arai Y., Kojima A. A case of lung cancer with alkaline phosphatase flare phenomenon during gefitinib therapy. Nihon Kokyuki Gakkai Zasshi. 2007;45(12):962–965. [PubMed] [Google Scholar]
- 12.Hashisako M., Wakamatsu K., Ikegami S., Kumazoe H., Nagata N., Kajiki A. Flare phenomenon following gefitinib treatment of lung adenocarcinoma with bone metastasis. Tohoku J Exp Med. 2012;228(2):163–168. doi: 10.1620/tjem.228.163. [DOI] [PubMed] [Google Scholar]
- 13.Itotani R., Takemura M., Inoue D., Takamatsu K., Isitoko M., Suzuki S. Effectiveness of liver hydrolysate composite (Proheparum®) on hepatotoxicity of gefitinib therapy in nonsmall cell lung cancer patients. Nihon Kokyuki Gakkai Zasshi. 2012;1(5):369–373. [Google Scholar]
- 14.Yoshimoto A., Kasahara K., Kimura H., Kita T., Fujimura M., Nakao S. Transient liver injury caused by gefitinib. Nihon Kokyuki Gakkai Zasshi. 2004;42(1):56–61. [PubMed] [Google Scholar]


