Abstract
This study presents the optimization of a simple HPLC-UV method for the determination of metformin in human plasma. Ion pair separation followed by UV detection was performed on deproteinized human plasma samples. The separation was carried out on a Discovery Reversed Phase C-18 column (250 × 4.6 mm, 5 μm) with UV detection at 233 nm. The mobile phase contained 34% acetonitrile and 66% aqueous phase. Aqueous phase contained 10 mM KH2PO4 and 10 mM sodium lauryl sulfate. Aqueous phase pH was adjusted to 5.2. The mobile phase was run isocratically. The flow rate of the mobile phase was maintained at 1.3 ml/min. The linearity of the calibration curve was obtained in the concentration range of 0.125–2.5 μg/ml and coefficient of determination (R2) was found to be 0.9951. The lowest limit of quantification and detection was 125 and 62 ng/ml respectively. No endogenous substances were found to interfere with the peaks of drug and internal standard. The intra-day and inter-day coefficient of variations was 6.97% or less for all the selected concentrations. The relative errors at all the studied concentrations were 5.60% or less. This method is time efficient and samples are easy to prepare with minimum dilution. So, it can be applied for monitoring metformin in human plasma.
Keywords: HPLC-UV method, Human plasma, Metformin hydrochloride, Protein precipitation
1. Introduction
Metformin was developed from a herb Galega officinalis and it was synthesized in 1922 in Dublin as a blood glucose lowering agent (Shenfield, 2013). It has been widely prescribed worldwide since it was introduced in clinical practice in 1957. Currently metformin has been used as the first line therapy in the treatment of type 2 diabetes mellitus patients (Nathan et al., 2009). Also, metformin is now used to treat polycystic ovary syndrome, gestational diabetes and is showing early promise as a treatment for cancer. It is advantageous in diabetic subjects who are overweight and also has further potential advantages in patients with the metabolic syndrome where hyperinsulinaemia is present (Campbel and Howlett, 1995). Major clinical benefits of metformin over sulfonylureas are that (1) therapeutic doses of metformin do not cause hypoglycemia, (2) metformin does not lower blood glucose in non-diabetic individuals and (3) metformin has direct beneficial effects on serum lipids and lipoproteins (Klip and Leiter, 1990). It reduces blood glucose levels, predominantly by improving hepatic and peripheral tissue sensitivity to insulin without affecting the secretion of insulin.
Chemically, metformin hydrochloride is N, N-dimethylimidocarbonimidic diamide hydrochloride (Klepser and Kelly, 1997) and has the structural formula as shown in Fig. 1.
Figure 1.
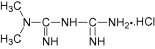
Chemical structure of metformin hydrochloride.
Metformin is a small highly polar molecule (pKa = 2.8, 11.5, log P octanol: water = −2.6) which has great solubility in water and poor solubility in lipids so it is very difficult to extract it from the aqueous plasma matrix (Georgita et al., 2010). There are many methods reported to analyze metformin hydrochloride in plasma by HPLC (AbuRuz et al., 2003; AbuRuz et al., 2005; Gabr et al., 2010; Ranetti et al., 2009; Yardimci et al., 2007; Yuen and Peh, 1998). Those reported methods use gradient mobile phases, use the solid phase extraction technique or have a very complex and multiple step protein extraction technique that make the analysis of large numbers of sample difficult, costly and time consuming. Some do not use internal standards while the others dilute the sample excessively and require nitrogen drying. So there is a need for a sensitive, fast and easy method to determine metformin hydrochloride in plasma. This research was conducted to optimize an easy, sensitive and time efficient method for the quantification of metformin hydrochloride in human plasma by HPLC using UV detection. The plasma samples are diluted minimally during sample pretreatment so the volume of sample required to be injected is small. The plasma sample requires only a one step protein precipitation that makes the method faster and easier.
2. Experimental
2.1. Reagents and chemicals
Perchloric acid 60% solution m/m, potassium dihydrogen phosphate, sodium dodecyl (lauryl) sulfate and acetonitrile were purchased from Ferak Germany, Merck Germany, ACROS Organics and VWR Prolabo Belgium respectively. Metformin hydrochloride was obtained from ABC chemicals and phenytoin sodium from FAGRON Belgium. The lyophilized human plasma was purchased from SIGMA–ALDRICH, Belgium.
2.2. Instrumentations
The LC system consisted of a pump (Merck Hitachi L-6200 intelligent pump) with auto sampler (Spectra SERIES AS 100) having 50 and 20 μl injectors. The detector used was a UV–vis (ELITE Lachrom Hitachi L-2400 model). The wavelength was set at 233 nm. The software used was Chromeleon. The column used was Discovery Reversed Phase C-18 (250 × 4.6 mm, 5 μm). Vortex-2 Genie and MiniSpin plus (Eppendorf) were used to vortex and centrifuge plasma samples respectively.
2.3. Mobile phase preparation
The mobile phase contained 34% acetonitrile and 66% aqueous phase. The aqueous phase contained 10 mM KH2PO4 and 10 mM sodium lauryl sulfate. The pH of aqueous phase was adjusted to 5.2 by using dilute orthophosphoric acid. The mobile phase was run isocratically. The flow rate of the mobile phase was maintained at 1.3 ml/min. Injection volumes were 20 μl. The mobile phase was degassed by using helium gas prior to its use. The HPLC column was kept at ambient temperature.
2.4. Preparation of standard and sample solutions
Stock solution of metformin hydrochloride was prepared by dissolving 20 mg of metformin hydrochloride in methanol (200 μg/ml) and the final volume was made to 100 ml with the same solvent. Similarly phenytoin sodium, the internal standard, was also prepared by dissolving 20 mg in 100 ml methanol (200 μg/ml). The stock solution of metformin hydrochloride was diluted with methanol to prepare working solutions having the concentrations of 25, 20, 10, 5, 2.5 and 1.25 μg/ml respectively.
2.5. Plasma sample preparation for the determination of metformin hydrochloride
380 μl of human plasma was transferred into a 1.5 ml eppendorf tube. 50 μl each of drug and internal standard solutions were added to the plasma and vortex mixed for 1 min. Then 20 μl of perchloric acid (60% m/m) was added and vortex mixed for 1 min and the mixture was centrifuged at 9400g for 3 min (Fig. 2). The supernatant layer was transferred into another tube and filtered through a 0.45 μm filter. A 20 μl of the filtrate was injected onto the HPLC column.
Figure 2.
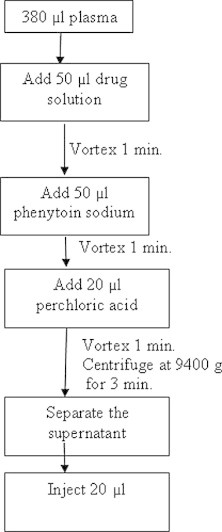
Sample preparation procedure.
3. Results and discussion
The developed HPLC method was optimized for the analysis of metformin hydrochloride in human plasma. Different mobile phases were tested to find the best condition to quantify metformin hydrochloride in plasma. Different ratios of methanol, acetonitrile, sodium lauryl sulfate and potassium dihydrogen phosphate were tried and the optimum mobile phase was finalized. Then the method was validated for selectivity, linearity, limit of quantification, accuracy, precision and recovery as per the international guidelines (FDA guideline, 2001).
3.1. Selectivity
Selectivity is the ability of an analytical method to differentiate and quantify the analyte in the presence of other components in the sample. Six plasma samples were chromatographed to check for endogenous components which might interfere with metformin hydrochloride and internal standard (phenytoin sodium). Spiked plasma samples representing a low (0.25 μg/ml), medium (1 μg/ml) and high (2.5 μg/ml) metformin hydrochloride concentration were analyzed to verify the selectivity of the method of analysis. Both the peaks of phenytoin sodium and metformin hydrochloride did not interfere with any endogenous components. There was also a very good resolution between the peaks of phenytoin and drug (Figs. 3 and 4). The resolution between metformin and phenytoin is 9.7 and the resolution between metformin and the closest matrix peak is 5.6. These values confirm that the method is selective. The mean retention time of phenytoin and metformin was found to be 7.56 ± 0.01 min and 9.93 ± 0.01 min respectively.
Figure 3.
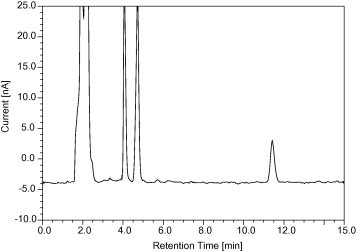
Chromatogram of blank plasma.
Figure 4.
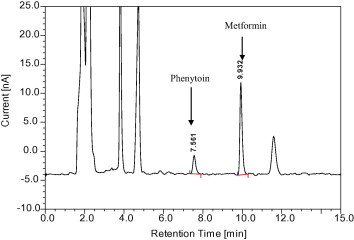
Chromatogram of plasma samples spiked with phenytoin sodium and metformin HCl.
3.2. Linearity
The linearity was determined from the constructed standard calibration curve. The upper limit of the range was taken as 2.5 μg/ml since the plasma concentration of metformin is lower than this value after its oral administration. Six non-zero samples (0.125, 0.250, 0.5, 1, 2, and 2.5 μg/ml) including the lower limit of quantification (LLOQ i.e. 0.125 μg/ml) were used to draw the standard calibration curve (n = 3).
The calibration curve was obtained by plotting chromatographic peak area ratios (metformin/phenytoin) versus concentration of metformin hydrochloride (Fig. 5). Samples were prepared and injected same day. The calibration curve was linear in the given range (R2 = 0.9951).
Figure 5.
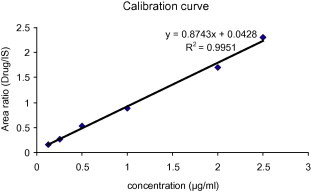
Calibration curve.
3.3. Lower limit of quantification (LLOQ) and lower limit of detection (LLOD)
The analyte concentration that produced a signal to noise ratio greater than 5 and 3 was considered as the LLOQ and LLOD respectively. The LLOQ and LLOD as per these criteria were found to be 0.125 and 0.062 μg/ml when 20 μl of sample was injected. But when the injection volume was increased to 50 μl, the LLOQ was further reduced to 0.031 μg/ml. So, this method was found to be sensitive enough to apply for bioavailability studies.
3.4. Accuracy and precision
The accuracy of the analytical method describes the closeness of the mean test results obtained by the method to the true value of the analyte whereas precision is the closeness of individual measures of an analyte when the procedure is applied repeatedly to multiple aliquots of a single homogeneous volume of biological matrix. For accuracy the mean value of three concentrations should be within 15% of the actual value and for the precision the coefficient of variation (CV) should not exceed 15% at each concentration level (FDA guideline, 2001).
The intra-day and inter-day degree of precision and accuracy of the method is expressed as coefficient of variation and relative error respectively (Table 1). The intra-day and inter-day coefficient of variations was 6.97% or less for all the selected concentrations. The relative errors at all the studied concentrations were 5.60% or less. These data indicate a considerable degree of precision and accuracy of the method both during the analytical run and between different runs. The relative error shows that the method is remarkably accurate which ensures that reliable results are obtained.
Table 1.
Intra-day and inter-day accuracy and precision data (n = 5).
| Conc. added (μg/ml) | Conc. calculated |
Std. deviation |
% Coefficient of variation (CV) |
% Relative error (RE) |
||||
|---|---|---|---|---|---|---|---|---|
| Intra-day | Inter-day | Intra-day | Inter-day | Intra-day | Inter-day | Intra-day | Inter-day | |
| 0.5 | 0.49 | 0.51 | 0.001 | 0.04 | 0.21 | 6.97 | −2.00 | 2.00 |
| 1 | 1.04 | 1.03 | 0.05 | 0.06 | 4.54 | 5.98 | 4.00 | 3.00 |
| 2.5 | 2.62 | 2.64 | 0.05 | 0.06 | 2.01 | 2.30 | 4.80 | 5.60 |
3.5. Recovery
Recovery is the detector response obtained from an amount of the analyte added to and extracted from the biological matrix, compared to the detector response obtained for the true concentration of the pure authentic standard (FDA guideline, 2001). Recovery pertains to the extraction efficiency of an analytical method within the limits of variability. A single stage extraction using 20 μl of 60% m/m perchloric acid was used for the protein precipitation of 380 μl plasma. This minimal volume does not dilute the drug which ultimately improved the sensitivity of this technique. This method gave a good recovery with a minimal time for extraction (Table 2).
Table 2.
Recovery of metformin hydrochloride at three concentrations (n = 3).
| Conc. added (μg/ml) | Mean recovery ± SD | %RSD |
|---|---|---|
| 0.5 | 102.32 ± 8 | 7.82 |
| 1 | 98 ± 3.09 | 3.12 |
| 2.5 | 105.89 ± 1.79 | 1.69 |
The metformin was found to be stable during storage and during all steps of the analytical method. For the protein extraction from plasma, acetonitrile and methanol were also tried but the protein precipitation was not complete and there were interfering peaks as well (Figs. 6 and 7). The ratio of plasma and acetonitrile was 1:1.5. Methanol was also used in the same ratio. As mentioned above, the precipitation of plasma proteins with organic solvents was insufficient. Increasing the volume of organic solvent would dilute the drug in the sample and adversely affect the sensitivity. Previous methods that used a protein precipitation or liquid–liquid extraction procedure with a column specifically designed for reversed phase chromatography used an injection volume of 100 μl or more (Gabr et al., 2010). So the modification of the previous method (Zarghi et al., 2003) by using perchloric acid reduced the dilution of the sample and also the volume of injection (20 μl) but the method still remained sensitive.
Figure 6.
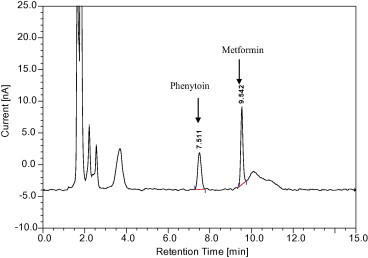
Chromatogram after plasma protein precipitation with acetonitrile.
Figure 7.
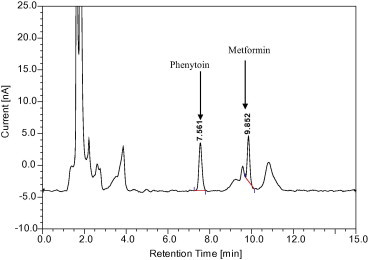
Chromatogram after plasma protein precipitation with methanol.
4. Conclusion
The optimized HPLC-UV method is selective, accurate, precise and repeatable. The method is linear over a wide range and utilizes a mobile phase which can be easily prepared. The column used is a widely available reversed phase C-18. The run time is short and protein precipitation technique is very simple. Even for an injection volume of 20 μl the method is quite sensitive. It can be concluded that the method is suitable for the routine quantification of metformin in human plasma.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication.
Acknowledgements
The authors are thankful to the Laboratory for Pharmaceutical Analysis KU Leuven, Leuven, Belgium for providing the necessary facilities to conduct research work. We would also like to acknowledge the support received from Erasmus Mundus Action 2 and Prof. Erwin Adams while conducting the research work.
Footnotes
Peer review under responsibility of King Saud University.
References
- AbuRuz S., Millership J., McElnay J. Determination of metformin in plasma using a new ion pair solid phase extraction technique and ion pair liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;798:203–209. doi: 10.1016/j.jchromb.2003.09.043. [DOI] [PubMed] [Google Scholar]
- AbuRuz S., Millership J., McElnay J. The development and validation of liquid chromatography method for the simultaneous determination of metformin and glipizide, gliclazide, glibenclamide or glimperide in plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;817:277–286. doi: 10.1016/j.jchromb.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Campbel I.W., Howlett H.C.S. Worldwide experience of metformin as an effective glucose lowering agent: a meta analysis. Diabetes Metabol. Rev. 1995;S1:s57–s62. doi: 10.1002/dmr.5610110509. [DOI] [PubMed] [Google Scholar]
- FDA guideline, 2001. Guidance for industry: Bioanalytical method validation, US, FDA. Rockville, MD.
- Gabr R.Q., Padwal R.S., Brocks D.R. Determination of metformin in human plasma an urine by high performance liquid chromatography using small sample volume and conventional octadecyl silane column. Pharm. Pharm. Sci. 2010;13:486–494. doi: 10.18433/j32c71. [DOI] [PubMed] [Google Scholar]
- Georgita C., Sora I., Albu F., Monciu C.M. Comparision of a LC/MS method with a LC/UV method for the determination of metformin in plasma samples. Farmacia. 2010;58:158–169. [Google Scholar]
- Klepser T.B., Kelly M.W. Metformin hydrochloride: an antihyperglycemic agent. Am. J. Health Syst. Pharm. 1997;54:893–903. doi: 10.1093/ajhp/54.8.893. [DOI] [PubMed] [Google Scholar]
- Klip A., Leiter L.A. Cellular mechanism of action of metformin. Diabetes Care. 1990;13:696–704. doi: 10.2337/diacare.13.6.696. [DOI] [PubMed] [Google Scholar]
- Nathan D.M., Buse J.B., Davidson M.B., Ferrannini E., Holman R.R., Sherwin R., Zinman B. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American diabetes association and the European association for the study of diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranetti M.C., Ionescu M., Hinescu L., Ionică E., Anuţa V., Ranetti A.E., Stecoza C.E., Mircioiu C. Validation of a HPLC method for the simultaneous analysis of metformin and gliclazide in human plasma. Farmacia. 2009;57:728–735. [Google Scholar]
- Shenfield G. Metformin: myths, misunderstandings, and lessons from history. Editorial. Austr. Prescr. 2013;36:38–39. [Google Scholar]
- Yardimci C., Ozaltin N., Gurlek A. Simultaneous determination of rosiglitazone and metformin in plasma by gradient liquid chromatography with UV detection. Talanta. 2007;72:1416–1422. doi: 10.1016/j.talanta.2007.01.042. [DOI] [PubMed] [Google Scholar]
- Yuen K.H., Peh K.K. Simple high-performance liquid chromatographic method for the determination of metformin in human plasma. J. Chromatogr. B. 1998;710:243–246. doi: 10.1016/s0378-4347(98)00117-0. [DOI] [PubMed] [Google Scholar]
- Zarghi A., Foroutan S.M., Shafaati A., Khoddam A. Rapid determination of metformin in human plasma using ion-pair HPLC. J. Pharm. Biomed. Anal. 2003;31:197–200. doi: 10.1016/s0731-7085(02)00608-8. [DOI] [PubMed] [Google Scholar]



