Abstract
Early life stress is a risk factor for developing functional pain disorders. The “limited bedding” (LB) model (Gilles et al., 1996) elicits psychological stress in the dam and her pups by providing minimal nesting material following delivery. Little is known about the effects of LB on visceral pain. Rats (female, male) were exposed to LB on postnatal days 2–9. Electromyographic visceromotor responses (VMR) were recorded at age 11–12 weeks during titrated colorectal distension. LB exposure resulted in significant visceral hyperalgesia in both sexes. Sex differences were demonstrated only in non-stressed controls, with females showing greater VMR. Our results prepare the way for use of the LB model in studying the development of visceral pain in adults with functional gastrointestinal disorders.
Keywords: visceral pain, abdominal pain, nociception, colorectal distension, early life stress, sex differences, nesting behavior
INTRODUCTION
Psychological stress during the early stages of life results in immediate and enduring effects on brain development and increases the risk of psychopathology in adult life. Recent work has highlighted an important role also for early life stress in the development of functional pain disorders [1], including functional gastrointestinal disorders and its associated visceral pain [2–4]. Preclinical studies have demonstrated that maternal separation during early life predisposes animals to visceral hyperalgesia as adults [5–7]. A second model of psychological stress, the “limited bedding” model was introduced by Gilles et al. [8] as an alternate model of maternal neglect and abuse that, unlike the maternal separation model, allows continuous presence of the mother. In the limited bedding model minimal nesting material is provided to the dam following delivery of her pups. Typically this results in increased maternal anxiety and decreased nurturing behavior (licking/grooming), increased rough handling, and increased pup vocalization [9,10]. Long-term consequences on the offspring include increased stress hormone release, reduced expression of hypothalamic corticotrophin releasing hormone, adrenal hypertrophy [11], decreased social and exploratory behavior [9,10], increased learned helplessness in the Porsolt swim test [9], impaired visual-spatial memory in the Morris Water maze [12], decreased dendritic branching in the CA1 area of the hippocampus [12], increased CRH-positive interneurons in the hippocampus [12], and increased c-fos expression in the amygdala in response to a stress challenge [9]. A recent report demonstrated that offspring also show alteration in mechanical nociceptive thresholds and prolongation of prostaglandin E2 hyperalgesia in skeletal muscle [13]. Little is known about the effects of limited bedding on visceral pain responses, which we explored in the current study.
METHODS
Animals
All experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee of the University of Southern California and are in accordance with the guidelines of the Committee for Research and Ethical Issues of the International Association or the Study of Pain. Adult female and male Wistar rats (3 month old) were purchased from Harlan Sprague Dawley (Indianapolis, IN, USA) and were housed in the vivarium on a 12-hour light/12-hour dark cycle with free access to water and rodent chow. Breeding was performed in the vivarium in separately housed breeding pairs (1 male, 1 female) for the production of male and female offspring.
Limited Bedding Model
The model was adapted from Gilles et al. [8]. One day after testing, plug-positive dams were individually housed under standard vivarium conditions. On postnatal day 2 litters and dams were transferred to a standard cage equipped with a steel grid (2.5 cm above the floor, 5 mm2 mesh). The only bedding material available was a paper towel (1 paper towel for 8–15 pups, ½ towel for <8 pups) that the dam would shred and uses to make her nest. The paper towel was replaced at postnatal day 4/5 (female pups n=11, male pups n=11). Control groups (female pups n=11, male pups n=10) were left undisturbed and maintained with standard woodchip bedding. On postnatal day 9 all litters and dams (limited bedding and control) were returned to standard housing conditions using woodchip bedding. Upon weaning on postnatal day 21, offspring were separately placed in same sex, social groups of 3, and housed until 10–11 weeks of age under standard vivarium conditions.
Surgical procedures
Animals at age 10–11 weeks were anesthetized (isoflurane 2% in 70% oxygen and 30% nitrous oxide). A telemetry transmitter (TA11-CTA-F40, Data Sciences Intl., St. Paul, MN, USA) was implanted subcutaneously on the dorsum of the animal just caudal to the scapula. A skin incision was made on the abdomen and electrodes of the transmitter were tunneled subcutaneously to the abdominal incision. Tips of the electrodes were bared, placed in parallel (0.5 cm apart), and stitched into the left external oblique musculature, just superior to the inguinal ligament. The receiver platform was linked via a data exchange matrix to a computer. Implants can be turned on and off with an external magnet and send a radiofrequency signal of electromyographic (EMG) activity to a receiver platform placed underneath the experimental cage on the day of recording. All animals were allowed to recover for seven days.
Assessment and quantification of the visceromotor response to colorectal distension
The rats were habituated to an uninflated colorectal balloon and the experiment cage for 45 min per day for three days prior to measurement of the visceromotor response (VMR). VMR to colorectal distension (CRD) was assessed as described before [14,15]. Briefly, under light isoflurane anesthesia (1.5% isoflurane × 3 min), a flexible latex balloon (length = 6 cm) was inserted intra-anally such that its caudal end was 1 cm proximal to the anus. The silicon tubing connecting the balloon and the barostat (Distender Series II, G&J Electronics Inc., Toronto, Canada) was fixed to the base of the tail with adhesive tape and covered by a stainless steel spring for protection against animal biting. Animals were allowed to recover for 30 min in the experiment cage, the floor of which was covered with bedding from the animal’s home cage. The CRD procedure consisted of two series of phasic distension to constant pressure of 10, 20, 40, and 60 mmHg with 20-s duration and 4-min interstimulus intervals. The VMR was quantified by measuring EMG activity in the external oblique musculature. EMG signals were recorded telemetrically at a sampling rate of 1 kHz, digitized and stored on a computer with the Dataquest ART 3.0 software (Data Sciences Intl., St. Paul, MN, USA). EMG waveforms were lowcut filtered at 20 Hz to eliminate movement interference, and then full-wave rectified. Area under the curve (AUC) was calculated for the 20-s distension period normalized by the 20-s before-distension baseline.
Estrus cycle staging would require daily vaginal lavage over 2 weeks, which would introduce additional stressors potentially confounding the “no-stress” comparison, as well as the sex differences comparison [16]. Therefore, we evaluated high or low estrogen states using uterine weights which change in response to estradiol levels during the 4- to 5-day estrus cycle of the rat [17].
Data analysis
To avoid ambiguities associated with the interpretation of a three-way ANOVA results (with Stress and Sex as the between-subjects factors, and CRD as the within-subjects factor with repeated measure), and given that the effect of increasing CRD levels is well known, we chose to apply ordinary two-way ANOVA (with Stress and Sex as the between-subjects factors) for individual CRD pressure level. Fisher’s Least Significant Difference (LSD) tests were performed with P < 0.05 considered statistically significant. Data were presented as mean ± SEM. All analyses were conducted using Prism (version 6.05, GraphPad Software, Inc., La Jolla, CA, USA).
RESULTS
Results are shown in figure 1. For 60-mmHg CRD, there were significant main effects of Stress (F1,39 = 12.97, P = 0.0009) and Sex (F1,39 = 6.057, P = 0.02), but no significant Sex × Stress interaction. Fisher’s LSD test revealed significant pairwise differences for Male/Control vs. Male/Stressed (P = 0.005, significant after Tukey’s correction for multiple comparisons), Female/Control vs. Female/Stressed (P = 0.04), Male/Control vs. Female/Control (P = 0.03), but not Male/Stressed vs. Female/Stressed (P = 0.2).
Figure 1. Viseromotor responses (VMR) to colorectal distension (CRD).
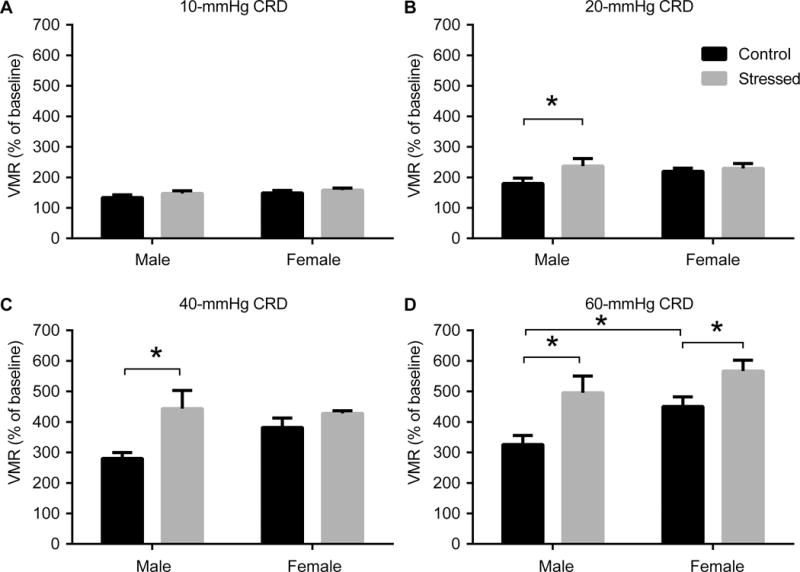
VMR (mean ± standard error) is shown for stressed female (n = 11), control female (n = 11), stressed male (n =11), and control male rats (n = 10). *: P < 0.05 (Fisher’s LSD test).
For 40-mmHg CRD, there were significant main effects of Stress (F1,37 = 7.841, P = 0.008), but not Sex (P = 0.3) or Sex × Stress interaction (P = 0.1). Fisher’s LSD test revealed significant pairwise differences for Male/Control vs. Male/Stressed (P = 0.004, significant after Tukey’s correction for multiple comparisons) and a trend for Male/Control vs. Female/Control (P = 0.07). For 20-mmHg CRD, there were no significant main effects or Sex × Stress interaction. Fisher’s LSD test revealed significant pairwise differences only for Male/Control vs. Male/Stressed (P = 0.03).
For 10-mmHg CRD, there were no significant main effects or Sex × Stress interaction. Fisher’s LSD test revealed no significant pair-wise differences. In female rats, there was no significant difference in uterine weights (P = 0.14) or in the correlation of the VMR and uterine weights (Female/Control R2 = 0.0059, Female/Stress R2 = 0.00113).
DISCUSSION
Our study demonstrated the presence of visceral hyperalgesia in adult animals with a prior history of exposure to limited bedding during postnatal days 2–9. These results confirm similar findings reported for the maternal separation rat model [5–7]. Differences in our study were most apparent at 60-mmHg of CRD in stressed animals compared to nonstressed, control animals. During 40-mmHg CRD the effect of stress was significant in males but not in females as the difference in females was masked by a higher VMR in the control animals.
Sex differences in the VMR response were demonstrated only in non-stressed controls, with females showing greater responses. These findings are consistent with a prior report in normal rats [18]. The higher VMR noted in female controls and a possible ceiling effect to the VMR in the stressed female animals at 60-mmHg of CRD, precluded us finding a significant VMR sex difference in the stressed animals, though a nonsignificant trend was apparent.
The VMR response to CRD has previously been shown to fluctuate with the estrus cycle in anesthetized [18,19] and in awake, restrained rats [20] (but see also [15,21]). In our study, there was no significant correlation of uterine weights with the VMR in both the stressed, as well as non-stressed females. We did not assess individual stages of the estrus cycle by vaginal lavage (daily × 2 wks.), as this would have had the potential to introduce additional stressors that could confound a comparison to the ‘no stress’ condition, as well as in revealing sex differences. Future work may need to examine the relationship of serum estradiol levels with the VMR in the limited bedding model.
The ‘limited bedding’ model is easy to implement and less time intensive, while still maintaining many of the abnormalities in behavior, stress hormone response, and neural changes observed in the ‘maternal separation’ model. The model has the unique advantage of inducing abnormal maternal care without separating the dam from her pups. This may approximate the clinical situation more closely, in so far as stress is in the presence, rather than in the absence of a caretaker. The model, furthermore, avoids the daily handling of pups which may itself alter stress hormone release by the hypothalamic-pituitary-adrenal axis [22].
A number of prior studies have documented that acute or subchronic stress in adult animals can elicit visceral hyperalgesia (e.g. [14]), possibly as a result of induced hyperexcitability of nociceptive dorsal root ganglia neurons [23], as well as central pain amplification [24]. A strong clinical argument can be made for the inclusion of measures of early life stress-induced visceral hyperalgesia in animal models of functional bowel disorders based on the understanding that early life adversity affects pain behavior in functional gastrointestinal disorders [2–4]. Our results prepare the way for use of the limited bedding model in preclinical studies on visceral pain, as has been suggested also by others [25].
Acknowledgments
The research was supported by United States National Institutes of Health grants P50DK064539 (Mayer)
References
- 1.Chaloner A, Greenwood-Van Meerveld B. Early life adversity as a risk factor for visceral pain in later life: importance of sex differences. Front Neurosci. 2013;7:13. doi: 10.3389/fnins.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beesley H, Rhodes J, Salmon P. Anger and childhood sexual abuse are independently associated with irritable bowel syndrome. British journal of health psychology. 2010;15:389–399. doi: 10.1348/135910709X466496. [DOI] [PubMed] [Google Scholar]
- 3.Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385–390. e381–383. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Gastrointestinal tract symptoms and self-reported abuse: a population-based study. Gastroenterology. 1994;107:1040–1049. doi: 10.1016/0016-5085(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 5.Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, et al. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 6.Bian ZX, Zhang M, Han QB, Xu HX, Sung JJ. Analgesic effects of JCM-16021 on neonatal maternal separation-induced visceral pain in rats. World J Gastroenterol. 2010;16:837–845. doi: 10.3748/wjg.v16.i7.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welting O, Van Den Wijngaard RM, De Jonge WJ, Holman R, Boeckxstaens GE. Assessment of visceral sensitivity using radio telemetry in a rat model of maternal separation. Neurogastroenterol Motil. 2005;17:838–845. doi: 10.1111/j.1365-2982.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- 8.Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatric neurology. 1996;15:114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raineki C, Cortes MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32:7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J Neuroendocrinol. 2001;13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivy AS, Rex CS, Chen Y, Dube C, Maras PM, Grigoriadis DE, et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green PG, Chen X, Alvarez P, Ferrari LF, Levine JD. Early-life stress produces muscle hyperalgesia and nociceptor sensitization in the adult rat. Pain. 2011;152:2549–2556. doi: 10.1016/j.pain.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Guo Y, Bradesi S, Labus JS, Maarek JM, Lee K, et al. Sex differences in functional brain activation during noxious visceral stimulation in rats. Pain. 2009;145:120–128. doi: 10.1016/j.pain.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman RL. A quantitative analysis of the physiological role of estradiol and progesterone in the control of tonic and surge secretion of luteinizing hormone in the rat. Endocrinology. 1978;102:142–150. doi: 10.1210/endo-102-1-142. [DOI] [PubMed] [Google Scholar]
- 18.Holdcroft A, Sapsed-Byrne S, Ma D, Hammal D, Forsling ML. Sex and oestrous cycle differences in visceromotor responses and vasopressin release in response to colonic distension in male and female rats anaesthetized with halothane. Br J Anaesth. 2000;85:907–910. doi: 10.1093/bja/85.6.907. [DOI] [PubMed] [Google Scholar]
- 19.Sapsed-Byrne S, Ma D, Ridout D, Holdcroft A. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res. 1996;742:10–16. doi: 10.1016/s0006-8993(96)00989-4. [DOI] [PubMed] [Google Scholar]
- 20.Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience. 2008;154:1562–1567. doi: 10.1016/j.neuroscience.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine-induced analgesia of visceral pain are supraspinally and peripherally mediated. Am J Physiol Regul Integr Comp Physiol. 2006;291:R307–314. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- 22.Meaney MJ, Bhatnagar S, Diorio J, Larocque S, Francis D, O’Donnell D, et al. Molecular basis for the development of individual differences in the hypothalamic-pituitary-adrenal stress response. Cell Mol Neurobiol. 1993;13:321–347. doi: 10.1007/BF00711576. [DOI] [PubMed] [Google Scholar]
- 23.Ochoa-Cortes F, Guerrero-Alba R, Valdez-Morales EE, Spreadbury I, Barajas-Lopez C, Castro M, et al. Chronic stress mediators act synergistically on colonic nociceptive mouse dorsal root ganglia neurons to increase excitability. Neurogastroenterol Motil. 2014;26:334–345. doi: 10.1111/nmo.12268. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Ocampo MA, Pang RD, Bota M, Bradesi S, Mayer EA, et al. Alterations in prefrontal-limbic functional activation and connectivity in chronic stress-induced visceral hyperalgesia. PLoS ONE. 2013;8:e59138. doi: 10.1371/journal.pone.0059138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy D, Tyler K, Ebajemito J, Greenwood-Van Meerveld B. Early life stress induced by neonatal limited nesting produces colonic and somatic hypersensitivity in adult male rats. 17th Neurogastroenterology & Motility Scientific Meeting; Huntington Beach, CA. 2013; abstract #50. [Google Scholar]


