Abstract
Context
Peer-group deviance is strongly associated with externalizing behaviors. We have limited knowledge of the sources of individual differences in peer-group deviance.
Objective
To clarify genetic and environmental contributions to peer-group deviance in twins from mid-childhood through early adulthood.
Design
Retrospective assessments using a life-history calendar. Analysis by biometric growth curves.
Setting
General community.
Participants
Members of male-male pairs from the population-based Virginia Twin Registry personally interviewed in 1998–2004 (n=1802).
Main Outcome Measure
Self-reported peer-group deviance at ages 8 to 11, 12 to 14, 15 to 17, 18 to 21, and 22 to 25 years.
Results
Mean and variance of peer-group deviance increased substantially with age. Genetic effects on peer-group deviance showed a strong and steady increase over time. Family environment generally declined in importance over time. Individual-specific environmental influences on peer-group deviance levels were stable in the first 3 age periods and then increased as most twins left home. When standardized, the heritability of peer-group deviance is approximately 30% at ages 8 to 11 years and rises to approximately 50% across the last 3 time periods. Both genes and shared environment contributed to individual differences in the developmental trajectory of peer-group deviance. However, while the correlation between childhood peer-group deviance levels and the subsequent slope of peer-group deviance over time resulting from genetic factors was positive, the same relationship resulting from shared environmental factors was negative.
Conclusions
As male twins mature and create their own social worlds, genetic factors play an increasingly important role in their choice of peers, while shared environment becomes less influential. The individual specific environment increases in importance when individuals leave home. Individuals who have deviant peers in childhood, as a result of genetic vs shared environmental influences, have distinct developmental trajectories. Understanding the risk factors for peer-group deviance will help clarify the etiology of a range of externalizing psychopathology.
Peers have broad influences on many aspects of behavior and are an important source of individual differences for a variety of human traits.1 In particular, exposure to high levels of peer-group deviance in childhood and adolescence is strongly associated with future drug use and other externalizing behaviors.2–4 Consequently, peer-group deviance plays a prominent role in developmental models for antisocial behavior.5–7 Understanding what makes individuals associate with prosocial vs antisocial friends will be critical in clarifying sources of individual differences in externalizing behaviors.
Whereas models of person-environment interaction traditionally emphasized the passive role of the individual (ie, the environment affecting the person), prior studies of peer-group deviance have suggested bidirectional effects (the environment and the person affecting each other).8–11 While social pressures to conform make adolescents adopt the behaviors of their peers (via peer influence), adolescents also seek out like-minded friends who share their own attitudes (via peer selection). To disentangle the effects of peer influence and selection, longitudinal studies are needed. Beginning with the seminal study by Kandel,8 most longitudinal studies suggest that both influence and selection processes are at work. However, even longitudinal studies of adolescents may be inadequate to resolve questions of causality, as individual, family, and social characteristics in childhood can predict peer-group characteristics in adolescence,12–15 clouding the issue of what causes initial peer selection.
A genetic strategy offers a complementary approach to disentangling mechanisms of peer influence and selection. During the past 2 decades, a growing number of studies have applied behavioral genetic models to analysis of environmental measures.16 If genetically influenced characteristics of an individual affect the type of friends selected, then measures of peer-group deviance should be heritable.
To date, behavioral genetic studies of peer-group deviance are scarce. Studies assessing differences in environments across sibling pairs have found consistent support for genetic influence on peer-group deviance. Specifically, adoptive siblings reported greater differences in peer-group deviance than nonadoptive siblings,17 and dizygotic (DZ) twins reported greater differences than monozygotic (MZ) twins.18,19 Traditional behavioral genetic studies of peer-group deviance have yielded less consistent results. Examining parental reports of peer-group deviance from a twin-family study of 10- to 18-year-old twins, Manke et al20 found that peer-group deviance was substantially heritable. In contrast, using twin and nontwin sibling self-reports of peer-group deviance from the same sample21 found that variation in peer-group deviance was influenced primarily by shared and nonshared environmental influences. Using self- and teacher-reported data from more than 1700 same-sex twin pairs aged 14 to 16 years, Walden et al22 found shared environmental factors to be considerably more important than genetic factors in accounting for variation in peer-group deviance. Cleveland et al23 found high levels of heritability for peer drinking and smoking using genetically informative data from the National Longitudinal Study of Adolescent Health. Finally, in 12-year-old Finnish twins, Rose24 found, after excluding friends shared by both twins, higher correlations for peer ratings of behavior problems in the friends unique to each member of MZ vs DZ twin pairs.
Whereas cross-study differences may explain some of these inconsistencies, there may also be systematic differences in the relative importance of genetic and environmental influences on peer-group deviance related to developmental context. Adolescence is associated with a marked increase in autonomy25,26 and a shift in socializing influences from parents to peers as children spend less time with family and more time with friends.27 As children become active agents in creating their social world, genetic influence on peer-group deviance may increase.
Because previous behavioral genetic research on peer-group deviance has either focused on a relatively narrow age range or combined individuals from different developmental stages, nothing is known about potential changes in the relative importance of genetic and environmental factors on variation in peer-group deviance. Therefore, we combined twin and developmental perspectives to elucidate the sources of individual differences in male peer-group deviance from midchildhood to early adulthood.
METHODS
SAMPLE
This report uses data collected in the third wave of interviews conducted in adult male-male twin pairs from the Virginia Twin Registry. This larger study has been described in detail elsewhere,28 and originally contained male-male and male-female twins born between 1940 and 1974. Initially, 6814 twins participated in the 1993–1996 interview (participation rate, 72.4%), with an 82.6% follow-up response rate for the second wave of interviews, conducted in 1994–1998. The third interview wave (1998–2004) was completed only in members of male-male twin pairs. Interviews were completed by 1796 twins, representing 75.1% of the entire sample and 77.8% of the eligible sample (excluding those who died and were lost to follow-up). Subjects were aged 24 to 62 years (mean age, 40.3 years [SD, 9.0 years]). The sample for the present analysis consisted of 469 MZ and 287 DZ twin pairs (including 2 triplet sets subdivided into all possible pairings) and 290 twins whose co-twin did not participate.
Most subjects were interviewed by telephone, with a small number interviewed in person. After a full explanation of the research protocol, verbal assent or signed informed consent was obtained before all interviews. This project was approved by the Committee for the Conduct of Human Research at Virginia Commonwealth University. Interviewers had a master’s degree in a mental health–related field or a bachelor’s degree in this area with 2 years of clinical experience. The 2 members of a twin pair were never interviewed by the same interviewer. Zygosity was assigned by a combination of self-report measures, photographs, and DNA polymorphisms.29
ASSESSMENT
To improve accuracy of retrospective recall, we developed a life-history calendar–based interview,30 which assessed a range of constructs including peer-group deviance for 5 age periods: 8 to 11, 12 to 14, 15 to 17, 18 to 21, and 22 to 25 years. These periods were assessed sequentially after the development of a calendar that traced major developmental events from ages 1 to 30 years, including whether or not the twin was living with his co-twin. Interviewers were scripted to begin each new period with specific memory prompts taken from events in the calendar, thereby cuing the respondent into the relevant “memory files.”
Peer-group deviance was assessed by mean levels of 12 items obtained from 2 validated instruments31,32 that assessed the proportion of the respondent’s friends who engaged in specific behaviors at each particular age. Friends were defined as “people who you would have seen regularly and spent time with in school and outside of school.” The twins were asked how many of their friends smoked cigarettes; drank alcohol; got drunk; had problems with alcohol; skipped or cut school a lot; cheated on school tests; stole anything or damaged property on purpose; had been in trouble with the law; smoked marijuana; used inhalants; used other drugs like cocaine, downers, or LSD (lysergic acid diethylamide); and sold or gave drugs to others. The 5 response options were none, a few, some, most, and all.
Four of the most deviant behavior items were excluded from the first assessment period (ages 8–11 years) when, during our pilot phase, several respondents reacted negatively to them. Two of the items related to school activities (skipped or cut school a lot and cheated on school tests) were not included in the 18 to 21– and 22 to 25–years assessments, as many friends would not then be in school. Cronbach standardized α coefficient for this scale across the 5 age groups (from youngest to oldest) was 0.82, 0.90, 0.90, 0.87, and 0.87, respectively.
Test-retest correlations in 141 subjects who were interviewed a mean of 29 days apart produced intraclass correlations for peer-group deviance at the 5 age groups of 0.42, 0.75, 0.81, 0.78, and 0.73, respectively. The low correlation in the 8 to 11–years period resulted from much lower between-subject variation in this (4.89) than other age periods (range, 20.75–33.43), while the within-individual across-time variation was quite similar in the 8 to 11–years group (6.63) vs other ages (range, 6.78–7.80).
To address whether our measures of peer deviance predicted future externalizing behaviors, we examined the association between our standardized peer-group deviance measure and cannabis use or cannabis abuse/dependence33 and the number of endorsed criteria A (adult behaviors) of antisocial personality disorder.33 Across the 5 time periods (8 to 11, 12 to 14, 15 to 17, 18 to 21, and 22 to 25 years), the odds ratios and P values for peer-group deviance that predicted cannabis use, calculated by logistic regression, were 1.09 (P = .09), 1.41 (P < .001), 1.73 (P < .001), 2.29 (P < .001), and 2.16 (P < .001), respectively. Predicting cannabis abuse or dependence produced similar results: 1.26 (P = .004), 1.77 (P < .001), 2.16 (P < .001), 2.86 (P < .001), and 2.70 (P < .001), respectively. The number of adult antisocial criteria was predicted by Poisson regression with the following β levels: 0.24 (P < .001), 0.33 (P < .001), 0.41 (P < .001), 0.46 (P < .001), and 0.48 (P < .001), respectively. As expected, our measure of peer-group deviance robustly predicted externalizing behaviors.
STATISTICAL ANALYSIS
Sporadic missing values, constituting less than 0.3% of data, were singly imputed using SAS PROC MI (SAS Institute Inc, Cary, NC) and a Markov chain Monte Carlo approach. Approximately 1% of the observations were lacking for a time period, because if the respondent replied that he had no friends during the specified age period, these were scored as missing.
The structurally missing items were simulated using PROC MIXED in SAS with Monte Carlo residuals. By assuming that the covariance structure of the data from the nearest time period with complete data is representative of the time periods with missing data, we estimated, using a hierarchical regression model, the response as well as the residual variance and covariance between MZ and DZ twins. Monte Carlo residuals were then generated from a bivariate normal distribution with these parameters and added to the predicted values to obtain the simulated values.
Preliminary analyses indicated a significant positive relationship between birth year and peer-group deviance, which differed across individual items. To account for these effects, we divided our sample into 6 similarly sized groups based on birth year. Peer-group deviance items were averaged for each cohort across all time periods, and each individual item centered around the cohort mean. Cohort-adjusted item scores were summed and the overall score was adjusted to have a minimum score of 0. The raw cohort-adjusted scores were then transformed to normality using the method of Blom34 and the normalized scores were rescaled to the original mean and variance.
Biometric latent growth curve analyses were performed in SAS PROC MIXED as described by McArdle.35 Model fit comparisons involving fixed effects were carried out using maximum likelihood, whereas model fit comparisons involving random effects were carried out using residual maximum likelihood. The fit statistics that were used included likelihood ratio χ2, Akaike information criterion,36 and the generalized coefficient of determination of Cox and Snell.37 Variance component models used in estimating biometric latent growth curve parameters and intraclass correlations were fit using residual maximum likelihood in PROCMIXED. Confidence intervals for variance components were constructed using a Satterthwaite approximation.38
RESULTS
DESCRIPTIVE DATA
The mean and variance of peer-group deviance scores and the intraclass correlations in MZ and DZ twins across the 5 time periods are presented in Table 1. Levels of peer-group deviance increase monotonically from ages 8 to 11, to 18 to 21 years, and then decline at ages 22 to 25 years. The variance of peer-group deviance nearly doubles from 8 to 11, to 12 to 14 years, increases more slowly until ages 18 to 21 years, and then stabilizes. The correlations for peer-group deviance are consistently higher in MZ than in DZ twin pairs. While the MZ twin correlations are stable over time at around 0.66, the resemblance in peer-group deviance in DZ twins declines with age. These results suggest that genetic factors contribute to levels of peer-group deviance and this influence increases over time. Table 1 also includes the percentage of twins in each age group living with their co-twin. A sharp drop in the proportion of cohabiting twins is seen between ages 15 to 17, and 18 to 21 years.
Table 1.
Means, Variances, and Intraclass Correlations of Peer-Group Deviance (PGD) and Percentage of Twins Cohabiting Across Different Ages
| Age, y | Mean PGD Score | Variance | MZ ICC | DZ ICC | Twins Cohabiting, % |
|---|---|---|---|---|---|
| 8–11 | 4.49 | 18.52 | 0.66 | 0.47 | 99.5 |
| 12–14 | 7.48 | 38.03 | 0.68 | 0.43 | 98.4 |
| 15–17 | 11.39 | 45.78 | 0.67 | 0.40 | 92.7 |
| 18–21 | 14.04 | 49.02 | 0.66 | 0.39 | 33.3 |
| 22–25 | 13.35 | 48.68 | 0.65 | 0.39 | 12.1 |
Abbreviations: DZ, dizygotic; ICC, intraclass correlation; MZ, monozygotic.
Inspection of the data revealed significant individual variability in the developmental pattern of peer-group deviance, with some individuals increasing in peer-group deviance across time, some decreasing over time, and others remaining relatively stable at either high, medium, or low levels of peer-group deviance. Given this variation in peer-group deviance trajectories, we calculated the slope of the peer-group deviance across the 5 time periods for each individual. These slopes were more highly correlated in MZ (0.60) than in DZ pairs (0.44), indicating that genetic factors also contribute to the rate of change in peer-group deviance over time. The correlation between the levels of peer-group deviance at ages 8 to 11 years (intercept) and the subsequent slope was –0.14, meaning that, compared with those with high levels of peer-group deviance in childhood, those with low levels had a slightly more rapid rise in peer deviance through adolescence and early adulthood.
BASIC REGRESSION MODEL
Our best-fit fixed-effects regression model for peer-group deviance, determined by the Akaike information criterion, included a linear effect of standardized age (β = 3.26, t7942 = 56.3; P < .001) and a spline on the effect of age impacting only the oldest age group (β = −4.15, t7942 = 29.6; P < .001). No significant effect was seen for year of birth (β = .07, t1044 = 0.5; P = .61) because this was statistically controlled by our use of cohort-adjusted item scores. However, significant interactions were seen between year of birth and the main effect of age (β = .48, t7942 = 8.3; P < .001) and the spline (β = −1.10, t7942 = 7.9; P < .001). The opposite signs of these 2 effects mean that older individuals have a less steep rise in peer-group deviance up to ages 18 to 21 years and a less steep decline between 18 to 21, and 22 to 25 years. Instead of the regression spline, we also examined a quadratic effect of age on peer-group deviance, but that did not provide as good of a fit.
RANDOM-EFFECTS GROWTH CURVE MODEL
A random-effects growth curve model adding additive genetic (A), shared environmental (C), and unique environmental (E) effects to the best-fitting fixed-effects model was fitted to the peer-group deviance data. The method we used35 parameterizes the model with unique effects of A, C, and E on the intercept; unique effects of A, C, and E on slopes; and A, C, and E effects common to both intercept and slope. The explanatory power of the model can be summarized by the generalized coefficient of determination,37 which was 25% for the fixed-effects only model and 54% for the full biometric growth curve model.
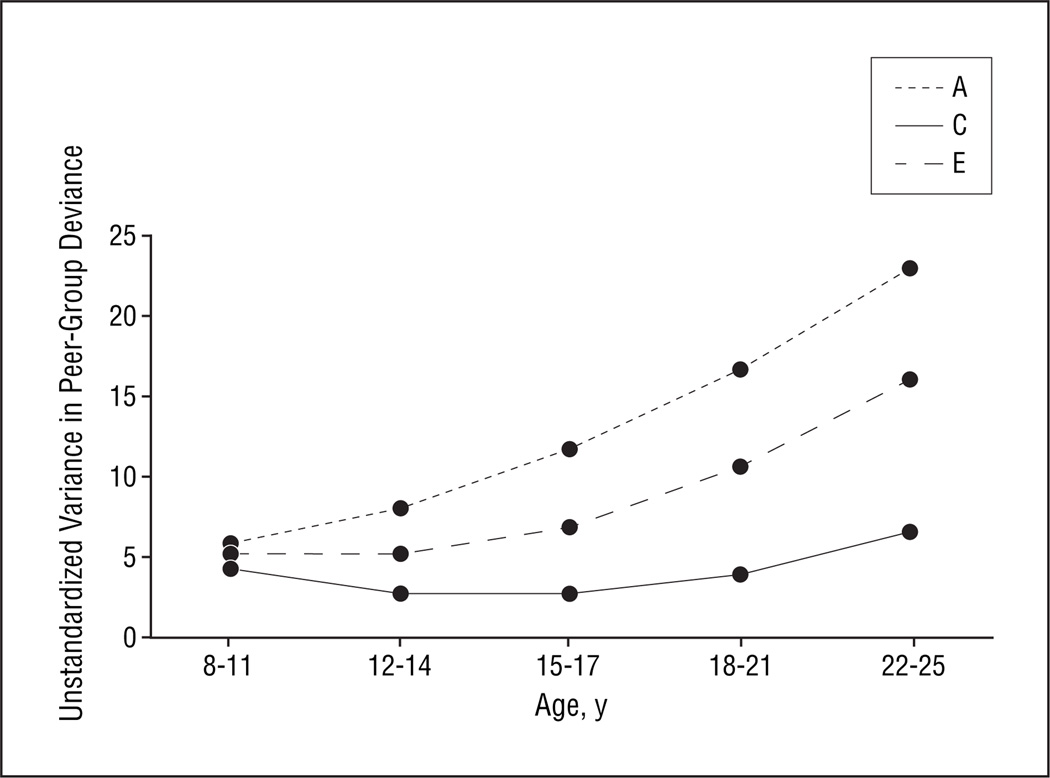
Figure 1 shows the raw genetic and environmental variance components for peer-group deviance at each age. The pattern of results differs considerably for genetic, shared environmental, and individual-specific environmental effects. Genetic variance in peer-group deviance increases substantially and monotonically across the 5 age periods. Variance in peer-group deviance resulting from shared environmental effects, by contrast, decreases across the first 3 age periods and then increases modestly at ages 18 to 21 and 22 to 25 years. Individual-specific environmental variance in peer-group deviance is relatively stable across the first 3 age periods but then increases sharply throughout the final 2 periods.
Figure 1.
Unstandardized additive genetic (A), shared or common environmental (C), and individual-specific environmental (E) variance for peer-group deviance across 5 time periods estimated from our best-fit growth curve model.
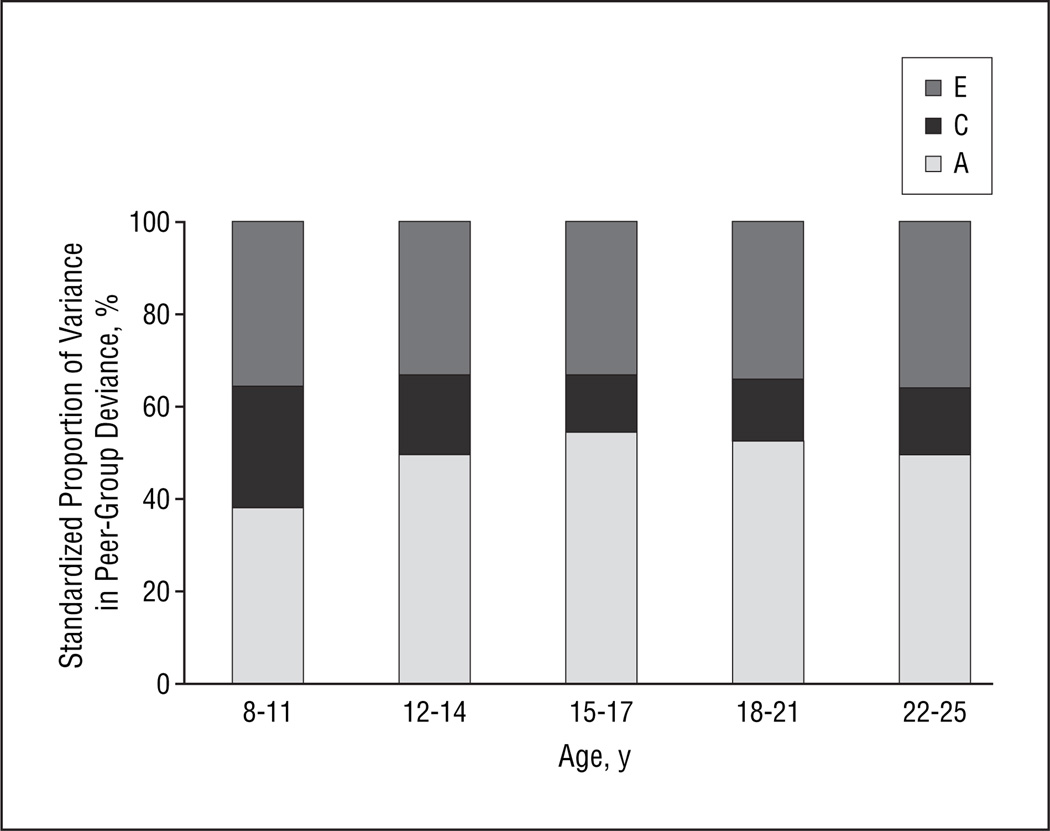
Standardized results are seen in Figure 2 and, with 95% confidence intervals, in Table 2. The most striking differences occur between ages 8 to 11 and 12 to 14 years, when the relative importance of additive genetic effects increases substantially and, conversely, the relative importance of shared environmental factors decreases. The relative role of individual-specific environment is rather stable across the entire time period, accounting for approximately one-third of the variance in peer-group deviance.
Figure 2.
Standardized additive genetic (A), shared or common environmental (C), and individual-specific environmental (E) variance for peer-group deviance across 5 time periods estimated from our best-fit growth curve model. The standardized additive genetic effects equal the heritability.
Table 2.
Parameter Estimates and 95% Confidence Intervals for the Standardized Full Model for Peer-Group Deviance
| Parameter Estimate (95% Confidence Interval) | |||
|---|---|---|---|
| Age, y | Additive Genetic Effects |
Shared Environmental Affects |
Individual-Specific Environmental Effects |
| 8–11 | 38.8 (28.6–55.6) | 27.0 (17.7–46.3) | 34.2 (23.2–55.1) |
| 12–14 | 50.7 (40.3–65.6) | 17.1 (9.3–41.0) | 32.2 (21.7–52.3) |
| 15–18 | 54.9 (46.7–65.4) | 12.5 (6.7–31.0) | 32.6 (24.1–46.3) |
| 18–21 | 53.5 (47.7–60.4) | 12.6 (8.1–22.4) | 33.9 (27.7–42.4) |
| 22–25 | 50.5 (46.5–55.2) | 14.3 (10.7–19.8) | 35.2 (30.7–40.7) |
Our model also estimates the sources of individual differences in the slope of peer-group deviance over time. Genetic factors account for 30.4% of the variation in individual trajectories, with shared and nonshared environmental factors accounting for 19.1% and 40.5%, respectively. Finally, we decomposed the within-individual correlation between peer-group deviance level at ages 8 to 11 years (the intercept) and the subsequent peer-group deviance slope. The genetic, shared environmental, and unique environmental contributions to this intercept-slope correlation were, respectively, 0.37, −0.62, and −0.23.
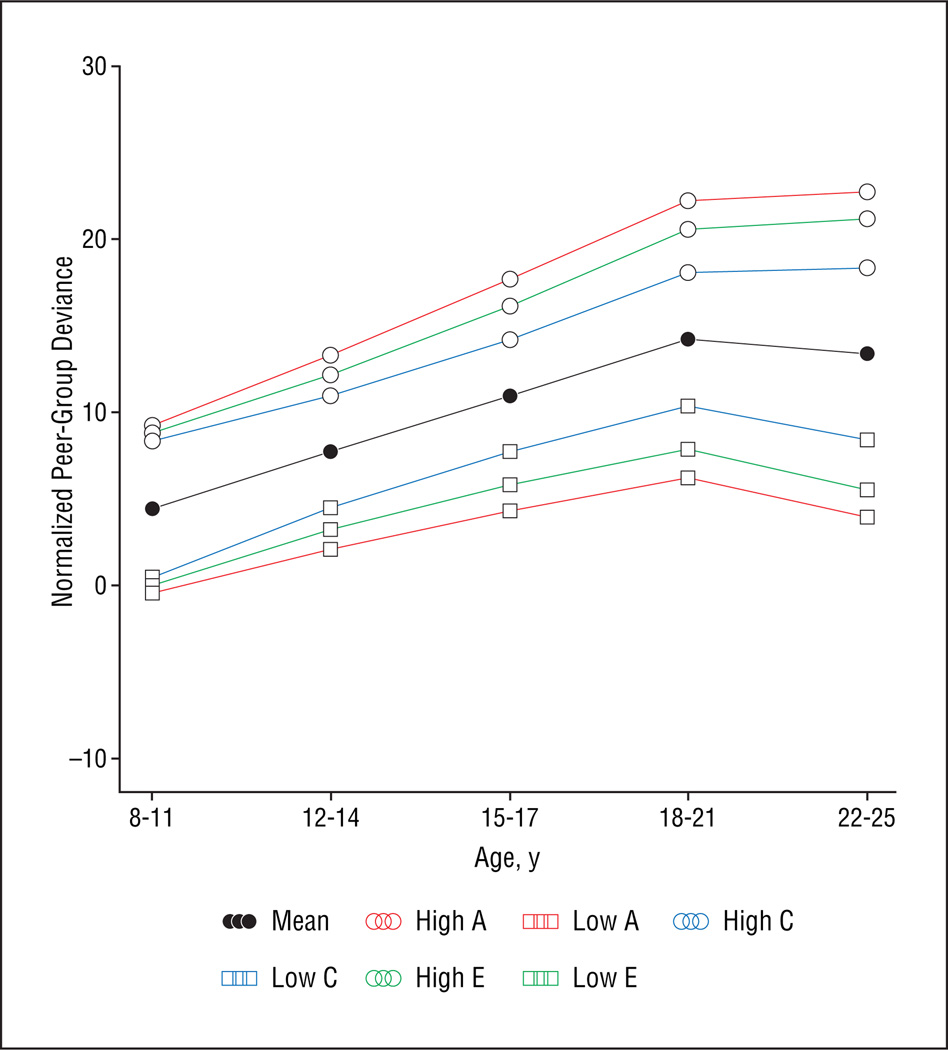
We illustrate this effect in Figure 3, where the results of our full random-effects growth curve model are used to plot the mean expected levels of peer-group deviance for individuals with hypothetically very high and very low levels of genetic, shared environmental, and unique environmental liability to peer-group deviance. As seen in Figure 3, despite similar levels of deviance in childhood, individuals with high levels of genetic liability to peer deviance have a more extreme trajectory over time than individuals whose high level of peer-group deviance stems largely from familial environmental sources. Those whose high levels of peer-group deviance resulted largely from unique environmental sources have intermediate values. The reverse effect is seen in those with low levels of peer-group deviance, in which those whose low level derives from a very low genetic liability have a trajectory that diverges most from the expected mean.
Figure 3.
Parameter estimates from the biometrical latent growth curve analysis were used to construct expected growth curve patterns for hypothetical individuals with different underlying liabilities with respect to genetic (A), shared or common environmental (C), and individual-specific environmental (E) variance for peer-group deviance. The mean curve was constructed by setting the deviation for each of these latent constructs at 0, while the upper and lower curves, respectively, were constructed by setting the deviations for A, C, and E at 1.96 or −1.96 while holding the others at 0, thus providing 97.5% and 2.5% percentiles for expected variation due to each of these sources of variance.
IMPACT OF POSSIBLE ENVIRONMENTAL BIASES
The greater resemblance in peer-group deviance for MZ vs DZ twins could arise because (owing to social expectations) MZ twins socialize with others together more frequently than DZ twins. We assessed the degree of friend sharing in each age period and this was consistently higher in MZ than DZ twins. We added this variable (F for friends) to our model as a specified form of shared environment.39 Adding this parameter rendered the impact of the background shared environment nonsignificant; thus, we obtained parameter estimates for an AFE model. The pattern of results was quite similar to those seen in Figures 1 and 2, the largest change being a reduction in genetic effect, particularly in the younger age groups. Examining the standardized results, the heritability of peer-group deviance was estimated across the 5 age groups (8–11, 12–14, 15–17, 18–21, and 22–25 years) to equal 13.8%, 31.6%, 43.0%, 46.6%, and 46.2%, respectively. The rising heritability of peer-group deviance with age seen in our analyses does not result from changes in the socialization patterns of MZ vs DZ twins. Moreover, if sharing friends is also partially driven by genetic factors, as would be suggested by the higher levels of sharing between MZ twins, then our analyses controlling for the effects of shared friends may actually underestimate the total genetic contribution to peer-group deviance.
COMMENT
This report sought to elucidate, from a developmental perspective, the sources of individual differences in peer-group deviance from midchildhood to early adulthood. Six findings are worthy of emphasis. First, our simple biometric growth curve model accounted for 54% of the variance of the development of peer-group deviance, supporting the explanatory power of this approach. Second, genetic factors contributed substantially to peer-group deviance, and this contribution increased as individuals aged. Third, in midchildhood, shared family environment was an important determinant of peer-group deviance, but its importance diminished over time. Fourth, individual-specific environment had an important impact on peer-group deviance that became more pronounced when twins started to live apart. Fifth, the rate of change in peer-group deviance over time was strongly influenced by familial factors, with genes playing a somewhat greater role than the shared environment. Sixth, genes and environment contributed to the correlation between initial levels of deviant peers in childhood and the subsequent trajectory of peer-group deviance in quite different ways.
CONTEXT
The present study demonstrates the value of using behavioral genetic strategies to better understand developmental changes in peer-group deviance. Our evidence that individual differences in peer-group deviance are heritable adds to a growing body of research demonstrating heritabilities of other putative environmental measures, including parenting, social support, and stressful life events.40
Genetic influence on environmental measures is indicative of a gene-environment correlation, of which Plomin et al41 delineated 3 possible forms: passive, reactive, and active. They defined the last of these as follows: “active genotype-environment correlation occurs as a result of the fact that a child is not merely the passive recipient of his environment. Rather, he contributes to his own environment and may active[ly] seek one related to his genetic propensities.”41
Building on this work, Scarr and McCartney42 proposed a developmental theory of childhood and adolescence in which an active genotype-environment correlation “becomes particularly important in later childhood and adolescence as individuals make their own environments.” Our finding that genetic influence on peer-group deviance increases over time supports their hypothesis. The pattern of developmental change that we saw for peer-group deviance—an increase in genetic variance and a decrease in the effects of shared environmental factors with aging—has also been observed in a number of genetically informative longitudinal studies with a range of externalizing behaviors, including smoking,43,44 drinking,44,45 and conduct disorder.46,47
Our findings that genetic factors influence both mean levels of peer-group deviance as well as change in peer-group deviance over time are supportive of prior nongenetic longitudinal research that suggests that a substantial proportion of the similarity between adolescents and their peer groups results from peer selection.8,10,48,49 Our results indicate that genetically influenced characteristics of the individual are related to the types of friendships that adolescents develop. Dishion and colleagues50 have deemed this the shopping model effect, whereby adolescents seek out friends who are more similar to them.
Although genetic factors play an increasing role in accounting for individual differences in peer-group deviance, environmental factors are also clearly implicated. One of the most interesting trends in this study was the marked increase in nonshared environmental influences at ages 18 to 21, and 22 to 25 years, which coincided with decreased rates of cohabitation.
Our study also found that shared environmental factors accounted for variation in peer-group deviance, especially during late childhood and early adolescence. Possible influences include a family’s socioeconomic status and neighborhood influences, both of which have been associated with peer-group deviance.12,14,51 Consistent with our results, Dishion et al51 found that 13- to 14-year-old antisocial boys typically found their close friends within their neighborhood block. Parental monitoring, an important mediating and moderating variable in theories describing the developmental interplay between peer-group deviance and delinquency,52,53 could also account for shared environmental effects, as same-age male twins are likely to receive similar levels of monitoring.
The shared environmental influence on peer-group deviance might also reflect the influence of shared peers.1 In our study, when similarity of friends was included in the model to test equal environment assumptions, the shared environmental effects effectively disappeared. In addition, previous work with our second wave sample showed that adding measures of twin similarity of friendships during childhood significantly reduced the shared environmental effect on conduct disorder behavior (K.C.J., unpublished data, July 2005).
The relationship detected between the genetic and environmental influences on the initial peer-group deviance (intercept) and subsequent changes in peer-group deviance (slope) led to an intriguing pattern of results. If high levels of peer-group deviance in childhood resulted largely from shared environmental factors, this predicted a relatively benign developmental course with modest growth in peer-group deviance over time. By contrast, if high levels of childhood peer-group deviance arose mostly from genetic effects, this predicted a steep increase in deviance of peers with increasing age. These patterns are consistent with prior hypotheses of developmental heterogeneity in life-course patterns on antisocial behavior,54,55 which predict different etiologies for different types of antisocial youth.
Specifically, early starter or life course–persistent delinquents are more likely than late starter or adolescence-limited delinquents to evidence early signs of temperamental difficulties and early problem behaviors,56–58 factors that have been shown to be heritable.59–61 In contrast, the primary mechanism associated with late-starting delinquents is associated with deviant peers. If similarity of peer groups accounts for the shared environmental influences found for peer-group deviance in the present study, this would suggest that individuals who show more benign courses of peer-group deviance and whose initial levels of peer-group deviance are environmentally influenced may represent the late-starting or adolescent-limited group of delinquents. By contrast, those with high levels of genetic liability on the initial level of peer-group deviance and the subsequent steep increase in peer-group deviance over time may represent the early starter or life course–persistent delinquents. These patterns are congruent with predictions that severe life course–persistent antisocial behavior is more likely to be influenced by genetic factors, whereas normative trajectories of antisocial behavior are more likely to be caused by environmental factors (K.C.J., unpublished data, August 2005).62,63
This study has implications not only for interpreting traditional studies of peer-group deviance and antisocial behavior based on nongenetic samples and for developing appropriate interventions and preventions, but also for the growing field of molecular genetics. Our field is beginning to identify individual genetic variants that impact risk for psychiatric and substance use disorders. Along with reviewed findings, our study suggests that the mechanisms by which some of these variants work will not be entirely reducible to “within the skin” physiological pathways. Rather, these genes will impact risk through “outside the skin” pathways, by altering the probability of exposure to pathogenic environments.64,65
An important focus for further research will be to clarify the degree to which genetic risk factors for peer-group deviance also impact the risk for externalizing behaviors, including drug use and abuse. As suggested for smoking behaviors,43 the selection of deviant peers may be an important mediating step between genetic risk factors and a range of externalizing disorders.
LIMITATIONS
The results of this report should be interpreted in the context of 5 methodologic limitations. First, our sample was white male twins born in Virginia. The degree to which these results would extrapolate to women, singletons, or other ethnic groups is unknown. Second, approximately one-fourth of the eligible twins for this study did not participate. Might this have biased our findings? Using data collected at the second interview wave, a multiple logistic regression analysis, correcting for the twin structure of the data, showed participation in the third wave to be strongly predicted by educational status but not by age; zygosity; cannabis use or abuse/dependence; or number of DSM-IV adult antisocial symptoms. With respect to vulnerability to the externalizing symptoms strongly predicted by peer-group deviance, this sample was likely to be broadly representative of the original twin cohort.
Third, could our results be viewed as a violation of the equal environment assumption in twin studies rather than evidence for outside the skin genetic pathways for peer selection? A violation of the equal environment assumption requires that the environment causes twins to be phenotypically similar.66 However, our results are more consistent with causal effects from person to the environment. While the present study supports the hypothesis of peer selection, we cannot rule out peer influences on behavior, as that would require a joint analysis of peer-group deviance with a separate measure of antisocial behavior, which we do not do in the present study.
Fourth, our measure of peer-group deviance was solely by self-report. Collecting objective measures of peer-group deviance would be infeasible in this kind of design. Prior studies of self-report peer-group deviance suggest that they may be subject to projection bias in which individuals exaggerate the congruence between themselves and their social environment.10 We cannot rule out the impact of such biases. However, given that 3 prior studies found heritability for peer delinquency orientation from parental report,20 self-report by peers,23 and peer behavior problems from classmate ratings,24 it is unlikely that our results were highly distorted by our method of assessment.
Lastly, information on peer-group deviance was collected retrospectively from adults. We cannot rule out the possibility that the pattern of our findings result from retrospective recall bias. We consider this unlikely. We demonstrated good to excellent short-term test-retest reliability for our measures of peer-group deviance. It is difficult to construct a plausible pattern of recall bias that would produce the observed results. Most importantly, we used a life-history calendar in our assessment of peer-group deviance. An accumulating body of evidence indicates that such methods, which reflect the structure of autobiographical memory and promote sequential retrieval within memory networks, substantially improve the completeness and accuracy of retrospective reports.30,67–69
Acknowledgments
Funding/Support: This study was supported in part by grants DA-011287 and MH-49492 from the National Institute of Health (NIH). The NIH played no direct role in the design or conduct of the study or in the collection, management, analysis, and interpretation of the data, and did not review or approve this manuscript.
Additional Contributions: Linda Corey, PhD, provided assistance with the ascertainment of twins from the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry (MATR). TheMATRhas received support from the NIH, the Carman Trust, and the W.M. Keck, John Templeton, and Robert Wood Johnson Foundations. Lenn Murrelle, PhD, and Patrick Sullivan, MD, contributed to the design of this study. Data collection was supervised by Sarah Powers, BA, Karen Hough, MS, and Frank Butera, MA. Indrani Ray, BA, assisted with database management.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Harris JR. The Nurture Assumption: Why Children Turn Out the Way They Do. New York, NY: Touchstone/Simon & Schuster; 2002. [Google Scholar]
- 2.Allen M, Donohue WA, Griffin A, Ryan D, Turner MM. Comparing the influence of parents and peers on the choice to use drugs. Crim Justice Behav. 2003;30(2):163–186. [Google Scholar]
- 3.Petraitis J, Flay BR, Miller TQ, Torpy EJ, Greiner B. Illicit substance use among adolescents. Subst Use Misuse. 1998;33(13):2561–2604. doi: 10.3109/10826089809059341. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins JD, Herrenkohl T, Farrington DP, Brewer D, Catalano RF, Harachi TW. A review of predictors of youth violence. In: Loeber R, Farrington DP, editors. Serious and Violent Juvenile Offenders: Risk Factors and Successful Interventions. London, England: Sage Publications Inc; 1998. pp. 106–146. [Google Scholar]
- 5.Patterson GR, DeBaryshe BD, Ramsey E. A developmental perspective on antisocial behavior. Am Psychol. 1989;44(2):329–335. doi: 10.1037//0003-066x.44.2.329. [DOI] [PubMed] [Google Scholar]
- 6.Coie JD, Miller-Johnson S. Peer factors and interventions. In: Loeber R, Farrington DP, editors. Child Delinquents: Development, Intervention, and Service Needs. London, England: Sage Publications Inc; 2001. pp. 191–209. [Google Scholar]
- 7.Farrington D. Childhood origins of antisocial behavior. Clin Psychol Psychother. 2005;12(3):177–190. [Google Scholar]
- 8.Kandel DB. Homophily, selection, and socialization in adolescent friendships. AJS. 1978;84(2):427–436. [Google Scholar]
- 9.Eiser JR, Morgan M, Gammage P, Brooks N, Kirby R. Adolescent health behaviour and similarity-attraction: friends share smoking habits (really), but much else besides. Br J Soc Psychol. 1991;30(pt 4):339–348. doi: 10.1111/j.2044-8309.1991.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 10.Kandel DB. The parental and peer contexts of adolescent deviance: an algebra of interpersonal influences. J Drug Issues. 1996;26(2):289–315. [Google Scholar]
- 11.Wills TA, Cleary SD. Peer and adolescent substance use among 6th–9th graders: latent growth analyses of influence versus selection mechanisms. Health Psychol. 1999;18(5):453–463. doi: 10.1037//0278-6133.18.5.453. [DOI] [PubMed] [Google Scholar]
- 12.Dishion TJ, Patterson GR, Stoolmiller M, Skinner ML. Family, school, and behavioral antecedents to early adolescent involvement with antisocial peers. Dev Psychol. 1991;27(1):172–180. [Google Scholar]
- 13.Fergusson DM, Woodward LJ, Horwood LJ. Childhood peer relationship problems and young people’s involvement with deviant peers in adolescence. J Abnorm Child Psychol. 1999;27(5):357–369. doi: 10.1023/a:1021923917494. [DOI] [PubMed] [Google Scholar]
- 14.Fergusson DM, Horwood LJ. Prospective childhood predictors of deviant peer affiliations in adolescence. J Child Psychol Psychiatry. 1999;40(4):581–592. [PubMed] [Google Scholar]
- 15.Simons RL, Whitbeck LB, Conger RD, Conger KJ. Parenting factors, social skills, and value commitments as precursors to school failure, involvement with deviant peers, and delinquent-behavior. J Youth Adolesc. 1991;20(6):645–664. doi: 10.1007/BF01537367. [DOI] [PubMed] [Google Scholar]
- 16.Plomin R, Reiss D, Hetherington EM, Howe G. Nature and nurture: genetic contributions to measures of the family environment. Dev Psychol. 1994;30(1):32–43. [Google Scholar]
- 17.Daniels D, Dunn J, Furstenberg FF, Jr, Plomin R. Environmental differences within the family and adjustment differences within pairs of adolescent siblings. Child Dev. 1985;56(3):764–774. [PubMed] [Google Scholar]
- 18.Baker LA, Daniels D. Nonshared environmental influences and personality differences in adult twins. J Pers Soc Psychol. 1990;58(1):103–110. doi: 10.1037//0022-3514.58.1.103. [DOI] [PubMed] [Google Scholar]
- 19.Pike A, Manke B, Reiss D, Plomin R. A genetic analysis of differential experiences of adolescent siblings across three years. Soc Dev. 2000;9(1):96–114. [Google Scholar]
- 20.Manke B, McGuire S, Reiss D, Hetherington EM, Plomin R. Genetic contributions to adolescents’ extrafamilial social interactions. Soc Dev. 1995;4(3):238–256. [Google Scholar]
- 21.Iervolino AC, Pike A, Manke B, Reiss D, Hetherington EM, Plomin R. Genetic and environmental influences in adolescent peer socialization: evidence from two genetically sensitive designs. Child Dev. 2002;73(1):162–174. doi: 10.1111/1467-8624.00398. [DOI] [PubMed] [Google Scholar]
- 22.Walden B, McGue M, Iacono WG, Burt S, Elkins I. Identifying shared environment contributions to early substance use: the importance of peers versus parents. J Abnorm Psychol. 2004;113(3):440–450. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]
- 23.Cleveland HH, Wiebe RP, Rowe DC. Sources of exposure to smoking and drinking friends among adolescents: a behavioral-genetic evaluation. J Genet Psychol. 2005;166(2):153–169. [PubMed] [Google Scholar]
- 24.Rose RJ. How do adolescents select their friends? a behavior-genetic perspective. In: Pulkkinen L, Avshalom C, editors. Paths to Successful Development: Personality in the Life Course. Cambridge, England: Cambridge University Press; 2002. [Google Scholar]
- 25.Bridges LJ. Autonomy as an element of developmental well-being. In: Bornstein M, Davidson L, Keyes CL, Moore KA, editors. Well-being: Positive Development Across the Life Course. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. pp. 167–175. [Google Scholar]
- 26.Steinberg L. Autonomy, conflict, and harmony in the family relationship. In: Feldman SS, Elliott GR, editors. At the Threshold: The Developing Adolescent. Cambridge, MA: Harvard University Press; 1990. pp. 255–276. [Google Scholar]
- 27.Larson R, Richards MH. Daily companionship in late childhood and early adolescence: changing developmental contexts. Child Dev. 1991;62(2):284–300. doi: 10.1111/j.1467-8624.1991.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 28.Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. 1st ed. New York, NY: Guilford Press; 2006. [Google Scholar]
- 29.Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry. 2000;57(3):261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- 30.Freedman D, Thornton A, Camburn D, Alwin D, Young-DeMarco L. The life history calendar: a technique for collecting retrospective data. Sociol Methodol. 1988;18(2):37–68. [PubMed] [Google Scholar]
- 31.Johnston LD, Bachman JG, O’Malley PM. Monitoring the Future: Questionnaire Responses From the Nation’s High School Seniors, 1981. Ann Arbor, MI: Institute for Social Research; 1982. p. 286. [Google Scholar]
- 32.Tarter RE, Hegedus A. The drug use screening inventory: its application in the evaluation and treatment of alcohol and drug abuse. Alcohol Health Res World. 1991;15(11):65–75. [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 34.Blom G. Statistical Estimates and Transformed Beta Variables. New York, NY: John Wiley & Sons Inc; 1958. [Google Scholar]
- 35.McArdle JJ. Latent curve analyses of longitudinal twin data using a mixed-effects biometric approach. Twin Res Hum Genet. 2006;9(3):343–359. doi: 10.1375/183242706777591263. [DOI] [PubMed] [Google Scholar]
- 36.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- 37.Cox DR, Snell EJ. Analysis of Binary Data. 2nd ed. London, England: Chapman & Hall; 1989. [Google Scholar]
- 38.Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics Bull. 1946;2(6):110–114. [PubMed] [Google Scholar]
- 39.Hettema JM, Neale MC, Kendler KS. Physical similarity and the equal-environment assumption in twin studies of psychiatric disorders. Behav Genet. 1995;25(4):327–335. doi: 10.1007/BF02197281. [DOI] [PubMed] [Google Scholar]
- 40.Kendler KS, Baker JH. Genetic influences on measures of the environment: a systematic review [published online ahead of print December 19, 2006] Psychol Med. 2007;37(5):615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- 41.Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychol Bull. 1977;84(2):309–322. [PubMed] [Google Scholar]
- 42.Scarr S, McCartney K. How people make their own environments: a theory of genotype greater than environment effects. Child Dev. 1983;54(2):424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- 43.White VM, Hopper JL, Wearing AJ, Hill DJ. The role of genes in tobacco smoking during adolescence and young adulthood: a multivariate behaviour genetic investigation. Addiction. 2003;98(8):1087–1100. doi: 10.1046/j.1360-0443.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- 44.Koopmans JR. TheGeneticsofHealth-RelatedBehaviors:AStudyofAdolescentTwins and Their Parents. Enschede, the Netherlands: Print Partners Ipskamp; 1997. [Google Scholar]
- 45.Rose RJ, Dick DM, Viken And RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: regional residency moderates longitudinal influences on alcohol use. Alcohol Clin Exp Res. 2001;25(5):637–643. [PubMed] [Google Scholar]
- 46.Lyons MJ, True WR, Eisen SA, Goldberg J, Meyer JM, Faraone SV, Eaves LJ, Tsuang MT. Differential heritability of adult and juvenile antisocial traits. Arch Gen Psychiatry. 1995;52(11):906–915. doi: 10.1001/archpsyc.1995.03950230020005. [DOI] [PubMed] [Google Scholar]
- 47.Jacobson KC, Prescott CA, Kendler KS. Sex differences in the genetic and environmental influences on the development of antisocial behavior. Dev Psychopathol. 2002;14(2):395–416. doi: 10.1017/s0954579402002110. [DOI] [PubMed] [Google Scholar]
- 48.Gordon RA, Lahey BB, Kawai E, Loeber R, Stouthamer-Loeber M, Farrington DP. Antisocial behavior and youth gang membership: selection and socialization. Criminology. 2004;42(1):55–88. [Google Scholar]
- 49.van den Bree MB, Pickworth WB. Risk factors predicting changes in marijuana involvement in teenagers. Arch Gen Psychiatry. 2005;62(3):311–319. doi: 10.1001/archpsyc.62.3.311. [DOI] [PubMed] [Google Scholar]
- 50.Dishion TJ, Patterson GR, Griesler PC. Peers adaption in the development of antisocial behavior: a confluence model. In: Huesmann LR, editor. Aggressive Behavior: Current Perspectives. New York, NY: Springer; 1994. pp. 61–95. [Google Scholar]
- 51.Dishion TJ, Andrews DW, Crosby L. Antisocial boys and their friends in early adolescence: relationship characteristics, quality, and interactional process. Child Dev. 1995;66(1):139–151. doi: 10.1111/j.1467-8624.1995.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 52.Patterson GR. Coercive Family Process. Eugene, OR: Castalia; 1982. [Google Scholar]
- 53.Snyder J, Dishion TJ, Patterson GR. Determinants and consequences of associating with deviant peers during preadolescence and adolescence. J Early Adolesc. 1986;6(1):29–43. [Google Scholar]
- 54.Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychol Rev. 1993;100(4):674–701. [PubMed] [Google Scholar]
- 55.Patterson GR, Forgatch MS, Yoerger KL, Stoolmiller M. Variables that initiate and maintain an early-onset trajectory for juvenile offending. Dev Psychopathol. 1998;10(3):531–547. doi: 10.1017/s0954579498001734. [DOI] [PubMed] [Google Scholar]
- 56.Lacourse E, Nagin DS, Vitaro F, Cote S, Arseneault L, Tremblay RE. Prediction of early-onset deviant peer group affiliation: a 12-year longitudinal study. Arch Gen Psychiatry. 2006;63(5):562–568. doi: 10.1001/archpsyc.63.5.562. [DOI] [PubMed] [Google Scholar]
- 57.Moffitt TE, Caspi A. Childhood predictors differentiate life-course persistent and adolescence-limited antisocial pathways among males and females. Dev Psychopathol. 2001;13(2):355–375. doi: 10.1017/s0954579401002097. [DOI] [PubMed] [Google Scholar]
- 58.Tremblay RE, Pihl RO, Vitaro F, Dobkin PL. Predicting early onset of male antisocial behavior from preschool behavior. Arch Gen Psychiatry. 1994;51(9):732–739. doi: 10.1001/archpsyc.1994.03950090064009. [DOI] [PubMed] [Google Scholar]
- 59.Saudino KJ. Behavioral genetics and child temperament. J Dev Behav Pediatr. 2005;26(3):214–223. doi: 10.1097/00004703-200506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmitz S, Fulker DW, Mrazek DA. Problem behavior in early and middle childhood: an initial behavior genetic analysis. J Child Psychol Psychiatry. 1995;36(8):1443–1458. doi: 10.1111/j.1469-7610.1995.tb01674.x. [DOI] [PubMed] [Google Scholar]
- 61.Bartels M, van den Oord EJ, Hudziak JJ, Rietveld MJ, van Beijsterveldt CE, Boomsma DI. Genetic and environmental mechanisms underlying stability and change in problem behaviors at ages 3, 7, 10, and 12. Dev Psychol. 2004;40(5):852–867. doi: 10.1037/0012-1649.40.5.852. [DOI] [PubMed] [Google Scholar]
- 62.DiLalla LF, Gottesman I. Heterogeneity of causes for delinquency and criminality: lifespan perspectives. Dev Psychopathol. 1989;1(4):339–349. [Google Scholar]
- 63.Taylor J, Iacono WG, McGue M. Evidence for a genetic etiology of early-onset delinquency. J Abnorm Psychol. 2000;109(4):634–643. doi: 10.1037//0021-843x.109.4.634. [DOI] [PubMed] [Google Scholar]
- 64.Kendler KS. Twin studies of psychiatric illness: an update. Arch Gen Psychiatry. 2001;58(11):1005–1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- 65.Kendler KS. Toward a philosophical structure for psychiatry. Am J Psychiatry. 2005;162(3):433–440. doi: 10.1176/appi.ajp.162.3.433. [DOI] [PubMed] [Google Scholar]
- 66.Kendler KS. Twin studies of psychiatric illness: current status and future directions. Arch Gen Psychiatry. 1993;50(11):905–915. doi: 10.1001/archpsyc.1993.01820230075007. [DOI] [PubMed] [Google Scholar]
- 67.Yoshihama M, Clum K, Crampton A, Gillespie B. Measuring the lifetime experience of domestic violence: application of the life history calendar method. Violence Vict. 2002;17(3):297–317. doi: 10.1891/vivi.17.3.297.33663. [DOI] [PubMed] [Google Scholar]
- 68.Cook LS, White JL, Stuart GC, Magliocco AM. The reliability of telephone interviews compared with in-person interviews using memory aids. Ann Epidemiol. 2003;13(7):495–501. doi: 10.1016/s1047-2797(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 69.Belli RF. The structure of autobiographical memory and the event history calendar: potential improvements in the quality of retrospective reports in surveys. Memory. 1998;6(4):383–406. doi: 10.1080/741942610. [DOI] [PubMed] [Google Scholar]