Abstract
Objective
To evaluate early cellular influences of bone morphogenetic protein (BMP)12 and BMP2 on equine superficial digital flexor tenocytes (SDFTNs) and equine bone marrow–derived mesenchymal stem cells (BMDMSCs).
Animals
9 adult clinically normal horses.
Procedures
BMDMSCs and SDFTNs were cultured in monolayer, either untreated or transduced with adenovirus encoding green fluorescent protein, adenovirus encoding BMP12, or adenovirus encoding BMP2. Cytomorphologic, cytochemical, immunocytochemical, and reverse transcriptase–quantitative PCR (RT-qPCR) analyses were performed on days 3 and 6. Genetic profiling for effects of BMP12 was evaluated by use of an equine gene expression microarray on day 6.
Results
BMDMSCs and SDFTNs had high BMP12 gene expression and remained viable and healthy for at least 6 days. Type l collagen immunocytochemical staining for SDFTNs and tenocyte-like morphology for SDFTNs and BMDMSCs were greatest in BMP12 cells. Cartilage oligomeric matrix protein, as determined via RT-qPCR assay, and chondroitin sulfate, as determined via gene expression microarray analysis, were upregulated relative to control groups in SDFTN-BMP12 cells. The BMDMSCs and SDFTNs became mineralized with BMP2, but not BMP12. Superficial digital flexor tenocytes responded to BMP12 with upregulation of genes relevant to tendon healing and without mineralization as seen with BMP2.
Conclusions and Clinical Relevance
Targeted equine SDFTNs may respond to BMP12 with improved tenocyte morphology and without mineralization, as seen with BMP2. Bone marrow–derived mesenchymal stem cells may be able to serve as a cell delivery method for BMP12.
The most common musculoskeletal injuries in racehorses between 1996 and 1998 involved either the SDFT or the suspensory ligament, which collectively comprised 46% of all the musculoskeletal injuries sustained.1 Several different treatments for SDFT injury exist, consisting of but not limited to medical management2,3 including controlled exercise,2,4,5 use of intralesional injections2,6–9 with in vitro and in vivo investigation into tissue engineering approaches on tendon healing,10–13 and surgical management such as proximal suspensory ligament desmotomy,14 tendon splitting,15,16 annular desmotomy,13 and fasciotomy.3 Presently, even with these treatment options, tendon injuries in equine athletes can be debilitating because of the high incidence of recurrence and reduced performance.1,8–10,17 Identification of a treatment with potential acceleration of tendon healing, increased return to performance, and decrease in reinjury rate is warranted and would be valuable.
In many species, evidence is accumulating concerning the benefits of BMDMSCs in treatment of tendon and ligament injuries.18–23 In vivo studies of MSC-seeded collagen gels and BMDMSCs in rabbits have revealed improvements in biomechanics and histologic features in early stages of tendon healing,22 as well as enhanced biomechanical characteristics after tendon healing.20–22 For National Hunt horses, BMDMSC treatment allowed 51% to return to racing with a 30% reinjury rate,17 compared with a 56% reinjury rate for horses not treated.2 Additionally, an 18% reinjury rate was observed in racehorses returned to full work following BMDMSC treatment at the site of SDFT injury.19 The BMDMSCs administered to collagenase-induced lesions of the SDFT in a recent in vivo study14 resulted in increased stiffness, compared with results for control animals. There are, however, limited equine cases with sufficient follow-up time to reveal substantial improvement of BMDMSC-treated horses when evaluated against horses with prolonged rehabilitation and controlled exercise.
The BMDMSC can serve not only as a treatment for tendon injury itself but also as a delivery vehicle for mediators of tissue regeneration (ie, growth factors). The BMDMSCs remain localized at the site of injection with a small degree of migration into surrounding healthy tissue and neither autologous nor allogenic MSCs result in an adverse immune response from the host.19 Ex vivo gene treatment by use of BMDMSCs allows for genetic manipulation of the cells in vitro with subsequent delivery to a specific anatomic site, resulting in local expression of desired therapeutic proteins.
The BMPs are a group of related proteins in the transforming growth factor-β superfamily known for osteoinductive capacity.24–26 Recombinant human BMP2 is well characterized and is the most studied BMP with potent osteoinductive capacity and ability to induce mineralization of BMDMSCs24 in vivo and in vitro; furthermore, this has been determined in many species, including horses.25,27 Bone morphogenetic protein 12, a human homologue of murine growth and differentiation factor-7, is in the BMP family and is related to other BMPs involved in the developmental processes of the musculoskeletal system,24 including regulating tissue differentiation,28,29 tendon healing, and tenogenesis.30 Unlike other BMPs, however, BMP12 does not have an obvious osteoinduction effect on tendon cells29–35 and is associated with accelerated healing and improved biomechanical quality of repairs in human patellar tendons,32 tendon laceration models in chickens and rats,31,35,36 gastrocnemius tendon models in rats and mice,33,37 and periodontal ligaments in dogs.38 Specifically, in rats, in vivo experimentation reveals that BMP12 induced formation of tendon- and ligament-like tissue36 and differentiated MSCs into tenocytes in vitro.29 Therefore, studies are warranted to evaluate the effect of BMP12 in specific and relevant equine tissues.
Bone morphogenetic protein 12 exogenously introduced into tendon cells in vitro induces up to 30% more type I collagen gene expression and protein production, compared with results for control groups.35,36 Type I collagen is a major constituent of tendon, and restoration of mature extracellular matrix is a limitation in tendon and ligament repair.39 Additionally, BMP12 could be delivered by gene therapy to a specific site, allowing for local expression and sustained release.25,27,40
The purpose of the study reported here was to investigate the early cellular effects of BMP12, compared with effects of BMP2, on equine SDFTNs and BMD-MSCs and determine whether BMP12 has potential for therapy in tendon healing in horses.
Materials and Methods
Study design
Bone marrow–derived mesenchymal stem cells were collected from the sternum of 3 adult horses and SDFTNs isolated from the left midmeta-carpal region of the SDFT of 6 adult horses. Isolated cells were expanded in monolayer and achieved approximately 75% confluency within 24 hours, at which time the BMDMSCs and SDFTNs were transduced with an adenoviral vector encoding either the GFP gene, (rh)BMP12 gene, or (rh)BMP2 gene. The groups were defined as untreated, GFP, BMP12, and BMP2. The groups were then allocated in triplicate in a 24-well plate (3 ×104 cells/well) for cytochemical analysis (von Kossa method for phosphate mineral and bone alkaline phosphatase activity) and immunocytochemical analysis (for type l collagen), allocated in duplicate in a 6-well plate (1 × 105 cells/well) for RNA extraction and RT-qPCR assay (for transgene production of β-actin, type I collagen, type III collagen, alkaline phosphatase, COMP, BMP12, and BMP2) and cytomorphologic examination, or expanded in a culture flask (seeded at 2 × 105 cells/flask) for equine-specific microarray analysis. Two time points (days 3 and 6) were evaluated.
Sample population
Grossly normal left forelimb SDFTs were aseptically harvested from 6 adult horses (age range, 10 to 22 years) of different breeds immediately following euthanasia for reasons other than superficial digital flexor tendonitis (eg, colic or neurologic disease). The BMDMSCs were obtained via bone marrow aspiration from the sternum27,41–43 from an additional 3 adult (age range, 10 to 18 years) clinically normal horses of various breeds. The bone marrow–aspiration procedure was approved by The Ohio State University Institutional Animal Use and Care Committee and was similar to that in previous studies.42,43 Three horses were sedated with xylazine hydrochloride (0.5 to 1.1 mg/kg, IV) for the duration of the procedure. The hair of the sternum was clipped and shaved. After an initial aseptic preparation, 5 mL of local anesthetic was infiltrated SC over the site of the bone marrow aspiration, and the site was prepared in an aseptic manner. A stab incision was made by use of a No. 15 blade. An 11-gauge, 4-inch Jamshedi needlea was used to collect 40 to 50 mL of bone marrow aspirate.
MSC isolation
The bone marrow sample was collected in two 35-mL syringes with heparin sodiumb (1,000 U/10 mL of aspirate). Following collection, 20 to 25 mL of the bone marrow aspirate from each horse was placed into separate 50-mL tubesc containing 30 mL of culture medium consisting of Dulbecco modified Eagle medium 1×b and F-12d in a 1:1 ratio supplemented with 10% heat-inactivated fetal bovine serume and 1% penicillin-streptomycind and placed on ice until isolation in the laboratory. The BMDMSCs were isolated via centrifugation of the bone marrow aspirates and cultured in monolayer as described.41,42
Nucleated cells were isolated in the laboratory via a described method.41,f Briefly, 20 mL of the diluted bone marrow was placed over 15 mL of density gradient solutionf and centrifuged at 400 × g for 30 minutes at 4°C. The cell layer was aspirated and washed with 30 mL of Gey balanced salt solutiong and centrifuged at 300 × g for 5 minutes. This wash was performed twice. The washed pellet was resuspended in 15 mL of culture media as described in a culture flask and placed in a 37°C, 5% CO2, 100% relative humidity incubator. Cells from different horses were kept separate for use in parallel experiments and incubated, with the medium being changed on the fifth day, removing any nonadherent cells. Medium change then took place every 2 days during expansion of the cells.
BMDMSC differentiation
At the time of initiating the experiment, pluripotency of the BMDMSCs was confirmed by culturing in controlled media for osteogenesis (dexamethasone with ascorbate), chondrogenesis (rh transforming growth factor-β1), and adipogenesis (dexamethasone with insulin and indo-methacin), according to the manufacturer’s protocol.h The cultures were stained with 2% alizarin red solutiong for evaluation of osteogenesis, oil red Og for evaluation of adipogenesis, and toluidine blueg for evaluation of chondrogenesis.
SDFTN isolation
A complete 8- to 10-cm cross section of the left SDFT in the midmetacarpal region was harvested aseptically immediately following euthanasia of each horse and placed into ice-cold medium as for MSC isolation. The tendon was minced (2 × 2-mm pieces) and treated with 0.3% collagenase IIi at 37°C in overnight. The a humidified atmosphere with 5% CO2 resulting cell suspension was centrifuged at 400 × g for 5 minutes in an unheated centrifuge, forming a cell pellet. The cell pellet was washed twice in Gey balanced salt solution,g resuspended in culture medium, and expanded in monolayer.
Generation of an adenoviral vector
A recombinant, replication-deficient, E-1A–defective adenovirus encoding cDNA for GFP,j human BMP12,k or human BMP2j was used. Adenoviral vectors were generated in the authors’ laboratory (AdGFP and AdBMP2)j or another institute (human BMP12)k and amplified (AdGFP,j AdBMP12,k and AdBMP2j) in the authors’ laboratory according to a described method.40 The viral titer was determined by use of a commercial adenovirus titer kit.j
Adenovirus gene transduction
The MOI (or number of vector particles per cell) that would result in a transduction efficiency of at least 80% for both BMDMSCs and SDFTNs was determined prior to initiation of this study. All cells used in this study, for both preliminary data and experimentation, were in their third passage. The preliminary experiment consisted of plating 30,000 cells/well (in a 24-well plate), resulting in approximately 75% confluency within 24 hours. At this confluency, BMDMSCs and SDFTNs were transduced with AdGFP (MOI of 10, 20, 50, 100, 500, and control). Transduction efficiency was recorded 48 hours after transduction via microscopic assessment. The MOI determined for BMDMSCs and SDFTNs was 100 and 500, respectively.
The cells were left untreated or were treated with AdBMP12, AdGFP, or AdBMP2 in Gey balanced salt solutiong (at an MOI of 100:1 for BMDMSCs and 500:1 for SDFTNs) for 2 hours, after which the solution was aspirated and fresh mediumd was added. The cells were placed in an incubator for completion of the study.
Transduction efficiency
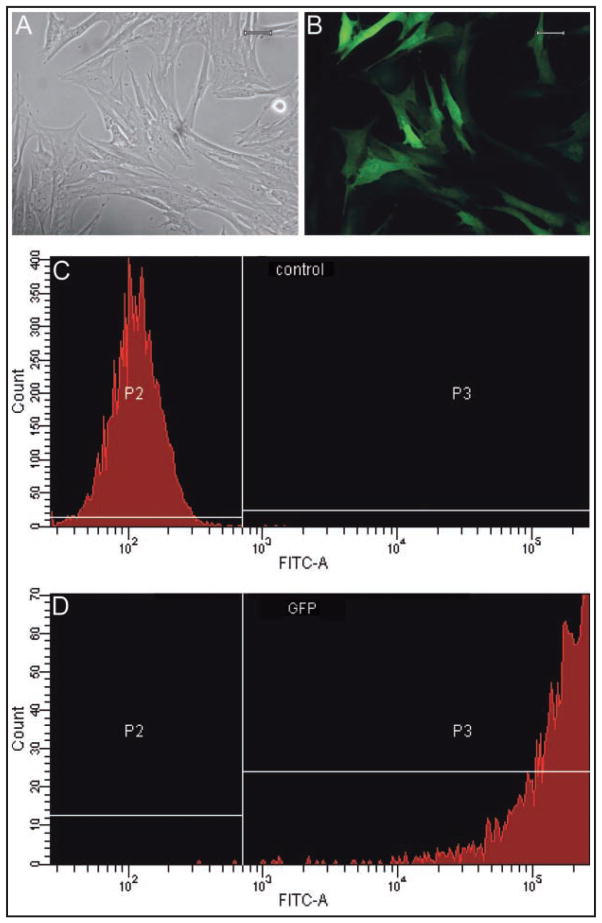
The AdGFP-transduced BMDMSCs and SDFTNs were examined via microscopic quantitative assessment of monolayer cell culture with fluoroscopy for transgene expression of GFP at 48 hours after transduction. Transduction efficiency for AdGFP-transduced BMDMSCs and SDFTNs was evaluated by counting cells in 3 microscopic fields under 20× magnification.m The total number of fluorescing cells was divided by the total number of cells per field, which yielded a transduction efficiency percentage. Quantitative confirmation of transduction efficiency for SDFTNs 48 hours after transduction was performed by use of fluorescent-activated cell sorting analysis.n Forward- and side-scatter parameters were optimized to eliminate dead cells and debris from the analysis. Green fluorescence protein was excited by an argon laser, and fluorescence was detected by use of a 30- to 530-nm bandpass filter in the FL1 channel.
Cytomorphology
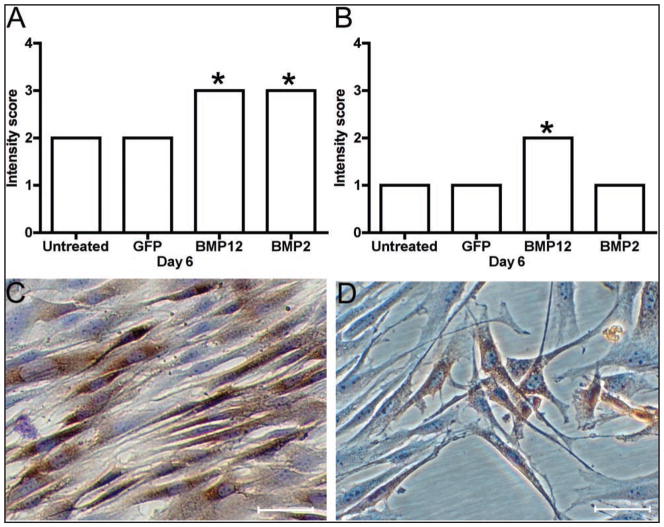
Cell morphology was scored on days 0, 3, and 6 to assess cell health. Three representative fields (objective, 20×) were assigned a score of 0 (> 20% rounded, dying, or detached), 1 (> 10% rounded, dying, or detached), 2 (BMDMSCs or SDFTNs with normal appearance seen as attached rounded cells with a smooth surface), 3 (moderately elongated cells), or 4 (markedly elongated cells), to correlate with increasing tenocyte-like appearance.
Cytochemical and immunocytochemical analysis
Monolayer cells were plated in triplicate in a 24-well culture plate (3 × 104 cells/well) on day –1. On day 0, at 75% confluency, the cells were transduced (untreated, AdGFP, AdBMP12, and AdBMP2). On days 3 and 6, the cells were stained for alkaline phosphatase activity and phosphate mineral (von Kossa method), and immunocytochemical analysis was performed for type I collagen. Alkaline phosphatase activity and type I collagen were scored for staining intensity (0 = no staining, 1 = faint to moderate staining, 2 = moderate staining, 3 = moderate to strong staining, 4 = strong staining) and extent (0 = 0%, 1 = 1% to 25%, 2 = 26% to 50%, 3 = 51% to 75%, 4 = 76% to 100%; where the percentage value refers to the proportion of cells in the corresponding field stained with type I collagen or alkaline phosphatase), and areas of extracellular phosphate mineralization were point counted and expressed as percentage of area mineralized.
Alkaline phosphatase
Cells were washed twice with PBS solution and fixed by use of citrate-buffered acetone for 30 seconds. Cells were then washed in distilled water for 45 seconds and kept at 4°C until the assay was started. An alkaline dye mixture,o containing a salt capsule dissolved in 48 mL of distilled water and 2 mL of phosphate alkaline solution, was prepared according to the manufacturer’s protocol26,o and added to the wells for 30 minutes at 23°C while protected from direct light. Cells were washed thoroughly in distilled water for 2 minutes and placed in Harris hematoxylin solutiong (diluted 1:1 with distilled water) for 1 minute. Cells were washed for 3 minutes with distilled water following counterstaining and analyzed for alkaline phosphatase activity by observing blue stain.
Von Kossa staining
For von Kossa staining,44 cell cultures were washed twice with 1× PBS solution.p Phosphate-buffered formalin fixative (4%) was placed on the cells for 20 minutes, and the cells were washed twice with 1× PBS solution and placed at 4°C. The cells were rinsed once with waterd and serially dehydrated in 70%, 95%, and 100% ethanol for 2 times each and air dried. Cells were rehydrated from 100% to 95% to 80% ethanol to tap water. The water was removed, 2% silver nitrate solutioni was added, and the cells were exposed to UV light for 20 minutes. The cells were rinsed with water. Sodium thiosulfated 5% (diluted in waterd) was added for 3 minutes, the plates were washed in tap water, and acid fuchsinc counterstain was added for 5 minutes. Following counterstaining, the plates were washed with tap water, washed twice with 95% ethanol, washed twice with 100% ethanol, and allowed to air dry prior to analysis.
Type I collagen immunocytochemical analysis
Cell cultures were washed with 1× PBS twice, and 4% phosphate-buffered formalin fixative was placed on the cells for 20 minutes at 4°C. Cells were washed in 1× PBS solution for 5 minutes and incubated for 20 minutes in 2.5% normal horse blocking serum.q The serum was aspirated, and a monoclonal anti-collagen type I (mouse IgG isotype)h antibody diluted in 2.5% normal horse blocking serumq was added to the cells at a 100:1 dilution for 30 minutes. Cells were rinsed in 1× PBS solution for 5 minutes and incubated for 30 minutes with reagent containing anti-mouse IgG (against heavy and light chains).q Cells were rinsed in 1× PBS solution for 5 minutes and incubated in diaminobenzidine substrate until desired stain intensity developed (10 minutes). Cells were rinsed in tap water and counter-stained in hematoxylin solutiong diluted (1:1) with distilled water.
RNA isolation and RT-qPCR assay
Transgene expression of untreated, GFP, BMP12, and BMP2 groups was quantified by use of RT-qPCR assay.45,r Total RNA (260 to 280 wavelength; optical density reading range, 1.8 to 2.1) was isolated from cell monolayers by use of an RNA extraction reagentd followed by DNase treatment.s Mean times change in gene expression on day 3 and day 6 after transduction was calculated relative to the untreated group and relative to the control gene encoding β-actin (units, 2−ΔΔCT).45,46 A sequence from each gene that is specific and unique to horses was purchasedt and used to design the primer sets for alkaline phosphatase, collagen I, collagen type III, COMP, and β-actin by use of software.r Primer sets for BMP12 and BMP2 were designed from the human sequence (Appendix). Following DNase treatment, cDNA was made from total RNA by use of a commercial reverse transcription kit,q and 2-step RT-qPCR assay was performed according to the manufacturer’s protocol.q
Gene expression analysis
Total RNA was isolatedc according to the manufacturer’s extraction protocol with minor modifications from untreated, GFP, and BMP12 monolayers for BMDMSCs and SDFTNs on day 6 for evaluation of gene expression by use of an equine-specific microarrayu,v and prepared for application to microarrays by use of established methods.25,45,w Each equine-specific microarray represented 3,098 unique genes, all of which have been annotated by Basic Local Assignment Search Tool and functionally classified.47
Protein expression analysis
Aliquots of media from the BMDMSC and SDFTN treatment groups were frozen at –80°C on days 0, 3, and 6. Production of BMP2 was quantified by use of a commercial quantitative sandwich enzyme immunoassay used to detect rhBMP2.w
Statistical analysis
Objective data were analyzed by use of a 1-way (treatment) ANOVA with a Bonferroni multiple comparison posttest analysis. Gaussian distribution was confirmed by use of a D’Agostino and Pearson omnibus normality test. Data that were non-parametrically distributed and scored data were then analyzed by use of Kruskal-Wallis and Dunn multiple comparison tests with a statistical software programx with significance set at P < 0.05. Microarray data were analyzed by use of software.y Array normalization was performed via quantile normalization; model-based expression indices were computed by use of only perfect match probes, and the log2 (model-based expression indices) values were then exported for further analysis via software.z A filtering step was performed to remove probe sets in which at least 75% of the arrays had expression values equal to or less than background value (ie, < log2[100]). This resulted in 1,501 probe sets included in analyses. A random-effects ANOVA, allowing for correlations in observations from the same horse, was fit to estimate the effect of treatment on gene expression in BMDMSCs. For the probe sets with differential expression (P < 0.005) among the 3 treatment groups and control group (untreated, AdGFP, vector control, and AdBMP12), pairwise comparisons were performed. Type I error was limited by use of the closure principle.48 Likewise, a random-effects ANOVA was used to estimate the effect of treatment on gene expression in SDFTNs. To accommodate for multiple comparisons and by accepted convention, microarray comparisons of multiple groups were considered significant at P < 0.005.
Results
Pluripotency was determined for BMDMSCs from each of the 3 horses by differentiation toward osteogenic (Figure 1), chondrogenic, and adipogenic pathways at passage 3. Transduction efficiency was > 90% for microscopic assessment of BMDMSC-GFP cells and SDFTN-GFP cells as well as fluorescent-activated cell sorting analysis of SDFTN-GFP cells 48 hours after transduction (Figure 2). Cytomorphologically, BMDMSC-BMP12 cells and SDFTN-BMP12 cells had greater cell elongation than did respective untreated, GFP, or BMP2 cells on day 3. Median score for BMDMSC-BMP12 cells and SDFTN-BMP12 cells was 3.0; median score for respective untreated, GFP, and BMP2 cells was 2.0. These scores persisted through day 6, except for the BMDMSC-GFP cells for which median score decreased to 1 (Figure 3). Via cytochemical and immunocytochemical analyses, alkaline phosphatase was not observed on day 3 or day 6 for untreated, GFP, or BMP12 groups of BMDMSCs and SDFTNs. Alkaline phosphatase transcript values were higher in the BMDMSC-BMP2 group relative to results for other groups (Figure 4), which was reflected in a higher staining intensity (P < 0.001) in the BMP2 group in both cell types on days 3 and 6 (Figure 5).
Figure 1.
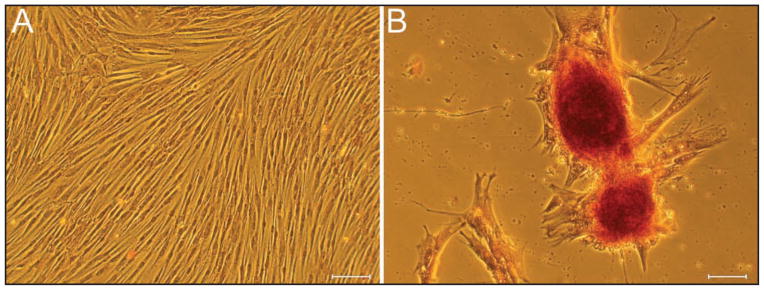
Photomicrographs of pluripotent equine BMDMSCs (A). The cells are undergoing mineralization, seen as alizarin red-stained nodules, in osteogenic differentiation media (B). Cytochemical stain; bars = 100 μm (A) and 20 μm (B).
Figure 2.
Photomicrographs of SDFTNs (A; light microscopy; bar = 20 μm) seen as GFP-positive cells via fluorescent microscopy (B; bar = 20 μm). Quantitative assessment of transduction efficiency via flow cytometry analysis; representative scatterplots of control SDFTNs (C) and SDFTN-GFP cells (D) are illustrated. Notice > 95% transduction efficiency in SDFTN-GFP samples. P2 = Region of cells lacking detectable fluorescent activity on the FITC-A channel. P3 = Region of detectable fluorescent activity in analyzed cells. FITC-A = Designated laser used to detect fluorescent activity. Y-axis = Channel numbers detected by use of the indicated laser. X-axis = Number of cells analyzed.
Figure 3.
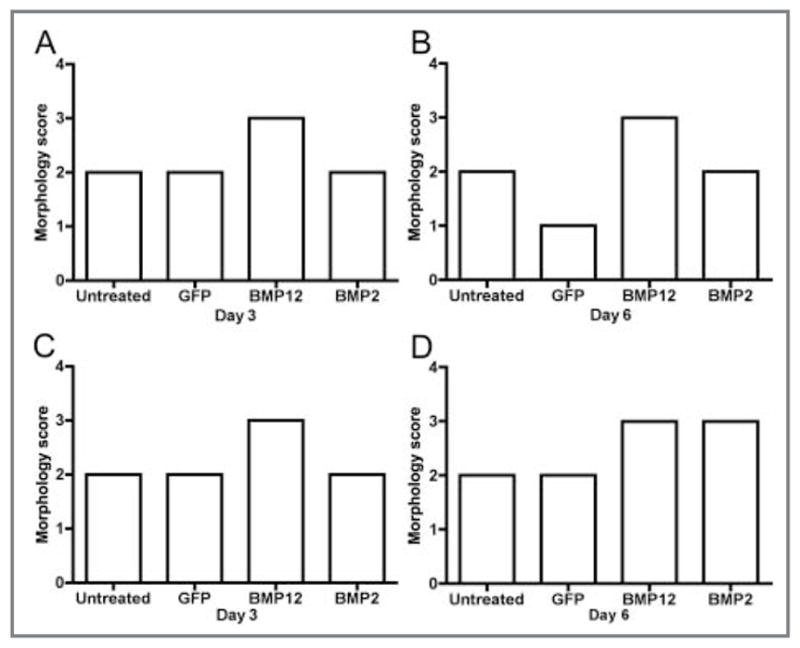
Morphology scores on cell culture days 3 and 6 for equine BMDMSCs (A, B) and SDFTNs (C, D) transduced with adenovirus encoding GFP, BMP12, or BMP2. In A, BMDMSC-BMP12 cells are significantly (P < 0.001) more elongated, compared with that of other groups. In B, BMDMSC-BMP12 cells are significantly more elongated, compared with that of BMDMSC -GFP cells (P < 0.001) and untreated control cells (P < 0.01), respectively. In C, SDFTN-BMP12 cells are significantly (P < 0.001) more elongated, compared with that of other groups. In D, SDFTN-BMP12 cells and SDFTN-BMP2 cells are significantly (P < 0.01) more elongated, compared with that of other groups.
Figure 4.
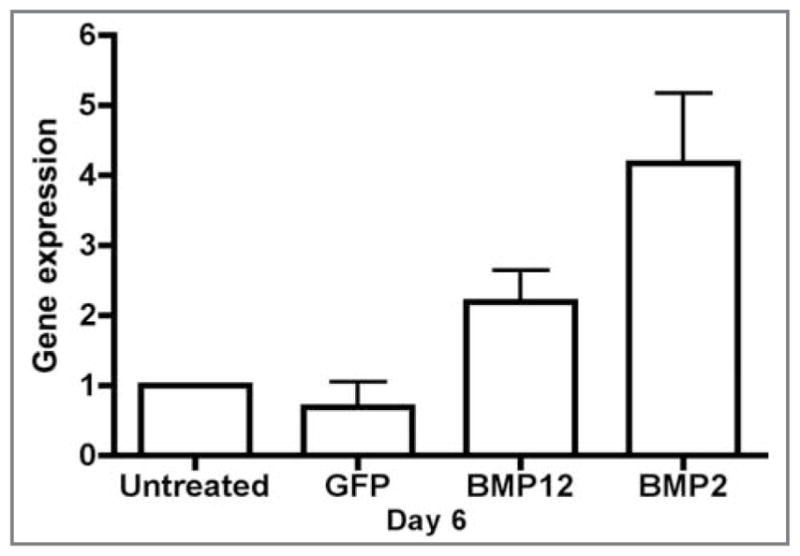
Graph of gene expression (via RT-qPCR assay) for alkaline phosphatase in equine BMDMSCs. On cell culture day 6, the value for BMDMSC-BMP2 cells was significantly (P < 0.01) greater, compared with results for other groups.
Figure 5.
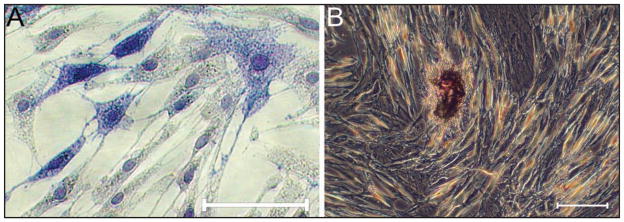
Photomicrographs of equine BMDMSC-BMP2 cells stained for alkaline phosphatase on cell culture day 3 (A; alkaline phosphatase stain; bar = 20 μm) and stained for focal mineral formation on day 6 (B; von Kossa stain; bar = 20 μm).
Percentage area mineralization was greater in the BMP2 group of both cell types with no detection of mineral on either day 3 or 6 in the untreated, GFP, or BMD-MSC-BMP12 groups (P < 0.001; Figure 5). Cell staining extent (BMDMSCs and SDFTNs) for type I collagen was greater for BMP12 and BMP2 groups, compared with results for untreated and GFP control cells (P < 0.001) for days 3 and 6, with the greatest extent of type I collagen staining of BMDMSCs observed for BMP12 on day 6. Intensity of cell staining was greater (P < 0.001) for BMP12 and BMP2 on days 3 (BMDMSCs and SDFTNs) and 6 (SDFTNs), compared with results for untreated and GFP control cells. However, the intensity of type I collagen staining was greatest compared with results for all other BMDMSC groups on day 6 for BMDMSC-BMP12 cells (P < 0.001; Figure 6).
Figure 6.
Type I collagen in equine SDFTNs (A, C) and BMDMSCs (B, D). On cell culture day 6, SDFTN-BMP12 and BMP2 cells had significantly (P < 0.001) greater type I collagen staining intensity, compared with that of the other groups (A). On cell culture day 6, BMDMSC-BMP12 cells had significantly (P < 0.001) greater staining intensity, compared with that of the other groups (B). C—Photomicrograph of SFTN-BMP12 cells containing type I collagen. Immunohistochemical stain; bar = 20 μm. D—Photomicrograph of BMD-MSC-BMP12 cells containing type I collagen. Immunohistochemical stain; bar = 20 μm.
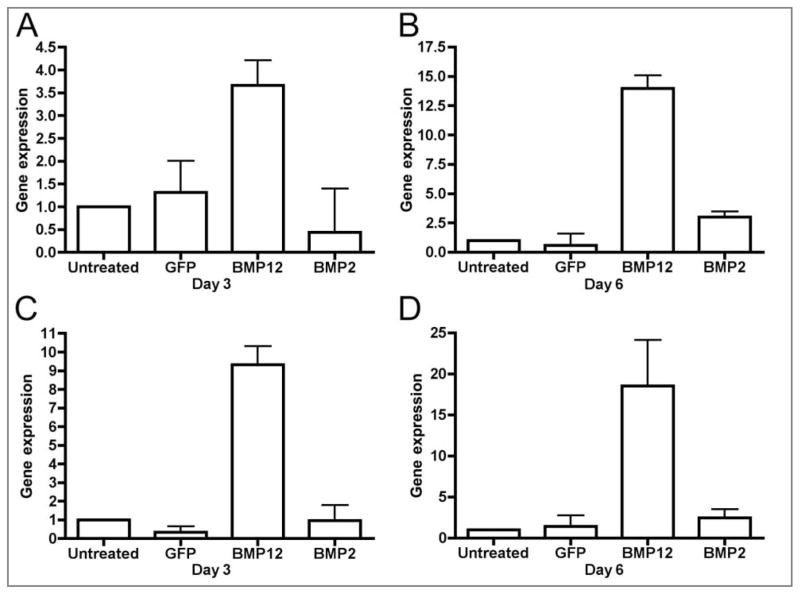
RT-qPCR assay
Expression of BMP12 was confirmed for BMDMSC–BMP12 cells and SDFTN-BMP12 cells and was greater than that for untreated cells (P < 0.01; Figure 7). Expression of BMP2 was confirmed for BMDMSC-BMP2 cells and SDFTN-BMP2 cells and was greater than that for untreated or GFP-treated cells (P < 0.001). The SDFTN-BMP12 cells had greater expression of COMP on day 6 (P < 0.001) than did other groups (Figure 8). There was no difference among groups in expression of COMP in BMDMSCs on days 3 or 6. Alkaline phosphatase expression was not evident on day 3 for either cell type but was greater on day 6 in BMDMSC-BMP2 cells, compared with results for control cells (P < 0.01). Type III collagen expression was greater in BMDMSC-BMP2 cells than in other groups on day 3 (P < 0.01).
Figure 7.
Gene expression of BMP12 in equine BMDMSCs (A, B) and SDFTNs (C, D). A—On cell culture day 3, gene expression was significantly greater in BMDMSC-BMP12 cells than in untreated control cells, BMDSMC-BMP2 cells (P < 0.01), or BMDMSC-GFP cells (P < 0.05). B—On cell culture day 6, gene expression was significantly (P < 0.001) greater in BMDMSC-BMP12 cells than in other groups. C—On cell culture day 3, gene expression was significantly (P < 0.001) greater in SDFTN-BMP12 cells, compared with that of other groups. D—On cell culture day 6, gene expression was significantly (P < 0.01) greater in SDFTN-BMP12 cells, compared that of with other groups.
Figure 8.
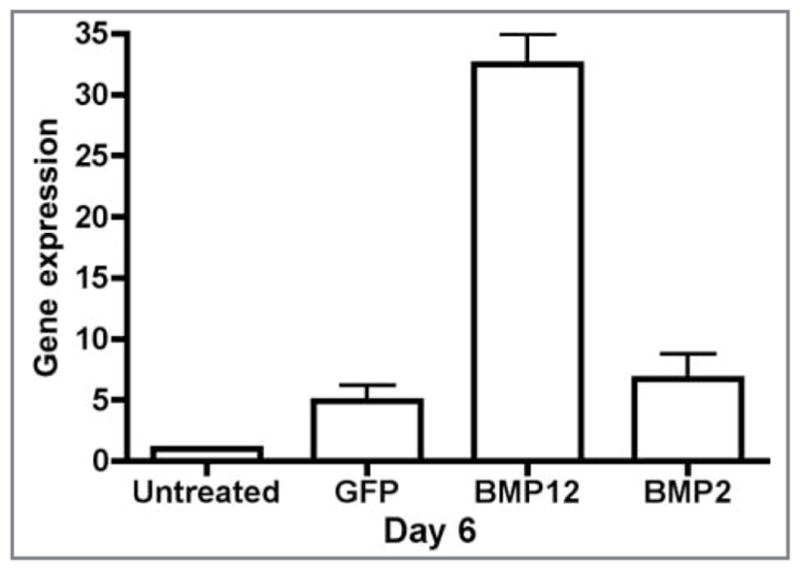
Gene expression of COMP in equine SDFTNs. On cell culture day 6, gene expression was significantly (P < 0.001) greater for SDFTN-BMP12 cells, compared with that of other groups.
Microarray gene expression analysis
Gene expression microarray analysis revealed significant upregulated differential gene expression of chondroitin sulfate, compared with that for GFP cells (P < 0.001), in SDFTNs. In addition, cellular trophic genes (eg, tumor necrosis factor, DEAD/H box, heat shock 90-kd protein 1, and peroxiredoxin) were upregulated in BMP12 cells, compared with results for GFP cells, on day 6 for BMDMSCs and SDFTNs (Tables 1 and 2).
Table 1.
Selected relevant genes with significant (P < 0.005) differential gene expression in equine BMDMSCs treated with AdBMP12, compared with cells treated with AdGFP.
| Equine Genbank accession No. | Sequence name | Biological function | Factor of change | P value |
|---|---|---|---|---|
| CD466560 | Myristoylated alanine-rich protein kinase C substrate | Calmodulin binding, actin filament binding | 1.85 | < 0.001 |
| CD528599 | Transcription elongation factor A | Regulation of transcription | 0.62 | < 0.001 |
| BI961271 | Ferritin, light polypeptide storage protein | Intracellular iron | 0.61 | < 0.001 |
| CD465526 | Glutamine synthase, mRNA | Metabolism of nitrogen | 0.61 | < 0.001 |
| CD467815 | Ganglioside activator protein | Innate immunity, lipid metabolism | 0.75 | 0.002 |
| BM780348 | Ubiquinol-cytochrome c reductase core protein l | Mitochondrial respiratory chain | 1.26 | 0.002 |
| BI961520 | Peroxiredoxin 1 | Antioxidant enzyme | 0.75 | 0.002 |
| BI961470 | Chondroitin sulfate proteoglycan 2 (versican) | Extracellular matrix proteoglycan | 4.35 | 0.002 |
| CD466435 | DEAD/H Box (Asp-Glu-Ala-Asp/His) | Positive regulation cell proliferation | 2.08 | 0.002 |
| BM781267 | Laminin receptor 1 | Mediate effects of laminin, extracellular matrix protein, cell adhesion molecule | 0.79 | 0.002 |
| BM780407 | Heat shock 90-kd protein 1, Alpha-like 3 | Stress proteins | 0.58 | 0.004 |
Table 2.
Selected relevant genes with significant (P < 0.005) differential gene expression in equine SDFTNs treated with AdBMP12, compared with results for control cells treated with AdGFP.
| Equine Genbank accession No. | Sequence name | Biological function | Factor of change | P value |
|---|---|---|---|---|
| 2170373 | Tumor necrosis factor | Cell survival and apoptosis | 1.19 | < 0.001 |
| CD465149 | Putative small membrane protein NID67 | Forming-regulating ion channels | 1.60 | 0.001 |
| CD465748 | Inhibitor of DNA binding 2, dominant negative helix-loop-helix protein | Protein binding, transcription repressor activity | 2.69 | 0.001 |
| 15082427 | DEAD/H (Asp-Glu-Ala-Asp/His) box | Positive regulation cell proliferation | 2.31 | 0.003 |
Protein expression analysis
Bone morphogenetic protein 2 protein expression was confirmed (mean ± SEM, 3,088 ± 837.2 pg/mL) for AdBMP2-transduced BMDMSCs and SDFTNs in monolayer culture.
Discussion
The in vitro study reported here effectively evaluated the early cellular influence of BMP12, compared with BMP2, on equine SDFTNs and BMDMSCs that may be used for transplantation. Results from previous studies29–32 suggest that BMP12 may play a pivotal role in early tendon healing. Cytomorphologic evaluation of SDFTN-BMP12 cells and BMDMSC-BMP12 cells revealed greater and accelerated cell elongation, compared with results for untreated, vector control, and BMP2 groups by day 3 and greater than results for GFP groups by day 6 (Figure 3), suggesting the ability to induce early differentiation of BMDMSCs and SDFTNs into tenocytes, which may be correlated with early tendon healing.28,33,34,36,37
Cytochemical analysis was performed for mineral and alkaline phosphatase, which are often used as markers for osteogenic differentiation.25,35 Unlike BMP2, treatment with BMP12 did not result in focal phosphate mineral formation of cells in culture. Previous research has used alkaline phosphatase expression as well as focal phosphate mineral formation to indicate osteogenic differentiation of BMDMSCs into osteoblasts by BMPs (BMP2 and BMP6) in vitro.25 Our findings supported earlier research in which, unlike other BMPs, BMP12 did not have obvious osteoinduction effects on tendon cells or mesenchymal stem cells,29,36 an important finding considering future applications of BMP12 in equine SDFTs.
Type I collagen immunohistochemical staining intensity and extent were increased by induction with BMP12 in SDFTNs and BMDMSCs (Figure 5), suggesting an early abundance and acceleration of type I collagen production in both cell types. These data, in concert with morphology scores and induction of COMP gene expression, suggest that BMP12 supported a tenogenic phenotype in both cell types. In contrast, the accelerated type I collagen expression in SDFTNs and BMDMSCs induced by BMP2, in concert with morphology scores, positive alkaline phosphatase expression, and focal mineral formation, suggested that BMP2 supported differentiation toward an osteocyte phenotype. Gene expression of prominent proteins associated with tendons, such as type I collagen and COMP, and decreased gene expression of type III collagen associated with bone formation, although not definitive markers for tenogenesis, are consistent with tendon cells and fibroblasts,20,49 suggesting differentiation of BMDMSC-BMP12 cells toward fibroblast-like tendon cells, rather than osteoblasts. In addition, this gene expression was also identified in SDFTN-BMP12 cells, compared with results for other SDFTN groups, suggesting an early influence on tendon differentiation, specific to tendon cells.
Gene expression microarray analysis revealed up-regulation of chondroitin sulfate proteoglycan 2 (versican) in SDFTNs in addition to other genes for cell proliferation, transcription, and matrix production (in BMDMSCs and SDFTNs) in BMP12-expressing cells, compared with control GFP-expressing cells. Chondroitin sulfate is an extracellular matrix glycosaminoglycan that has been linked to compressed regions of normal tendon, suggesting a function in a specific non-tensile biomechanical environment.31 The upregulation of chondroitin sulfate in BMP12-expressing cells is compatible with glycosaminoglycan extracellular matrix production. The biochemical structure of tendon incorporates proteoglycans and chondroitin sulfate and may be associated with tensile and compressive forces during loading of tendon.50 The role of BMP12 in supporting this extracellular matrix glycosaminoglycan seen in compressed zones of tendon is unknown. Microarray analysis has been used in vivo to determine potential tendon-associated genes.51–53 For example, type I collagen α–2 and insulin-like growth factor were determined to be genes expressed in tendon and associated with recovery from overuse in rat supraspinatus tendons.52
Microarray analysis performed on rat tissues53 sought to identify genes specific to tendon to verify whether MSCs had differentiated. Tissues harvested from rats included tendons (gastrocnemius and patellar), paratenon, ligament, cartilage, bone, and bone marrow. Results revealed a small number of genes specific to tendon and ligaments, and reverse transcription PCR assay confirmed that expression of the gene tenomodulin was specific to only tendons and ligaments. Scleraxis, which has also been referenced as a widely accepted tendon-specific gene, was not expressed on the rat microarray used in that study, and neither tenomodulin nor scleraxis was present on the equine microarray used in our study.
It is important to note that the microarray analysis performed on samples in this experiment included 3 horses for BMDMSCs and 3 horses for SDFTNs. The lack of upregulation of genes noted in the microarray, compared with results of the RT-qPCR analysis, could be attributable to this smaller sample size of SDFTNs, compared with that for RT-qPCR analysis performed on 6 horses. Also, a more stringent P value (P < 0.005) was set for the microarray data because of the large number of genes being analyzed statistically. Importantly, however, many genes of relevance to tendon healing were found to be significantly upregulated on the microarray. For SDFTNs, COMP, IGF-1, fibroblast growth factor, and tumor necrosis factor were all upregulated. For BMDMSCs, biglycan and fibronectin were upregulated and are both important proteins in extracellular matrix biology. Additionally, upregulation of other genes observed on the microarray indicated creation of an environment trophic to cell growth and health. Reverse transcriptase–quantitative PCR analysis is a more robust measure of gene expression and may be able to detect more subtle changes in gene expression than does microarray analysis, but it is limited in the scale of transcription analysis.
Gene expression observed via RT-qPCR assay revealed significant upregulation of COMP in SDFTN-BMP12 groups, compared with that for untreated, GFP-treated, and BMP2-treated SDFTN groups on day 6, supporting a tenocyte phenotype. Cartilage oligomeric matrix protein has been identified as an essential mediator in tendon growth, playing a role in organization of the collagen matrix.8,54,55 The present study evaluated only adult horses (> 10 years old), and BMP12-treated SDFTNs had a significant increase in COMP in these cells on day 6, suggesting that COMP may serve not only as an important mediator in tendon growth but also in tendon healing. The increased expression of COMP at the site of injury could potentially allow for healing tendon to better respond to and resist load. An increase in COMP gene expression was not observed in BMDMSCs. This could be the result of sample size or suggests that BMDMSC-BMP12 cells, if used as a BMP12 gene delivery vehicle, could act by releasing BMP12 in a paracrine manner on resident tendon cells instead of accelerating the differentiation of BMDMSCs into tendon cells. Our study suggests that BMP12 may induce BMDMSCs to produce type 1 collagen and that this may occur in vivo. Robust gene expression of BMP12 and BMP2 was detected for BMDMSCs and SDFTNs, compared with results for groups that were not transduced with the gene on day 3 and 6. The significant production of the BMP-12 gene produced by BMDMSC-BMP12 cells supports the concept that BMDMSCs can serve as a delivery vehicle of the protein to sites of tendon injury.
Transduction with adenovirus results in a cellular inflammatory event.25,40 Bone morphogenetic protein 2 overrides this inflammatory event, sustaining cell health, despite use of adenovirus as a delivery vehicle.24 Bone morphogenetic protein 12, as observed in the present study, was also able to create a supportive environment for cell growth despite the presence of the adenovirus. Further, cytomorphologic evaluation revealed that BMP12 created a more supportive environment than did either GFP or BMP2.
Early osteogenic differentiation was observed in BMP2-expressing cells in the form of mineralization, as well as gene expression for alkaline phosphatase and type III collagen.25,34 Osteogenic differentiation was not observed at day 3 or day 6 for BMP12-expressing cells. Previous research in rats indicates that implants containing BMP12 at concentrations 20 times as high as necessary for bone induction with an osteogenic BMP did not induce bone formation; rather, the implants resulted in development of tendon with neonatal characteristics.27
The present study has set a foundation for future investigation into the effects of BMP12 and early tendon healing in response to tendon injury in horses. Further studies are necessary to confirm lack of mineralization in vivo during short- and long-term use. Limitations of the present study include the duration (6 days) and use of in vitro monolayer cells. Duration of the study was selected on the basis of published experiments with BMP2, in which cell expression changes were identifiable within days.25 Our goal was to determine the early influences on these cells as proof of feasibility for use in vivo. Because of the short-term nature of the study, it remains unknown whether the early cellular influence of BMP12 is sustained. In addition, the lack of osteogenic potential of BMP12 requires further investigation in vivo. Finally, BMDMSCs and SDFTNs were transduced with AdBMP12 in this study. Future treatment, however, would rely on the SDFTNs at the site of injury responding to BMP12 protein produced by the gene delivery vehicle; additional studies will be needed to determine whether this gene delivery system will augment tendon healing in vivo.
Acknowledgments
Supported by The Comparative Orthopedic Molecular Medicine and Applied Research Laboratory, Department of Veterinary Clinical Sciences, The Ohio State University. Drs. Santangelo and Bertone were supported by NIH grant numbers F32AR053805 and KO-8AR4920101A2, respectively, from the National Institute of Arthritis and Muscoloskeletal and Skin Diseases. Dr. Santangelo is presently funded by a GlaxoSmithKline & ACVP/STP coalition Graduate Residency Fellowship.
The authors thank D. Spencer Smith, Marc Hardman, and Amy Stark for technical and statistical assistance.
Abbreviations
- AdBMP
Adenovirus encoding bone morphogenetic protein
- AdGFP
Adenovirus encoding green fluorescence protein
- BMDMSC
Bone marrow–derived mesenchymal stem cell
- BMP
Bone morphogenetic protein
- COMP
Cartilage oligomeric matrix protein
- GFP
Green fluorescence protein
- MOI
Multiplicity of infection
- MSC
Mesenchymal stem cell
- rh
Recombinant human
- RT-qPCR
Reverse transcriptase–quantitative PCR
- SDFT
Superficial digital flexor tendon
- SDFTN
Superficial digital flexor tenocyte
Appendix
Primers used for RT-qPCR analysis in a study of effects of BMP12 and BMP2 on equine (eq) SDFTNs and BMDMSCs in vitro.
| Primer | Forward sequence | Reverse sequence |
|---|---|---|
| eqAlkaline phosphatase | 5′-TGGAGCTTCAGAAGCTCAACAC-3′ | 5′-CCATCCCATCTCCCAGGAA-3′ |
| (rh)BMP12 | 5′-CATGTCCTCGTATTGCTTGTAGACA-3′ | 5′-CAGCCCCATCAGCATCCT-3′ |
| (rh)BMP2 | 5′-AAAACGTCAAGCCAAACACAAA-3′ | 5′-GTCACTGAAGTCCACGTACAAAGG-3′ |
| eqβ-Actin | 5′-GGGCATCCTGACCCTCAAG-3′ | 5′-TCCATGTCGTCCCAGTTGGT-3′ |
| eqCollagen type I | 5′-CTGTGATTTCTCTACTGGCGAAA-3′ | 5′-CCAGTTCTTGGCTGGGATGT-3′ |
| eqCollagen type III | 5′-CAGACGCATATTTGGCATGGT-3′ | 5′-CAGACGCATATTTGGCATGGT-3′ |
| eqCOMP | 5′-GAGATCGTCCAAACAATGAACAG-3′ | 5′-GCCATTGAAGGCCGTGTAA-3′ |
Footnotes
Jamshidi Needle, Terumo Corp, Tokyo, Japan.
Abraxis Pharmaceutical Products, Schaumburg, Ill.
VWR, Bridgeport, NJ.
Gibco Life Technologies, Grand Island, NY.
Atlanta Biologicals, Lawrenceville, Ga.
GE Healthcare, Piscataway, NJ.
Sigma Aldrich, St Louis, Mo.
Lonza Walkersville Inc, Walkersville, Md.
Gibco Life Technologies, Gaithersburg, Md.
Comparative Orthopedic Laboratory, College of Veterinary Medicine, The Ohio State University, Columbus, Ohio.
Genetics Institute Inc, Cambridge, Mass.
Adeno-XTM Rapid Titer Kit, Clontech Laboratories, Mountain View, Calif.
Olympus CK40 inverted fluorescent microscope with Olympus DP12 digital camera, Olympus America Inc, Melville, NY.
FACSCalibur Flow Cytometer, Becton-Dickinson, Franklin Lakes, NJ.
Procedure No. 85, Sigma-Aldrich, St Louis, Mo.
Bio-Rad Laboratories Inc, Hercules, Calif.
ImPRESS Reagent Kit, Vector Laboratories, Burlingame, Calif.
ABI Prism with Primer Express Software, version 2.0.0, Applied Biosystems, Foster City, Calif.
Deoxyribonuclease I, Amplification Grade, Invitrogen, Carlsbad, Calif.
Operon Biotechnologies Inc, Huntsville, Ala.
Custom Equine Gene Chip, Affymetrix Inc, Santa Clara, Calif.
Microarray-Genetics Core Lab, Dorothy M. Davis Heart and Lung Institute, The Ohio State University Medical Center, Columbus, Ohio.
Human BMP-2 Quantitative ELISA Kit, R&D Systems, Minneapolis, Minn.
Prism, version 4.0a, GraphPad Software Inc, San Diego, Calif.
Harvard University, Cambridge, Mass.
SAS Institute Inc, Cary, NC.
This manuscript represents a portion of a thesis submitted by Dr. S. J. Murray to the Department of Veterinary Clinical Sciences, The Ohio State University, as partial fulfillment of the requirements for a Master of Science degree.
Presented in abstract form at the 18th Annual Scientific Meeting of the American College of Veterinary Surgeons, San Diego, October 2008.
References
- 1.Williams R, Harkins L, Hammond C, et al. Racehorse injuries, clinical problems and fatalities recorded on British racecourses from flat racing and national hunt racing during 1996, 1997 and 1998. Equine Vet J. 2001;33:478–486. doi: 10.2746/042516401776254808. [DOI] [PubMed] [Google Scholar]
- 2.Dyson SJ. Medical management of superficial digital flexor tendonitis: a comparative study of 219 horses (1992–2000) Equine Vet J. 2004;36:415–419. doi: 10.2746/0425164044868422. [DOI] [PubMed] [Google Scholar]
- 3.Ross MW. Superficial digital flexor tendonitis. In: Ross MW, Dyson SJ, editors. Diagnosis and management of lameness in the horse. Philadelphia: Saunders; 2003. pp. 628–643. [Google Scholar]
- 4.Dowling BA, Dart AJ, Hodgson DR, et al. Superficial digital flexor tendonitis in the horse. Equine Vet J. 2000;32:369–378. doi: 10.2746/042516400777591138. [DOI] [PubMed] [Google Scholar]
- 5.Gillis C. Rehabilitation of tendon and ligament injuries. 43rd Annu Meet Am Assoc Equine Pract. 1997;43:306–309. [Google Scholar]
- 6.Marr CM, Love S, Boyd JS, et al. Factors affecting the clinical outcome of injuries to the superficial digital flexor tendon in National Hunt and point-to-point racehorses. Vet Rec. 1993;132:476–479. doi: 10.1136/vr.132.19.476. [DOI] [PubMed] [Google Scholar]
- 7.Spurlock SL. Treatment of acute superficial flexor tendon injuries in performance horses with high molecular weight sodium hyaluronate. J Equine Vet Sci. 1999;19:338–344. [Google Scholar]
- 8.Genovese RL. Sonographic response to intralesional therapy with beta-aminopropionitrile fumurate for clinical tendon injuries in horses. 38th Annu Meet Am Assoc Equine Pract. 1992;38:265–272. [Google Scholar]
- 9.Reef VB, Genovese RL, Davis WM. Initial long-term results of horses with superficial digital flexor tendonitis treated with intralesional beta-aminopropionitrile fumurate. 43rd Annu Meet Am Assoc Equine Pract. 1997;43:301–305. [Google Scholar]
- 10.Haupt JL, Donnelly BP, Nixon AJ. Effects of platelet-derived growth factor-BB on the metabolic function and morphologic features of equine tendon in explant culture. Am J Vet Res. 2006;67:1595–1600. doi: 10.2460/ajvr.67.9.1595. [DOI] [PubMed] [Google Scholar]
- 11.Dahlgren LA, van der Meulen MC, Bertram JE, et al. Insulin-like growth factor-l improves cellular and molecular aspects of healing in a collagenase-induced modle of flexor tendonitis. J Orthop Res. 2002;20:910–919. doi: 10.1016/S0736-0266(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 12.Schnabel LV, Mohammed HO, Miller BJ, et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007;25:230–240. doi: 10.1002/jor.20278. [DOI] [PubMed] [Google Scholar]
- 13.Henninger RW, Bramlage LR, Bailey M, et al. Effects of tendon splitting on experimentally experimentally-induced acute equine tendinitis. Vet Comp Orthop Traumatol. 1992;5:1–9. [Google Scholar]
- 14.Schnabel LV, Lynch ME, van der Meulen MC, et al. Mesenchymal stem cells and insulin-like growth factor–l gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J Orthop Res. 2009;10:1392–1398. doi: 10.1002/jor.20887. [DOI] [PubMed] [Google Scholar]
- 15.Nixon AJ, Sams AE, Ducharme NG. Endoscopically assisted annular ligament release in horses. Vet Surg. 1993;22:501–507. doi: 10.1111/j.1532-950x.1993.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 16.Hogan PM, Bramlage LR. Transection of the accessory ligament of the superficial digital flexor tendon for treatment of tendinits: long term results in 61 standardbred racehorses (1985–1992) Equine Vet J. 1995;27:221–226. doi: 10.1111/j.2042-3306.1995.tb03066.x. [DOI] [PubMed] [Google Scholar]
- 17.Guest DJ, Smith MR, Allen RW. Monitoring the fate of autologous and allogenic mesenchymal progenitor cells injected into the superficial digital flexor tendon of horses: preliminary study. Equine Vet J. 2008;40:178–181. doi: 10.2746/042516408X276942. [DOI] [PubMed] [Google Scholar]
- 18.Fortier LA. Stem cells: classifications, controversies, and clinical applications. Vet Surg. 2005;34:415–423. doi: 10.1111/j.1532-950X.2005.00063.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith RKW. Mesenchymal stem cell therapy for equine tendinopathy. Disabil Rehabil. 2008;30:1752–1758. doi: 10.1080/09638280701788241. [DOI] [PubMed] [Google Scholar]
- 20.Young R, Butler D, Weber W, et al. Use of mesenchymal stem cells in a collagen matrix for achilles tendon repair. J Orthop Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 21.Awad H, Butler D, Boivin G, et al. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5:267–277. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 22.Chong AK, Ang AD, Goh JC, et al. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit Achilles tendon model. J Bone Joint Surg Am. 2007;89:74–81. doi: 10.2106/JBJS.E.01396. [DOI] [PubMed] [Google Scholar]
- 23.Zachos TA, Bertone AL. Growth factors and their potential therapeutic applications for healing of musculoskeletal and other connective tissues. Am J Vet Res. 2005;66:727–738. doi: 10.2460/ajvr.2005.66.727. [DOI] [PubMed] [Google Scholar]
- 24.Fortier LA, Smith RK. Regenerative medicine for tendinous and ligamentous injuries of sport horses. Vet Clin North Am Equine Pract. 2008;24:191–201. doi: 10.1016/j.cveq.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Zachos TA, Shields KM, Bertone AL. Gene-mediated osteogenic differentiation of stem cells by bone morphogenetic proteins-2 and -6. J Orthop Res. 2006;24:1279–1291. doi: 10.1002/jor.20068. [DOI] [PubMed] [Google Scholar]
- 26.Chang H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Ishihara A, Shields KM, Litsky AS, et al. Osteogenic gene regulation and relative acceleration of healing by adenoviral-mediated transfer of human BMP-2 or -6 in equine osteotomy and ostectomy models. J Orthop Res. 2008;26:746–771. doi: 10.1002/jor.20585. [DOI] [PubMed] [Google Scholar]
- 28.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Chen Z, Piao Y. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng. 2005;100:418–422. doi: 10.1263/jbb.100.418. [DOI] [PubMed] [Google Scholar]
- 30.Lou J, Tu Y, Ludwig FJ, et al. Effect of bone morphogenetic protein-12 gene transfer on mesenchymal progenitor cells. Clin Orthop Relat Res. 1999;369:333–339. doi: 10.1097/00003086-199912000-00035. [DOI] [PubMed] [Google Scholar]
- 31.Seeherman HJ, Archambault JM, Rodeo SA, et al. rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J Bone Joint Surg Am. 2008;90:2206–2219. doi: 10.2106/JBJS.G.00742. [DOI] [PubMed] [Google Scholar]
- 32.Fu SC, Wong YP, Chan BP. The roles of bone-morphogenetic protein (BMP) 12 in stimulating the proliferation and matrix production of human patellar tendon fibroblasts. Life Sci. 2003;72:2965–2974. doi: 10.1016/s0024-3205(03)00169-3. [DOI] [PubMed] [Google Scholar]
- 33.Majewski M, Betz O, Ochsner PE, et al. Ex vivo adenoviral transfer of bone morphogenetic protein 12 (BMP-12) cDNA improves Achilles tendon healing in a rat model. Gene Ther. 2008;15:1139–1146. doi: 10.1038/gt.2008.48. [DOI] [PubMed] [Google Scholar]
- 34.Lou J, Tu Y, Burns M, et al. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19:1199–1202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 35.Inada M, Katagiri T, Akiyama S. Bone morphogenetic protein-12 and -13 inhibit terminal differentiation of myoblasts, but do not induce their differentiation into osteoblasts. Biochem Biophys Res Commun. 1996;222:317–322. doi: 10.1006/bbrc.1996.0742. [DOI] [PubMed] [Google Scholar]
- 36.Wolfman NM, Hattersly G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-β gene family. J Clin Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikic B, Bierwert L, Tsou D. Achilles tendon characterization in GDF-7 deficient mice. J Orthop Res. 2006;24:831–841. doi: 10.1002/jor.20092. [DOI] [PubMed] [Google Scholar]
- 38.Wikesjö UM, Sorensen RG, Kinoshita A, et al. Periodontal repair in dogs: effect of recombinant human bone morphogenetic protein-12 (rhBMP-12) on regeneration of alveolar bone and periodontal attachment. J Clin Periodontol. 2004;31:662–670. doi: 10.1111/j.1600-051X.2004.00541.x. [DOI] [PubMed] [Google Scholar]
- 39.Hayaskhi K, Frank JD, Dubinsky C, et al. Histologic changes in ruptured canine cranial cruciate ligament. Vet Surg. 2003;32:269–277. doi: 10.1053/jvet.2003.50023. [DOI] [PubMed] [Google Scholar]
- 40.Ishihara A, Zachos TA, Bartlett JS, et al. Evaluation of permissiveness and cytotoxic effects in equine chondrocytes, synovial cells, and stem cells in response to infection with adenovirus 5 vectors for gene delivery. Am J Vet Res. 2006;67:1145–1155. doi: 10.2460/ajvr.67.7.1145. [DOI] [PubMed] [Google Scholar]
- 41.Vidal MA, Kilroy GE, Johnson JR, et al. Cell growth characteristics and differentiation frequency of adherent equine bone marrow-derived mesenchymal stromal cells: adipogenic and osteogenesis capacity. Vet Surg. 2006;35:601–610. doi: 10.1111/j.1532-950X.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 42.Smith RKW, Korda M, Blunn GW, et al. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet J. 2003;35:99–102. doi: 10.2746/042516403775467388. [DOI] [PubMed] [Google Scholar]
- 43.Shaer BD, Orsini JA. Biopsy techniques. In: Orsini JA, Divers TJ, editors. Manual of equine emergencies: treatment and procedures. 3. Philadelphia: Saunders; 2008. pp. 27–28. [Google Scholar]
- 44.Bonewald LF, Harris SE, Rosser J, et al. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int. 2003;72:537–547. doi: 10.1007/s00223-002-1057-y. [DOI] [PubMed] [Google Scholar]
- 45.Santangelo KS, Johnson AL, Ruppert AS, et al. Effects of hyaluronan treatment on lipopolysaccharide-challenged fibroblast-like synovial cells. Arthritis Res Ther. 2007;9:R1–R11. doi: 10.1186/ar2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 47.Gu W, Bertone AL. Generation and performance of an equine-specific large-scale gene expression microarray. Am J Vet Res. 2004;65:1664–1673. doi: 10.2460/ajvr.2004.65.1664. [DOI] [PubMed] [Google Scholar]
- 48.Marcus R, Peritz E, Gabriel K. On closed testing procedures with special reference to ordered analysis of variance. Biometrika. 1976;63:655–660. [Google Scholar]
- 49.Richardson LE, Dudhia J, Clegg PD, et al. Stem cells in veterinary medicine—attempts at regenerating equine tendon after injury. Trends Biotechnol. 2007;25:409–416. doi: 10.1016/j.tibtech.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact. 2005;5:22–34. [PubMed] [Google Scholar]
- 51.Yoon JH, Brooks R, Hwan Kim Y, et al. Proteoglycans in chicken gastrocnemius tendons change with exercise. Arch Biochem Biophys. 2003;412:279–286. doi: 10.1016/s0003-9861(03)00064-x. [DOI] [PubMed] [Google Scholar]
- 52.Jelinsky SA, Lake SP, Archambault JM, et al. Gene expression in rat supraspinatus tendon recovers from overuse with rest. Clin Orthop Relat Res. 2008;466:1612–1617. doi: 10.1007/s11999-008-0270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Archambault JM, Jelinksy SA, Lake SP, et al. Rat supraspinatus tendon expresses cartilage markers with overuse. J Orthop Res. 2007;25:617–624. doi: 10.1002/jor.20347. [DOI] [PubMed] [Google Scholar]
- 54.Smith RKW, Birch HL, Goodman S, et al. The influence of ageing and exercise on tendon growth and degeneration—hypothesis for the initiation and prevention of strain-induced tendinopathies. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1039–1050. doi: 10.1016/s1095-6433(02)00148-4. [DOI] [PubMed] [Google Scholar]
- 55.Smith RKW, Gerard M, Dowling B, et al. Correlation of cartilage oligomeric protein (COMP) levels in equine tendon with mechanical properties: a proposed role for COMP in determining function-specific mechanical characteristics of locomotor tendons. Equine Vet J Suppl. 2002;34:241–244. doi: 10.1111/j.2042-3306.2002.tb05426.x. [DOI] [PubMed] [Google Scholar]