Abstract
Desmoplakin (DP) anchors the intermediate filament cytoskeleton to the desmosomal cadherins and thereby confers structural stability to tissues. In this study, we present a patient with extensive mucocutaneous blisters, epidermolytic palmoplantar keratoderma, nail dystrophy, enamel dysplasia, and sparse woolly hair. The patient died at the age of 14 years from undiagnosed cardiomyopathy. The skin showed hyperplasia and acantholysis in the mid- and lower epidermal layers, whereas the heart showed extensive fibrosis and fibrofatty replacement in both ventricles. Immunofluorescence microscopy showed a reduction in the C-terminal domain of DP in the skin and oral mucosa. Sequencing of the DP gene showed undescribed mutations in the maternal and paternal alleles. Both mutations affected exon 24 encoding the C-terminal domain. The paternal mutation, c.6310delA, leads to a premature stop codon. The maternal mutation, c.7964 C to A, results in a substitution of an aspartic acid for a conserved alanine residue at amino acid 2655 (A2655D). Structural modeling indicated that this mutation changes the electrostatic potential of the mutated region of DP, possibly altering functions that depend on intermolecular interactions. To conclude, we describe a combination of DP mutation phenotypes affecting the skin, heart, hair, and teeth. This patient case emphasizes the importance of heart examination of patients with desmosomal genodermatoses.
INTRODUCTION
Desmosomes are specialized intercellular adhesive junctions found in simple and stratified epithelium, and are vital for the integrity and stability of these tissues (for review, see Getsios et al., 2004). Although desmosomes are traditionally considered as epithelial junctions, they are also found in the intercalated discs of cardiomyocytes in specialized hybrid structures of desmosomes and adherens junctions, called area composita. The cardiac desmosome has at least two proposed roles: supporting structural stability and regulating transcription of genes involved in adipogenesis and apoptosis (for review, see Awad et al., 2008). In addition, desmosomes may participate in maintaining proper electrical conductivity through regulation of gap junctions (Oxford et al., 2007; Saffitz et al., 2007). The desmosomal complex of proteins includes the armadillo repeat-containing proteins plakoglobin (PG) and plakophilins; the desmosomal cadherins, desmocollins 1–3 and, desmogleins (Dsg) 1–4; and the plakins, desmoplakin (DP), envoplakin, periplakin, plectin, bullous pemphigoid antigen 1, and microtubule actin cross-linking factor. DP is a major scaffolding component of the desmosomal plaque and serves to anchor the cytoskeleton to the plasma membrane (for review, see Jefferson et al., 2004). Similar to other plakin family proteins, DP is composed of two globular domains flanked by a central coiled-coil rod domain that facilitates homodimerization (Green et al., 1990). The N-terminal region binds to plakophilin 1 and PG and is required for desmosome assembly, whereas the C-terminal region, which contains the plakin repeat domains, facilitates interactions with intermediate filaments (IFs) (Stappenbeck and Green, 1992; Stappenbeck et al., 1993; Kouklis et al., 1994; Cowin and Burke, 1996; Kowalczyk et al., 1997; Smith and Fuchs, 1998). DP exists in two isoforms, DPI (predicted 310 kDa, reported 240–285 kDa) and DPII (predicted 238 kDa, reported 210–225 kDa), generated by alternative splicing (Green et al., 1988; Virata et al., 1992), with DPII having a truncated rod domain. Both DP isoforms are expressed in all desmosome-containing tissues with the exception of the heart, in which only DPI protein is expressed; although DPII mRNA has been detected by reverse transcriptase-PCR (Uzumcu et al., 2006).
Heritable mutations in the DP gene have recently been reported to cause cardiomyopathies and keratodermas (for review, see Lai-Cheong et al., 2007). Dominant mutations of DP can result in arrhythmogenic right ventricular dilation/cardiomyopathy (ARVC/D) without skin involvement (Rampazzo et al., 2002; Bauce et al., 2005; Norman et al., 2005) or striate palmoplantar keratoderma (PPK) without heart involvement (Armstrong et al., 1999; Whittock et al., 1999). ARVC/D is a condition defined by extensive myocardial fibrosis in the right ventricle in which arrhythmias arise. PPK patients develop extensive hyperplasia and hyperkeratosis in the palms of the hands and soles of the feet.
In exons coding for the DP C-terminal tail, a total of seven different homozygous or compound heterozygous recessive mutations have been identified (Table 1). The mutation 7901delG in the DP gene causes a frameshift and subsequent truncation of the DP protein with partial loss of the keratin-binding domain (Norgett et al., 2000). The phenotype includes dilated left ventricular cardiomyopathy, woolly hair, and striate PPK, but no skin fragility. The homozygous mutation p.G2375R in exon 24 caused ARVC/D, woolly hair, dry skin, and acantholytic skin lesions in palms, soles, and knees (Alcalai et al., 2003). The mutation p.R2366C, together with a nonsense mutation in the N-terminal W domain, resulted in acantholytic disease, combined with woolly hair and PPK, but no signs of cardiac involvement (Whittock et al., 2002). Compound heterozygous recessive mutations c.6079C>T and c.6370delTT together led to neonatally lethal acantholytic disease. Thus, the C-terminal tail is essential in the functioning of DP, but the genotype–phenotype correlations with DP mutations are poorly understood.
Table 1.
Desmoplakin gene mutations affecting the C-terminal domain of desmoplakin
| Clinical symptoms | Inheritance | Exon(s) | Sequence changes | Amino acid change | Reference |
|---|---|---|---|---|---|
| Woolly hair/PPK/left ventricular cardiomyopathy | Recessive | 24/24 | 7901delG 7901delG | Frameshift and PTC | Norgett et al. (2000) |
| Woolly hair, skin fragility | Recessive | 15/24 | 1990C>T 7096C>T | Q664X/R2366C | Whittock et al. (2002) |
| ARVC/D, woolly hair, dry skin, acantholysis in palms, soles, and knees | Recessive | 24 | 7402G<C | G2375R | Alcalai et al. (2003) |
| ARVC/D | Dominant | Intron 3 splicing | Skipping of exon 4 | PTC | Bauce et al. (2005) |
| Lethal acantholytic EB | Recessive | 24/24 | 6079C>T 6370delTT | R1934X/PTC | Jonkman et al. (2005) |
| ARVC/D | Dominant | 24 | 8501G>A | R2834H | Yang et al. (2006) |
| ARVC/D | Dominant | 24 | 7516G>A | R2339Q | Yu et al. (2008) |
| Woolly hair/PPK/biventricular cardiomyopathy | Recessive | 18/23 | 2516del4/ 3971del4 | Frameshift and PTC | Tanaka et al. (2009) |
| Woolly hair/PPK/mucocutaneous blistering/biventricular cardiomyopathy | Recessive | 24/24 | 7964C>A 6310delA | A2655D/PTC | This study |
Abbreviations: ARVC/D, arrhythmogenic right ventricular dilation/cardiomyopathy; PPK, palmoplantar keratoderma; PTC, premature termination codon.
The C-terminal domain of DP interacts with the IF cytoskeleton, and the crystal structure of subdomains B and C has been determined (Choi et al., 2002). The C-terminal domain is composed of three homologous subdomains, designated A, B, and C, flanked by intervening sequences of varying lengths (Green et al., 1990; Choi et al., 2002). It has been proposed that these distinct subdomains and flanking sequences mediate binding to different IF proteins (Stappenbeck and Green, 1992; Stappenbeck et al., 1993; Kouklis et al., 1994; Kowalczyk et al., 1997; Meng et al., 1997; Fontao et al., 2003). In the heart, DP interacts with the IF protein desmin at the intercalated disc (Lapouge et al., 2006). Thus, mutations affecting the region coding for the C-terminal domain could lead to a disturbed interaction between the desmosomal plaque and the desmin IF cytoskeleton, thereby disrupting the structural integrity of the intercalated disc. Mutations in the regions encoding the DP C-terminal tail show a genotype–phenotype correlation in terms of cardiac involvement; however, the clinical consequences of mutations affecting other regions of the DP gene have been more unpredictable.
In this study, we report a compound heterozygous patient with a missense mutation and a single-nucleotide deletion in the DP gene affecting both DPI and DPII isoforms. The resulting clinical manifestations in diverse tissues highlight the significance of the C-terminal tail of this desmosomal plaque protein and its role in cell adhesion and survival. These previously unreported DP mutations also add to a genotype–phenotype correlation in this group of inherited ectodermal dysplasia disorders.
RESULTS
Clinical features
Our female patient was born of Finnish descent in 1994 to nonconsanguineous parents. The parents and two siblings showed no skin or cardiac symptoms. The pregnancy and delivery were reported as normal, but skin fragility became apparent on postnatal day 1. Superficial blisters and skin erosions, 0.5–1.5 cm in diameter, appeared within 12 hours after birth on the patient's buttocks, back, abdomen, thighs, legs, and ears. Mucosal blistering in the mouth and esophagus was noted a week after birth and persisted for a year. During her first 2–3 years, she had persistent blistering in various skin areas, including the scalp and face. The blisters healed without scarring or milia formation. At the age of 6–8 years, the blistering tendency slowly diminished and blisters only developed after severe mechanical stress. Her growth was delayed compared with a standard deviation of −3 during the first 2 years, but by puberty she had reached a height with a standard deviation of only −0.5. By the age of 14 years, she had reached a height of 164 cm, which is the average female height in Finland.
The patient showed PPK with localized hyperkeratotic areas (Figure 1a) and minimal palmar involvement. Toenails detached easily under mechanical stress and some nails developed secondary dystrophy with thickening (Figure 1b). The plantar hyperkeratosis showed a blistering tendency, especially in areas exposed to mechanical stress (Figure 1b). The patient suffered from focal hyperhidrosis with constant profuse sweating in her hands and feet. The patient's scalp hair was sparse, especially during the first years of life, and she had a high frontal hairline. Her hair was blond and woolly and rarely grew longer than 10–15 cm (Figure 1c). Body hair was also very sparse and eyelashes were extensively curved. Teeth abnormalities were noted once the deciduous teeth erupted. They were suspected to have opacity, but mineralization was considered normal. Enamel defects were detected in several teeth in both the deciduous and permanent dentition (Figure 1d). The number and shape of her teeth, however, appeared normal. The patient had no outward signs or symptoms of cardiac involvement, despite being an active dancer and soccer player. At the age of 14 years and 3 months, she died in her sleep. After the diagnosis of cardiomyopathy, a cardiology consultation was offered to the family. Only the father agreed to an examination, which did not reveal abnormalities.
Figure 1. Clinical manifestations of the patient with novel mutations in the desmoplakin (DP) gene.
(a) Palmoplantar keratoderma, hyperkeratosis, and erosion on a site of a blister; (b) dystrophic second and fifth toenails; (c) sparse and woolly scalp hair; (d) and enamel dysplasia and horizontal ridging of the teeth.
The heart shows macroscopic abnormalities
The autopsy showed widespread left and right ventricular cardiomyopathy, but no additional organ abnormalities. In a normal female of 15–20 years, the average heart weighs 196 g. In this case, the patient heart was 310 g, over 150% of the weight of the control. The pericardial sack, valves, endocardium, and coronary arteries appeared normal. The left ventricle was dilated, with a wall thickness of 12–13 mm. The posterior and lateral walls of the left ventricle were firm and nodular, and the cut surface showed spotty hemorrhages and whitish areas. The thickness of the right ventricular wall was 4–5 mm, and the cut surface was reddish brown and smooth.
Skin lesions show desmosomal disruption
Light microscopy of periodic acid Schiff-stained lesional skin showed extensive suprabasal acantholysis with widening of the intercellular space (spongiosis) in the spinous layer. Some cell–cell separation between basal keratinocytes (row of tombstones) was also observed (Figure 2a). There was evidence of hyperkeratosis (Figure 2a), but terminal differentiation appeared normal. Electron microscopy of the esophagus showed sparse desmosomes with an abnormally thin inner plaque. The diameter of the affected desmosomes was 110.6 ± 44.7 nm (n = 11), essentially considered normal for desmosomes in the spinous cell layer (Figure 2b and c). However, IFs were rarely observed interacting with the desmosomal plaques. Furthermore, the plasma membrane appeared undulated and stretched, resulting in the rupture of the inter-desmosome plasma membrane, leaving intact desmosomes despite extensive acantholysis (Figure 2b and c).
Figure 2. Morphological analysis shows keratinocyte disadhesion.
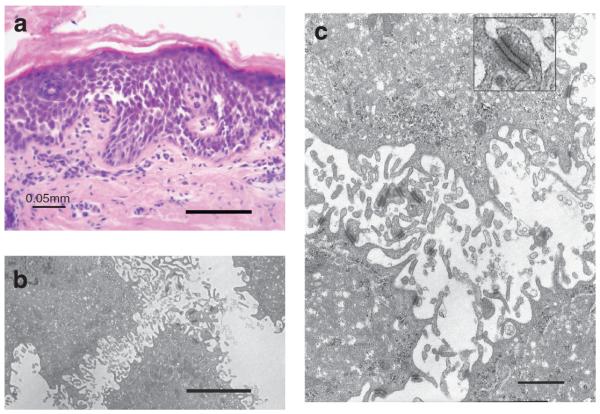
(a) Periodic acid-Schiff staining of the affected skin and (b and c) transmission electron microscopy of the affected oral mucosa. Orthokeratotic hyperkeratosis (arrows), acanthosis in the mid- and lower epidermal layers, and widened intercellular spaces in the upper epidermal layers. The remaining desmosomes appear intact. Inset, in panel c, intact desmosome. Bars=100 μm (a), 2 μm (b), and 0.5 μm (c).
Immunohistochemistry of skin and oral mucosa suggests aberrant DP expression
Paraffin-embedded skin and cryosections of oral mucosa were available for analyses. The expression and localization of DP and other desmosomal proteins were examined by immunohistochemistry using a panel of antibodies (Abs) recognizing different epitopes (Figure 3a). A schematic presentation of the structure of DP and the sites recognized by the different Abs is shown in Figure 3a. DP mix was a commercially available mixture of three different monoclonal Abs with uncharacterized antigenic sites. Staining with DP mix showed an intense immunosignal at cell–cell borders in the epidermis of the patient and of healthy control persons (Figure 3b). In contrast, Ab 10F6 detecting the epitopes located in the C-terminal subdomain A showed a markedly reduced immunoreaction in the patient's epidermis (Figure 3b). Abs 2C8 and 2A5, specific for the C-terminal subdomain A and for parts of subdomains B and C, respectively, were used for studying the cryosections of oral mucosa (Figure 3b). The patient's sample showed a substantially decreased intensity compared with normal oral mucosa, and widened intercellular gaps as a sign of disrupted cell–cell adhesion (Figure 3b). Analysis of the patient's skin with Abs against desmosomal cadherin Dsg1 and the desmosomal plaque protein PG showed staining patterns and intensity similar to those seen in control skin (Figure 3c). A slight decrease in the adherens junction protein β-catenin was noted in the affected skin, probably because of formalin fixation and paraffin embedding. Oral mucosa, examined in frozen sections, showed a normal staining intensity for β-catenin (Figure 3c).
Figure 3. Immunohistochemical staining of the epidermis and oral epithelium using a panel of desmoplakin (DP)-specific antibodies (Abs).
(a) Schematic diagram of DP depicts regions of antibody recognition. (b) All antibodies showed cell surface staining throughout the normal adult epidermis (upper panel). In patient's skin, the multiclonal epitope antibody (DP mix) shows significant but disrupted staining of DP in the skin, whereas the C-terminal antibody 10F6 does not detect appreciable levels of DP. Two different antibodies (2C8 and 2A8) detecting parts of the C-terminal domain of DP show clear reduction in patient's oral mucosa compared with normal oral epithelium. Typical cell-cell border staining of desmoglein 1 (Dsg1) and plakoglobin (PG) (c) is observed in both control and patient's skin. Staining for β-catenin in formalin-fixed, paraffin-embedded patient's skin is reduced when compared with that of control epidermis (possibly because of formalin fixation). However, β-catenin expression does not seem to be altered in patient's oral mucosa when compared with that of the control section. Bar=50μm.
Heart shows severe myocardial abnormalities
Masson's trichrome staining of paraffin-embedded heart tissue showed abnormalities in both the right (Figure 4a) and left (Figure 4b) ventricular walls. Fibrosis could be seen in all parts of the myocardium, but in focal areas of both ventricles, the fibrotic tissue had largely replaced myocytes. Fatty replacement of myocytes could be detected focally (Figure 4c). A higher magnification showed shrunken myocytes, which had cytoplasmic vacuoles and pycnotic nuclei, suggesting ongoing apoptosis (Figure 4d). Very few inflammatory cells were present. Immunostaining for connexin 43 showed a clear reduction and even disappearance of protein in both ventricles in areas affected by cardiomyopathy (Figure 4f and g). A normal ordered pattern of connexin 43 gap junctions was seen in apparently normal cardiomyocytes (Figure 4e).
Figure 4. Widespread right and left ventricular cardiomyopathy.
Masson's trichrome staining of the (a) right and (b–d) left ventricles showing loss of cardiomyocytes (pink/red) with replacement by fibrous tissue (blue/gray/green). Degenerating cardiomyocytes embedded in extensive deposits of collagenous connective tissue and fat tissue (c), with dense “pycnotic” nuclei, suggestive of apoptosis (arrows in d), as compared with the slender and spindle-shaped nuclei in normal cells (arrowheads in d). Connexin 43 containing gap junctions in normal heart (e) and in the patient's right ventricle (f). (g) In the patient's left ventricle, the expression of connexin 43 was clearly reduced. Immunostaining of normal heart (h, top panels) and patient's myocardial sample (h, lower panels) for desmosomal proteins using antibodies to desmoplakin (DP) (DP mix), PG (11E4), and desmoglein 2 (Dsg2) (Rb5) shows disorganization of intercalated disks in the patient's sample, whereas the expression levels of desmosomal components do not show significant differences. Blue: DAPI-stained nuclei. Bars=0.5μm (a and b), 50 μm (c), 20 μm (d), 100 μm (e–g), and 25μm (h).
The expression and localization of desmosomal components in the patient's heart tissue were examined using Abs to DP, PG, and Dsg2 (Figure 4h), and the labeling pattern was compared with that of normal heart (Figure 4h, top panel). The results did not show significant differences in the expression levels of DP, PG, and Dsg2, whereas the intercalated disks in the patient's heart were arranged less regularly compared with that of the control (Figure 4h, lower panel).
Sequencing of the DP gene reveals two mutations
As the structural and protein data suggested a possible mutation in the DP gene, all exons of the DP gene were sequenced to identify potential pathogenic mutations. Two mutations were identified in exon 24 of the DP gene (GenBank accession no. m77830) (Figure 5a). The paternal mutation c.6310delA is a deletion of an A at nucleotide position 6310 in the DP gene, which causes a frameshift and creates a downstream STOP codon resulting in a prematurely truncated protein (amino acids 1–2,103 + 12 missense) (Figure 5a). The frameshift changed the wild-type amino acids from TVSVSEAIKKNL to QYLFQKPSRKI, ending at amino acid 2115, followed by a STOP codon. The maternal mutation, c.7964 C to A, results in a substitution of an aspartic acid for an alanine at amino acid 2655 in the DP protein (p.A2655D). A survey of 100 alleles in unrelated control individuals showed the presence of alanine exclusively at position 2655, indicating that this amino-acid change is not a common polymorphism. Genetic screening showed that both surviving siblings are carriers of the paternal mutation (Figure 5b).
Figure 5. Novel compound heterozygous mutations in desmoplakin (DP) gene.
Nucleotide sequencing of PCR-amplified genomic DNA of the patient, father, mother, and control corresponding to exon 24 of the DP gene (a). Arrows indicate the positions of identified mutations. Pedigree of the family with novel compound heterozygous mutations (b). Father and all three offspring harbor a c.6310delA mutation, whereas only the deceased proband inherited the maternal mutation, p.A2655D (b). A schematic structure of DP showing three functional domains: plakophilin/plakoglobin-binding domain (plakin), central rod domain, and intermediate filament-binding plakin repeat domain (PRD). Arrows indicate mutation sites. Predicted paternal and maternal DP proteins based on sequence analysis; the sites of mutations indicated in red (c). Western analysis of normal human skin and paternal skin using two different DP antibodies shows classical DP doublets and truncation of DP (d). Molecular analysis of the electrostatic potential (EP) of the surface of the wild-type (WT) and mutant fragments (e). The EP range for WT is (+)227.9/(−)247.7, whereas that of the mutant is (+)216.9/(−)269.4. Color-coded panel indicates differences in the EP and lipophilic potential (LP): red, highly positive EP; blue, highly negative EP; brown, highly hydrophobic; and blue, highly hydrophilic.
The structural domains of DP include the N-terminal desmosome-binding domain (amino acids: 1–1056), the central coiled-coil rod oligomerization domain (amino acids: 1057–1945), and the C-terminal IF-binding domain (amino acids: 1946–2871) (Figure 5c). The C-terminal domain is further divided into three functional subdomains, A, B, and C, each comprising 4.5 copies of the plakin repeat domain. The maternal amino-acid substitution mutation is localized to the beginning of the C subdomain (Figure 5c). The paternal mutation (c.6310delA) is located within the genomic DNA sequence encoding for the A subdomain. This results in a truncated protein product, in which 144 amino acids of the A subdomain are present and the remainder of the C-terminal tail are missing.
As mentioned above, the predicted size of the two alternative spliced DP isoforms are of 310 and 238 kDa, with a difference of 70 kDa. However, western blot analysis often detects a doublet of 250 and 210 kDa with a difference of only 40 kDa. In this study, we used the DP mix Ab and by western blot analysis detected a single 250 kDa band in normal human skin. In addition to the 250-kDa band, we detected several bands ranging in size from 180 to 205 kDa in the paternal skin (Figure 5d). We reprobed the same membrane with the Ab 10F6 raised against DP subdomain A and showed both 250 and 210 kDa bands in normal and paternal skin (Figure 5d). We did not detect any truncated products from paternal skin using this Ab, possibly because of low Ab sensitivity. It is not clear why the Ab DP mix only detected 250 kDa DPI and not 210 kDa DPII. Nevertheless, the presence of lower bands suggests that the paternal mutation leads to truncated protein products. As such, a product would not be detected if mediated by nonsense-mediated RNA decay.
DP mutations predict changes in the DP protein
In an attempt to understand the mechanisms contributing to the cell adhesion defects observed in the patient skin and heart, we observed more closely the mutated DP C-terminal domain harboring the mutation A2655D. Sequence analysis showed that the A2655D mutation site is adjacent to the fold region, which has been reported to be a binding site for IF proteins (Choi et al., 2002). A structural analysis of this region showed no major changes (data not shown), but an analysis of the physicochemical properties of the molecular surfaces showed that the A2655D substitution decreased the electrostatic potential (EP) of a region encompassing the mutation site (Figure 5e). In particular, the EP values (expressed in kcal mol−1) for the region encompassing the A2566 residue ranged from (+)227.9 to (−)247.7. In contrast, the correcorresponding EP values for the analogous region encompassing the D2655 site ranged from (+)216.9 to (−)269.4. Although these changes are relatively small, clinical features suggest that this slight alteration in EP may critically impair DP binding to the IFs contributing to the multiorgan phenotype. At the same time, the lipophilic potential of the mutant site of the protein was not altered.
DISCUSSION
In this study, we report a patient who inherited two mutations in the C-terminal domain of the DP gene. Together, these previously undescribed mutations contributed to a fatal syndrome, including all characteristics thus far described for DP mutations: woolly hair, blistering skin disease, PPK, and left and right ventricular cardiomyopathy, which led to the demise of the patient at the age of 14 years. In addition, she had dental anomalies. At the protein level, the expression of the C-terminal domain of DP was found decreased in the epidermis and oral mucosa, which is in accordance with the putative consequences of the mutations at the protein level. In the heart, the levels of PG and Dsg2 were similar to those in normal myocardium. Reduced levels of PG at intercalated disks have recently been reported to be specific for ARVC/D (Asimaki et al., 2009), suggesting that our PG Ab recognized epitopes that were not detected by the previous study or that the heart disease of our patient cannot be classified as ARVC/D. Interestingly, the Carvajal syndrome is characterized by PPK, woolly hair, and left ventricular cardiomyopathy, and is caused by mutations resulting in the ablation of subdomain C of DP (Carvajal-Huerta, 1998). Furthermore, similar to our patient, skin phenotypes are typically restricted to regions of mechanical stress. Furthermore, homozygous missense mutations in subdomain B are reported in cases of Carvajal-like syndrome (Alcalai et al., 2003) in which PPK, woolly hair, blisters, and ARVC/D are reported. Our reported paternal mutation results in the loss of much of the C-terminus, whereas the maternal mutation is postulated to reduce the C-terminal binding function. Taken together with the biventricular involvement observed in this patient, our data suggest similarities to these syndromes, and may account for the normal levels of PG observed in our patient.
The consequences of the combination of the two mutations may affect several known functions of desmosomes, including cell adhesion, differentiation, morphogenesis, and adhesion-independent signaling. Defects in cell–cell adhesion have been shown to perturb the integrity of the epidermis, as shown by acantholysis, and detachment of nails under mechanical stress. Correspondingly, myocardial injury could be explained by a mechanistic model in which the DP–desmin interaction is perturbed, resulting in decreased cardiomyocyte adhesion. A recent model suggests that myocardial fibrosis results from alterations in the desmosome-mediated Wnt/β-catenin signaling pathway and apoptosis (Garcia-Gras et al., 2006; Martin et al., 2009; for review, see Awad et al., 2008). The results of this study also suggest that DP has a role in keratinocyte differentiation, as shown by the development of palmoplantar hyperkeratosis, particularly in response to mechanical stress. Similarly, DP is suggested to participate in the morphogenesis of hair follicles, as evidenced by the presence of woolly hair and a distinct growth pattern on scalp hair. Enamel opacity was suspected in the deciduous teeth of the patient, and later, several permanent teeth showed yellowish spots. Enamel is produced by ameloblasts, specialized epithelial cells with desmosomes, having roles in the production, resorption, and degradation of the enamel matrix (Matthiessen and Rømert, 1980; Sasaki et al., 1984). We speculate that defective desmosomal adhesion may have either affected amelogenesis or predisposed the patient's teeth to damage from other toxic factors such as antibiotics.
Our patient had inherited two different mutations of the DP gene. The maternal mutation translates into a protein with an amino-acid substitution (A2655D) in subdomain C of the C-terminal domain of the DP. The paternal mutation, also in the region encoding the C-terminal domain, is a single-nucleotide deletion that results in a premature stop codon in subdomain A. Thus, the paternal allele reported here translates into a truncated DP protein product lacking much of the C-terminal tail. As these mutations occur downstream of the predicted alternative splice site that generates DPII mRNA, both DPI and DPII isoforms are predicted to possess the corresponding changes at the protein level. Before this study, only four pairs of mutations affecting the C-terminal domain of DP have been reported in literature (Lai-Cheong et al., 2007). The two homozygous recessive pairs resulted in phenotypes, which included cardiomyopathies (Norgett et al., 2000; Alcalai et al., 2003) together with woolly hair and PPK. The remaining two compound heterozygous mutation pairs each include a mutation upstream of the ABC domain; cardiac involvement was not reported in either case (Whittock et al., 2002; Jonkman et al., 2005). In the paper by Whittock et al. (2002), the authors describe two patients with severe mucocutaneous findings without cardiac symptoms by the ages of 4 and 17 years. The younger patient had both mutations upstream of the C-terminal domain, whereas the older patient had a missense mutation in exon 24, similar to the present case. It is not known whether she developed cardiac symptoms later. However, this comparison emphasizes the critical role of the conserved alanine 2655, which was mutated in the maternal allele in our subject.
Although the phenotype–genotype correlations of DP mutations are not well understood, the present and previous studies suggest that the truncation of the C-terminal domain predisposes to cardiomyopathy. The C-terminal domain of DP has been shown to interact with the IF cytoskeleton. On the basis of these observations, the DP mutations identified in this patient are predicted to disrupt the interactions between DP and the IF cytoskeleton both in the skin and heart. As both mutations are located in exon 24, 3′ of the last exon–exon border, the mRNAs would not likely be subjected to nonsense-mediated mRNA decay (Nagy and Maquat, 1998). Indeed, we detected truncated DP protein products in the paternal skin (Figure 5d).
The maternal mutation is an amino-acid substitution (A2655D) in subdomain C of the DP and in the presence of the paternal allele is unable to confer a normal desmosome function. Alanine 2655 is a highly conserved amino acid (Table 2) in a groove lined with basic residues in subdomain C. This conserved groove is speculated to be an important recognition site for binding to IFs, including cardiac vimentin and desmin (Choi et al., 2002). Although this mutation does not significantly alter the three-dimensional structure of DP, structural modeling indicates changes in the EP of the mutated region. The change may seem subtle but the clinical phenotypes suggest that it alters IF-binding functions that depend on electrostatically driven intermolecular interactions (Choi et al., 2002). These results show the importance of the correct amino-acid sequence of the plakin repeat domain for the DP functions and suggest potentially devastating effects of mutations that occur in this specific region.
Table 2.
Conservation of the alanine residue at amino acid 2655 between species
| Species | Sequence |
|---|---|
| 2655 | |
| ↓ | |
| Human | IVDSITGQRLLEAQACTGGII |
| Mouse | IVDSITGQRLLEAQACTGGII |
| Rat | IVDSITGQRLLEAQACTGGII |
| Chimpanzee | IVDSITGQRLLEAQACTGGII |
| Horse | IVDSITGQRLLEAQACTGGII |
| Chicken | IVDSITGQRLLEAQACTGGII |
We believe that identification of more DP mutations and additional structural/functional analyses are needed before we can accurately predict the clinical manifestations of a given mutation. Meanwhile, if a child with skin symptoms of desmosomal genodermatosis is examined, the possibility of cardiac involvement should be considered, even in the absence of overt heart-related symptoms.
MATERIALS AND METHODS
Tissue material and histology
Medical records and archival tissue material were used with permission from the Turku University Central Hospital and the Department of Forensic Medicine (University of Turku, Finland), Department of Dermatology (University Medical Center Freiburg, Germany), and Freiburg Institute for Advanced Studies, School of Life Sciences (Lifenet, Freiburg, Germany). Family members provided informed consent for the collection and analysis of DNA material, and approval for publication of data. Material was collected according to the Declaration of Helsinki Principles. Control tissue slides were obtained from BioChain Institute (Hayward, CA). Unless otherwise indicated, all chemicals were from Sigma (St Louis, MO) or Fisher Scientific (Waltham, MA). For histology, tissues were fixed overnight at room temperature in 10% formalin solution. Tissues were processed for paraffin embedding, sectioned (4 μm), mounted on glass slides, and stained with hematoxylin and eosin periodic acid Schiff or Masson's trichrome.
For transmission electron microscopy, skin and esophagus biopsies were fixed in 3% glutaraldehyde in 0.1 m Millonig phosphate buffer, pH 7.4, at 4 °C for 4 hours, and post-fixed with 1% OsO4 0.1 m in veronal acetate buffer. Samples were dehydrated in a graded ethanol series and embedded in Epon 812, Shell Chemical Corp., Cleveland, Ohio. Ultrathin sections were cut on coated copper grids and stained with uranyl acetate and lead citrate. The sections were examined using a Jeol, Tokyo, Japan. 1200SX electron microscope and photographed.
Abs
Abs used for immunostaining of the skin, esophagus, and heart sections were the following: DP mix, a “cocktail” of mAbs DP-2.15, DP-2.17, and DP-2.20 purchased from American Research Products (Belmont, MA); Abs 2C8 and 10F6 were generated using recombinant 6x His-tagged DP amino acids 1,960–2,151, and 2A5 was raised against amino acids 2,343–2,822 (Johnson et al., 1993). Abs 6F9 (β-catenin), 27B2 (Dsg1), and 11E4 (PG) were from our laboratory (Wahl, 2002). Ab for connexin 43 was purchased from Cell Signaling Technology (cat. no. 3512, Beverly, MA). Ab dilutions for immunofluorescence using OCT frozen tissues were 1:100 for 6F9, 1:100 for 27B2, 1:800 for 11E4, 1:800 for 2C8, 1:800 for 2A5, and 1:800 for 10F6. Ab dilutions for immunostaining on paraffin tissues were 1:20 for 6F9, 1:4 for 27B2, 1:20 for 11E4, 1:2 for 10F6, 1:2 for DP mix, and 1:50 for connexin 43. Alexa Fluor-conjugated secondary Abs (488 and 594 nm) were purchased from Invitrogen (at dilution of 1:400; Eugene, OR).
Immunohistochemistry and western analysis
For immunostaining, we prepared OCT-fixed tissue sections (5 μm) or formalin-fixed and paraffin-embedded tissue sections (5 μm) as previously described (Mahoney et al., 2006). Briefly, OCT sections were fixed in methanol (−20 °C) for 15 minutes, permeabilized with 1% Triton-X-100 in PBS for 5 minutes, and incubated in blocking buffer (5% normal goat serum, 1% BSA, and 0.02% TX-100 in PBS) for 1 hour at room temperature. Tissues were incubated overnight with primary Abs at 4 °C and with secondary Abs for 1 hour at room temperature. For formalin-fixed, paraffin-embedded sections, tissues were deparaffinized in 100% xylene (5 minutes; twice), 100% ethanol (5 minutes; twice), 95% ethanol (5 minutes; twice), 75% ethanol (2 minutes), 50% ethanol (2 minutes), and H2O (2 minutes). Antigen retrieval was performed by the microwave method with sodium citrate buffer (pH 6.0), followed by digestion with trypsin (Sigma) for 10 minutes at 42 °C. Tissues were incubated in primary and secondary Abs in blocking buffer. Nuclei were stained with DAPI (100 ng ml−1) before being mounted for viewing. All images were acquired using a Hamamatsu monochromatic digital camera (Phase 3 Imaging Systems, Glen Mills, PA; C4742-95). Direct immunofluorescence with FITC-conjugated Abs to human IgG, IgA, IgM, and complement C3 and C1q was performed twice as part of the diagnostic procedure. The results were first nonspecific and the second time showed negative results.
For western analysis, DP was extracted in 9 m urea in lysis buffer from normal skin and a punch biopsy from the proband's father. Proteins were separated in 5% SDS–PAGE gel and immunoblotted with DP mix Ab at a dilution of 1:100.
DNA extraction
DNA was extracted from 100 normal human controls and living relatives of the patient through buccal swabs (Cell Projects, Kent, UK). The patient's genomic DNA was extracted from tissue previously fixed and mounted in a paraffin block. A 5×5 mm2 slice of tissue was extracted and incubated with xylene for 10 minutes. The xylene was removed by three subsequent incubations in 100, 80, and 70% ethanol and the sample was air-dried. The pellet was suspended in TE buffer, pH 8.0 (Tris-HCl 50 mM, EDTA 1 mM) and digested with Proteinase K (1 mg ml−1) at 60 °C. Genomic DNA was further purified by standard phenol chloroform extraction.
PCR and sequencing
Conditions and primers for generating PCR products spanning all exons of the coding regions and flanking intronic sequences of DP have been described previously (Whittock et al., 1999). Additional primers were designed to screen for mutation 6310delA: forward—5′-AATCAGCCCAGAATCCACAG-3′, and reverse—5′-TATCTCGGG GATCATTCAGG-3′. PCR was carried out using Qiagen Taq polymerase and Q buffer (Qiagen, Valencia, CA), according to the manufacturer's instructions. The patient sample and two controls (normal human DNA and distilled water) were PCR amplified simultaneously. PCRs contained 200 ng DNA as template and 100 ng of each primer in a final volume of 50 μl. Cycling conditions for all primer pairs were 94 °C for 5 minutes, followed by 41 cycles of denaturation at 94 °C for 1 minute; annealing temperature for a particular primer pair (range: 55–60 °C) for 1 minute; extension at 72 °C for 1 minute; and finally at 72 °C for 5 minutes. The PCR products of the patient and control samples were sequenced forward and reverse two times. In all, 100 control alleles were studied to rule out the possibility that the mutation might be a frequent polymorphism. DNA sequencing was performed using an ABI Prism 377 or ABI 3100 automated sequencer (Perkin-Elmer-Cetus, Foster City, CA).
Structural modeling of the A2655D mutation site
A computer model of the region spanning the A2655D mutation site was generated by the Sybyl7.3 program (Tripos, St Louis, MO) on the basis of coordinates from the Protein Data Base (1lm5). Molecular surfaces were generated using the MOLCAD module of the SYBYL program (Tripos Inc, St Louis, MO) (Connolly, 1983). The same module was used to calculate the EP (physical unit: kcal mol−1) of the analyzed molecular surfaces (Warwicker and Watson, 1982).
ACKNOWLEDGMENTS
We thank the family members of the patient for their cooperation and participation in our study. This research was supported by grants from the National Institutes of Health to Mỹ G. Mahoney (AR055251), James K. Wahl III (DE016905), Andrzej Fertala (AR049537-06), and Jouni Uitto (P01AR38923); an Epidermolysis Bullosa Network grant from the German Ministry for Education and Research to Leena Bruckner-Tuderman; a grant from the Finnish Academy to Juha Peltonen; and grants from the Finnish Medical Foundation and the Southwest Finland Hospital District to Sirkku Peltonen.
Abbreviations
- Ab
antibody
- ARVC/D
arrhythmogenic right ventricular dilation/cardiomyopathy
- DP
desmoplakin
- Dsg
desmoglein
- EP
electrostatic potential
- IF
intermediate filament
- PG
plakoglobin
- PPK
palmoplantar keratoderma
Footnotes
CONFLICT OF INTEREST The authors state no conflict of interest.
REFERENCES
- Alcalai R, Metzger S, Rosenheck S, et al. A recessive mutation in desmoplakin causes arrhythmogenic right ventricular dysplasia, skin disorder, and woolly hair. J Am Coll Cardiol. 2003;42:319–27. doi: 10.1016/s0735-1097(03)00628-4. [DOI] [PubMed] [Google Scholar]
- Armstrong DK, McKenna KE, Purkis P, et al. Haploinsufficiency of desmoplakin causes a striate subtype of palmoplantar keratoderma. Hum Mol Genet. 1999;8:143–8. doi: 10.1093/hmg/8.1.143. [DOI] [PubMed] [Google Scholar]
- Asimaki A, Tandri H, Huang H, et al. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–84. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]
- Awad MM, Calkins H, Judge DP. Mechanisms of disease: molecular genetics of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008;5:258–67. doi: 10.1038/ncpcardio1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauce B, Basso C, Rampazzo A, et al. Clinical profile of four families with arrhythmogenic right ventricular cardiomyopathy caused by dominant desmoplakin mutations. Eur Heart J. 2005;26:1666–75. doi: 10.1093/eurheartj/ehi341. [DOI] [PubMed] [Google Scholar]
- Carvajal-Huerta L. Epidermolytic palmoplantar keratoderma with woolly hair and dilated cardiomyopathy. J Am Acad Dermatol. 1998;39:418–21. doi: 10.1016/s0190-9622(98)70317-2. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Park-Snyder S, Pascoe LT, et al. Structures of two intermediate filament-binding fragments of desmoplakin reveal a unique repeat motif structure. Nat Struct Biol. 2002;9:612–20. doi: 10.1038/nsb818. [DOI] [PubMed] [Google Scholar]
- Connolly ML. Solvent-accessible surfaces of proteins and nucleic acids. Science. 1983;221:709–13. doi: 10.1126/science.6879170. [DOI] [PubMed] [Google Scholar]
- Cowin P, Burke B. Cytoskeleton-membrane interactions. Curr Opin Cell Biol. 1996;8:56–65. doi: 10.1016/s0955-0674(96)80049-4. [DOI] [PubMed] [Google Scholar]
- Fontao L, Favre B, Riou S, et al. Interaction of the bullous pemphigoid antigen 1 (BP230) and desmoplakin with intermediate filaments is mediated by distinct sequences within their COOH terminus. Mol Biol Cell. 2003;14:1978–92. doi: 10.1091/mbc.E02-08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gras E, Lombardi R, Giocondo MJ, et al. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–21. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol. 2004;5:271–81. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- Green KJ, Goldman RD, Chisholm RL. Isolation of cDNAs encoding desmosomal plaque proteins: evidence that bovine desmoplakins I and II are derived from two mRNAs and a single gene. Proc Natl Acad Sci USA. 1988;85:2613–7. doi: 10.1073/pnas.85.8.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Parry DA, Steinert PM, et al. Structure of the human desmoplakins. Implications for function in the desmosomal plaque. J Biol Chem. 1990;265:2603–12. [PubMed] [Google Scholar]
- Jefferson JJ, Leung CL, Liem RK. Plakins: goliaths that link cell junctions and the cytoskeleton. Nat Rev Mol Cell Biol. 2004;5:542–53. doi: 10.1038/nrm1425. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Lewis JE, Li D, et al. P- and E-cadherin are in separate complexes in cells expressing both cadherins. Exp Cell Res. 1993;207:252–60. doi: 10.1006/excr.1993.1191. [DOI] [PubMed] [Google Scholar]
- Jonkman MF, Pasmooij AM, Pasmans SG, et al. Loss of desmoplakin tail causes lethal acantholytic epidermolysis bullosa. Am J Hum Genet. 2005;77:653–60. doi: 10.1086/496901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouklis PD, Hutton E, Fuchs E. Making a connection: direct binding between keratin intermediate filaments and desmosomal proteins. J Cell Biol. 1994;127:1049–60. doi: 10.1083/jcb.127.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk AP, Bornslaeger EA, Borgwardt JE, et al. The amino-terminal domain of desmoplakin binds to plakoglobin and clusters desmosomal cadherin-plakoglobin complexes. J Cell Biol. 1997;139:773–84. doi: 10.1083/jcb.139.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai-Cheong JE, Arita K, McGrath JA. Genetic diseases of junctions. J Invest Dermatol. 2007;127:2713–25. doi: 10.1038/sj.jid.5700727. [DOI] [PubMed] [Google Scholar]
- Lapouge K, Fontao L, Champliaud MF, et al. New insights into the molecular basis of desmoplakin- and desmin-related cardiomyopathies. J Cell Sci. 2006;119:4974–85. doi: 10.1242/jcs.03255. [DOI] [PubMed] [Google Scholar]
- Mahoney MG, Hu Y, Brennan D, et al. Delineation of diversified desmoglein distribution in stratified squamous epithelia: implication in diseases. Exp Dermatol. 2006;15:101–9. doi: 10.1111/j.1600-0625.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- Martin ED, Moriarty MA, Byrnes L, et al. Plakoglobin has both structural and signalling roles in zebrafish development. Dev Biol. 2009;327:83–96. doi: 10.1016/j.ydbio.2008.11.036. [DOI] [PubMed] [Google Scholar]
- Matthiessen ME, Rømert P. Ultrastructure of the human enamel organ. I. External enamel epithelium, stellate reticulum, and stratum inter-medium. Cell Tissue Res. 1980;205:361–70. doi: 10.1007/BF00232278. [DOI] [PubMed] [Google Scholar]
- Meng JJ, Bornslaeger EA, Green KJ, et al. Two-hybrid analysis reveals fundamental differences in direct interactions between desmoplakin and cell type-specific intermediate filaments. J Biol Chem. 1997;272:21495–503. doi: 10.1074/jbc.272.34.21495. [DOI] [PubMed] [Google Scholar]
- Nagy E, Maquat LE. A rule for termination-codon position withing introl-containing genes: when nonsense affects RNA abundance. Trends Biochem Sci. 1998;23:198–9. doi: 10.1016/s0968-0004(98)01208-0. [DOI] [PubMed] [Google Scholar]
- Norgett EE, Hatsell SJ, Carvajal-Huerta L, et al. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet. 2000;9:2761–6. doi: 10.1093/hmg/9.18.2761. [DOI] [PubMed] [Google Scholar]
- Norman M, Simpson M, Mogensen J, et al. Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation. 2005;112:636–42. doi: 10.1161/CIRCULATIONAHA.104.532234. [DOI] [PubMed] [Google Scholar]
- Oxford EM, Musa H, Maass K, et al. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res. 2007;101:703–11. doi: 10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- Rampazzo A, Nava A, Malacrida S, et al. Mutation in human desmoplakin binding domain to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2002;71:1200–6. doi: 10.1086/344208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffitz JE, Hames KY, Kanno S. Remodeling of gap junctions in ischemic and nonischemic forms of heart disease. J Membr Biol. 2007;218:65–71. doi: 10.1007/s00232-007-9031-2. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Segawa K, Takiguchi R, et al. Intercellular junctions in the cells of the human enamel organ as revealed by freeze-fracture. Arch Oral Biol. 1984;29:275–86. doi: 10.1016/0003-9969(84)90101-8. [DOI] [PubMed] [Google Scholar]
- Smith EA, Fuchs E. Defining the interactions between intermediate filaments and desmosomes. J Cell Biol. 1998;141:1229–41. doi: 10.1083/jcb.141.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappenbeck TS, Bornslaeger EA, Corcoran CM, et al. Functional analysis of desmoplakin domains: specification of the interaction with keratin versus vimentin intermediate filament networks. J Cell Biol. 1993;123:691–705. doi: 10.1083/jcb.123.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappenbeck TS, Green KJ. The desmoplakin carboxyl terminus coaligns with and specifically disrupts intermediate filament networks when expressed in cultured cells. J Cell Biol. 1992;116:1197–209. doi: 10.1083/jcb.116.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Lai-Cheong JE, Café ME, et al. Novel truncating mutations in PKP1 and DSP cause similar skin phenotypes in two Brazilian familes. Br J Dermatol. 2009;160:692–7. doi: 10.1111/j.1365-2133.2008.08900.x. [DOI] [PubMed] [Google Scholar]
- Uzumcu A, Norgett EE, Dindar A, et al. Loss of desmoplakin isoform I causes early onset cardiomyopathy and heart failure in a Naxos-like syndrome. J Med Genet. 2006;43:e5. doi: 10.1136/jmg.2005.032904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virata ML, Wagner RM, Parry DA, et al. Molecular structure of the human desmoplakin I and II amino terminus. Proc Natl Acad Sci USA. 1992;89:544–8. doi: 10.1073/pnas.89.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl JK., III Generation of monoclonal antibodies specific for desmoglein family members. Hybrid Hybridomics. 2002;21:37–44. doi: 10.1089/15368590252917629. [DOI] [PubMed] [Google Scholar]
- Warwicker J, Watson HC. Calculation of the electric potential in the active site cleft due to alpha-helix dipoles. J Mol Biol. 1982;157:671–9. doi: 10.1016/0022-2836(82)90505-8. [DOI] [PubMed] [Google Scholar]
- Whittock NV, Ashton GH, Dopping-Hepenstal PJ, et al. Striate palmoplantar keratoderma resulting from desmoplakin haploinsufficiency. J Invest Dermatol. 1999;113:940–6. doi: 10.1046/j.1523-1747.1999.00783.x. [DOI] [PubMed] [Google Scholar]
- Whittock NV, Wan H, Morley SM, et al. Compound heterozygosity for non-sense and mis-sense mutations in desmoplakin underlies skin fragility/woolly hair syndrome. J Invest Dermatol. 2002;118:232–8. doi: 10.1046/j.0022-202x.2001.01664.x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Bowles NE, Scherer SE, et al. Desmosomal dysfunction due to mutations in desmoplakin causes arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Res. 2006;99:646–55. doi: 10.1161/01.RES.0000241482.19382.c6. [DOI] [PubMed] [Google Scholar]
- Yu CC, Yu CH, Hsueh CH, et al. Arrhythmogenic right ventricular dysplasia: clinical characteristics and identification of novel desmosome gene mutations. J Formos Med Assoc. 2008;107:548–58. doi: 10.1016/S0929-6646(08)60168-0. [DOI] [PubMed] [Google Scholar]






