Abstract
Mutations in COL2A1 produce a spectrum of disorders whose hallmark feature is alterations in skeletal development. Attempts to counteract the effects of collagen mutations at the molecular level have been relatively ineffective due to the inability to selectively suppress a mutant allele, and failure to deliver a sufficient number of cells expressing wild-type collagen. Moreover, these approaches are hampered because the minimal therapeutic conditions that would allow extracellular matrix remodeling and recovery of cells from stress are not known. Here, we employed a tetracycline-inducible system for expressing the R789C or R992C collagen II mutants, allowing us to decrease the production of mutant proteins by 25, 50, 75, or 100% with respect to their initial production. Through analysis of intracellular and extracellular parameters we have shown that affected cell/matrix systems are able to recover from mutation-induced aberrations only when 100% expression of mutant collagens is shut off, but not if the expression of small amounts of mutant molecules persists in the system. Our data suggest that efficient remodeling of tissues affected by the presence of thermolabile collagen mutants may depend on their complete elimination rather than on partial reduction.
Keywords: collagen mutations, collagen II, cartilage, spondyloepiphyseal dysplasia, endoplasmic reticulum stress
Introduction
Mutations in genes encoding fibrillar collagens are associated with pathological changes of the involved tissues, and the clinical consequences of such mutations range from mild to lethal [Royce and Steinmann, 2002]. The mechanisms responsible for transmitting the effects of a mutation from a collagen gene to the tissue level are complex and include changes in the structure of individual mutant molecules as well as alterations in their secretion and assembly into fibrils. In addition, due to their atypical intracellular accumulation, some mutants may also trigger endoplasmic reticulum (ER) stress, a process that can lead to apoptosis [Chung et al., 2009; Hintze et al., 2008; Ito et al., 2005].
The two predominant approaches to counteract the dominant negative effects of collagen mutations are: (1) gene therapy through suppressing expression of a mutant allele and (2) therapy by cell delivery. For the first approach, linear antisense oligonucleotides and hammerhead ribozymes have been employed to selectively suppress mutated genes in cell culture systems [Dawson and Marini, 2000; Peace et al., 2005; Wang and Marini, 1996]. These approaches have been to some extent effective in cell culture models, but testing them at the organismal level has proven difficult due to the low efficiency of sustained delivery of these suppressors into affected tissues.
Gene therapy by cell delivery has been pursued for more than a decade to treat diseases caused by mutations in collagens. The main strategy has been to employ multipotent mesenchymal stem cells that have the ability to generate structures resembling certain characteristics of bone, cartilage, and muscle tissue [Prockop, 1997]. In the pioneering experiments performed in a mouse model of osteogenesis imperfecta (OI), a disease caused by mutations in collagen I, marrow stromal cells from wild-type mice were infused into the OI mice [Pereira et al., 1995, 1998]. A small increase in the mineral content and amount of normal collagen in the femur was reported without indication of any significant incorporation of implanted cells into the tissue [Pereira et al., 1995, 1998]. In those studies, the authors determined that the percentage of donor cells in primary cultures derived from the targeted bone tissue ranged from 2% to 7%. The percentages of donor cells detected in primary cultures from other tissues varied, with 19% found in lung, 0.6% in brain, and 0% in aorta and heart [Pereira et al., 1998]. Similarly, a relatively low percentage of donor-derived cells were detected in cell cultures obtained from bone explants of an OI patient who received bone marrow transplants from a healthy donor [Horwitz et al., 1999, 2001, 2002].
Previous studies have demonstrated that the success of genetic therapies for collagen diseases will ultimately depend on increasing the ratio of wild-type (WT) to mutant collagen, and on creating a mosaic arrangement of cells with groups producing WT collagen and creating pockets of normal tissue. However, it is unclear what would be the minimal increase in WT collagen or in the number of exogenous cells necessary to improve tissue structures damaged by the presence of mutant molecules.
In the study presented here, focusing on mutations in COL2A1 (MIM# 120140), we have attempted to determine the minimal change in the ratio of WT to mutant collagen II needed to reduce the negative effects caused by the presence of mutant collagen. Specifically, we investigated the consequences of the net reduction of mutant collagen molecules in a controlled model system that represents an affected cartilaginous tissue. Here, our main focus has been on the R789C (p.R989C) and R992C (p.R1192C) collagen II mutations, which are associated with spondyloepiphyseal dysplasia (SED) [Chan et al., 1993; Donahue et al., 2003].
At the molecular level, the effects of mutations that introduce cysteine residues into the collagen II triple-helical domain share a number of similarities with those caused by other mutation types. For instance, the presence of cysteine residues may be associated with decreased thermostability of mutants, their excessive intracellular accumulation, and aberrations in the formation of homotypic or heterotypic fibrils [Fertala et al., 1997, 2001; Steplewski et al., 2004a, b, 2005]. Additionally, the presence of cysteine residues, absent in the WT collagen II triple helix, may potentially result in the formation of intramolecular and intermolecular disulfide bonds among collagen II chains as well as atypical complexes with other proteins in the intracellular or extracellular compartments [Chung et al., 2009; Fertala et al., 1997].
By employing a controlled experimental system in which expression of mutant collagen II variants in a chondrocytic cell line is regulated by a tetracycline-responsive (Tet) promoter, we have demonstrated that the measurable attenuation of both intracellular and extracellular aberrations caused by the presence of these collagen II mutants is only possible by switching off their expression entirely. The results presented here suggest that reducing the pathological intracellular and extracellular consequences of the presence of collagen mutants with intracellular accumulation may not be possible by employing approaches that only partially reduce the amount of mutant collagen molecules in affected tissues.
Materials and Methods
Collagen II Mutants and Their Nomenclature
The amino acid substitutions are named according to the literature, with amino acid residues numbered from the first glycine of the collagen triple helix. At their initial text appearances the mutations are also numbered from the ATG start codon following the journal and HGVS guidelines (www.hgvs.org) and are listed in parentheses (with a “p” included in the mutation name).
The mutants selected for the presented study are associated with severe forms of SED [Bleasel et al., 1995, 1996a,b; Chan et al., 1993; Donahue et al., 2003; Hoornaert et al., 2006; Knowlton et al., 1990; Olsen, 1995]. Our previous studies have demonstrated that at the molecular and cellular levels, the R789C and R992C mutations alter the structure and thermostability of individual collagen molecules, cause abnormal assembly of collagen fibrils, trigger ER stress, and increase the rate of apoptosis of cells harboring these mutants [Chung et al., 2009; Hintze et al., 2008; Ito et al., 2005; Steplewski et al., 2004a,b].
Unconditional and Tet-Regulated Expression of Procollagen II Variants
The experimental system employed here has been characterized by us in detail elsewhere [Chung et al., 2009; Hintze et al., 2008; Ito et al., 2005]. Briefly, these experiments utilize two sets of engineered cells: one constantly expressing green fluorescent protein (GFP)-tagged procollagen II variants (Pro-GFP) and a second group conditionally expressing the same variants in an inducible manner (Pro-GFPTet). The chondrosarcoma cell line SW-1353 (ATCC; HTB-94) has been employed for expression of these variants. These cells express endogenous WT procollagen II and a number of other cartilage-specific macromolecules, as well as respond to many stress stimuli in a way similar to that of primary chondrocytes [Chung et al., 2009; Gebauer et al., 2005; Hintze et al., 2008; Ito et al., 2005].
Expression of Collagen II Variants in High-Density Cell Cultures
Cells expressing WT, R789C, or R992C variants were cultured in high density to facilitate formation of a cohesive extracellular matrix (ECM). For the biochemical and histological assays, two systems of high-density cultures have been employed: one is based on growing cells on plastic dishes and the other is based on growing cells in three dimensional (3D) conditions in the form of spherical clusters, as described [Chung et al., 2009; Hintze et al., 2008; Ito et al., 2005]. Our previous studies have demonstrated that these two systems are comparable in that cells grown in both monolayers and 3D respond in the same way to the presence of mutant collagen II molecules and assemble complex matrices in the extracellular space [Chung et al., 2009; Hintze et al., 2008; Ito et al., 2005]. All cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% Tet-approved fetal bovine serum (Clontech Laboratories, Palo Alto, CA) and 40 μg/ml of L-ascorbic acid phosphate magnesium salt (Wako, Japan). Induction of Tet-responsive genes in the Pro-GFPTet cells was initiated through addition of 1 μg/ml of doxycycline (Dox; Clontech Laboratories) to cell culture media.
Explanation of Experimental Model
Two experimental paradigms were employed using a system that is based on two sets of cells: one which unconditionally expresses Pro-GFP variants, and a second group which conditionally expresses corresponding Pro-GFPTet variants (Fig. 1). First, we studied the intracellular and extracellular consequences of switching off expression of the R789C and R992C mutants completely (100%-OFF) in models formed by Pro-GFPTet variants after removing doxycycline (Dox) from cell culture media (Fig. 1 and Table 1). Thus, this model represents ideal rescue conditions in which the cell/matrix system formed in the presence of mutant molecules (100%-ON) is able to undergo self-remodeling in the complete absence of those molecules (100%-OFF) while still producing endogenous WT collagen molecules.
Figure 1.
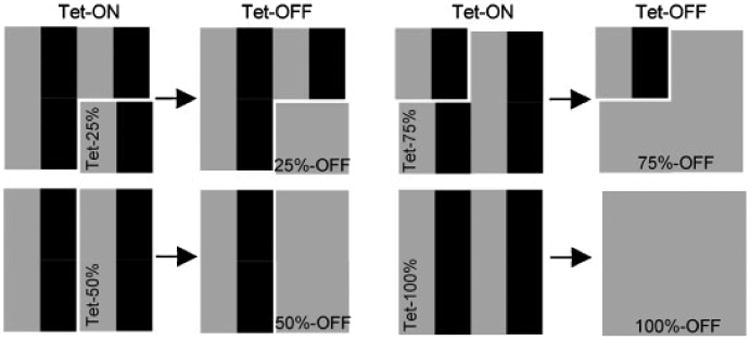
A schematic illustrating an experimental strategy to completely eliminate or partially reduce production of R789C or R992C collagen II in cell/matrix systems. Individual blocks represent cell compositions used in described experiments. Moreover, black color are exogenous collagen II mutants, expressed unconditionally (Pro-GFP) or in a Tet-dependent fashion (Pro-GFPTet), while gray color are endogenous WT collagen II. Tet-ON columns represent conditions in which expression of exogenous collagen II variants was induced by Dox. Note: in the ON conditions, all cells present in a system were producing collagen II variants at 100% capacity. In the Tet-ON columns, segments described as Tet-25%, Tet-50%, Tet-75%, and Tet-100% represent the percentage of cells in which expression of Pro-GFPTet variants was Tet-dependent. Tet-OFF columns represent conditions in which expression of the Pro-GFPTet variants but not Pro-GFP variants was switched off by omitting Dox from cell culture media. In the Tet-OFF columns, segments described as 25%-OFF, 50%-OFF, 75%-OFF, and 100%-OFF represent subpopulations of cells in which expression of the Pro-GFPTet variants was switched off. Note that regardless of conditions, production of endogenous WT collagen II and exogenous Pro-GFP variants whose expression was not Tet-dependent was constantly active.
Table 1. Seeding Percentages, Contributions of Exogenous Collagen II Variants, and Text Notations of Experimental Groups.
| aPro-GFPTet(%) | bExpression of mutant collagens in ON conditions (%) | cExpression of mutant collagens in OFF conditions (%) | dActual contribution of mutants to total collagen II pool (%) | Text notation |
|---|---|---|---|---|
| 100 | 100 | 0 | 0 | 100%-OFF |
| 75 | 100 | 25 | 7 | 75%-OFF |
| 50 | 100 | 50 | 15 | 50%-OFF |
| 25 | 100 | 75 | 23 | 25%-OFF |
The percentage of cells expressing Pro-GFPTet of the total 100% of cells seeded.
Note that in the presence of Dox, all cells were producing collagen II variants at 100% capacity.
The percentages of Pro-GFP collagen II variants constantly expressed in the presence and absence of Dox (±). Note that all text notations refer the %OFF of Pro-GFPTet cells unless otherwise indicated.
Previous work has shown that stably transfected cells do not express endogenous and exogenous collagen at the same levels, but rather that GFP-tagged procollagen contributes about 30% to the total collagen pool. Consequently, we predict that by shutting off production of exogenous procollagen in 25, 50, 75, and 100% of cells, we are effectively altering the contribution of mutant molecules to the levels indicated.
In the second model, we studied the intracellular and extracellular consequences of partially reducing the amount of mutant molecules in cell/matrix systems that were initially formed in the presence of mutant molecules (100%-ON). Unlike the first model, consisting only of cells expressing Pro-GFPTet variants, the second model consists of a mixture of cells expressing Pro-GFP and Pro-GFPTet variants (Fig. 1). Consequently, in this system, removing Dox from cell culture media switches off production of mutant variants only in the Pro-GFPTet subpopulation of cells while the production of unconditional Pro-GFP variants continues uninterrupted. Within this model, three experimental groups with different percentages of Pro-GFP and Pro-GFPTet -producing cells were prepared: (1) a group with 75% and 25%Tet (25%-OFF), (2) a group with 50% and 50%Tet (50%-OFF), and (3) a group with 25% and 75%Tet (75%-OFF), respectively. Thus, in this model we are able to modulate the WT:mutant collagen ratio through switching off expression in a specific subpopulation of cells, allowing us to subsequently evaluate the intracellular and extracellular consequences of such modulations. Moreover, by switching off the expression of mutant chains in only a certain subpopulation of cells, we are able to create a mosaic pattern of cells expressing WT collagen II, a spatial arrangement similar to that predicted for cell-based therapies.
Previous work has shown that stably transfected SW-1353 cells do not express endogenous and exogenous collagen at the same levels, but rather that the exogenous GFP-tagged procollagen contributes about 30% to the total collagen pool [Ito et al., 2005]. Therefore, we can predict that by shutting off production of exogenous procollagen in 25, 50, 75, and 100% of cells, we are effectively decreasing the amount of mutant molecules to 23, 15, 7, and 0% of their initial content, respectively (Table 1).
Analyzing the Effects of Decreasing the Amount of R789C and R992C Collagen in Cell/Matrix Systems
Previous work has shown that after 2 weeks of postconfluent cell culture, the ECM is fully developed and the consequences of the presence of mutant molecules within the cell and the ECM are readily detectable [Chung et al., 2009; Hintze et al., 2008; Ito et al., 2005]. To determine whether the cells and surrounding matrix have the ability to remodel themselves after completely or partially suppressing expression of mutant collagen, we employed a 4-week system whereby cells grown in high density were treated with Dox for the first 2 weeks and then cultured for an additional 2 weeks in its absence (2ON/ 2OFF). As intracellular degradation of misfolded collagen proceeds quite rapidly, it is predicted that the length of a 2OFF period is adequate to clear the intracellular collagen accumulated during a subsequent 2ON period [Bienkowski et al., 1986]. This notion is further supported indirectly by our observation that the GFP signal seen in cells expressing Pro-GFPTet variants in the presence of Dox is reduced to baseline levels within 48 hr after removing this compound from cell culture media (unpublished observation). In addition to the 2ON/2OFF group, control groups included cells cultured for 2 weeks in the presence of Dox (2ON), 4 weeks in the presence of Dox (4ON), and 4 weeks in the absence of Dox (4OFF).
Ensuring the Fidelity of Tet-Regulated Expression of Pro-GFPTet Variants in Long-Term Culture
Expression of Pro-GFPTet variants in the presence or absence of Dox was determined by Western blot assays and reverse-transcriptase PCR (RT-PCR), as described [Chung et al., 2009; Hintze et al., 2008]. The long-term stability of expression levels was monitored to ensure defined ratios of Pro-GFP and Pro-GFPTet remained the same after 4 weeks in culture (see Supp. Materials and Methods).
Intracellular Response to Maximal or Partial Reduction of the R789C or R992C Mutants
The relative amounts of protein disulfide isomerase (PDI), whose elevated concentrations are associated with ER stress, were analyzed as described [Chung et al., 2009; Hintze et al., 2008]. Analysis of intracellular indicators of apoptosis included Western blot assays of the relative amounts of cleaved poly (ADP-ribose) polymerase (cPARP) and microscopic assays of fragmented DNA with TdT-mediated dUTP Nick-End Labeling (TUNEL; In Situ Cell Death Detection Kit, TMR Red; Roche, Indianapolis, IN), as described [Chung et al., 2009; Hintze et al., 2008].
The relative amounts of PDI and cPARP were quantified by measuring the intensities of pixels of specific bands with EZQuant computer software (EZQuant, Ltd., Tel-Aviv, Israel) and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) detected with mouse anti-GAPDH monoclonal antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), as described [Chung et al., 2009; Hintze et al., 2008]. In all Western blot assays, bands were detected by chemiluminescence with the use of a bioimaging system (EpiChemi, UVP Inc., San Gabriel, CA). The linear range of measured pixel intensities has been ensured by employing a sequential integration mode of operation of the bioimaging system.
Apoptotic indices of cells were determined by TUNEL assays employing fluorescence microscopy, as described [Chung et al., 2009; Hintze et al., 2008]. In brief, the apoptotic index was defined as the percentage of TUNEL-positive nuclei out of the total number of 4′, 6-diamidino-2-phenylindole (DAPI)-stained nuclei.
Analysis of ECM Components
Western blot assays were employed to analyze changes in the amount of macromolecules deposited in cell layers formed during high-density culture. The following parameters were analyzed by Western blot assays: (1) the relative amount of exogenous, GFP-tagged collagen II using a mouse anti-GFP antibody; (2) the relative amount of total collagen II with mouse anti-collagen II antibodies (Millipore, Bedford, MA); and (3) the relative amount of fibronectin (FN) with a chicken anti-tissue FN antibody (Molecular Probes, Inc., Eugene, OR). The relative amount of FN was calculated with respect to β-actin detected with a monoclonal mouse anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO). The rationale for assaying FN was that a previous study demonstrated that thermolabile collagen II mutants alter not only formation of collagenous matrices but also influence the assembly of FN fibrils [Ito et al., 2005]. Western blot measurements were normalized to internal controls as described.
In addition to collagen II and FN, changes in the content of glycosaminoglycans were analyzed with the Blyscan Sulfated Glycosaminglycans kit according to the manufacturer's protocol (Biocolor, County Antrim, UK). In those assays, the relative amount of glycosaminoglycans was normalized to total DNA content determined with the use of the Quant-iT PicoGreen dsDNA Assay kit (Invitrogen, Carlsbad, CA).
Microscopic Assays of ECMs
Microscopy was employed to determine the extent of ECM formation. For these assays, we visualized readily-detectable FN matrices by employing immunofluorescence, as described [Ito et al., 2005]. Subsequently, the surface area of the deposits was measured by EZQuant image analysis software. In these assays, the results from each viewing area were normalized to the total area occupied by DAPI-stained nuclei.
To ensure the structural integrity of the cell/matrix assemblies, we analyzed the gross morphology of constructs expressing collagen II mutants. These constructs were grown in 3D as described and were then analyzed by histology [Ito et al., 2005]. Specifically, the overall morphology and cellularity of constructs was evaluated by hematoxylin and eosin (H&E) staining, while glycosaminoglycans were stained with Alcian blue. Moreover, deposition of collagen II in the extracellular matrices of the 3D constructs was analyzed by immunostaining with a rabbit anti-human collagen II antibody (MD Biosciences, Inc., St. Paul, MN).
Fluorescence Recovery after Photobleaching (FRAP) Assays
Diffusional mobility of the R992C mutant was analyzed by FRAP assays, as described (for details, see Supp. Materials and Methods) [Ito et al., 2005].
Cell Migration Assays
The overall impact of complex changes in the structure/ composition of the ECM formed in layers of cells cultured in the presence or the absence of collagen mutants on cell behavior was analyzed by monitoring cell migration, a characteristic that largely depends on cell–matrix interactions. The motility of cells was analyzed with use of an agarose drop assay, as described (for details, see Supp. Materials and Methods) [Ito et al., 2006].
Data Analysis
Quantitative data were collected from a number of independent experiments defined as those originating from seeding cells and culturing them in the presence and then the absence of Dox according to the described strategies, collecting biological materials, and analyzing them using the described assays.
Aside from microscopic assays of FN deposits, comparisons of the analyzed parameters from cells expressing WT collagen II with cells expressing the R789C or R992C mutants were already reported by us elsewhere [Chung et al., 2009; Hintze et al., 2008]. Thus, the main comparisons here were performed within groups of cells expressing the same collagen II variant but with varying percentages of Pro-GFP and Pro-GFPTet and cultured in the presence or absence of Dox. Such comparisons allow direct analysis of the intracellular and extracellular effects of partial reduction of mutant collagen molecules at various levels.
Western blot data are presented as ratios of pixel intensities of bands representing an analyzed protein and a corresponding internal control. The statistical significance of differences between the means of analyzed groups was evaluated by employing the Student's t-test (GraphPad Prism v. 5.03, GraphPad Software, Inc., San Diego, CA). Data is presented such that analyzed protein from any given group was compared to the corresponding control group cultured in 4ON conditions. Note that regardless of the seeding percentage, expression of any given collagen II variant is considered maximal (i.e., 100%-ON) in the 4ON conditions due to complete induction of Pro-GFPTet genes in addition to constant expression of Pro-GFP genes, making the 4ON group a suitable control for all other groups. Therefore, an analyzed parameter from cells expressing the R789C mutant in the 25%-OFF group and cultured in 2ON, 4OFF, or 2ON/2OFF conditions was compared to the group expressing R789C in the same 25%-OFF group but in 4ON conditions. Based on these considerations, we first normalized analyzed proteins to internal controls. The obtained ratio was then expressed as a percentage of the ratio calculated for the same group cultured in 4ON conditions. For each specific percentage group, the results were plotted on separate graphs.
For microscopy-based assays of extracellular FN produced by cells expressing Pro-GFP variants, the total surface area occupied by FN fibrils formed by cells producing WT collagen cultured in 4ON conditions was considered 100%. The surface area of FN deposits produced by cells expressing the R789C or R992C mutants and cultured in 2ON, 4ON, 2ON/2OFF, and 4OFF conditions was expressed as percentages of this base value. For analyses of FN deposits formed in systems in which expression of collagen II mutants was only partially switched off, we analyzed groups cultured in 4OFF conditions. In these experiments, a group expressing WT collagen served as control.
Results
Experimental Model to Study Responses of Cell–Matrix Systems to Varying Amounts of Selected Collagen Mutants
The cellular and extracellular consequences of modulating the content of the R789C and R992C collagen II mutants were studied in a model consisting of SW-1353 cells engineered to express these variants either continuously or in a Tet-dependent fashion.
Fidelity of Tet-Regulated Expression of Collagen II Variants in Long-Term Cultures
Expression of Pro-GFPTet variants was monitored by Western blot and RT-PCR assays. These and earlier studies have demonstrated that conditional expression of these variants is strictly regulated by the presence of Dox in cell culture media. Background expression of Pro-GFPTet variants seen in the absence of Dox was expected due to residual amounts of tetracycline present in cell culture media or marginal nonspecific activation of the Tet promoter (Supp. Fig. S1) [Gossen et al., 1995; Hintze et al., 2008]. The initial ratios of cells expressing Pro-GFP and Pro-GFPTet variants were steady throughout the duration of the experiments (Supp. Fig. S2).
Formation of Cell/Matrix Assemblies in Long-Term Cultures
The ability of the employed cells to form cohesive, matrix-rich structures was confirmed by analyses of three dimensional (3D) constructs formed in high density cell culture. Histological analyses of these constructs demonstrate the formation of collagen II-rich and proteoglycan-rich matrices deposited within the extracellular space of all analyzed constructs (Fig. 2) [Ito et al., 2005].
Figure 2.

Histological analysis of 3D constructs. Constructs were analyzed for gross morphology and cellularity by staining with hematoxylin and eosin (H&E), for the presence of collagen II (Coll-II) by immunostaining, and for the presence of glycosaminoglycans by staining with Alcian blue (Alc). Symbols: WT, R789C, R992C, groups expressing wild-type collagen II or specific mutants, respectively. Bars presented for the low and the high magnifications correspond to 0.1 and 0.2 mm, respectively.
Analysis of Total and Exogenous Collagen II Variants After Switching Off Their Expression in 25, 50, 75, or 100% of Cells
We were able to selectively determine the relative amounts of exogenous collagen II in cell layers by employing an anti-GFP antibody (Fig. 3) for immunoblotting. Similarly, we were able to establish total collagen content, that is, the sum of endogenous WT collagen II and exogenous collagen II variants, using an anti-collagen II antibody. Note that assays of cell layers detect molecules present in the ECM as well as the intracellular compartments. The relative amounts of Pro-GFPTet variants in the layers formed in the 2ON and 4ON conditions were similar in all groups, thereby indicating the formation of a plateau within a 2-week period. When cultured in the 4OFF or 2ON/2OFF conditions, the total amount of GFP-positive procollagen II was significantly less than in ON conditions. As expected, in the system consisting of various percentages of cells expressing the Pro-GFPTet variants, the percent decrease in the expression of exogenous procollagen produced in the 4OFF and 2ON/2OFF conditions corresponds to the seeding percentage of cells expressing each Pro-GFPTet variant. The amount of total collagen II accumulated in the cell layers formed in the 4OFF or 2ON/2OFF conditions was less than in those formed in the ON conditions, which is likely due to switching off production of the exogenous Pro-GFPTet variants (Fig. 3). Likewise, the relative amounts of total collagen II detected with anti-collagen II antibodies mirrored the ON or OFF conditions of cell cultures, but in a less precise way (Fig. 3). Such an apparent discrepancy was most likely a result of the fact that the anti-collagen II antibody recognizes the triple-helical region of a collagen II molecule and is able to bind to all elements of the collagen II pool; that is, both soluble and insoluble collagen. Because varying amounts of the insoluble fraction may be lost during sample preparation, it is possible that not all collagen II molecules are accounted for in these assays. In contrast, the GFP-tagged variants detected represent the soluble fraction whose preservation during sample preparation and detection is more consistent among analyzed groups. Despite some discrepancies between the results of collagen II-specific and GFP-specific assays, however, the overall trend in the production of collagen II is consistent with the applied ON or OFF cell culture conditions [Ito et al., 2005].
Figure 3.

Graphic representations of Western blot analyses of exogenous Pro-GFP variants and total collagen II. Data represent experiments in which the percentage of cells expressing Pro-GFPTet variants ranged from 100% to 25%. Individual points represent mean values for Pro-GFP/ GAPDH or collagen II/GAPDH ratios, expressed as the percent of the corresponding ratio obtained for the control 4ON conditions (considered to be 100% due to exogenous Pro-GFP variants being produced at maximal levels). Groups with statistically significant differences between relevant means (i.e., 2ON, 2ON/2OFF, and 4OFF vs. 4ON; P <0.05) are indicated with asterisks. Symbols: WT, R789C, R992C, groups expressing wild-type collagen II or specific mutants, respectively; GFP, Pro-GFPTet/Pro-GFP variants; 2ON, 4ON, 2ON/2OFF, and 4OFF describe specific regimes of cell cultures; 100, 75, 50, and 25% in the upper row indicates groups with a specific percentage of cells expressing Pro-GFPTet variants. Values in the lower row describe the percent contribution of mutant chains to the total pool of collagen II. Dotted lines represent the value obtained for the control 4ON conditions.
Effects of Decreasing Production of Pro-GFPTet Variants on Intracellular Markers of ER Stress and Apoptosis
The ability of cell/matrix constructs formed in the presence of the R789C or R992C mutants to recover from the consequences imposed by those mutants was analyzed by culturing these constructs in 2ON/2OFF conditions. In this set of experiments, cells expressing Pro-GFPTet variants were first cultured in the presence of Dox and then in its absence, either partially or completely eliminating the production of exogenous collagen II variants. Analyses of PDI have demonstrated that its relative amounts are higher in cells expressing the R789C and R992C mutants grown in 2ON and 4ON conditions than in corresponding cells cultured in 4OFF conditions (Fig. 4). At the end of culture, the relative amount of PDI was not significantly reduced in groups in the 2ON/2OFF conditions and remained elevated in comparison to the corresponding 4OFF group. In cells expressing the R992C mutant, the changes observed for PDI among analyzed groups were more apparent than in corresponding groups expressing the R789C mutant. No significant differences were observed among cells expressing WT collagen in any conditions.
Figure 4.

Graphic representations of Western blot-based assays of cPARP and PDI. Individual points represent mean values for cPARP or PDI normalized to GAPDH and expressed as the percent of the corresponding ratio obtained for the control 4ON conditions (note that in 4ON conditions, exogenous collagen II variants were produced at maximal levels). Groups with statistically significant differences between relevant means (i.e., 2ON, 2ON/2OFF, and 4OFF vs. 4ON; P<0.05) are indicated with asterisks. Symbols: WT, R789C, R992C, groups expressing wild-type collagen II or specific mutants, respectively; 2ON, 4ON, 2ON/2OFF, 4OFF describe specific regimes of cell cultures; 100, 75, 50, and 25% in the upper rows indicate groups with a specific percentage of cells expressing Pro-GFPTet variants in a Tet-dependent fashion. Values in the lower row describe the percent contribution of mutant chains to the total pool of collagen II. Dotted lines represent the value obtained for 4ON conditions, considered 100%.
Western blot assays of cPARP have demonstrated that no significant differences were seen among groups expressing the WT procollagen variant. In contrast, cells expressing the R789C or R992C Pro-GFPTet mutants were characterized by elevated levels of cPARP. Two weeks after switching off expression of the R789C and R992C mutants in the 100%-OFF group, the relative amounts of cPARP decreased, returning to the baseline levels seen in corresponding 4OFF groups and groups expressing WT Pro-GFPTet (Fig. 4). A similar pattern of changes was observed for the apoptotic indices determined for cells expressing the R789C mutant (Fig. 5). Changes in the apoptotic indices of cells expressing the R992C mutant were less apparent.
Figure 5.

Graphic representation of the apoptotic indices of cells expressing 100% Pro-GFPTet variants as determined by TUNEL assays. Individual bars represent mean values for apoptotic indices. The statistical significance of differences between specific means are indicated. Symbols: WT, R789C, R992C, groups expressing wild type collagen II or specific mutants, respectively; 2ON, 4ON, 2ON/2OFF, and 4OFF describe specific regimes of cell cultures.
Unlike groups in which the expression of mutant collagen was completely eliminated, those with only a partial reduction of mutant molecules did not show a decrease in the relative amounts of cPARP in the 2ON/2OFF groups (Fig. 4). Similarly, when compared to the group constantly cultured in ON conditions, no changes were observed in the relative amounts of PDI in groups that had only a partial reduction (Fig. 4).
Deposition of FN in the Presence or Absence of R789C and R992C Mutants
The effects of reducing the amount of R789C or R992C collagen on the deposition of the ECM macromolecules were analyzed by monitoring FN. Fibronectin is a relevant indicator of potential changes because this protein contributes to the extracellular milieu formed by complex interactions of various macromolecules, because it colocalizes with collagenous fibrillar networks and because the formation of FN networks may be affected by the presence of thermolabile collagen II mutants [Dzamba et al., 1993; Ito et al., 2005; Little and Chen, 1982; Velling et al., 2002]. Immunofluorescence microscopy was employed to image extracellular FN fibrils in cells expressing 100% Pro-GFPTet variants. Immunofluorescence of cells expressing WT Pro-GFPTet demonstrated abundant deposits that appear to closely surround the cell surfaces (Fig. 6). FN deposits produced by cells harboring R789C or R992C mutants and cultured in the 4OFF and 2ON/2OFF conditions displayed similar morphology. In contrast, FN deposits produced by cells expressing R789C or R992C in the 4ON condition appear less dense than their 4OFF counterparts (Fig. 6).
Figure 6.

Representative immunofluorescence images of the FN network surrounding cells expressing 100% Pro-GFPTet variants. The upper images (A, B, and C) represent those that were used to analyze the surface area occupied by the FN network. The bottom panels (D) are enlarged images displaying more detailed characteristics of FN deposits formed by analyzed cells cultured in 4ON and 4OFF conditions. Symbols: WT, R789C, R992C, groups expressing wild-type collagen II or specific mutants, respectively; FN, FN; 2ON, 4ON, 2ON/2OFF, and 4OFF describe specific regimes of cell cultures.
In agreement with a previous report, Western blot assays of the 100% Pro-GFPTet group have demonstrated that the overall amount of FN in the cell layers of WT, R789C and R992C groups was similar regardless of cell culture conditions (Fig. 7A) [Ito et al., 2005]. However, microscopic analyses of the surface areas occupied by extracellular FN deposits demonstrated differences among the groups. Specifically, cells expressing mutant ProGFPTet variants in the 4ON condition had significantly less FN deposited in the extracellular space when compared to the WT 4ON group. The surface area of FN fibrils surrounding cells expressing R789C or R992C in the 4OFF or 2ON/2OFF conditions was comparable to that of cells in the WT 4ON condition (Fig. 7B). However, in groups in which the amount of mutant collagen was only partially reduced, the total area occupied by FN fibrils was significantly less when compared to the corresponding matrices formed by cells expressing WT collagen II in 4OFF conditions (Fig. 7C).
Figure 7.
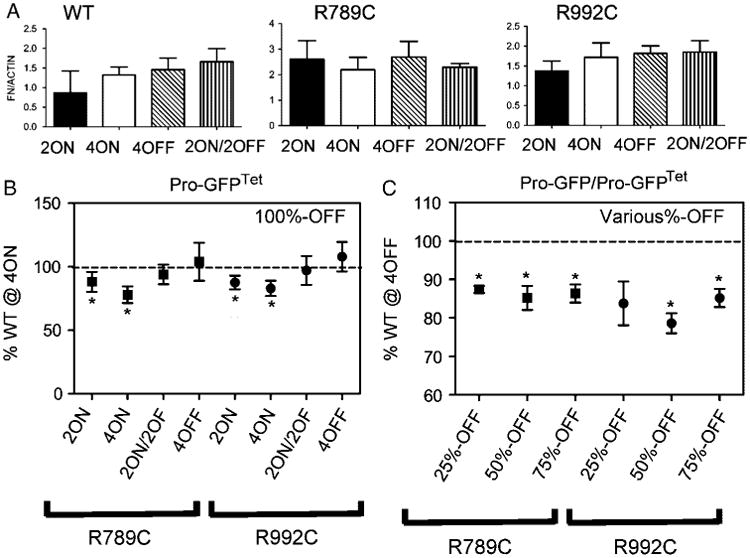
Analyses of FN in cell/matrix systems formed in the presence of the R789C or R992C collagen II mutants after completely or partially reducing expression of those mutants. Western blot-based analyses (A) of the 100% Pro-GFPTet group were performed to evaluate the total amount of FN in the cell layers. Microscopic analysis of FN fibrils (Fig. 6) deposited by cells expressing R789C or R992C after complete (B) or partial (C) reduction of expression of those mutants were also performed. Individual bars represent mean values (A). In the 100%-OFF group (B), comparisons were performed between R789C or R992C cultured in specific ON/OFF conditions and the group expressing WT collagen II cultured in 4ON conditions. In this control group the surface area occupied by FN was considered 100%. For groups in which the expression of analyzed mutants was switched off in a specific subpopulation of cells (C), comparisons were done between each group cultured in the 4OFF conditions and the value obtained for WT collagen II in the 4OFF conditions, considered 100%. Groups with statistically significant differences between relevant means (P <0.05) are indicated with asterisks. Symbols: WT, R789C, R992C, groups expressing wild-type collagen II or specific mutants, respectively; 2ON, 4ON, 2ON/2OFF, and 4OFF describe specific regimes of cell cultures; 25%-OFF, 50%-OFF, and 75%-OFF indicate groups with a specific percentage of cells expressing Pro-GFPTet variants. Dotted lines represent values (considered 100% production) for the surface areas of FN deposited by cells expressing the WT collagen II in 4ON conditions (A) or 4OFF conditions (B).
Diffusional Mobility of the Thermolabile R992C Pro-GFP Mutant
The intracellular diffusional mobility of fluorophore-tagged proteins decreases as a result of their interactions with various macromolecules [Reits and Neefjes, 2001]. The results of FRAP assays indicate that in comparison to the Pro-GFP WT collagen II, the diffusional mobility of the R992C mutant was reduced (Supp. Fig. S3). This data is consistent with previous work that demonstrated that the diffusional mobility of the R789C mutant is also reduced due to its interaction with FN [Ito et al., 2005].
Alterations in Cell Motility in Response to Switching Off Expression of Collagen Mutants
Migration assays have determined that the average distance of migration of untransfected SW-1353 cells was decreased on an ECM formed by R789C or R992C cells in the ON conditions. This decrease is not observed in cells migrating on a WT-derived ECM, or on R789C and R992C-derived ECMs formed in the OFF conditions (Supp. Fig. S4).
Analysis of Glycosaminoglycans
Total production of glycosaminoglycans in 3D constructs and high-density cell layers was analyzed by histology (Fig. 2) and by biochemical assays (not shown). No observable changes were seen, indicating that the overall deposition of glycosaminoglycans was unaffected by the presence of mutant procollagen.
Discussion
Among mutations in fibrillar collagens, those that cause a decrease in the thermostability of the triple helix are associated with distinct pathomechanisms that not only involve formation of structurally inferior mutant molecules, which may have a limited ability to form critical fibrillar structures, but may also cause complex alterations in cellular function due to atypical intracellular retention [Chung et al., 2009; Hintze et al., 2008; Ito et al., 2005]. Although the decreased thermostability of certain collagen II mutants cannot be the sole factor in determining the severity of a disease, of note is the observation that the presence of the thermostable R75C (p.R275C) and R519C (p.R719C) collagen II mutants is associated with a relatively mild form of SED and mild SED with early-onset osteoarthritis, respectively [Bleasel et al., 1995, 1998; Fertala et al., 1997; Steplewski et al., 2004b; Williams et al., 1993]. In contrast, the thermolabile R789C and R992C collagen II mutants are associated with severe skeletal malformations [Chan et al., 1993; Chung et al., 2009; Donahue et al., 2003; Steplewski et al., 2004b].
It has been suggested that regardless of the mutation type, a decrease in the total collagen content is a common characteristic of affected tissues [Royce and Steinmann, 2002]. Aside from mutations causing haploinsufficiency, the leading concept explaining this phenomenon was that of “collagen suicide,” which suggests that the presence of mutant α chains in collagen molecules causes their premature degradation [Prockop, 1984]. Together, collagen suicide and the fact that most spontaneous mutations in collagens affect only one allele form the premise of therapeutic approaches. Specifically, two major techniques to increase the amount of WT collagen in affected tissues have emerged: (1) specific inhibition of the mutant allele and (2) cell therapy [Forlino and Marini, 2000]. In the first approach, it is assumed that blocking a mutant allele would result in the production of only WT collagen chains, thereby eliminating collagen suicide and ultimately increasing the amount of collagen in affected tissues. In the second approach, the goal is to not only increase the amount of WT collagen molecules, thereby effectively decreasing the relative amount of molecules harboring mutant α chains, but also to create a mosaic with centers of production of normal tissue. Although some of these methods have been tested in cell culture, mouse models or in patients, none of them were scrutinized with the intent of determining the extent of reduction of the amount of mutant α chains or the number of cells needed to change the direction of processes occurring in the presence of mutant molecules from pathological to physiological.
To address this problem, here we employed a biologically relevant experimental system in which the formation of cell/ matrix structures was initiated in the presence of mutant molecules and then continued after completely eliminating or partially reducing expression of mutant collagen. The consequences of reducing the amount of mutant molecules in cell/ matrix structures were followed by measurements of intracellular and extracellular parameters that have previously been shown to change in the presence of the R789C and R992C mutants [Chung et al., 2009; Hintze et al., 2008].
Quantitative studies of PDI, cPARP, and DNA fragmentation, indicators of ER stress and apoptosis, suggest that these processes are significantly attenuated in systems expressing thermolabile R789C or R992C collagen II mutants only after the production of those mutants is switched off completely. In contrast, partial reduction of mutant molecules neither decreases the extent of apoptosis nor reduces ER stress in the conditions examined. Of particular interest was the observation made after analysis of the cPARP data that only switching off 100% expression of the R789C or R992C mutants led to a decrease in the cleavage of PARP. The extent of apoptosis was unchanged in groups in which the content of mutant protein was reduced to 23, 15, or 7% of the total collagen pool (see Table 1). Although it was expected that in subpopulations of cells with continuing expression of R789C or R992C apoptotic processes would persist, the initial prediction was that once Dox was removed, the majority of cells expressing the Pro-GFPTet variant would then be able to recover. Because our data demonstrates a persistence of apoptosis in groups with only partial reduction of mutant collagen, we postulate that even in the subpopulation of cells in which the expression of collagen II mutants was switched off, the processes leading to cell death could still continue due to alterations within the ECM caused by continuing production of Pro-GFP mutants by that subpopulation of cells. Interestingly, there were also no apparent differences in the expression of PDI in groups in which the relative amounts of mutants were consistently at 23, 15, or 7% (4OFF conditions) when compared to the groups that initially had 100% expression of mutant collagen followed by a reduction to 23, 15, or 7% of this initial amount (2ON/2OFF conditions).
We followed the changes in ECM structure through microscopic assays of the FN network, which is an appropriate indicator of changes because its secretion and assembly is directly affected by the presence of thermolabile collagen II mutants [Ito et al., 2005]. The results of FN assays indicate that the overall structure and organization of the ECM did not improve after partially reducing the amount of collagen II mutants. However, the overall structure of matrices initially formed in the presence of these specific mutants improved after switching off their production completely. The observed improvement in the structure and composition of the matrices assembled in the absence of mutant collagens is further supported by observations of enhanced mobility of cells on the surfaces of such matrices.
Our study suggests that the presence of R789C and R992C mutants changes the cellular behavior not only through excessive intracellular accumulation of mutant molecules, but also by affecting the properties of the ECM relevant to cell survival activities. The presented studies, however, did not specifically address which downstream elements of the critical ECM-dependent cell signaling mechanism could be potentially affected in the system employed here, but the dependency of cell survival on the correct composition and structure of the ECM is well established. For instance, it has been demonstrated that in the absence of collagen II, chondrocytes undergo apoptosis [Yang et al., 1997]. Moreover, cartilage homeostasis is regulated in part through α5β1 integrin, a receptor also present in SW-1353 cells, which mediates the interactions of chondrocytes with FN. Changes in FN-dependent signaling influence a number of processes in chondrocytes and may impact their survival and migration characteristics [Burton-Wurster et al., 1997; Durr et al., 1993; Gebauer et al., 2005; Martin and Buckwalter, 1998]. Although there appear to be no significant differences in the total production of FN as demonstrated by Western blot, specific analysis of the ECM through immunofluorescence microscopy displayed differences in the deposition patterns and morphology of the FN network. We have previously demonstrated that the relatively slow diffusional mobility of the Pro-GFP R789C mutant was a result of the formation of abnormal FN-mutant collagen II complexes [Ito et al., 2005]. Data presented here indicate that there are alterations in the structure of the extracellular deposits of FN without apparent changes in its total content in analyzed cell layers formed by cells expressing both R789C and R992C mutations. Taken together with the FRAP data reported here for R992C, these observations may indicate that atypical binding to FN and perhaps other macromolecules may be a common characteristic of thermolabile collagen II mutants.
Similarly, there were no significant changes observed in the total amount of proteoglycans or the extent of sulfation of glycosaminoglycans in all analyzed groups. Thus, these results further indicate that alterations in the amount of the major extracellular macromolecules may not be the only cause of pathological changes triggered by the presence of collagen mutants [Chung et al., 2009; Hintze et al., 2008; Ito et al., 2005].
Although the employed experimental system provides an adequate model for measuring intracellular and extracellular changes occurring in the presence or the absence of mutant collagen, it also has some limitations inheritably associated with cell-based models. Thus, the relevance of our observations for native tissues harboring collagen mutations is, at present, unclear. First, despite the fact that SW-1353 cells express a number of macromolecules typical for chondrocytes and respond in a similar way to many physiological and pathological factors, the system we employed does not mimic all characteristics of native cartilage. Second, most mutations in collagen genes cause single amino acid substitutions, so, at the protein level, it is difficult to distinguish mutant chains from the wild-type ones and determine their actual contribution to the total collagen pool. In support of our results, however, are certain observations that suggest that a very small percentage of mutant molecules seen in the extracellular space may cause significant pathological effects. For instance, an earlier study of a thermolabile collagen I mutant in which the endogenous matrix metalloproteinase 1 (MMP-1)-cleavage site had been disrupted made it possible to determine the percentage of MMP-1-resistant mutant α1(I) chains in the total pool of collagen I. Even though the mutant α1 chains contributed only about 10% to the total pool of collagen present in the extracellular space, the clinical consequences of mutations were severe [Cabral et al., 2002]. Another recent study involving collagen VII mutants demonstrated that coexpression of WT:mutant collagen in a 1:1 ratio led to a large decrease in thermostability, and only by increasing the ratio to 9:1 was thermal stability significantly increased to near WT values [Fritsch et al., 2009].
Our results demonstrating the negative effects of relatively small amounts of R789C and R992C mutants are somewhat in contrast with those obtained for the R519C collagen II mutant extracted from the tissue of an affected patient [Eyre et al., 1991]. The authors determined that the mutant chains contributed about 25% to the total pool of extracellular collagen II. Despite the relatively large contribution of mutant collagen to the total pool, no evidence of severe chondrodysplasia was present in affected patients [Katzenstein et al., 1990; Knowlton et al., 1990]. It is not clear why the R519C mutant does not produce a severe form of chondrodysplasia, but of note is the observation that at the molecular level, the R519C mutation does not cause major changes to the triple-helical structure. Specifically, it has been determined that only a relatively small percentage of mutant chains form dimers stabilized by disulfide bonds, and that the thermostability of mutant molecules is normal [Eyre et al., 1991; Fertala et al., 1997, 2001]. Still, it has been determined that the ability of the R519C mutant to form fibrils in vitro is significantly altered, thereby suggesting that aberrations of fibril formation could be part of the pathomechanism associated with the development of early onset osteoarthritis in patients harboring the R519C mutation [Adachi et al., 1999; Fertala et al., 1997].
Collectively, our study presented here indicates that even relatively small amounts of certain mutant collagens can affect cell behavior, ECM structure, and alter interactions between affected collagen fibrils and other matrix molecules. From a biological standpoint, this phenomenon indicates that native collagen-rich cell–matrix systems are highly specialized and cannot adapt well to the presence of mutant molecules, especially those that cause significant structural changes that lead to reduced thermostability of the collagen triple helix.
Based on the study presented here, we postulate that regardless of the mutation type or the total amount of mutant molecules present in the ECM, the aim of future approaches to attenuate the harmful effects of mutations in collagens should be to develop methods to block expression of a mutant allele completely, reduce the content of mutant molecules in the total pool of collagen to a background level, or to improve their structure by employing chemical chaperones or other molecules that may increase the total amount of stable collagen molecules incorporated into the ECM.
Acknowledgments
We are grateful to Dr. Maria Y. Covarrubias for her assistance with confocal microscopy assays performed at the Bioimaging Facility of the Kimmel Cancer Center.
Contract grant sponsor: The National Institutes of Health; Contract grant number: 5R01AR049537-08 (to A.F).
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Adachi E, Katsumata O, Yamashina S, Prockop DJ, Fertala A. Collagen II containing a Cys substitution for Arg-alpha1–519. Analysis by atomic force microscopy demonstrates that mutated monomers alter the topography of the surface of collagen II fibrils. Matrix Biol. 1999;18:189–196. doi: 10.1016/s0945-053x(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Bienkowski RS, Curran SF, Berg RA. Kinetics of intracellular degradation of newly synthesized collagen. Biochemistry. 1986;25:2455–2459. doi: 10.1021/bi00357a024. [DOI] [PubMed] [Google Scholar]
- Bleasel JF, Bisagni-Faure A, Holderbaum D, Vacher-Lavenu MC, Haqqi TM, Moskowitz RW, Menkes CJ. Type II procollagen gene (COL2A1) mutation in exon 11 associated with spondyloepiphyseal dysplasia, tall stature and precocious osteoarthritis. J Rheumatol. 1995;22:255–261. [PubMed] [Google Scholar]
- Bleasel JF, Holderbaum D, Brancolini V, Moskowitz RW, Considine EL, Prockop DJ, Devoto M, Williams CJ. Five families with arginine 519-cysteine mutation in COL2A1: evidence for three distinct founders. Hum Mutat. 1998;12:172–176. doi: 10.1002/(SICI)1098-1004(1998)12:3<172::AID-HUMU4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bleasel JF, Holderbaum D, Brancolini V, Moskowitz RW, Haqqi TM, Considine E, Prockop DJ, Devoto M, Williams CJ. Arg519-Cys mutation in COL2A1: evidence for multiple founders. Ann N Y Acad Sci. 1996a;785:215–218. doi: 10.1111/j.1749-6632.1996.tb56265.x. [DOI] [PubMed] [Google Scholar]
- Bleasel JF, Holderbaum D, Mallock V, Haqqi TM, Williams HJ, Moskowitz RW. Hereditary osteoarthritis with mild spondyloepiphyseal dysplasia—are there “hot spots” on COL2A1? J Rheumatol. 1996b;23:1594–1598. [PubMed] [Google Scholar]
- Burton-Wurster N, Lust G, Macleod JN. Cartilage fibronectin isoforms: in search of functions for a special population of matrix glycoproteins. Matrix Biol. 1997;15:441–454. doi: 10.1016/s0945-053x(97)90018-4. [DOI] [PubMed] [Google Scholar]
- Cabral WA, Fertala A, Green LK, Korkko J, Forlino A, Marini JC. Procollagen with skipping of alpha 1(I) exon 41 has lower binding affinity for alpha 1(I) C-telopeptide, impaired in vitro fibrillogenesis, and altered fibril morphology. J Biol Chem. 2002;277:4215–4222. doi: 10.1074/jbc.M109048200. [DOI] [PubMed] [Google Scholar]
- Chan D, Taylor TK, Cole WG. Characterization of an arginine 789 to cysteine substitution in alpha 1 (II) collagen chains of a patient with spondyloepiphyseal dysplasia. J Biol Chem. 1993;268:15238–15245. [PubMed] [Google Scholar]
- Chon JH, Wang HS, Chaikof EL. Role of fibronectin and sulfated proteoglycans in endothelial cell migration on a cultured smooth muscle layer. J Surg Res. 1997;72:53–59. doi: 10.1006/jsre.1997.5168. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Jensen DA, Gawron K, Steplewski A, Fertala A. R992C (p.R1192C) Substitution in collagen II alters the structure of mutant molecules and induces the unfolded protein response. J Mol Biol. 2009;390:306–318. doi: 10.1016/j.jmb.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Marini JC. Hammerhead ribozymes selectively suppress mutant type I collagen mRNA in osteogenesis imperfecta fibroblasts. Nucleic Acids Res. 2000;28:4013–4020. doi: 10.1093/nar/28.20.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue LR, Chang B, Mohan S, Miyakoshi N, Wergedal JE, Baylink DJ, Hawes NL, Rosen CJ, Ward-Bailey P, Zheng QY, Bronson RT, Johnson KR, Davisson MT. A missense mutation in the mouse Col2a1 gene causes spondyloepiphyseal dysplasia congenita, hearing loss, and retinoschisis. J Bone Miner Res. 2003;18:1612–1621. doi: 10.1359/jbmr.2003.18.9.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durr J, Goodman S, Potocnik A, von der Mark H, von der Mark K. Localization of beta 1-integrins in human cartilage and their role in chondrocyte adhesion to collagen and fibronectin. Exp Cell Res. 1993;207:235–244. doi: 10.1006/excr.1993.1189. [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Wu H, Jaenisch R, Peters DM. Fibronectin binding site in type I collagen regulates fibronectin fibril formation. J Cell Biol. 1993;121:1165–1172. doi: 10.1083/jcb.121.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre DR, Weis MA, Moskowitz RW. Cartilage expression of a type II collagen mutation in an inherited form of osteoarthritis associated with a mild chondrodysplasia. J Clin Invest. 1991;87:357–361. doi: 10.1172/JCI114994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertala A, Ala-Kokko L, Wiaderkiewicz R, Prockop DJ. Collagen II containing a Cys substitution for arg-alpha1–519. Homotrimeric monomers containing the mutation do not assemble into fibrils but alter the self-assembly of the normal protein. J Biol Chem. 1997;272:6457–6464. doi: 10.1074/jbc.272.10.6457. [DOI] [PubMed] [Google Scholar]
- Fertala A, Sieron AL, Adachi E, Jimenez SA. Collagen II containing a Cys substitution for Arg-alpha1–519: abnormal interactions of the mutated molecules with collagen IX. Biochemistry. 2001;40:14422–14428. doi: 10.1021/bi0109109. [DOI] [PubMed] [Google Scholar]
- Forlino A, Marini JC. Osteogenesis imperfecta: prospects for molecular therapeutics. Mol Genet Metab. 2000;71:225–232. doi: 10.1006/mgme.2000.3039. [DOI] [PubMed] [Google Scholar]
- Fritsch A, Spassov S, Elfert S, Schlosser A, Gache Y, Meneguzzi G, Bruckner-Tuderman L. Dominant-negative effects of COL7A1 mutations can be rescued by controlled overexpression of normal collagen VII. J Biol Chem. 2009;284:30248–30256. doi: 10.1074/jbc.M109.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer M, Saas J, Sohler F, Haag J, Soder S, Pieper M, Bartnik E, Beninga J, Zimmer R, Aigner T. Comparison of the chondrosarcoma cell line SW1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to IL-1beta. Osteoarthritis Cartilage. 2005;13:697–708. doi: 10.1016/j.joca.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Hintze V, Steplewski A, Ito H, Jensen DA, Rodeck U, Fertala A. Cells expressing partially unfolded R789C/p.R989C type II procollagen mutant associated with spondyloepiphyseal dysplasia undergo apoptosis. Hum Mutat. 2008;29:841–851. doi: 10.1002/humu.20736. [DOI] [PubMed] [Google Scholar]
- Hoornaert KP, Dewinter C, Vereecke I, Beemer FA, Courtens W, Fryer A, Fryssira H, Lees M, Mullner-Eidenbock A, Rimoin DL, Siderius L, Superti-Furga A, Temple K, Willems PJ, Zankl A, Zweier C, De Paepe A, Coucke P, Mortier GR. The phenotypic spectrum in patients with arginine to cysteine mutations in the COL2A1 gene. J Med Genet. 2006;43:406–413. doi: 10.1136/jmg.2005.035717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–1231. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- Ito H, Rucker E, Steplewski A, McAdams E, Brittingham RJ, Alabyeva T, Fertala A. Guilty by association: some collagen II mutants alter the formation of ECM as a result of atypical interaction with fibronectin. J Mol Biol. 2005;352:382–395. doi: 10.1016/j.jmb.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Ito H, Steplewski A, Alabyeva T, Fertala A. Testing the utility of rationally engineered recombinant collagen-like proteins for applications in tissue engineering. J Biomed Mater Res A. 2006;76:551–560. doi: 10.1002/jbm.a.30551. [DOI] [PubMed] [Google Scholar]
- Katzenstein PL, Malemud CJ, Pathria MN, Carter JR, Sheon RP, Moskowitz RW. Early-onset primary osteoarthritis and mild chondrodysplasia. Radiographic and pathologic studies with an analysis of cartilage proteoglycans. Arthritis Rheum. 1990;33:674–684. doi: 10.1002/art.1780330510. [DOI] [PubMed] [Google Scholar]
- Knowlton RG, Katzenstein PL, Moskowitz RW, Weaver EJ, Malemud CJ, Pathria MN, Jimenez SA, Prockop DJ. Genetic linkage of a polymorphism in the type II procollagen gene (COL2A1) to primary osteoarthritis associated with mild chondrodysplasia. N Engl J Med. 1990;322:526–530. doi: 10.1056/NEJM199002223220807. [DOI] [PubMed] [Google Scholar]
- Little CD, Chen WT. Masking of extracellular collagen and the co-distribution of collagen and fibronectin during matrix formation by cultured embryonic fibroblasts. J Cell Sci. 1982;55:35–50. doi: 10.1242/jcs.55.1.35. [DOI] [PubMed] [Google Scholar]
- Martin JA, Buckwalter JA. Effects of fibronectin on articular cartilage chondrocyte proteoglycan synthesis and response to insulin-like growth factor—I. J Orthop Res. 1998;16:752–757. doi: 10.1002/jor.1100160618. [DOI] [PubMed] [Google Scholar]
- Olsen BR. Mutations in collagen genes resulting in metaphyseal and epiphyseal dysplasias. Bone. 1995;17:45S–49S. doi: 10.1016/8756-3282(95)00208-u. [DOI] [PubMed] [Google Scholar]
- Peace BE, Florer JB, Witte D, Smicun Y, Toudjarska I, Wu G, Kilpatrick MW, Tsipouras P, Wenstrup RJ. Endogenously expressed multimeric self-cleaving hammerhead ribozymes ablate mutant collagen in cellulo. Mol Ther. 2005;12:128–136. doi: 10.1016/j.ymthe.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Pereira RF, Halford KW, O'Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci USA. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ. Osteogenesis imperfecta: phenotypic heterogeneity, protein suicide, short and long collagen. Am J Hum Genet. 1984;36:499–505. [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Reits EA, Neefjes JJ. From fixed to FRAP: measuring protein mobility and activity in living cells. Nat Cell Biol. 2001;3:E145–E147. doi: 10.1038/35078615. [DOI] [PubMed] [Google Scholar]
- Royce PM, Steinmann B. Connective tissue and its heritable disorders: molecular, genetic, and medical aspects. New York: John Wiley; 2002. [Google Scholar]
- Steplewski A, Brittingham R, Jimenez SA, Fertala A. Single amino acid substitutions in the C-terminus of collagen II alter its affinity for collagen IX. Biochem Biophys Res Commun. 2005;335:749–755. doi: 10.1016/j.bbrc.2005.07.139. [DOI] [PubMed] [Google Scholar]
- Steplewski A, Ito H, Rucker E, Brittingham RJ, Alabyeva T, Gandhi M, Ko FK, Birk DE, Jimenez SA, Fertala A. Position of single amino acid substitutions in the collagen triple helix determines their effect on structure of collagen fibrils. J Struct Biol. 2004a;148:326–337. doi: 10.1016/j.jsb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Steplewski A, Majsterek I, McAdams E, Rucker E, Brittingham RJ, Ito H, Hirai K, Adachi E, Jimenez SA, Fertala A. Thermostability gradient in the collagen triple helix reveals its multi-domain structure. J Mol Biol. 2004b;338:989–998. doi: 10.1016/j.jmb.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377–37381. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- Vernon RB, Gooden MD. An improved method for the collagen gel contraction assay. In Vitro Cell Dev Biol Anim. 2002;38:97–101. doi: 10.1290/1071-2690(2002)038<0097:AIMFTC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Vernon RB, Sage EH. A novel, quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvasc Res. 1999;57:118–133. doi: 10.1006/mvre.1998.2122. [DOI] [PubMed] [Google Scholar]
- Wang Q, Marini JC. Antisense oligodeoxynucleotides selectively suppress expression of the mutant alpha 2(I) collagen allele in type IV osteogenesis imperfecta fibroblasts. A molecular approach to therapeutics of dominant negative disorders. J Clin Invest. 1996;97:448–454. doi: 10.1172/JCI118434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CJ, Considine EL, Knowlton RG, Reginato A, Neumann G, Harrison D, Buxton P, Jimenez S, Prockop DJ. Spondyloepiphyseal dysplasia and precocious osteoarthritis in a family with an Arg75→Cys mutation in the procollagen type II gene (COL2A1) Hum Genet. 1993;92:499–505. doi: 10.1007/BF00216458. [DOI] [PubMed] [Google Scholar]
- Yang C, Li SW, Helminen HJ, Khillan JS, Bao Y, Prockop DJ. Apoptosis of chondrocytes in transgenic mice lacking collagen II. Exp Cell Res. 1997;235:370–373. doi: 10.1006/excr.1997.3692. [DOI] [PubMed] [Google Scholar]


