Abstract
Endostatin (ES), the C-terminal fragment of collagen XVIII known for its anti-angiogenic properties, is associated with neurological diseases in mammals. In this study, we investigated the effect of ES on nerve growth factor (NGF)-induced neuronal differentiation, migration, neuritogenesis, and neurite extension. ES partially inhibited PC12 cell differentiation and cerebellar granule cell migration. In addition, neurite outgrowth was inhibited in a concentration-dependent manner. This effect was also matrix-dependent, as we observed better inhibition on PC12 cells grown on collagen compared to laminin matrices. Furthermore, we observed partial NGF depletion by collagen and ES, but not by laminin suggesting that NGF–matrix interactions may be important for promoting neuritogenesis, competitive inhibition by ES or low affinity matrix impairs PC12 differentiation and neurite outgrowth. Finally, using a biosensor technique, we demonstrated a direct interaction between NGF and ES suggesting the mechanism of action of ES may involve NGF sequestration. In conclusion, our study demonstrates the inhibitory effect of ES on different steps of neurogenesis including cell differentiation and migration and neuritogenesis by NGF sequestration. Such sequestration may compromise brain repair following injury, but also may play important role in axon finding as well as a potent therapeutical target in diseases involving abnormal elevated neurotrophic growth factor levels. Taken together, this study raises the consideration of ES as a double-edge sword that carries both deleterious and putative therapeutical effects.
Keywords: Endostatin, Nerve growth factor, Neurite outgrowth, Collagen, Laminin
1. Introduction
Neurons constitute a remarkably conserved cell type through animal lineage. An important feature displayed by neuronal cells is their ability to establish contacts with surrounding cells by formation of synapses linking axon to dendrites. The development of these cellular structures occurs by neurite formation and subsequent neurite outgrowth. Although the literature describes the important roles played by signaling molecules such as semaphorins or ephrins on axonal guidance (Bolsover et al., 2008; Roth et al., 2009), the modulation of such features by extracellular matrix (ECM) components remains less well understood. Until recently, ECM has been considered to be mostly proteoglycan complex with restricted scaffold function. Endostatin (ES) (O’Reilly et al., 1997), an anti-angiogenic, 20 kDa C-terminal cleavage product of the heparan sulfate proteoglycan, collagen XVIII, surrounds endothelial and epithelial cells of the central nervous system. Although the importance of ES in the central nervous system has been highlighted in studies pertaining to the maintenance of ocular structure (for review see (Marneros and Olsen, 2005)), the possible effect of ES on neurons still remains poorly understood. Interestingly, different studies performed in Caenorhabditis elegans (Ackley et al., 2001) and Drosophila melanogaster (Meyer and Moussian, 2009) highlight the positive role played by ES homologs on axonal guidance. On the other hand, ES has also been negatively associated with certain neurological diseases in mammals. Other studies suggest the involvement of ES in Alzheimer’s disease by showing ES deposits among amyloid plaques around neurons (Deininger et al., 2002) and cerebral vasculature (Gebbink et al., 2004; van Horssen et al., 2002). In addition, Tian et al. (2007) demonstrated elevated plasma levels of ES following stroke insult, whereas other studies (Hou et al., 2010; Kranenburg et al., 2003) noted the deposition of ES around apoptotic neurons. In addition to these neurological diseases, increased ES levels following traumatic brain injury (Zhang et al., 2007) as well as in Down syndrome patients have been reported (Zorick et al., 2001). Taken together, these studies would seemingly suggest a negative role on neurite outgrowth in mammalian cells. In this study, we investigated the effect of ES on neurogenesis using PC12 cells and cerebellar granular neurons as in vitro models of neurite outgrowth (Greene and Tischler, 1976; Vaudry et al., 2002) and neuronal migration (Bix and Clark, 1998; Clark et al., 1995), respectively.
2. Results
2.1. ES inhibits neurite outgrowth, PC12 cell differentiation and granular neuron migration
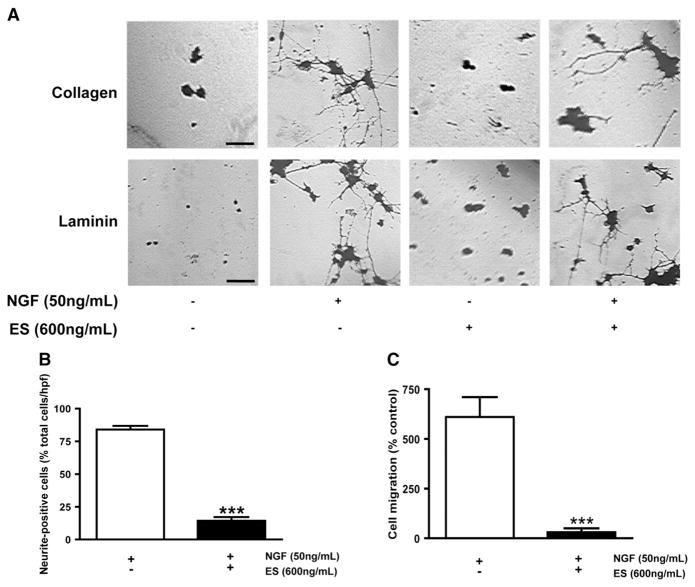
We first investigated the global effect of ES treatment over NGF-induced neurite outgrowth in PC12 cells by microscopy (Fig. 1A). The presence of NGF (50 ng/mL) was necessary to induce neurite-positive cells after 6 days of culture on both collagen and laminin. ES alone (600 ng/mL) was unable to induce neurite outgrowth in PC12 cells on both matrices. Interestingly, ES inhibited NGF-induced neurite outgrowth. This inhibition was further investigated by quantifying the average number of neurite-positive cells in the absence or presence of ES (Fig. 1B). We observed that NGF alone induced differentiation in PC12 population with an average value of 84.15±2.82% neurite-positive cells/hpf. Addition of ES significantly decreased (***P<0.0001) this average value to 14.44±2.77% suggesting that ES inhibits PC12 cell differentiation. We further investigated the effect of ES on cell migration (Fig. 1C) using primary culture of rat cerebellar granule cells, a technique classically used as an in vitro model of cell migration (Bix and Clark, 1998; Burgoyne and Cambray-Deakin, 1988; Clark et al., 1995). In the absence of NGF as a chemoattractant, cell migration was negligible and served as the negative control. The presence of NGF dramatically induced cerebellar granule cell by showing an increase of 613.2±50.6%. Further addition of ES completely abrogated granule cell migration (***P=0.0006), showing a value of only 15.6±6.17%. In conclusion, we observed that ES inhibits PC12 cell differentiation, neuritogenesis, and granular neuron migration.
Fig. 1.
ES inhibits neurite outgrowth, cell differentiation and cell migration. (A) Representative micrograph picture of PC12 cells grown for 6 days. Pictures represent a 100× magnification. (B) Presence of ES inhibits PC12 cell differentiation. N=6, ***P<0.001 versus NGF alone. (C) ES inhibits cerebellar granule cell migration. N=6, ***P<0.001.
2.2. ES inhibits PC12 cell differentiation in a concentration-dependent manner
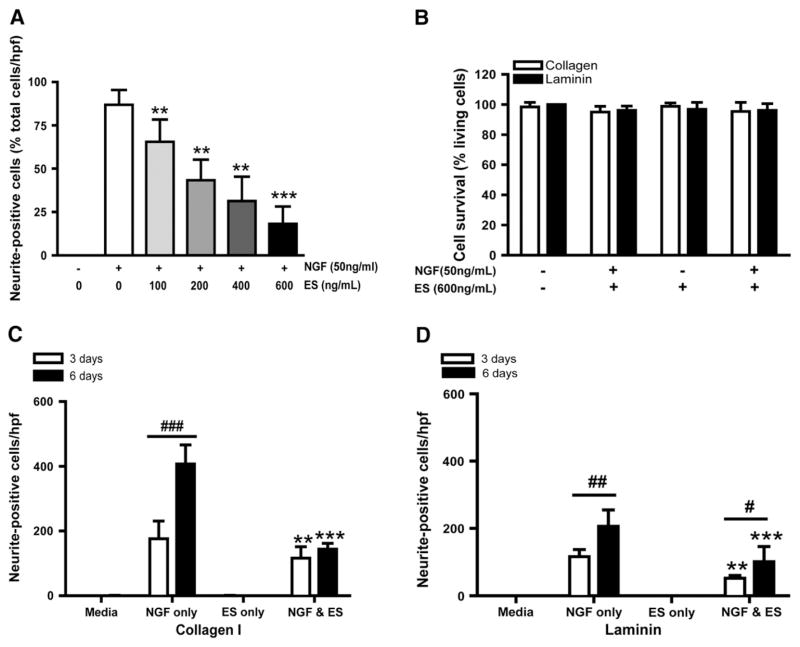
We next investigated if ES inhibition was dose dependent. Surprisingly, Fig. 2A demonstrates that relatively low ES concentrations (100 ng/mL) could inhibit the number of PC12 neurite-positive cells. Specifically, the average number of neurite-positive cells decreased from 86.87±8.48% to 65.52± 12.86%. Maximum inhibition was obtained at a concentration of 600 ng/mL (***P<0.0001) by showing the presence of only 18.09±10.08% of neurite-positive cells. This concentration of ES did not effect PC12 cell survival (Fig. 2B) demonstrating that ES’ inhibitory effects were unlikely due to cytotoxicity. In summary, these experiments demonstrate that ES inhibits PC12 cell differentiation in a dose-dependent fashion, without affecting cell survival.
Fig. 2.
ES activity is concentration-dependent, substrate-independent and not cytotoxic (A) ES activity was proportional to its concentration. Increased concentration in ES (from 100 to 600 ng/mL) resulted in progressive decrease in neurite-positive cells after 3 days of culture. N=6, **P<0.01 versus NGF-treated group. (B) Absence of decreased cell survival after 6 days of continuous exposure to ES in absence or presence of NGF, both on collagen (white bars) or laminin (black bars) substrates. N=4. ES inhibits PC12 differentiation into neurite-positive cells at 3 days (white bars) and 6 days (black bars) on cells grown on collagen (C) or laminin (D). N=4. **P<0.01, ***P<0.01 versus NGF-treated group. #P<0.05, ##P<0.01, ###P<0.001 in comparison between NGF and NGF/ES treatment.
2.3. ES inhibits NGF-induced PC12 differentiation on both collagen and laminin substrates
NGF-induced PC12 differentiation constitutes the initial step in neurite outgrowth. In the next series of experiments, we quantified the number of PC12 cells undergoing cell differentiation (Fig. 2C and D) on different substrates. Medium and ES groups demonstrated a complete absence of neurite-positive cells on collagen-coated wells (Fig. 2C). As expected, NGF was required to induce neurite-positive cells at 3 days (175.50±55.51 positive cells/well), reaching a maximum after 6 days of culture (407.30±58.59). ES provided significant inhibition after 3 days (115.50±35.87 positive cells/area, ***P<0.0001) and 6 days (144.40±17.53) demonstrating that ES inhibits NGF-induced PC12 differentiation. As observed with collagen, laminin did not elicit PC12 differentiation in both control and ES groups (Fig. 2D). Despite NGF-induced formation of neurite-positive cells, their absolute numbers were significantly lower than those grown on collagen either after 3 or 6 days of culture (116.30±21.45 and 206.80±48.45 respectively) in accordance with previous literature (Attiah et al., 2003). As on collagen, ES was able to partially inhibit NGF activity by decreasing the mean number of neurite-positive cells present at both 3 days (52.00±8.75, **P=0.0024) and 6 days (101.2±44.89, ***P=0.0009). These results suggest the possible inhibition of NGF-induced PC12 differentiation by ES. Furthermore, a comparison between the effect of collagen and laminin over ES inhibitory activity showed a slight difference in term of ES inhibition efficiency. ES abrogated a further increase in differentiating cells on collagen matrix between 3 and 6 days, but did not do so well on cells grown on laminin. This suggests that interactions between ES and the surrounding matrix may play an important role in modulating its activity.
2.4. ES inhibits neurite formation on both collagen and laminin substrates
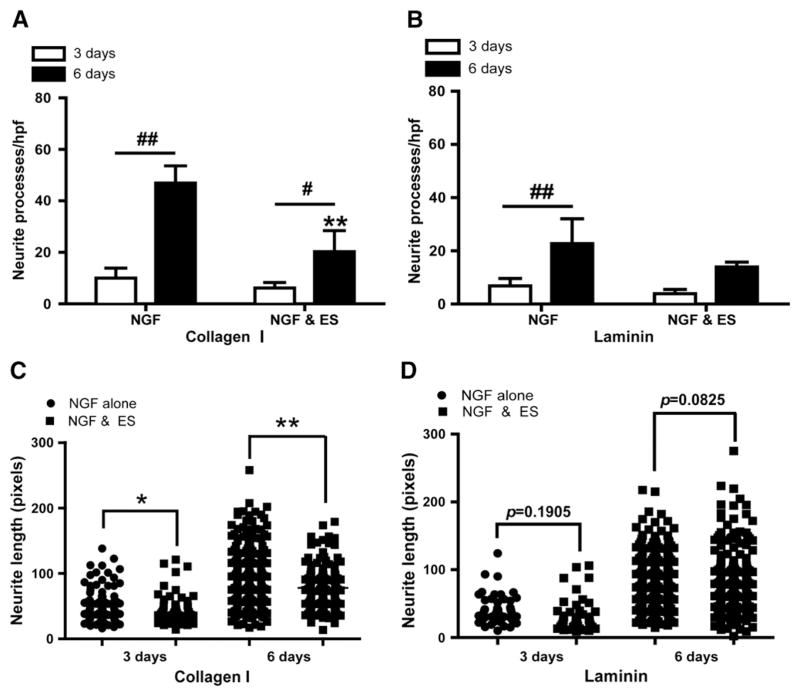
We next quantified the amount of neurites formed in differentiating PC12 cells to determine whether ES inhibited neurite outgrowth on collagen and laminin. On collagen-coated wells (Fig. 3A), ES did not markedly inhibit neurite outgrowth until 6 DIV, reaching a value of 20.20±8.25 neurites/hpf compared to NGF-treated group (46.96±6.63 neurites/hpf, **P=0.0002). However, on laminin matrix (Fig. 3B), ES did not significantly inhibit NGF-induced neurite outgrowth at either 3 DIV (6.77±2.77 and 3.87±1.56 neurites/hpf, P=0.1350) or 6 DIV (22.6±9.24 and 13.80±1.94 neurites/hpf, P=0.0696). A direct comparison between values obtained at 6 days shows that the inhibition rate reached a value of 60% on collagen, whereas it barely reached a value of 40% on laminin. Again these results demonstrate that ES inhibits neurite outgrowth on both matrices, although its inhibitory efficiency is favored on collagen over laminin matrix.
Fig. 3.
ES inhibits neurite formation and elongation. Inhibition of neurite formation occurs already at 3 days (white bars) compared to NGF-only treated cells on both collagen (A) and laminin (B). Note the persistent inhibition at 6 days (black bars). N=4, **P<0.01 versus NGF-treated group. #P<0.05, ##P<0.01 in comparison between NGF and NGF/ES treatment. Scatter-plot of neurite length measured in five random fields on neurite grown on collagen (C) and laminin (D). N=4, *P<0.05, **P<0.01 versus NGF-treated group.
2.5. ES inhibits neurite outgrowth on both collagen and laminin matrices
Finally, we investigated whether ES also influenced neurite length by measuring neurite length within a large population of neurites (Fig. 3C and D) grown on collagen and laminin matrices. On collagen matrix (Fig. 3C), ES treatment resulted in shorter neurites after 3 DIV which was further exacerbated after 6 DIV. Interestingly, ES was again less inhibitory of neurite length formed on a laminin substrate (Fig. 3D), as no significant differences were observed between the two groups at 3 days, whereas a tendency of shorter neurites in ES-treated was observed at 6 DIV. Taken together, these results demonstrate that ES may also inhibit neurite extension in addition to neurite outgrowth in PC12 cells. As in neurite outgrowth, such inhibition was more efficient on collagen than laminin substrates.
2.6. Interaction between ES and collagen is partially responsible for the inhibitory effect on PC12 cell neurite outgrowth
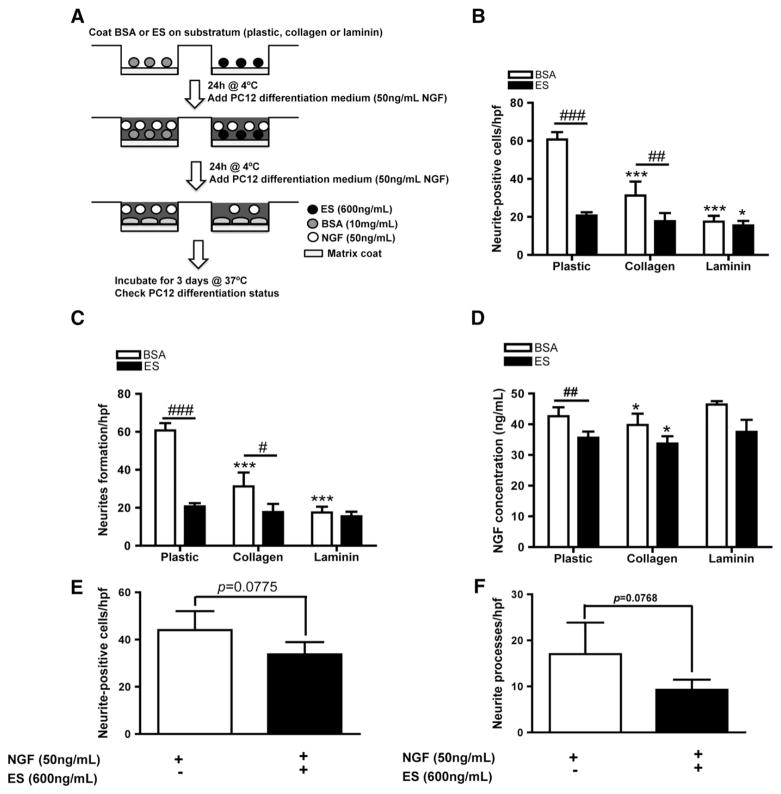
Following our previous observations, we designed an NGF sequestration assay (Fig. 4A) to investigate whether ES as well as collagen and laminin are able to deplete soluble NGF present in the medium. Using this model, we investigated the impact of NGF depletion on neurite outgrowth using PC12 cell differentiation as our functional assay (Fig. 4B and C). Incubation of NGF medium on a plastic surface coated with BSA had minimal effects on PC12 differentiation and neurite outgrowth, as maximal values for both neurite-positive cells (55.8±6.10/hpf) and neurite formation (60.67±3.9/hpf) were obtained. However, the presence of ES on the plastic surface significantly depleted NGF, inasmuch as we noted a significant decrease in the average numbers of neurite-positive cells (26.53±4.22/hpf, #P=0.0024) and neurites (20.57±1.80/hpf, #P<0.001), suggesting the possibility that bound ES can natively sequestrate NGF and decrease its bioavailability. Interestingly, collagen as a coating surface was also able to significantly reduce the ability of the differentiation medium to induce neuritogenesis in PC12 cells (***P<0.001), yielding values of 35.53±2.91 neurite-positive cells/hpf and 31.20±7.46 neurites/hpf. Again, ES was better in reducing NGF activity compared to collagen (18.47±2.68 cells/hpf and 17.80±4.20 neurites/hpf), suggesting a higher affinity of ES for NGF compared to collagen. Surprisingly, laminin treatment resulted in the lowest values in both formation of neurite-positive cells (21.27±2.36/hpf) and neurites (17.53±3.57/hpf). ES appeared less active on laminin as values observed (16.33±2.10 cells/hpf and 15.53±2.44 neurites/hpf) were lower than those obtained on ES coated directly on plastic, suggesting that laminin may have the highest affinity towards NGF but also inhibits ES activity. We further investigated this phenomenon by measuring the amount of NGF present in depleted medium by ELISA (Fig. 4D). In the absence of a coating substrate, we found an average NGF value of 42.64±2.87 ng/mL. The presence of collagen significantly reduced NGF to 34.46 ±1.44 ng/mL (P=0.0117) whereas the presence of laminin did not result in significant differences to plastic, with a value of 46.44±1.06 ng/mL. ES coated wells significantly reduced NGF concentration to 36.62±0.54 ng/mL. The presence of collagen contributed to further reduced NGF concentration to 31.67 ±1.07 ng/mL (*P=0.021), while the presence of laminin resulted in an average value of 39.51±1.01 ng/mL. In conclusion, collagen outperforms laminin as an ECM substrate to promote neurite outgrowth by increased NGF binding capacity. Competition between ES and collagen for binding NGF, or the presence of an ECM with poor binding capacity towards NGF (as observed with laminin) may explain the discrepancy in promoting neuritogenesis as observed between collagen and laminin, but also the apparent loss of ES inhibitory activity on PC12 cells grown on laminin substrate.
Fig. 4.
Both Collagen and ES can bind NGF present in medium, while laminin does not. (A) Schematic representation of NGF-depletion experiment. BSA or ES were coated directly on plastic or jointly with collagen or laminin for 24 h. Medium containing NGF was incubated in the presence of these substrates for 6 h; an aliquot of incubated medium was kept for ELISA, while the remaining amount was transferred to serum-starved PC12 medium and allowed to differentiate in it for 3 days. ES and collagen partially depleted NGF from incubation medium. Note the significant difference between collagen and ES for the formation of neurite-positive cells (B) and neurite formation (C). N=4, *P<0.05 and ***P<0.01 versus plastic-treated group, ##P<0.01, ###P<0.001 in comparison between BSA and ES groups. (D) ELISA for NGF showed a significant decrease in NGF when medium was incubated in presence of ES or collagen. Note the quasi-absence of NGF depletion by laminin. N=4, *P<0.05 versus plastic group, ##P<0.01 between BSA and ES. The presence of ES bound to collagen resulted in most of the inhibitory effect on PC12 cell differentiation as observed in quantifying neurite-positive cells (E) and neurite formation (F). N=4.
2.7. ES bound to collagen shows similar activity to soluble ES
Next, we investigated the possible inhibitory effect of collagen on ES activity by growing PC12 cells on a collagen-ES coated surface and compared this surface to collagen without ES (Fig. 4E and F). The presence of immobilized ES decreased the average number of neurite-positive cells (Fig. 4E) compared to collagen alone (33.80±5.19 and 44±8.06, respectively), as well as the average number of neurite formed (Fig. 4F) with values of 17±3.4 neurites/hpf in collagen alone versus 9.26 neurites/hpf on collagen-ES coated surfaces. Taken together, these data confirm that ES may inhibit neurite outgrowth by NGF sequestration and subsequent depletion from the medium, as such interactions appear to be unaffected by its binding to collagen.
2.8. ES directly binds to NGF
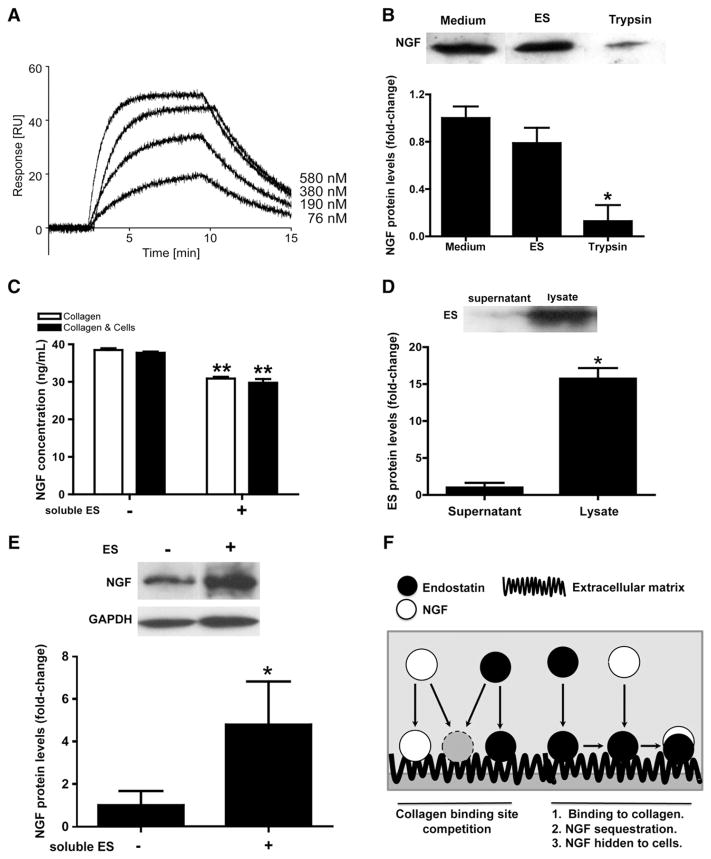
Finally, we performed biosensor experiments to demonstrate whether NGF sequestration by ES involved direct interactions between the two entities. Biosensor analysis (Fig. 5) demonstrated a direct interaction between NGF over immobilized ES, with concentration ranges varying from 76 nM to 580 nM. We determined kon and koff values of 1.7×106 M−1 s−1 and 2.5 s−1 respectively, as well as an average Kd value of 1.5×10−7 M. These results demonstrate that ES and NGF are potential binding partners and suggest that ES sequesters and inhibits NGF activity via direct interaction.
Fig. 5.
(A) Representative biosensor interaction plot obtained for immobilized ES. Free NGF was added at concentrations ranging from 76 nM to 580 nM. Biosensor shows a direct interaction between NGF and ES. Note the maximal response obtained at a sub-micromolar range 5.8×10−7 M, reflecting the relative affinity of ES for NGF. (B) ES does not degrade NGF. NGF alone (500 ng/mL) or in presence of ES (5 μg/mL) were incubated for 6 h at 37 °C. No NGF degradation comparable to trypsin digestion treatment (0.25%) was observed in ES-NGF group. N=3. *P<0.05. (C) NGF sequestration occurs in presence of cells. ES and collagen are responsible of most of the sequestration. Serum-free cells were starved 24 h before addition of NGF±ES. Cells were incubated for 6 h at 37 °C. Supernatant were collected and spinned-down. Note the capacity of soluble ES to decrease NGF. NGF concentration was determined by ELISA kit. N=3, *P<0.01. (D) ES is mostly associated with the extracellular matrix. Equal amount of protein (20 μg) were loaded. Note the quasi-absence of ES in the supernatant whereas it is strongly present in lysates (cells & collagen). (E) Matrix-bound ES still sequestrates NGF, even in a cellular environment. Note the significant increase of NGF protein levels in lysates from ES-treated group versus non-treated group. N=3, *P<0.01. (F) Schematic representation of ES interaction with NGF and collagen (extracellular matrix). Note the presence of two hypotheses: the first one suggests a direct competition between NGF and ES for a collagen binding site and may be involved at the very early stages of the experiments (<2 h), the second one represents the most likely situation where ES binds to the matrix (collagen).
2.9. ES inhibitory effects are not related to NGF degradation
As ES directly binds NGF, one possible hypothesis for ES effects on NGF is that ES binds to and degrades NGF. To exclude such a hypothesis, we investigated changes in NGF stability following co-incubation with ES by Western blot (Fig. 5B). Remarkably, in the presence of ES, NGF levels were not significantly different compared to NGF alone (0.78±0.13 versus 1.00±0.09, respectively, P=0.2076). However, co-incubation of NGF with trypsin, a carboxypeptidase, resulted in a significant decrease in NGF band density (0.12 ±0.13, *P=0.0127), thus reflecting a consequent cleavage. These results demonstrate that ES is unable to degrade NGF and further support that ES’s inhibitory effects described in this study is driven solely by a sequestration mechanism.
2.10. ES–matrix interactions are largely responsible of NGF sequestration
In order to validate that the direct interaction between NGF and ES observed in Fig. 5A naturally occurs within a cellular environment, we firstly demonstrated, by ELISA, the capacity of soluble ES to deplete NGF present in the medium in the presence of cells and/or the surrounding extracellular matrix (Fig. 5C). As previously observed (Fig. 4D), the presence of collagen was able to partially sequestrate a certain fraction of soluble NGF present in the differentiation medium, as we measured an average value of 38.51±0.48 ng/mL. The presence of both cells and collagen resulted in a slight decrease compared to collagen alone, reaching a value of 37.74±0.33 ng/mL, suggesting that the cellular contribution in NGF sequestration is negligible. Again, the presence of soluble ES significantly depleted soluble NGF from the supernatants under both acellular (30.93 ng/mL, **P<0.0001) and cellular (29.80±0.98 ng/mL, **P=0.0002) environments. These results demonstrate that the binding of NGF by soluble ES is not affected by collagen or by the presence of PC12 cells. Taken together, our results demonstrate that the binding of NGF by ES observed in a defined environment also naturally occurs in a cellular environment. The presence of either cells or surrounding extracellular matrix does not disturb such interactions.
2.11. Soluble ES binds to collagen matrix and binds NGF
As we observed that soluble ES maintained its capacity to sequestrate NGF but also bind to collagen, it is important to know which ES form results in most of the biological effects described in this study. We firstly investigated the distribution of soluble ES between the liquid (supernatant) and solid (extracellular matrix and cells) phases by western-blot (Fig. 5D). After 6 h of incubation, soluble ES in the supernatant was negligible, as only a faint ES-positive band was detected. Indeed, most of the soluble ES appears to have bound to the cellular background (extracellular matrix and cells), as we observed a significant increase in ES band density in the lysate compared to the supernatant (*P<0.0001), demonstrating that soluble ES rapidly binds to the surrounding extracellular matrix, suggesting that matrix-bound ES may represent the active form responsible for the biological effects observed in our experiments.
As ES mostly binds to the extracellular matrix, we verified if such binding was associated with an increase in NGF sequestration from the cellular background (Fig. 5E). As expected, the presence of collagen as an extracellular matrix was able to immobilize a modest amount of NGF, as seen as the immuno-reactive positive band observed on western-blot. Interestingly, the presence of ES in the medium (600 ng/mL) significantly increased (approximately 5-fold) the total amount of NGF retained by collagen (*P=0.0364), demonstrating that matrix-bound ES in a cellular environment is capable of capturing and sequestering NGF. This further supports our proposed mechanism of action that ES inhibit neuritogenesis by NGF sequestration.
3. Discussion
Neuronal differentiation, migration and outgrowth are crucial steps of neurogenesis during development and brain repair following injury. The presence of bioavailable neurotrophic and neuroprotective growth factors following a spatio-temporal pattern is crucial for establishment of proper cell interactions. In this study, we showed the inhibitory effect of ES on neuron differentiation, migration and neurite outgrowth using PC12 and cerebellar granular neurons. The growth factor NGF was necessary to induce the different features observed, as the different extracellular matrices used (collagen, laminin and ES) were not by themselves able to induce such features, as expected (Achyuta et al., 2009; Attiah et al., 2003). Untreated NGF has been shown to natively bind to collagen (Bhang et al., 2009) and laminin (Sun et al., 2009b). The reversible binding and release of NGF, as a soluble form, increased neuritogenesis in PC12 cells over time. Different studies have highlighted that NGF-TrkA signaling induces different signaling pathways in a time-dependent manner (Saragovi et al., 1998; Zhang et al., 2000). Early NGF exposure stimulates cell proliferation whereas prolonged exposure induces cell differentiation and eventually neuritogenesis. In our study, we noticed that NGF-induced cell differentiation and neuritogenesis was greater in cells grown on collagen compared to laminin. In two distinct studies, Sun and colleagues (Sun et al., 2009a,b) determined a Kd value for NGF-collagen and NGF-laminin binding values of 8.9×10−7 M and 6.25×10−4 M respectively. In regards to these respective Kd values and the discrepancy observed between collagen and laminin over neuritogenesis and NGF sequestration, we can speculate that interactions between NGF and the surrounding ECM may be crucial to induce cell differentiation and neuritogenesis in PC12 cells. Collagen appeared to bind NGF more efficiently than laminin. Therefore any increase of NGF binding to a determined ECM substrate should improve neuritogenesis. Different studies pinpointed that increasing NGF binding to collagen and laminin substrate improved neurite outgrowth in PC12 cells (Achyuta et al., 2009; Sun et al., 2009a,b) and therefore would agree with our current finding and working hypothesis. However, evidence of direct binding of NGF to chondroitin/dermatan-sulfate proteoglycans (CSPGs/DSPGs), a major class of proteoglycans present in the CNS, remains unclear though it has been described that their ability to bind certain secreted growth factors such as pleiotrophin and hepatocyte growth factor plays important role as a guide for neuritogenesis and axon guidance (Sugahara and Mikami, 2007). Further investigations focusing on the effects of such matrices on NGF binding and PC12 neurite outgrowth would definitely further contribute to the impact of our study.
In our study, we primarily focused on the extracellular effects of ES on PC12 cell morphogenesis. ES is known to bind to cell surfaces by direct interactions with α5β1 and αvβ3 integrins (Faye et al., 2009b). However, our group was not able to show a direct binding of ES over cell surface as well as a positive expression of α5β1 in PC12 cells, agreeing with the study of Berger et al. (2006), suggesting that ES-mediated effect on neuritogenesis may involve extracellular alterations rather triggering inhibition by a direct binding to its cell surface receptors.
We showed that the presence of collagen did not inhibit ES biological activity on neurite outgrowth and NGF sequestration. In this study, we used human recombinant ES present as a monomer (less than 1% of ES existed as a dimer or aggregate as determined by silver stained SDS-PAGE gel analysis per the manufacturer). Oligomerization and formation of disulfide-bonds between two ES are known to influence ES activity, including cell motility (Kuo et al., 2001) and fibrillogenesis (He et al., 2006). We did not detect significant ES aggregation with time in our experiments (data not shown) suggesting that monomeric ES was responsible for the effects reported here. Whether oligomerized/aggregated ES is also capable of similar effects on NGF-mediated effects on neurons will be investigated in future experiments.
We demonstrated by biosensor analysis a direct interaction between ES and NGF with a Kd value of 1.5×10−7 M, showing an ES affinity towards NGF that is roughly four times higher than collagen. We can therefore speculate a direct competition between ES and collagen towards NGF binding, as suggested in the summarizing diagram (Fig. 5F). Importantly, we observed that pre-incubation of PC12 cells with ES prior to the addition of NGF was necessary to observe the inhibitory effects; simultaneous co-incubation of NGF with ES (data not shown) did not significantly inhibit cell differentiation and neuritogenesis, supporting the idea that ES and collagen directly compete for NGF binding. Subsequent interactions between NGF and ES may mask NGF binding sites towards its receptor, but not when NGF is bound to collagen. Substantial experiments done on crystallized ES-NGF and collagen-NGF complexes may help us to better understand the spatial interactions between these different entities. Our results suggest the possibility that ES may have two distinct binding sites for collagen and NGF, respectively, as we observed that ES was able to simultaneously bind to collagen and NGF. Further studies are necessary to identify the different binding sites present in ES and ultimately provide us with more information about this unique capacity of ES to sequestrate NGF. In addition, differences in binding affinities between ES-collagen and ES-laminin may explain the differences observed. ES has been described to directly interact with these two proteins using SPR technique (Faye et al., 2009a), however the Kd values of such interactions have not yet been reported.
In this study, we primarily focused on NGF sequestration by ES and its outcome on cell migration and neurite outgrowth. Such cellular processes occur during development as well as a physiological response following cerebral injury such as stroke. We can therefore consider that any factor that can disturb such repair mechanism to be deleterious. As acute stroke is known to transiently deplete NGF in the injured area (Lee et al., 1998), the presence of ES may dramatically compromise brain recovery and functional outcome. On the other hand, the capacity of ES to sequestrate NGF may play a previously unrecognized additional role (in addition to its anti-angiogenic properties) to treat diseases involving abnormal elevated levels of NGF such as glioblastoma (Singer et al., 1999), but may also contribute to appropriate neuronal wiring and establishment of novel neuronal connections. Indeed, deletion of an ES homolog in invertebrates often resulted in axon wiring impairment (Ackley et al., 2001; Kuo et al., 2001; Meyer and Moussian, 2009), with subsequent overexpression providing partial rescue. Expression of ES in apoptotic neurons following cerebral ischemia (Hou et al., 2010) may be considered as a regulatory mechanism to avoid connection between intact and injured neurons.
We restricted our study to NGF, but other neurotrophins such as brain-derived nerve growth factor (BDNF) or neutrophin-3 and -4 (NT-3 and -4), known to play important roles during physiological and pathological states (Sofroniew et al., 2001), as well as other neuroprotective factors such as vascular endothelial growth factor (VEGF) or erythropoietin (Epo), might also merit further study with ES. A complementary study following the similar experimental approach used by Faye et al. (2009a,b) to establish the interaction network between ES and the variety of growth factors described in the literature would likely sharpen this double-edged sword that represents ES in physiological and pathological states.
4. Experimental procedures
4.1. Cell culture
PC12 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Cells were routinely cultivated in RPMI-1640 (Invitrogen, Carlsbad, CA) supplemented with 10% horse serum (Gemini Bio-Products, Sacramento, CA), 5% fetal bovine serum (Gemini), 1% antibiotic–antimycotic (AA, Invitrogen). Prior to each experiment, cells were serum-starved for 24 h by incubating them with RPMI containing 1% serum and 1% AA. Cerebellar granule cells were obtained from P8 Sprague–Dawley rat pups (Harlan Laboratories, Houston, TX) following a previously described protocol (Clark et al., 1995). Briefly, rat pups were anesthetized with methoxyflurane (Metofane, Pitman-Moore, Mundelein, IL) according to approval of Texas A&M College of Medicine’s animal use guidelines. The cerebellum was removed, stripped of meninges, and digested with papain and then triturated to obtain single cerebellar granule cells.
4.2. Endostatin preparation
Human recombinant ES (Cell Sciences, Canton, MA) was purchased as a solution titrating 5 mg/mL. As per the manufacturer, ES was produced in Pichia pastoris, purity was defined as 99% of total ES detected on SDS-PAGE was present as a monomer form, remaining amounts were either ES dimers or aggregate. Stock solution was aliquoted and stored at −80 °C prior usage within the experiments.
4.3. PC12 cell differentiation
Tissue-culture plates were coated with either rat-tail collagen I solution at 50 μg/mL (BD Biosciences, San Jose, CA, USA) or mouse laminin-1 solution at 100 μg/mL (Invitrogen) for 2 h at 37 °C. Coated plates were washed twice with PBS before usage. PC12 were seeded at a cell density of 5×104 cells/well and serum-starved for 24 h. Cell differentiation was obtained by incubating PC12 cells with PC12 differentiation medium (RPMI-1640, 1% FBS, 1% AA) complemented with NGF (BD Biosciences) at a concentration of 50 ng/mL in serum-free medium. For investigating the effects of ES on PC12 differentiation, cells were incubated in serum-free medium containing 600 ng/mL of ES (Cell Science). In the experiment conducted in the presence of both NGF and ES, PC12 cells were pre-incubated 2 h with ES prior to addition of NGF. Cells were maintained in the same medium for 3 days or 6 days, respectively. Cellular differentiation was defined as a PC12 cell presenting an average neurite outgrowth length higher or equal to twice its soma diameter.
4.4. Cell migration assay
Cell migration was performed using cerebellar granule cells seeded within a modified Boyden Chamber (Neuroprobe, Gaithersburg, MD). Migration across a collagen I-coated poly-carbonate membrane (PVD-free, 8×8 μm pore size) towards NGF (50 ng/mL), in the absence or presence of 600 ng/mL of ES. Migratory cells were quantified after 8 h by counting the number of cells present on the bottom side of the chamber.
4.5. Cell survival
Medium was removed from cells grown on collagen or laminin and replaced with 100 μl of 0.25% Trypsin/EDTA solution (Invitrogen) and incubated for 5 minutes at 37 °C. Trypsin treatment was partially stopped by addition of 100 μl of complete PC12 medium. An aliquot was taken and mixed with an equal volume of 0.4% Trypan Blue solution (Invitrogen). Cell survival rate was determined by dividing average number of living cells by the total number of cells (living and dead cells).
4.6. Morphological analysis
PC12 cells grown on 24-well plates coated with collagen or laminin were briefly washed with PBS and fixed with 4% paraformaldehyde solution. Cells were counterstained using 0.1% crystal violet staining and washed twice with PBS. Cells were observed using an inverted microscope using a 10× objective (Vistavision, VWR, Sugar Land, TX). Images were acquired with a 2.0 megapixel CCD camera using the Motic Image Plus 2.0 software (Motic, Richmond, BC). Images were processed using ImageJ NIH software 1.43.
4.7. ES-NGF binding assay
ES-NGF binding assays were carried out on an optical biosensor (IAsys; Affinity Sensors, UK). In brief, following the activation of a surface of a carboxylate biosensor’s chip (IAsys; Affinity Sensors, UK) by an injection of a 1:1 mixture of 0.1 M N-hydroxysuccinimide and 0.4 M N-ethyl3-(3-dimethylaminopropyl) carbodiimide (Pierce), ES dissolved in phosphate buffered saline (PBS) was allowed to bind to the activated surface until a response plateau was reached. The residual active groups were blocked by an injection of 1 M Tris–HCl (pH 8.5). Nonspecific binding sites were blocked by the injection of a solution of 1% bovine serum albumin (BSA) dissolved in PBS. Excess BSA was removed by washing a biosensor chip with PBS containing 0.05% Tween-20 (PBST). A cuvette with immobilized ES was primed with PBST at 25 °C for 15 min. A sample containing free NGF was added to the cuvette, and proteins were allowed to interact for 7 min. (association phase). Subsequently, the sample was removed, and PBST without NGF was added to the cuvette for an additional 5 min (dissociation phase). To ensure complete dissociation, after each assay, the surface of each cuvette was regenerated by washing it and equilibrating with PBST. To determine affinities, NGF was added at concentrations ranging from 7.6×10−8 to 5.8×10−7 M. Association and dissociation data recorded by a biosensor were then analyzed by the CLAMP global fitting program according to Myszka et al. (1998). Employing a simple bimolecular model, for each assay, the kon and koff were obtained, and the Kd values were calculated from a ratio of koff/kon.
4.8. NGF sequestration assays
NGF sequestration assays were designed to investigate the effect of NGF depletion from PC12 differentiation medium by ECM fragments. A miniature sketch (Fig. 4A) explains the procedure. 24-well plates were coated with 200 μL/well of ES solution (600 ng/mL) alone or in presence of collagen (50 μg/mL) or laminin (100 μg/mL) for 24 h at 4 °C. Coated wells were washed three times with sterile distilled water and were then incubated with 500 μL of PC12 differentiation medium supplemented with NGF for 6 h at 37 °C. 100 μL of NGF-depleted medium was used to determine NGF concentration by ELISA using a NGF ELISA Kit (Chemikine, Milipore), while the remaining media was added to serum-starved PC12 cells. Cells were incubated for 3 days at 37 °C. Morphological analysis was performed after 3 days of culture following the protocol described above.
Collagen-ES double coating was achieved by the addition of 600 ng/mL of ES to 50 μg/mL of collagen. Coating was done overnight at 4 °C. Plates were washed three times with sterile distilled water and seeded with PC12 cells in the presence of PC12 differentiation medium containing NGF. Morphological analysis was performed after 3 days of culture following the protocol described above.
4.9. NGF depletion in a cellular environment
To demonstrate the ability of ES to bind NGF in a cellular environment, ES (600 ng/mL) was dissolved in serum-free medium and pre-incubated in the presence or absence of cells for 2 h at 37 °C on collagen-coated cell culture dishes. After such pre-incubation, NGF (50 ng/mL) was added and further incubated for 6 h. Media was removed and centrifuged for 10 minutes at 14.000 rpm. The pellet was discarded and the supernatant was used to determine NGF concentration by ELISA technique (Milipore).
4.10. Western-blot
Cells and extracellular matrix bound to cell culture dishes were harvested by RIPA buffer (G Biosciences, Maryland Heights, MO) supplemented with a protease inhibitor cocktail (Sigma-Aldrich) and centrifuged for 10 min at 14,000 rpm. Protein concentration was determined by Coomassie-blue assay using BSA as a standard (Bio-Rad, Hercules, CA). Equal amounts of proteins (40 μg) were separated by SDS-PAGE, followed by transfer onto a nitrocellulose membrane and blocking with TBS containing 5% non-fat dry milk. Membranes were then incubated with a rabbit anti-NGF antibody (1:1000, Abcam, Cambridge, MA) or with a rabbit anti-ES antibody (1:1000, Abcam), followed by incubation with horseradish peroxidase goat anti-rabbit antibody (1:5000, GenTex, Irvine, CA). Detection was performed using an ECL kit (Thermo Scientific, Rockford, IL).
4.11. NGF stability
NGF (500 ng) was incubated alone or in presence of 6 μg of ES for 6 h at 37 °C in 100 μl of serum-free medium. Co-incubation of 500 ng of NGF with 250 μg of trypsin was used as a positive control for NGF degradation. Equal volumes of samples (40 μl) were separated by SDS-PAGE and transferred on a nitrocellulose membrane. Western-blot against NGF was run according to the protocol mentioned above.
4.12. Statistical analysis
Data are presented as Mean±S.D. of at least three independent experiments. One-way and two-way ANOVA analysis was performed using the statistical package included in Prism 4.0 (Graphpad Software, LaJolla, CA).
Acknowledgments
This research was funded by Texas A&M College of Medicine Intramural funding to G.B., NIH 5R01AR049537-06 and 5R01AR01AR054876-03 to A.F.
Footnotes
The respective authors of this manuscript declare no conflict of interest.
References
- Achyuta AK, et al. Synergistic effect of immobilized laminin and nerve growth factor on PC12 neurite outgrowth. Biotechnol Prog. 2009;25:227–234. doi: 10.1002/btpr.58. [DOI] [PubMed] [Google Scholar]
- Ackley BD, et al. The NC1/endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance. J Cell Biol. 2001;152:1219–1232. doi: 10.1083/jcb.152.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attiah DG, et al. Characterization of PC12 cell proliferation and differentiation-stimulated by ECM adhesion proteins and neurotrophic factors. J Mater Sci Mater Med. 2003;14:1005–1009. doi: 10.1023/a:1026363018805. [DOI] [PubMed] [Google Scholar]
- Berger I, et al. VEGF receptors on PC12 cells mediate transient activation of ERK1/2 and Akt: comparison of nerve growth factor and vascular endothelial growth factor. J Negat Results Biomed. 2006;5:8. doi: 10.1186/1477-5751-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhang SH, et al. The effect of the controlled release of nerve growth factor from collagen gel on the efficiency of neural cell culture. Biomaterials. 2009;30:126–132. doi: 10.1016/j.biomaterials.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Bix GJ, Clark GD. Platelet-activating factor receptor stimulation disrupts neuronal migration In vitro. J Neurosci. 1998;18:307–318. doi: 10.1523/JNEUROSCI.18-01-00307.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolsover S, et al. Axonal guidance molecules and the failure of axonal regeneration in the adult mammalian spinal cord. Restor Neurol Neurosci. 2008;26:117–130. [PubMed] [Google Scholar]
- Burgoyne RD, Cambray-Deakin MA. The cellular neurobiology of neuronal development: the cerebellar granule cell. Brain Res. 1988;472:77–101. doi: 10.1016/0165-0173(88)90006-9. [DOI] [PubMed] [Google Scholar]
- Clark GD, et al. Platelet-activating factor produces neuronal growth cone collapse. NeuroReport. 1995;6:2569–2575. doi: 10.1097/00001756-199512150-00029. [DOI] [PubMed] [Google Scholar]
- Deininger MH, et al. Aberrant neuronal and paracellular deposition of endostatin in brains of patients with Alzheimer’s disease. J Neurosci. 2002;22:10621–10626. doi: 10.1523/JNEUROSCI.22-24-10621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye C, et al. The first draft of the endostatin interaction network. J Biol Chem. 2009a;284:22041–22047. doi: 10.1074/jbc.M109.002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye C, et al. Molecular interplay between endostatin, integrins, and heparan sulfate. J Biol Chem. 2009b;284:22029–22040. doi: 10.1074/jbc.M109.002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink MF, et al. Do antiangiogenic protein fragments have amyloid properties? Blood. 2004;104:1601–1605. doi: 10.1182/blood-2004-02-0433. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, et al. Deficiency of disulfide bonds facilitating fibrillogenesis of endostatin. J Biol Chem. 2006;281:1048–1057. doi: 10.1074/jbc.M507745200. [DOI] [PubMed] [Google Scholar]
- Hou Q, et al. Endostatin expression in neurons during the early stage of cerebral ischemia is associated with neuronal apoptotic cell death in adult hypertensive rat model of stroke. Brain Res. 2010;1311:182–188. doi: 10.1016/j.brainres.2009.11.033. [DOI] [PubMed] [Google Scholar]
- Kranenburg O, et al. Recombinant endostatin forms amyloid fibrils that bind and are cytotoxic to murine neuroblastoma cells in vitro. FEBS Lett. 2003;539:149–155. doi: 10.1016/s0014-5793(03)00218-7. [DOI] [PubMed] [Google Scholar]
- Kuo CJ, et al. Oligomerization-dependent regulation of motility and morphogenesis by the collagen XVIII NC1/endostatin domain. J Cell Biol. 2001;152:1233–1246. doi: 10.1083/jcb.152.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, et al. Expression of nerve growth factor and trkA after transient focal cerebral ischemia in rats. Stroke. 1998;29:1687–1696. doi: 10.1161/01.str.29.8.1687. discussion 1697. [DOI] [PubMed] [Google Scholar]
- Marneros AG, Olsen BR. Physiological role of collagen XVIII and endostatin. FASEB J. 2005;19:716–728. doi: 10.1096/fj.04-2134rev. [DOI] [PubMed] [Google Scholar]
- Meyer F, Moussian B. Drosophila multiplexin (Dmp) modulates motor axon pathfinding accuracy. Dev Growth Differ. 2009;51:483–498. doi: 10.1111/j.1440-169X.2009.01111.x. [DOI] [PubMed] [Google Scholar]
- Myszka DG, et al. Equilibrium analysis of high affinity interactions using BIACORE. Anal Biochem. 1998;265:326–330. doi: 10.1006/abio.1998.2937. [DOI] [PubMed] [Google Scholar]
- O’Reilly MS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Roth L, et al. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66:649–666. doi: 10.1007/s00018-008-8518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragovi HU, et al. A TrkA-selective, fast internalizing nerve growth factor-antibody complex induces trophic but not neuritogenic signals. J Biol Chem. 1998;273:34933–34940. doi: 10.1074/jbc.273.52.34933. [DOI] [PubMed] [Google Scholar]
- Singer HS, et al. Mitogenesis in glioblastoma multiforme cell lines: a role for NGF and its TrkA receptors. J Neurooncol. 1999;45:1–8. doi: 10.1023/a:1006323523437. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, et al. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Mikami T. Chondroitin/dermatan sulfate in the central nervous system. Curr Opin Struct Biol. 2007;17:536–545. doi: 10.1016/j.sbi.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Sun W, et al. The effect of collagen-binding NGF-beta on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury model. Biomaterials. 2009a;30:4649–4656. doi: 10.1016/j.biomaterials.2009.05.037. [DOI] [PubMed] [Google Scholar]
- Sun W, et al. Improvement of sciatic nerve regeneration using laminin-binding human NGF-beta. PLoS ONE. 2009b;4:e6180. doi: 10.1371/journal.pone.0006180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian HL, et al. Increased protein and mRNA expression of endostatin in the ischemic brain tissue of rabbits after middle cerebral artery occlusion. Neurosci Bull. 2007;23:35–40. doi: 10.1007/s12264-007-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Horssen J, et al. Collagen XVIII: a novel heparan sulfate proteoglycan associated with vascular amyloid depositions and senile plaques in Alzheimer’s disease brains. Brain Pathol. 2002;12:456–462. doi: 10.1111/j.1750-3639.2002.tb00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, et al. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002;296:1648–1649. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J Neurosci. 2000;20:5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, et al. Dexamethasone transiently attenuates up-regulation of endostatin/collagen XVIII following traumatic brain injury. Neuroscience. 2007;147:720–726. doi: 10.1016/j.neuroscience.2007.04.052. [DOI] [PubMed] [Google Scholar]
- Zorick TS, et al. High serum endostatin levels in Down syndrome: implications for improved treatment and prevention of solid tumours. Eur J Hum Genet. 2001;9:811–814. doi: 10.1038/sj.ejhg.5200721. [DOI] [PubMed] [Google Scholar]