Abstract
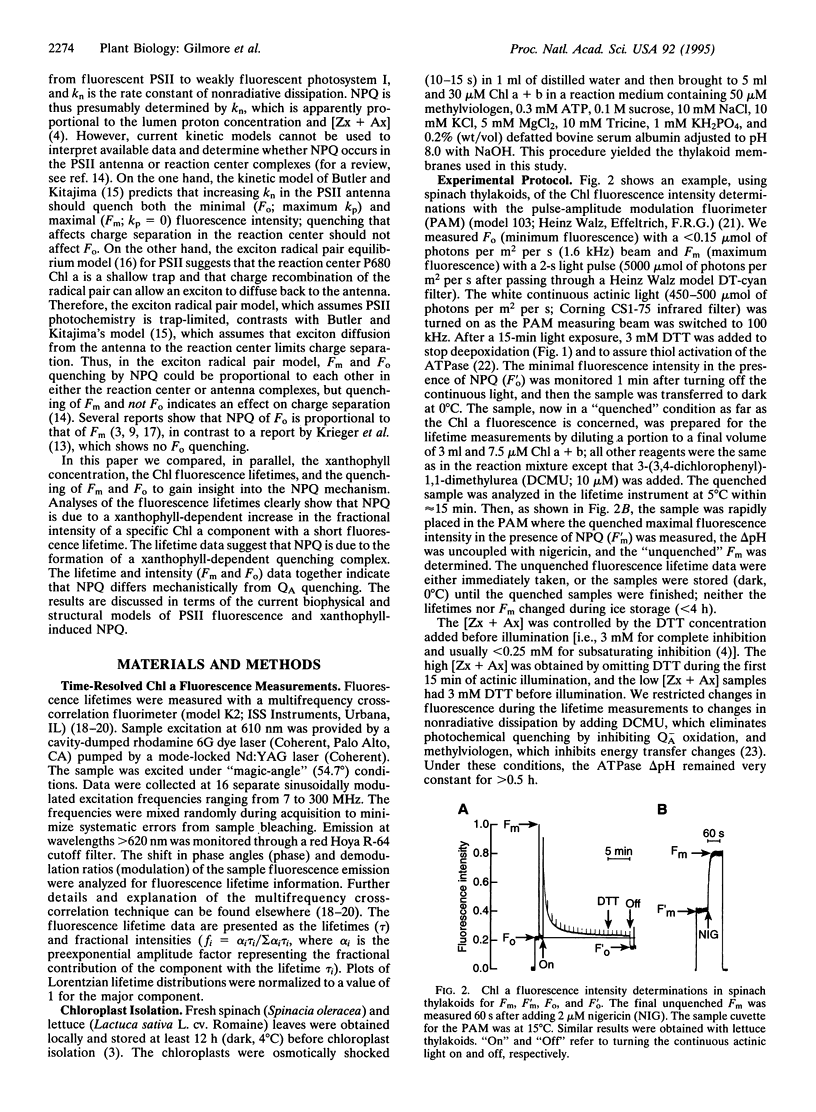
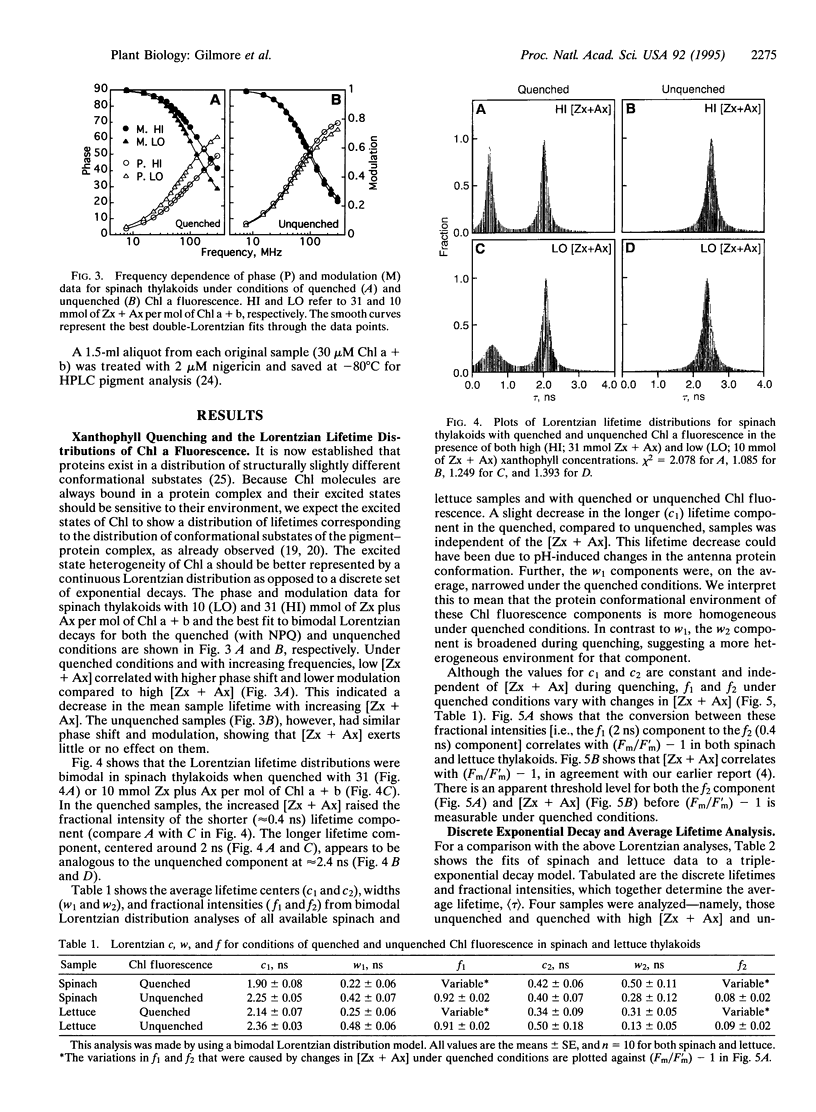
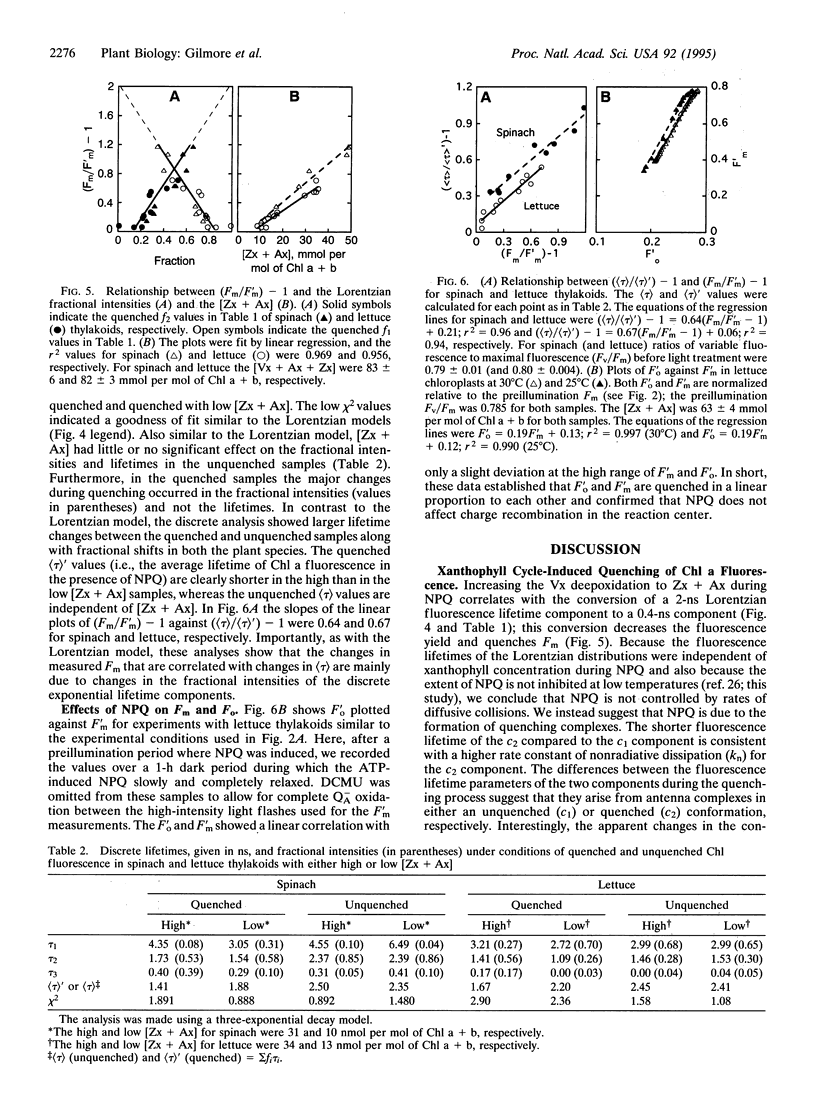
Excess light triggers protective nonradiative dissipation of excitation energy in photosystem II through the formation of a trans-thylakoid pH gradient that in turn stimulates formation of zeaxanthin and antheraxanthin. These xanthophylls when combined with protonation of antenna pigment-protein complexes may increase nonradiative dissipation and, thus, quench chlorophyll a fluorescence. Here we measured, in parallel, the chlorophyll a fluorescence lifetime and intensity to understand the mechanism of this process. Increasing the xanthophyll concentration in the presence of a pH gradient (quenched conditions) decreases the fractional intensity of a fluorescence lifetime component centered at approximately 2 ns and increases a component at approximately 0.4 ns. Uncoupling the pH gradient (unquenched conditions) eliminates the 0.4-ns component. Changes in the xanthophyll concentration do not significantly affect the fluorescence lifetimes in either the quenched or unquenched sample conditions. However, there are differences in fluorescence lifetimes between the quenched and unquenched states that are due to pH-related, but nonxanthophyll-related, processes. Quenching of the maximal fluorescence intensity correlates with both the xanthophyll concentration and the fractional intensity of the 0.4-ns component. The unchanged fluorescence lifetimes and the proportional quenching of the maximal and dark-level fluorescence intensities indicate that the xanthophylls act on antenna, not reaction center processes. Further, the fluorescence quenching is interpreted as the combined effect of the pH gradient and xanthophyll concentration, resulting in the formation of a quenching complex with a short (approximately 0.4 ns) fluorescence lifetime.
Full text
PDF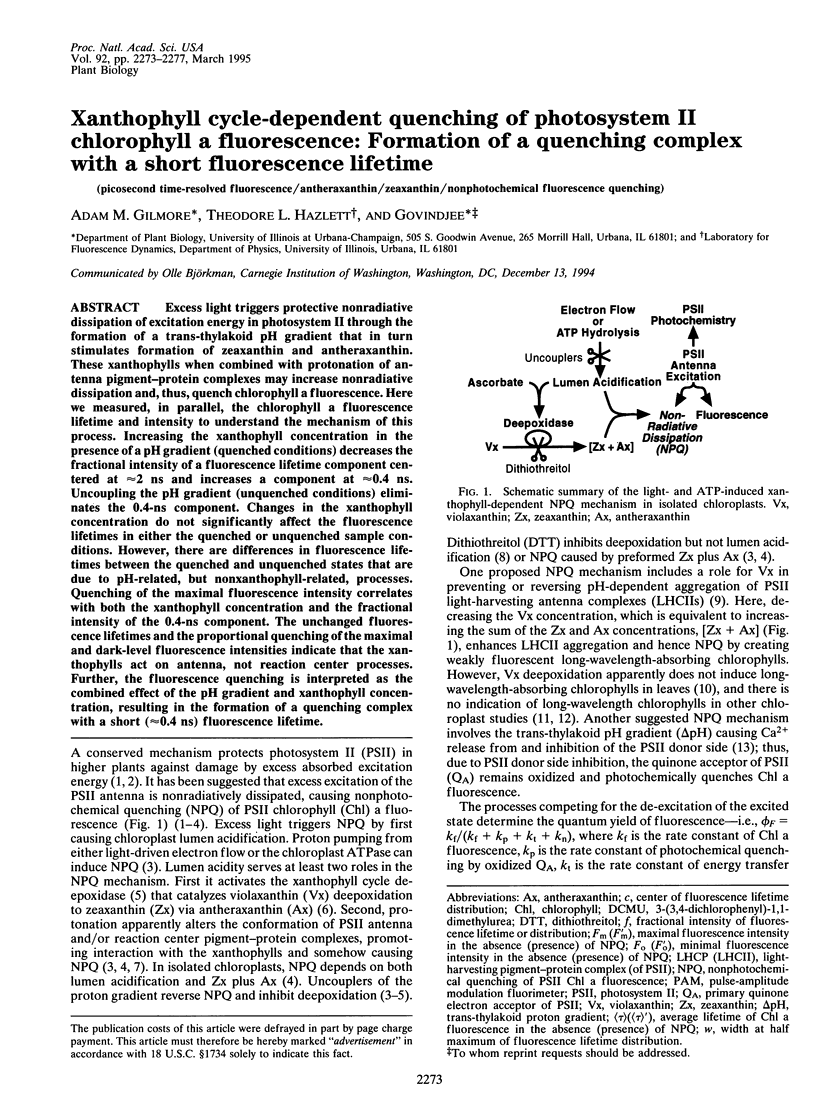

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcala J. R., Gratton E., Prendergast F. G. Fluorescence lifetime distributions in proteins. Biophys J. 1987 Apr;51(4):597–604. doi: 10.1016/S0006-3495(87)83384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi R., Pineau B., Dainese P., Marquardt J. Carotenoid-binding proteins of photosystem II. Eur J Biochem. 1993 Mar 1;212(2):297–303. doi: 10.1111/j.1432-1033.1993.tb17662.x. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Kitajima M. Fluorescence quenching in photosystem II of chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):116–125. doi: 10.1016/0005-2728(75)90210-8. [DOI] [PubMed] [Google Scholar]
- Crofts A. R., Yerkes C. T. A molecular mechanism for qE-quenching. FEBS Lett. 1994 Oct 3;352(3):265–270. doi: 10.1016/0014-5793(94)00976-7. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Gilmore A. M., Mohanty N., Yamamoto H. Y. Epoxidation of zeaxanthin and antheraxanthin reverses non-photochemical quenching of photosystem II chlorophyll a fluorescence in the presence of trans-thylakoid delta pH. FEBS Lett. 1994 Aug 22;350(2-3):271–274. doi: 10.1016/0014-5793(94)00784-5. [DOI] [PubMed] [Google Scholar]
- Gilmore A. M., Yamamoto H. Y. Dark induction of zeaxanthin-dependent nonphotochemical fluorescence quenching mediated by ATP. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1899–1903. doi: 10.1073/pnas.89.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee, Van de Ven M., Cao J., Royer C., Gratton E. Multifrequency cross-correlation phase fluorometry of chlorophyll a fluorescence in thylakoid and PSII-enriched membranes. Photochem Photobiol. 1993 Sep;58(3):438–445. doi: 10.1111/j.1751-1097.1993.tb09587.x. [DOI] [PubMed] [Google Scholar]
- Govindjee, van de Ven M., Preston C., Seibert M., Gratton E. Chlorophyll a fluorescence lifetime distributions in open and closed photosystem II reaction center preparations. Biochim Biophys Acta. 1990 Feb 2;1015(2):173–179. doi: 10.1016/0005-2728(90)90017-x. [DOI] [PubMed] [Google Scholar]
- Horton P., Black M. T. Light-dependent quenching of chlorophyll fluorescence in pea chloroplasts induced by adenosine 5'-triphosphate. Biochim Biophys Acta. 1981 Mar 12;635(1):53–62. doi: 10.1016/0005-2728(81)90006-2. [DOI] [PubMed] [Google Scholar]
- Horton P., Ruban A. V., Walters R. G. Regulation of Light Harvesting in Green Plants (Indication by Nonphotochemical Quenching of Chlorophyll Fluorescence). Plant Physiol. 1994 Oct;106(2):415–420. doi: 10.1104/pp.106.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter G. F., Thornber J. P. Biochemical composition and organization of higher plant photosystem II light-harvesting pigment-proteins. J Biol Chem. 1991 Sep 5;266(25):16745–16754. [PubMed] [Google Scholar]
- Schatz G. H., Brock H., Holzwarth A. R. Kinetic and Energetic Model for the Primary Processes in Photosystem II. Biophys J. 1988 Sep;54(3):397–405. doi: 10.1016/S0006-3495(88)82973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolove P. M., Marsho T. V. Ascorbate-independent carotenoid de-epoxidation in intact spinach chloroplasts. Biochim Biophys Acta. 1976 May 14;430(2):321–326. doi: 10.1016/0005-2728(76)90088-8. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO H. Y., NAKAYAMA T. O., CHICHESTER C. O. Studies on the light and dark interconversions of leaf xanthophylls. Arch Biochem Biophys. 1962 Apr;97:168–173. doi: 10.1016/0003-9861(62)90060-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. Y., Kamite L., Wang Y. Y. An Ascorbate-induced Absorbance Change in Chloroplasts from Violaxanthin De-epoxidation. Plant Physiol. 1972 Feb;49(2):224–228. doi: 10.1104/pp.49.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]



