Abstract
The aim of the present study was to optimize the polyethylenimine (PEI)-mediated transfection method in order to simplify the efficient production of lentiviral vectors (LvVs), and to compare the CaPO4- and PEI-mediated transfection methods for producing LvVs. Different titration methods of LvV stocks, as well as different culture media, culture durations, cell densities and DNA quantities were compared to obtain an optimized procedure for the production of LvVs. Optimization of the production method for LvVs was achieved using PEI-mediated transient transfections. Serum-free Opti-MEM® was used to directly produce LvVs that could be harvested 48 h after transfection. Furthermore, a cell density of 15×106 cells/10-cm plate and a DNA concentration of 1X were selected for the optimum production of LvVs. The optimized LvV titration method was simple and direct; it involved LvVs carrying fluorescent reporters, which proved to be faster than the standard methods but equally as sensitive. In conclusion, a scalable process for production of LvVs by PEI-mediated transfection was established and optimized. The optimized PEI-mediated transfection method was easy to use, as well as providing greater reliability with a higher degree of reproducibility and consistency. Despite using less DNA, the PEI-mediated transfection method resulted in viral titers that were the same as those achieved using the CaPO4-mediated method.
Keywords: lentivirus, vector production, polyethylenimine, calcium phosphate
Introduction
Lentiviral vectors (LvVs) have emerged as an important tool for transgene delivery and gene therapy. LvVs offer various advantages for gene therapy due to their ability to infect quiescent, slowly dividing or non-dividing cells, such as hematopoietic stem cells, neurons and glial cells; their ability to integrate into the host cell genome, resulting in long-term transgene expression; and their large packaging capacity (1). Integrated LvVs do not induce an inflammatory or immune response, which allows long-term in vivo maintenance of transgene expression in a variety of tissue types.
Standard LvV production typically relies on the transient transfection of human embryonic kidney cells (HEK 293T) with a packaging plasmid, an envelope glycoprotein-encoding plasmid and a lentiviral transfer vector plasmid (2). Following transfection, lentiviral particles (LvPs) are produced and released into the culture supernatant of the HEK 293T cells. Although novel methods of producing of LvVs have been developed (3), and various transfection protocols using specific transfection reagents, such as Lipofectamine® 2000 (4), SuperFect® (5), FuGENE® 6 and GeneJammer (6), have been successfully applied to the production of LvVs, high costs have hindered their extensive use. Thus, the transient transfection method using CaPO4 precipitation remains the most common method used for the production of LvVs (7). The main limitations of the CaPO4-mediated method of transient transfection are the large quantities of DNA required and the dependency of the method on an optimal pH of HEPES buffer. This pH can drift over time, resulting in large variability in transfection efficiency and significant batch-to-batch variation of the LvV titers (8). Furthermore, despite their safety and the marked progress that has been made in the development of lentiviral packaging systems, the average range of titers of crude lentiviral vector stocks produced is between 106 and 108 transduction units (TU) per ml (9–10). This range is useful for in vitro applications; however, the quantities are not high enough for in vivo use. For in vivo applications, large volumes of medium, large numbers of cells and large amounts of plasmid DNA are required to prepare sufficient stocks of crude LvVs. In addition, the relatively low transduction efficiency of LvVs means that the production of high-titer, high-quality LvV stocks is difficult and costly (11). Thus, improved methods for the consistent production of high-titer LvVs are required for the feasible use of LvVs in transgene delivery and gene therapy. Previously, the polyethylenimine (PEI)-mediated transient transfection method for LvV production was described (12); it uses a transfection reagent to produce recombinant viral vectors, such as adeno-associated viral vectors (AVVs) (13–14) or lentiviral vectors (12,15). There are various advantages of producing LvVs via the PEI-mediated transfection method. First, the relatively low cost of PEI allows it to be widely used in the research laboratory. Second, the chemical stability and pH independence of PEI ensures consistent high transfection efficiency (16–17). Finally, PEI has been demonstrated to be effective in transducing adherent and suspension cells (18–19).
The aim of the present study was to optimize the PEI-mediated transfection method in order to simplify the efficient production of LvVs, and to compare the CaPO4- and PEI-mediated transfection methods for producing LvVs. The PEI-mediated method was identified to be simple to use and more reliable, with a high degree of reproducibility and consistency. Despite using less DNA, the PEI-mediated transfection method resulted in viral titers that were the same as those achieved using the CaPO4-mediated method.
Materials and methods
Experimental cell lines
HEK 293T cells and murine fibroblastic NIH 3T3 cells were stocked in Dr Barbara C. Vanderhyden’s laboratory at the University of Ottawa (Ottawa, Ontario, Canada)and cultured in HyClone™ Dulbecco’s modified Eagle medium (DMEM)/high glucose (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). The cells were maintained at 37°C and 5% CO2 in a humidified incubator (VWR International, LLC., Atlanta, GA, USA) and were harvested at 80–90% confluence.
Plasmid constructs
The present study used a lentiviral transfer vector plasmid (pWPI) containing enhanced green fluorescent protein (eGFP), a pCMV-dR8.74 packaging plasmid and a pCAG4-Eco envelope plasmid, as previously described (20–21). pWPI and pCMV-dR8.74 plasmids were provided by Dr. D Trono (Swiss Federal Institute of Technology in Lausanne, Lausanne, Switzerland) and pCAG4-Eco plasmids were provided by Dr. A Nienhuis (St. Jude Children’s Research Hospital, Memphis, TN, USA).
Preparation of PEI stocks
A 7.5 mM PEI stock solution was prepared by dissolving 0.15 g PEI (molecular weight, 40 kDa; Polysciences Inc., Warrington, PA, USA) in 245 ml tissue culture grade water. Once prepared, the PEI stock was stored at 4°C (22).
PEI-mediated transfections were performed to generate lentiviral vectors
Lentiviral vectors were generated by the transient cotransfection of HEK 293T cells with a three-plasmid system. The lentiviral transfer vector plasmid (pWPI; 2 μg), packaging plasmid (pCMV-dR8.74; 1.3 μg) and envelope plasmid (pCAG4-Eco; 1.25 μg) DNA were mixed in 150 mM NaCl. Concurrently, 7.5 mM PEI stock was diluted 6-fold with 150 mM NaCl to yield a final PEI concentration of 1.25mM, using the formula previously described by Reed et al (13), and 0.38 ml was added to the 0.38 ml DNA (4.55 μg total) from above. Transfections were also performed using 2× DNA (9.10 μg) and 3× DNA (13.65) solutions. DNA/PEI mixtures were incubated at room temperature for ≥10 min. The HEK 293T cells were trypsinized, washed twice with 1X phosphate buffered saline (PBS) and resuspended (2×106 cells/ml) in serum-free Opti-MEM® (Invitrogen Life Technologies, Inc., Carlsbad, CA, USA). The DNA/PEI mixture was added to 7.5 ml suspended cells, and immediately plated onto a 10 cm and incubated at 37°C in a 5% CO2 humidified atmosphere. The supernatants containing LvVs were collected and stored at −80°C.
CaPO4-mediated transfections were performed to generate LvVs
HEK 293T cells (2×106 cells/well) were seeded in each 10 cm culture dish and incubated at 37°C in a 5% CO2 humidified atmosphere. After 24 h, transfer vector plasmid (pWPI; 20 μg), packaging plasmid (pCMV-dR8.74; 15 μg) and envelope plasmid (pCAG4-Eco; 6 μg) were diluted with 250 μl sterile double distilled water (Barnstead™ Nanopure™ ultrapure water purification system; Thermo Fisher Scientific, Inc.). An equal volume of 0.5 M CaCl2 was added. The DNA/CaCl2 mixture was added to 2X HBS (500 μl; 0.28 M NaCl, 0.05 M HEPES and 1.5 mM Na2HPO4; optimal pH range, 7.00–7.28) and incubated at room temperature for 30 min. Subsequently, the transfection mixture was applied to culture plates containing fresh culture media and incubated at 37°C in a 5% CO2 humidified atmosphere. After 48 h, the supernatants containing LvVs were collected and stored at −80°C.
Optimal HBS pH was determined for the production of LvVs in HEK 293T cells
To empirically determine the optimal pH of HBS for use in the CaPO4-mediated transfections, trial experiments (pH range, 7.0–7.28) were conducted. Following the infection of NIH 3T3 cells (described below), the LvVs produced from the transfected cells was quantified using the direct titration method.
An appropriate number of NIH 3T3 cells were seeded in a culture plate and incubated at 37°C in a 5% CO2 humidified atmosphere. After 24 h, all of the culture medium was removed and virus medium containing polybrene was added to the well. The culture plate was centrifuged twice at room temperature and incubated at 37°C in a 5% CO2 humidified atmosphere.
Titration of LvV stocks
Direct titration method
NIH 3T3 cells (2×105 cells/ml) were trypsinized and resuspended in DMEM containing 10% FBS and polybrene was added to a final concentration of 8 μg/ml. The NIH 3T3 cells (1 ml) and viral supernatant (5–50 μl) were transferred into a 3 ml snap-top tube and mixed. The cell solution containing the virus was immediately seeded in individual wells of six-well plates and incubated at 37°C in a 5% CO2 humidified atmosphere. After 24 h, each well was supplemented with 1 ml fresh culture media. Three days after infection, the NIH 3T3 cells were trypsinized and the number of fluorescence-positive cells was determined using flow cytometry (Epics® XL™ flow cytometer; Beckman Coulter, Inc., Mississauga, ON, Canada).
Delayed titration method
NIH 3T3 cells (2×105 cells/well) were seeded in six-well plates and incubated at 37°C in a 5% CO2 humidified atmosphere. After 24 h, the culture medium was removed and 1 ml fresh DMEM containing 10% FBS, 8 μg/ml polybrene and 5–50 μl viral supernatant were added to each well. After 24 h, each well was supplemented with 1 ml fresh culture medium. Three days after infection, the NIH 3T3 cells in each well were trypsinized and the number of fluorescence-positive cells was determined using flow cytometry (Epics XL flow cytometer; Beckman Coulter, Inc.).
Titer formula
The following formula was used to convert the percentage of eGFP-expressing cells for a specific dilution into TU: TU/ml =[(% of cells expressing eGFP/100) × total number of NIH 3T3 cells at time of infection/volume of virus stock added (ml) (23).
Flow cytometry
For immunostaining, cells were trypsinized and washed twice with 1X PBS. After determining cell number (Vi-Cell XR cell viability analyzer; Beckman Coulter, Inc.), 0.5–1×106 cells were resuspended in 1 ml cold PBS or 100% formalin, protected from light and measured using an Epics XL flow cytometer (Beckman Coulter, Inc.) immediately or stored at 4°C for future use. A total of 1×104–1×105 events were analyzed in each sample and the resulting data were analyzed using Summit software (version 4.3; Dako North America, Inc., Carpinteria, CA, USA).
Statistical analyses
Statistical analyses were performed using GraphPad Prism statistical software (GraphPad Software, Inc., La Jolla, CA, USA). Data was expressed as the mean ± standard error of the mean of three independent experiments performed in triplicate. The probability of significant differences between two groups or multiple groups was determined using Student’s t-test or analysis of variance, respectively. P<0.05 was considered to indicate a statistically significant difference.
Results
Optimization of lentivirus production
Although various commercial transfection reagents are available, the selection of a suitable transfection system was vital for the success of the present study. Virus production in serum-free conditions is ideal due to the possible future uses of virus purification and concentration. The key to successful LvV production is the efficient transfection of HEK 293T cells. For LvVs expressing fluorescent reporters, transfection efficiencies can be easily determined by the direct analysis of fluorescence in the transfected HEK 293T cells. Although more labor-intensive, the analysis of LvV production by viral titration using flow cytometry allows for a more precise determination of the optimal transfection method, choice of culture medium and timing of LvV harvest that cannot be adequately determined by looking solely at the transfection efficiencies of the HEK 293T cells. Therefore, prior to optimizing the transfection methods for the production of LvV, two titration methods were investigated.
Comparison of different titration methods of LvV stocks
Two methods were evaluated for the determination of the LvV titer produced from the transfected HEK 293T cells. The first method (direct titration method) was performed as follows: Vector stock (5 μl) was immediately added to 1 ml culture medium containing 2×105 freshly trypsinized NIH 3T3 cells suspension in a six-well plate. After 24 h, an additional 1 ml culture medium was added to each well. The second method (delayed titration method) was performed as follows: NIH 3T3 cells (2×105) were plated in each well of a six-well plate. After 24 h, the media was removed and replaced with 1 ml culture medium containing 5 μl vector stock. After 24 h, an additional 1 ml culture medium was added to each well of the six-well plates. Three days after transduction of the NIH 3T3 cells, the percentage of eGFP-expressing NIH 3T3 cells was measured using flow cytometry and titers were calculated. As demonstrated by Fig. 1, the titer produced by direct titration was significantly higher than that obtained by delayed titration, which involved infected cells which had been plated 24 h earlier. These results indicated that the direct titration method, which is faster and simpler to perform, is a sensitive and convenient method for viral titration.
Figure 1.
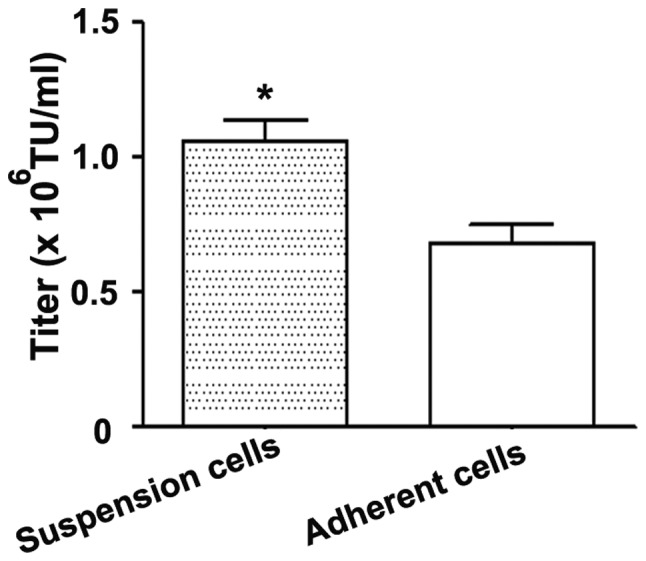
Comparison between the direct (suspension cells) and delayed (adherent cells) titration methods of LvV stocks. Titers of LvVs were determined 48 h after transduction of NIH3T3 cells. The calculated LvV titer was significantly higher for NIH3T3 cells infected in suspension when compared with cells infected while adhering to cell culture plates (n=3; *P<0.001). LvV, lentiviral vector; TU, transduction units.
Effect of different culture media and culture durations on the efficiency of PEI-mediated transfection
In the initial production trial, HEK 293T cells were seeded at a density of 1×107 cells/10-cm plate to produce LvVs. To quantify the amount of LvVs produced, the direct titration method was performed by adding 50 μl viral supernatant to each well containing NIH 3T3 cells. As demonstrated in Fig. 2, using serum-free Opti-MEM as the culture medium produced a higher titer compared with using DMEM, regardless of whether LvVs were harvested 48 or 72 h after transfection. Notably, the titers of LvV were not significantly different at 48 and 72 h post-transfection in the Opti-MEM or DMEM groups. These results indicated that serum-free Opti-MEM may be used directly to produce LvVs and that LvVs in the culture medium can be harvested 48 h after transfection, increasing the speed of the process.
Figure 2.
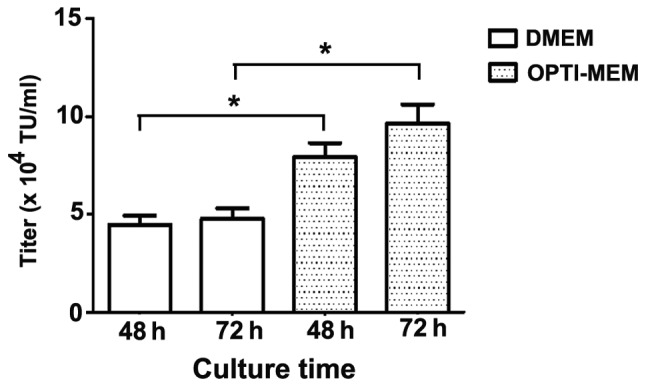
Effect of different culture media and times on the production of LvVs. Titers of LvV were obtained using polyethylenimine-mediated transient transfection with the human embryonic kidney 293T cells (cell density, 1×107 cells/10-cm plate). The direct titration method was used and the dilution factor of lentivirus was 1:20. *P<0.001. LvV, lentiviral vector; TU, transduction units; DMEM, Dulbecco’s modified Eagle medium.
Influence of cell density on the efficiency of PEI-mediated transfection
The optimal cell density for LvV production in 10-cm plates was investigated. Transient transfections were performed at low and high cell densities, from 5×106 to 40×106 cells/10-cm plate and the eGFP-expressing cells were observed under a fluorescent microscope (Fig. 3). Although the cells could achieve a density of >40×106 cells/10-cm plate, the percentage of eGFP-expressing cells did not increase beyond this point when increasing the cell density during the transient transfection.
Figure 3.
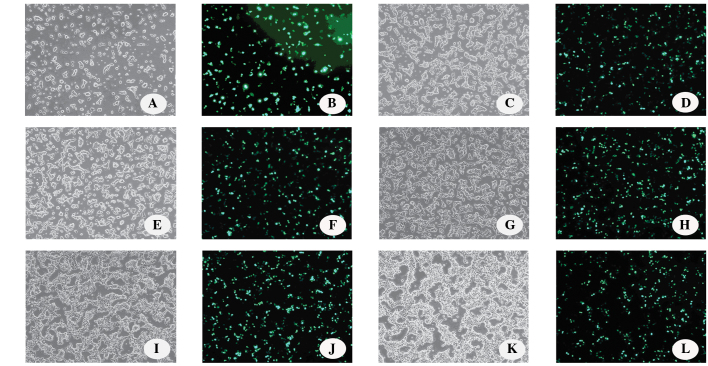
Effect of cell density on transfection efficiency in 10-cm plates was assessed by determining the number of green fluorescent protein-expressing cells by fluorescence microscopy (Axiovert 100 Fluorescence Microscope, Zeiss, Oberkochen, Germany; magnification, ×100). The cell densities evaluated were as follows: (A and B) 5×106 cells/10-cm plate; (C and D) 10×106 cells/10-cm plate; (E and F) 15×106 cells/10-cm plate; (G and H) 20×106 cells/10-cm plate; (I and J) 30×106 cells/10-cm plate; and (K and L) 40×106 cells/10-cm plate (magnification, ×100).
As demonstrated in Fig. 4, vector titers were dependent on cell density and a significant increase in vector titers was achieved by increasing the cell density from 10×106 cells/10-cm plate to 15×106 cells/10-cm plate. However, increasing the cell density to ≥20×106 cells/10-cm plate did not significantly increase the titer volume. Thus, 15×106 cells/10-cm plate was selected as the optimum cell density for the production of LvVs in the subsequent experiments.
Figure 4.
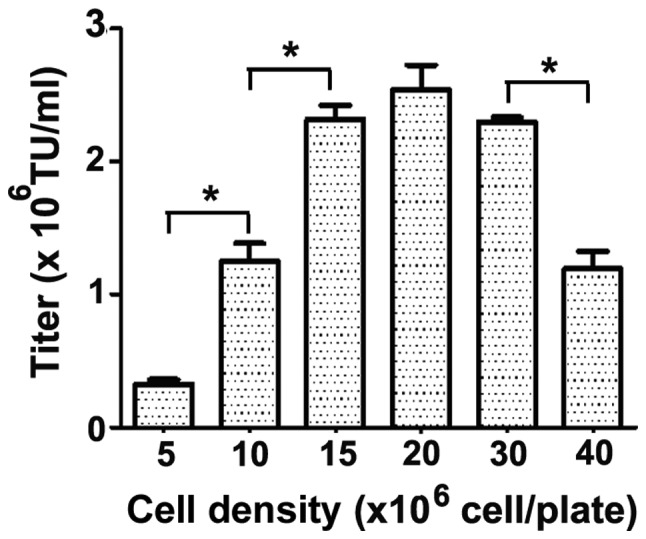
Optimization of cell densities for lentiviral vector production using 10-cm culture dishes. Experiments were conducted at low and high cell densities in serum-free Opti-MEM®. At densities of 5–15×106 cells/10-cm plate, an increase in cell density resulted in higher viral titers at transient transfection. *P<0.001. TU, transduction units.
Optimization of DNA quantity for PEI-mediated LvV production
Based on the results of the previous experiments, Opti-MEM medium and a HEK 293T cell density of 15×106 cells/10-cm plate was selected to investigate the effects of altered DNA concentrations on the titers of LvV produced. The plasmid DNA solution (containing 4.55, 9.10 or 13.65 μg plasmid DNA) was prepared as described above. As demonstrated in Fig. 5, the LvV titer was not significantly different when different plasmid DNA concentrations were used. This indicates that the DNA concentration is not a key factor in the present experiment; thus, the DNA concentration could be standardized for future experiments (4.55 μg plasmid DNA mixture mixed with 150 mM NaCl; final volume, 380 μl).
Figure 5.
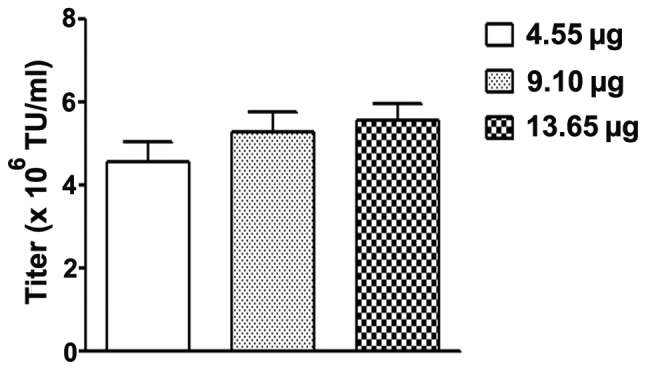
Optimization of DNA concentration for polyethylenimine-mediated LvV production in Opti-MEM®. LvVs were produced using 15×106 HEK 293T cells per 10-cm plate and a total amount of 4.55 μg, 9.1 μg and 13.65 μg plasmid DNA mixture was added to 150 mm NaCl to a final volume of 380 μl, respectively. LvV, lentiviral vector; TU, transduction units.
Optimization of the pH for LvV production using CaPO4
The pH of HBS was critical during CaPO4-mediated transient transfections. In the direct titration method, 7.00 and 7.05 were identified to be the optimum pH of HBS for CaPO4-mediated transient transfection (Fig. 6). These results indicated that the preferred HBS pH for LvV production using CaPO4 is 7.00. Both the direct observation of the percentage of eGFP-expressing HEK 293T cells by flow cytometry and the determination of LvV titers confirmed the optimal pH of HBS to be ~7.00. It was noted, however, that an HBS pH of 7.28 was effective at transfecting the HEK 293T cells based on direct flow cytometry, but produced a markedly lower LvV titer compared with at the optimal pH of 7.00.
Figure 6.
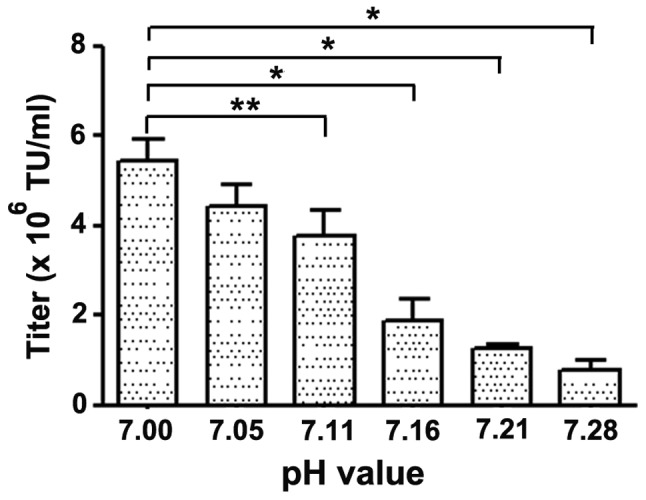
Optimization of the pH of the HBS buffer using the directed titration method. *P<0.001 and **P<0.05. TU, transduction units.
Comparison of LvV production using PEI- and CaPO4-mediated transient transfection
The LvV titers produced by PEI- or CaPO4-mediated transfection were compared following the transfection of HEK 293T cells. As demonstrated in Fig. 7A, the LvV titers produced were not significantly different between the PEI- and CaPO4-mediated transfection methods.
Figure 7.
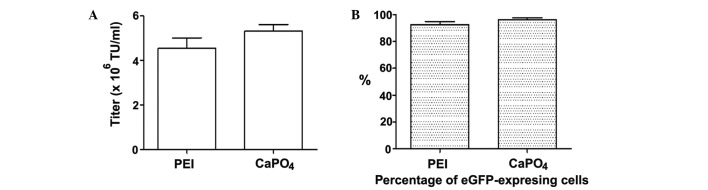
Comparison of lentiviral vector (LvV) production using PEI- and CaPO4-mediated transient transfection. (A) Comparison of the titers of LvV and (B) the percentage of eGFP-expressing cells 48 h after transfection produced by PEI-or CaPO4-mediated transfection in human embryonic kidney 293T cells. TU, transduction units; PEI, polyethylenimine; eGFP, enhanced green fluorescent protein.
The percentages of eGFP-expressing cells 48 h after transfection were determined by fluorescent microscopy observation of the culture plates or by analysis using a flow cytometer. No observable difference was identified in the proportion of eGFP-expressing cells between the PEI- and CaPO4-mediated transient transfection culture plates. Flow cytometry data confirmed that 92.25±2.80% of HEK 293FT cells transfected with PEI expressed eGFP, whereas 96.15±1.44% of the CaPO4-transfected cells were eGFP-positive. The percentages of eGFP fluorescent cells in the PEI- and CaPO4-transfected groups were not statistically different (Fig. 7B).
Discussion
In the present study, the protocols for LvV production using PEI-mediated transient transfection were optimized. Vector titers were increased almost 100-fold after using these protocols and were similar to those achieved using CaPO4-mediated transient transfection. The protocol was simple, pH-independent, economic, reproducible and consistent.
Generally, two different methods are used to produce LvVs: The delayed titration method (which involves the development of stable vector packaging cell lines) and the transient titration method. Although several stable vector packaging cell lines have been generated (24), their use has various limitations. Firstly, the development of a stable vector packaging cell line requires a relatively long period of selection and characterization (25). Secondly, transgenes or vector components may carry toxicities that are not compatible with the development of a stable packaging cell line (26). Additionally, for each desired vector pseudotype, a new packaging cell line must be developed and, finally, stable packaging cell lines often produce relatively low vector titers and lack stability over prolonged culture periods, limiting their long-term applications (10). As a consequence, transient transfections of plasmids constitute a faster and simpler approach to LvV production and avoid the time-consuming development of stable packaging cell lines (27). In addition, various transgenes and envelope glycoproteins can easily be substituted in the transient transfection vector systems (28). Thus, the production of LvVs by transient transfection remains the most widely used technique and has been applied in the present study.
While the transient transfection method was used in all of the experiments in the present study, it was important to standardize a convenient system for the routine production of LvVs. PEI-mediated transient transfection, a simple and effective method, was investigated for the transient transfection of HEK 293T cells for the efficient production of LvV.
In the present study, various conditions, including the type of medium, harvest period, cell density, amount of plasmid DNA and titer method were evaluated and optimized to achieve the most favorable PEI-mediated transient transfection protocol. The resultant protocol significantly increased LvV titers by almost 100-fold compared with the non-optimized conditions. Furthermore, vector titers similar to those achieved using the CaPO4-mediated transient transfection method were obtained in the serum and protein supplement-free Opti-MEM medium. In addition to the advantages of using serum-free media, the PEI-mediated transfection method is simpler than the CaPO4 method in that the solutions are simple to prepare, not dependent on pH and transfected cells do not require their medium to be changed 24 h after transfection. Also, the PEI-mediated method requires approximately ten-fold less DNA to achieve similar amounts of LvV production to the CaPO4-mediated method. In the present study, PEI-mediated transient transfection demonstrated a high degree of reproducibility and consistency in its LvV production compared with CaPO4-mediated transfection. For example, the pH of the HBS used in the CaPO4-mediated method changes during storage, making it necessary to periodically re-prepare the buffer using the abovementioned empirical approach. Therefore, the use of the PEI-mediated transfection method simplifies the transfection procedure, significantly reduces the quantity of plasmid DNA and shortens the time required for LvV production.
Although the present study considered various factors during the optimization of PEI-mediated transient transfection for LvV production, there are numerous other parameters that could also be examined. For example, the ratio of PEI nitrogen to DNA phosphorous (N/P ratio) (29) and the quantity of DNA (22) have previously been demonstrated as important parameters for transient transfection. The PEI volume per plate used in the present study was calculated based on the protocol previously described by Kuroda et al (22). However, the PEI volume per plate may vary, perhaps in accordance with the equation described by Reed et al (13). The present study determined that 4.55 μg DNA mixture was adequate for the transfection of 1.5×107 cells (0.3 μg DNA/106 cells). This quantity is similar to that described in previous studies where 0.4–0.6 μg total DNA/106 cells was used (22,30). Additional optimization of the amount of DNA used for PEI-mediated transfection could possibly be undertaken.
The use of different cell densities during PEI-mediated transient transfection resulted in significantly different titers (22) and the optimal cell density varied when different types of vectors were used. Based on the results obtained by Kuroda et al (22), LvV titers were relatively unaffected by 1–2×107 HEK 293T cells/15-cm plate and the highest vector titers were obtained at a cell density of 1×107 cells/15-cm plate. In the initial experiments of the present study, relatively lower titers were obtained when the HEK 293T cell density was 1×107 cells/10-cm plate. This data confirms that the cell density is one of the defining conditions in optimizing the process of LvV production.
Furthermore, the present study did not evaluate the effect of the addition of nutrients on the efficiency of virus production. For example, the addition of peptones has been reported to lead to superior transfection efficiencies using PEI-mediated transfection (31–32).
The production of LvV in serum-free medium has previously been described (22). In two related studies, the PEI-mediated transfection method for LvV production was described, using Opti-MEM reduced serum medium (15) and serum-free, chemically defined (FreeStyle™) medium (12). By contrast, the present study used DMEM/high glucose and serum-free Opti-MEM reduced serum medium to directly culture HEK 293T cells. In previous studies, the LvV in the culture medium were typically harvested 48 h after transfection (13,22,33). Consistent with this, the present study identified that the optimal conditions of LvV production involved PEI-mediated transient transfection, serum-free medium and a 48-h incubation.
Although HEK 293T cells have been widely used for the production of LvV, including in the present study, HeLa cells and XDC293 cells have also been used as packaging cell lines for the production of recombinant viruses (13). In the present protocol, the titers of infective LvP were assessed by their ability to transfer the eGFP gene to the NIH 3T3 cell. This was similar to a study by Salmon and Trono (7), who recommended that the optimization of transfection protocols use a plasmid encoding GFP when establishing vector production procedures (7). Different LvV titration methods, including the determination of transgenic mRNA levels, correlate well with TU and can be used for the functional titration of non-fluorescent transgenes (34). Similarly, the quantification of LvV in the absence of fluorescent markers can be determined by quantitative polymerase chain reaction. The present study used NIH3T3 cells to titer LvV due to their restricted ability to infect only murine cells; however, HeLa cells have also been used as target cells for measuring titers of LvV pseudotyped with the vesicular stomatitis virus (VSV) G glycoprotein, allowing the infection of human cells (7). Although not all LvV titration methods were optimized, the present study provided a simple and efficient titration method for future use.
An additional consideration when using PEI-mediated transient transfection are the reported toxicities (30). The efficiency and cytotoxicity depend on the molecular weight of PEI. Smaller PEIs are non-cytotoxic but less efficient; for example, Toledo et al (33) obtained a high LvV titer (~1×107 TU/ml) using branched 25 kDa PEI. Furthermore, cross-linked PEIs have been reported to enhance gene delivery efficiency (29). In the present study, which used 40 kDa PEI, no cytotoxicity was observed or evaluated; however, the vector titers were good enough for use in future transduction experiments.
For the optimization of CaPO4-mediated transient transfection, only the pH of HBS was optimized, as other parameters had previously been optimized in the laboratory. However, the titer of CaPO4-mediated transient transfection was affected by the structure of the envelope plasmid; the titer obtained from the envelope protein of VSV G glycoprotein was ~10-fold higher than from the envelope protein of rabies G glycoprotein (35). In addition, the titer of CaPO4-mediated transient transfection was affected by the concentration of CaPO4, the time of complex formation (36) and the cell cycle (37–38).
In the present study, similar titers of LvV were produced from PEI- and CaPO4-mediated transient transfection. Similarly, although Toledo et al (33) obtained a high LvV titer using PEI, the PEI-mediated transfection method did not demonstrate a higher titer compared with the CaPO4-mediated transfection method. By contrast, for the production of recombinant AVVs in HEK293 and HeLa cells, linear PEI was a better transfection reagent than CaPO4 (13). The vector titers achieved by transient transfection in the present study were relatively low compared with the typical titers achieved in previous reports (106–107 TU/ml) (22,30). This may have been due to the choice, for biosafety considerations, to pseudotype the LvV with the ecotropic envelope protein. The use of this envelope protein has consistently been demonstrated to result in lowered titers when compared with the VSV G glycoprotein (22,33).
Although numerous other factors may be optimized to improve LvV production, it is apparent that the PEI-mediated transfection method is the most cost-effective option (19). In addition to improved transfection efficiencies, LvV titers may be improved by the processing of cell culture supernatants to purify and concentrate the virus using serum-free media (22), physical concentration (11), ultracentrifugation (28), HYPERFlask® vessels (39) or filtration approaches (40), such as diafiltration (15). Although the LvV produced in the present study was not purified, the use of the PEI-mediated transfection method and protein-free media should allow this route to be pursued if required.
In conclusion, the present study developed, described and optimized a scalable process for the production of LvV by PEI-mediated transfection. Interest in the use of LvV for human gene therapy is high and it has even undergone phase I clinical trials to investigate its possible use in the treatment of human immunodeficiency virus (41). However, large-scale LvV production remains a challenge for clinical applications. Thus, additional developments in LvV production and purification strategies will be essential to improve the cost-effectiveness of LvV use in clinical studies.
Acknowledgements
The present study was supported by grants from the Natural Science Fund Program of Guangxi Province (grant nos. 2011GXNSFC018020 and 2012GXNSFAA053163).
References
- 1.Quinonez R, Sutton RE. Lentiviral vectors for gene delivery into cells. DNA Cell Biol. 2002;21:937–951. doi: 10.1089/104454902762053873. [DOI] [PubMed] [Google Scholar]
- 2.Vigna E, Naldini L. Lentiviral vectors: excellent tools for experimental gene transfer and promising candidates for gene therapy. J Gene Med. 2000;2:308–316. doi: 10.1002/1521-2254(200009/10)2:5<308::AID-JGM131>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Segura MM, Garnier A, Durocher Y, Ansorge S, Kamen A. New protocol for lentiviral vector mass production. Methods Mol Biol. 2010;614:39–52. doi: 10.1007/978-1-60761-533-0_2. [DOI] [PubMed] [Google Scholar]
- 4.Shin KJ, Wall EA, Zavzavadjian JR, et al. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc Natl Acad Sci USA. 2006;103:13759–13764. doi: 10.1073/pnas.0606179103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman JE, Huentelman MJ, Kasparov S, et al. Efficient large-scale production and concentration of HIV-1-based lentiviral vectors for use in vivo. Physiol Genomics. 2003;12:221–228. doi: 10.1152/physiolgenomics.00135.2002. [DOI] [PubMed] [Google Scholar]
- 6.Kosaka Y, Kobayashi N, Fukazawa T, et al. Lentivirus-based gene delivery in mouse embryonic stem cells. Artif Organs. 2004;28:271–277. doi: 10.1111/j.1525-1594.2004.47297.x. [DOI] [PubMed] [Google Scholar]
- 7.Salmon P, Trono D. Production and titration of lentiviral vectors. Curr Protoc Neurosci. 2006;37:4.21.1–4.21.24. doi: 10.1002/0471142301.ns0421s37. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Welsh MJ. Enhancement of calcium phosphate-mediated transfection by inclusion of adenovirus in coprecipitates. Gene Ther. 1999;6:676–682. doi: 10.1038/sj.gt.3300857. [DOI] [PubMed] [Google Scholar]
- 9.Mochizuki H, Schwartz JP, Tanaka K, Brady RO, Reiser J. High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. J Virol. 1998;72:8873–8883. doi: 10.1128/jvi.72.11.8873-8883.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther. 2005;5:387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segura MM, Kamen A, Garnier A. Downstream processing of oncoretroviral and lentiviral gene therapy vectors. Biotechnol Adv. 2006;24:321–337. doi: 10.1016/j.biotechadv.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Segura MM, Garnier A, Durocher Y, Coelho H, Kamen A. Production of lentiviral vectors by large-scale transient transfection of suspension cultures and affinity chromatography purification. Biotechnol Bioeng. 2007;98:789–799. doi: 10.1002/bit.21467. [DOI] [PubMed] [Google Scholar]
- 13.Reed SE, Staley EM, Mayginnes JP, Pintel DJ, Tullis GE. Transfection of mammalian cells using linear polyethylenimine is a simple and effective means of producing recombinant adeno-associated virus vectors. J Virol Methods. 2006;138:85–98. doi: 10.1016/j.jviromet.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Durocher Y, Pham PL, St-Laurent G, et al. Scalable serum-free production of recombinant adeno-associated virus type 2 by transfection of 293 suspension cells. J Virol Methods. 2007;144:32–40. doi: 10.1016/j.jviromet.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Geraerts M, Michiels M, Baekelandt V, Debyser Z, Gijsbers R. Upscaling of lentiviral vector production by tangential flow filtration. J Gene Med. 2005;7:1299–1310. doi: 10.1002/jgm.778. [DOI] [PubMed] [Google Scholar]
- 16.Boussif O, Lezoualc’h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pham PL, Kamen A, Durocher Y. Large-scale transfection of mammalian cells for the fast production of recombinant protein. Mol Biotechnol. 2006;34:225–237. doi: 10.1385/MB:34:2:225. [DOI] [PubMed] [Google Scholar]
- 18.Derouazi M, Girard P, Van Tilborgh F, et al. Serum-free large-scale transient transfection of CHO cells. Biotechnol Bioeng. 2004;87:537–545. doi: 10.1002/bit.20161. [DOI] [PubMed] [Google Scholar]
- 19.Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30:E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao DS, Li L, Garson K, Vanderhyden BC. OPCML gene transferred by recombinant lentiviruses in vitro and its inhibition to ovarian cancer cells. Zhonghua Fu Chan Ke Za Zhi. 2006;41:333–338. (In Chinese) [PubMed] [Google Scholar]
- 21.Jounai N, Okuda K, Kojima Y, et al. Contribution of the rev gene to the immunogenicity of DNA vaccines targeting the envelope glycoprotein of HIV. J Gene Med. 2003;5:609–617. doi: 10.1002/jgm.391. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda H, Kutner RH, Bazan NG, Reiser J. Simplified lentivirus vector production in protein-free media using polyethylenimine-mediated transfection. J Virol Methods. 2009;157:113–121. doi: 10.1016/j.jviromet.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Kahl CA, Marsh J, Fyffe J, Sanders DA, Cornetta K. Human immunodeficiency virus type 1-derived lentivirus vectors pseudotyped with envelope glycoproteins derived from Ross River virus and Semliki Forest virus. J Virol. 2004;78:1421–1430. doi: 10.1128/JVI.78.3.1421-1430.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kafri T, van Praag H, Ouyang L, Gage FH, Verma IM. A packaging cell line for lentivirus vectors. J Virol. 1999;73:576–584. doi: 10.1128/jvi.73.1.576-584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ni Y, Sun S, Oparaocha I, et al. Generation of a packaging cell line for prolonged large-scale production of high-titer HIV-1-based lentiviral vector. J Gene Med. 2005;7:818–834. doi: 10.1002/jgm.726. [DOI] [PubMed] [Google Scholar]
- 26.Sinn PL, Sauter SL, McCray PB., Jr Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors - design, biosafety, and production. Gene Ther. 2005;12:1089–1098. doi: 10.1038/sj.gt.3302570. [DOI] [PubMed] [Google Scholar]
- 27.Mitta B, Rimann M, Fussenegger M. Detailed design and comparative analysis of protocols for optimized production of high-performance HIV-1-derived lentiviral particles. Metab Eng. 2005;7:426–436. doi: 10.1016/j.ymben.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T, Flake AW. Optimized large-scale production of high titer lentivirus vector pseudotypes. J Virol Methods. 2004;122:131–139. doi: 10.1016/j.jviromet.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Thomas M, Ge Q, Lu JJ, Chen J, Klibanov AM. Cross-linked small polyethylenimines: while still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivo. Pharm Res. 2005;22:373–380. doi: 10.1007/s11095-004-1874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X, Hia HC, Goh PE, Yap MG. High-density transient gene expression in suspension-adapted 293 EBNA1 cells. Biotechnol Bioeng. 2008;99:108–116. doi: 10.1002/bit.21537. [DOI] [PubMed] [Google Scholar]
- 31.Pham PL, Perret S, Doan HC, et al. Large-scale transient transfection of serum-free suspension-growing HEK293 EBNA1 cells: peptone additives improve cell growth and transfection efficiency. Biotechnol Bioeng. 2003;84:332–342. doi: 10.1002/bit.10774. [DOI] [PubMed] [Google Scholar]
- 32.Pham PL, Perret S, Cass B, et al. Transient gene expression in HEK293 cells: peptone addition posttransfection improves recombinant protein synthesis. Biotechnol Bioeng. 2005;90:332–344. doi: 10.1002/bit.20428. [DOI] [PubMed] [Google Scholar]
- 33.Toledo JR, Prieto Y, Oramas N, Sánchez O. Polyethylenimine-based transfection method as a simple and effective way to produce recombinant lentiviral vectors. Appl Biochem Biotechnol. 2009;157:538–544. doi: 10.1007/s12010-008-8381-2. [DOI] [PubMed] [Google Scholar]
- 34.Geraerts M, Willems S, Baekelandt V, Debyser Z, Gijsbers R. Comparison of lentiviral vector titration methods. BMC Biotechnol. 2006;6:34. doi: 10.1186/1472-6750-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiser J. Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther. 2000;7:910–913. doi: 10.1038/sj.gt.3301188. [DOI] [PubMed] [Google Scholar]
- 36.Sakoda T, Kasahara N, Kedes L, Ohyanagi M. Calcium phosphate coprecipitation greatly enhances transduction of cardiac myocytes and vascular smooth muscle cells by lentivirus vectors. Exp Clin Cardiol. 2007;12:133–138. [PMC free article] [PubMed] [Google Scholar]
- 37.Trobridge G, Russell DW. Cell cycle requirements for transduction by foamy virus vectors compared to those of oncovirus and lentivirus vectors. J Virol. 2004;78:2327–2335. doi: 10.1128/JVI.78.5.2327-2335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Rourke JP, Newbound GC, Kohn DB, Olsen JC, Bunnell BA. Comparison of gene transfer efficiencies and gene expression levels achieved with equine infectious anemia virus- and human immunodeficiency virus type 1-derived lentivirus vectors. J Virol. 2002;76:1510–1515. doi: 10.1128/JVI.76.3.1510-1515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kutner RH, Puthli S, Marino MP, Reiser J. Simplified production and concentration of HIV-1-based lentiviral vectors using HYPERFlask vessels and anion exchange membrane chromatography. BMC Biotechnol. 2009;9:10. doi: 10.1186/1472-6750-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koldej R, Cmielewski P, Stocker A, Parsons DW, Anson DS. Optimisation of a multipartite human immunodeficiency virus based vector system; control of virus infectivity and large-scale production. J Gene Med. 2005;7:1390–1399. doi: 10.1002/jgm.803. [DOI] [PubMed] [Google Scholar]
- 41.McGarrity GJ, Hoyah G, Winemiller A, et al. Patient monitoring and follow-up in lentiviral clinical trials. J Gene Med. 2013;15:78–82. doi: 10.1002/jgm.2691. [DOI] [PubMed] [Google Scholar]