Abstract
The aim of this study was to investigate the dosimetric benefits between intensity-modulated radiotherapy (IMRT) and conventional radiotherapy (CR) among patients receiving breast-conserving surgery. A dosimetric comparison of IMRT and CR was evaluated in 20 patients with early-stage breast cancer using a three-dimensional treatment planning system. The prescribed mammary gland dose was completed in 25 fractions with a total dose of 5,000 cGy. Homogeneity of the planning target volume (PTV), irradiation dose and volume of organs at risk (OARs) were evaluated through a dose-volume histogram. For the homogeneity of PTV, the average volume receiving 95% of the prescribed dose in the IMRT plan was similar to that in the CR plan (97 vs. 96%, respectively). With regard to normal tissue sparing in OARs, the ipsilateral lung V20 in the IMRT and CR plans was 27.8 and 20.8%, respectively. The mean dose and V30 of the heart for five patients were 598.4 versus 348.3 cGy and 10.06 versus 5.3%, respectively. The mean dose sparing the heart or lung was markedly reduced in the IMRT plan compared with the CR plan. The results of the current study demonstrated that whole breast IMRT improves PTV dose distribution and improves normal tissue sparing in OARs.
Keywords: breast cancer, intensity-modulated radiotherapy, breast-conserving surgery, radiotherapy
Introduction
Breast-conserving surgery may eradicate macroscopic diseases that have been detected and palpated in females with early-stage breast cancer (1,2). However, certain microscopic tumor foci may remain in the conserved breast, leading to local recurrence and/or life-threatening distant metastases. The administration of adjuvant radiotherapy following breast-conserving surgery is effective in reducing the risk of locoregional recurrence and distant metastases in patients with early-stage breast cancer (1,2). Postoperative radiation treatment in patients with breast cancer is conventionally delivered using external beam radiation therapy, which is determined by rectangular tangential fields. With this radiotherapy technique, an appreciable dose within the irradiated volume may be administered, and the dose delivered to the lung and heart may be higher than predicted (3).
Over the past decade, there has been a rapid increase in the utilization of advanced radiation delivery technologies for the curative management of numerous types of solid cancer. Radiation patterns have shifted from conventional two-dimensional (2D) radiotherapy to a more developed three-dimensional (3D) approach in treating breast cancer (4,5). However, whether intensity-modulated radiotherapy (IMRT) is superior to traditional 3D radiation delivery remains unknown.
In recent years, the use of IMRT has been greatly improved, as the beam intensity profile has been conformed to the chest wall or delineated target volume, resulting in reduced radiation dose variations and sparing of organs at risk (OAR)(6–9). The shape of the IMRT plan can be optimized on the basis of geometrical parameters, including the shape of the breast and thoracic wall, or dosimetric parameters using inverse planning. Consequently, it has not always been possible to establish a satisfactory compromise between the dose delivered to the target volume or the clinical tumor volume (CTV) and the dose delivered to the OARs (10,11). Compared with conventional rectangular tangential fields, the dose distribution conforms more to the target volume when 3D data are available and conformal treatment fields are used. This approach reduces the dose to OARs. Intensity modulation may be considered as an additional step and allows for greater freedom in improving the dose distribution compared with the combination of open and wedged beams. This may result in a further improvement of the dose distribution in the target volume and the OARs. Inverse planning provides a method for minimizing the dose to OARs, whilst maintaining adequate target coverage.
The majority of IMRT studies have shown a potential clinical benefit in sparing OARs and improving dose homogeneity over the target volume, as compared with rectangular tangential fields without conformal blocks (12–14). However, the implementation of IMRT in clinical practice requires additional resources for patients with breast cancer in the adjuvant setting, as the IMRT plan is more time-consuming and complex. Therefore, it is useful to identify patients with a medical necessity for the IMRT plan. Several studies have compared a number of forms of IMRT, including forward and inverse methods, with conventional radiotherapy (CR) (15–17). However, the relative improvement attributable to intensity modulated irradiated fields compared with conformed fields is not yet known for the irradiation of patients with early-stage breast cancer. Therefore, a clinical trial was initiated to investigate radiation dosimetry of IMRT, assessed by changes in breast appearance and discomfort in patients with early stage breast cancer.
Patients and methods
Eligibility
A total of 20 patients under the care of the Department of Radiation Oncology, the First Affiliated Hospital of Anhui Medical University (Hefei, China), with early-stage breast cancer (T1–2N0M0; stage I or IIA), according to 7th edition of American Joint Committee on Cancer (18), between July 2008 and October 2009 were included in this study. Patients with no previous malignancies, complete microscopic excision of tumors and histological confirmation of breast cancer underwent breast-conserving surgery (16 patients with invasive ductal carcinoma, two with invasive lobular carcinoma, one with intraductal carcinoma, and one with medulla carcinoma). Radiotherapy was prescribed for the whole breast, and written informed consent was obtained prior to the trial. The study was approved by the ethics committee of The First Affiliated Hospital of Anhui Medical University, and was conducted in accordance with the declaration of Helsinki.
Patient positioning and localization
Localization of the treatment volume and the field geometry was conducted by an Acuity™ simulator (Varian Medical Systems, Inc., Palo Alto, CA, USA) according to laser mark points on the bodies of the patients. The computed tomography (CT) results were applied to this retrospective treatment planning study. CT slices were acquired every 5 mm, with the patient lying in a supine position. Patients were positioned in the supine position on an angled board with the arms abducted to 90°, such that the sternum was horizontal. All patients were positioned with the arms resting on an armrest placed above the head equivalent to the treatment position. The patients were immobilized about the shoulders and upper arms, by applying a vacuum air cushion across the shoulders. This position has been demonstrated to facilitate treatment planning of tangential fields without the arms extending into the treatment fields and changing the shape of the breast significantly (19). The CT scan included the complete left and right lung, breast, heart and liver. The median separation distance between the most medial and lateral aspects of the breast was 21.1 cm (range, 18.0–26.5 cm), which was confirmed in the current study. All graphic files of the CT scans were transmitted to the Topslane treatment planning system (Xops 2.0; Shanghai Topslane Medical Technology Co., Ltd., Shanghai, China) for further analysis.
Target volume outline
The CTV was delineated by a radiation oncologist according to surface mark points and CT scan files, which were optimized to visualize the glandular tissue of patients that had received breast-conserving surgery. The radiation oncologist contoured the CTV based on the CT scan. The CTV was assumed to start 3–5 mm below the skin and delineated smoothly with an anterior margin of 0.5 cm beneath the skin, a posterior margin of the chest wall surface, a medial margin of the body midline and a lateral margin of midaxillary line. A planning target volume (PTV) was generated by expanding the CTV by 7 mm isotropically, with the exception of the direction towards the skin surface, where expansion stopped at 5 mm below the skin, to account for the uncertainty in the patient set-up and CTV delineation. The cranial extent of the heart included the infundibulum of the right ventricle, right atrium and right atrium auricle, but excluded the pulmonary trunk, ascending aorta and superior vena cava. The lowest external contour of the heart was the caudal border of the myocardium, with the pericardium excluded. The contours of the lungs and skin were automatically outlined.
CR plan
The gantry and collimator angles of the tangential fields were selected using the beam’s eye view option on the 3D treatment planning system (3DTPS; Xops 2.0). The edges of the tangential fields were non-divergent, to minimize the irradiated lung volume to a margin of 1.5–2.0 cm. In the conformal plans, an automatically generated conformal wedge block around the PTV, with a margin of 1.5–2.0 cm, was added to these fields. The treatment planning was performed using the 3DTPS (Xops 2.0). The optimum wedge angle and beam weights were calculated using the optimization module. In the context of an equivalent beam weight of the tangential fields, the goal of the optimization module was to obtain a homogeneous dose, while maintaining a low dose in the lungs and heart.
IMRT plan
Using the same gantry angles as applied in the CR plans, tangential 6-MV photon beam intensity profiles were calculated using the inverse planning program (radio-SOFT 1.0; Apache Technologies Inc., Dayton, OH, USA). Following sequencing, the segment weights were refined with the optimization module using the same objective score function as the CR plan. From the PTV to normal tissues, the final dose distribution was calculated to inversely optimize the beam intensity profiles, which in turn was converted to a segmental sequence. Thus, the same dose calculation algorithm was used for all rectangular, conformal and step-and-shoot IMRT plans. The maximal dose in the IMRT plan was ≤105% of the prescribed dose; the ipsilateral lung (V20, volume of lung receiving >20 Gy) was ≤25% and the heart was V50 ≤50% in patients with left breast cancer. The prescription doses for both plans are as follows: total dose 5000 cGy/25,200 cGy/times, five times per week, a total of 25 times.
Statistical analysis
Dosimetric comparisons between the tumors and OARs were completed based on the following parameters from a dose-volume histogram (DVH): D95, maximal dose at 95%; Dmax, dose received by ≤1% volume of the PTV; Dmin, dose received by ≥99% volume of the PTV; and Dmean of the PTV, V30, V20, V10 and V5 of the lung (fraction of the lung volume receiving >30, 20, 10 or 5 Gy, respectively) and V30, V40 and V50 of the heart (fraction of the heart volume receiving >30, 40 or 50 Gy, respectively) in patients with left breast cancer. Statistical analyses were performed using SPSS, version 16.0 (SPSS. Inc., Chicago, IL, USA). Student’s paired, two-tailed t-tests were used to determine statistical significance. P≤0.05 was considered to indicate a statistically significant difference.
Results
Dosimetric comparison between the PTV in IMRT and CR
An adequate dose coverage of the mammary glands and lymph nodes in the IMRT and CR plans was achieved in the majority of patients. For example, 95% of the PTV of the mammary glands was delivered by ≥95.4% of the prescribed dose. Similarly, the CR PTV was 95% in the CR plan, as the partial PTV was located in a low-dose region of the tangential fields. Of note, the volume of the low-dose region in the breast PTV was observed to correlate with the breast tissue thickness at the medial beam edge of the tangential fields.
DVH plots of the two modalities for the PTV of a typical patient are illustrated in Figs. 1 and 2. D95, Dmax, Dmin and Dmean values are presented in Table I. No significant difference in Dmean was observed between the IMRT and CR (P=0.326). Furthermore, IMRT acquired a significantly lower Dmax (P=0.015), but a higher Dmin (P=0.031), as compared with the CR. Accordingly, the IMRT improved dosimetric homogeneity more efficiently, without dosimetric hot and cold spots (Fig. 3).
Figure 1.
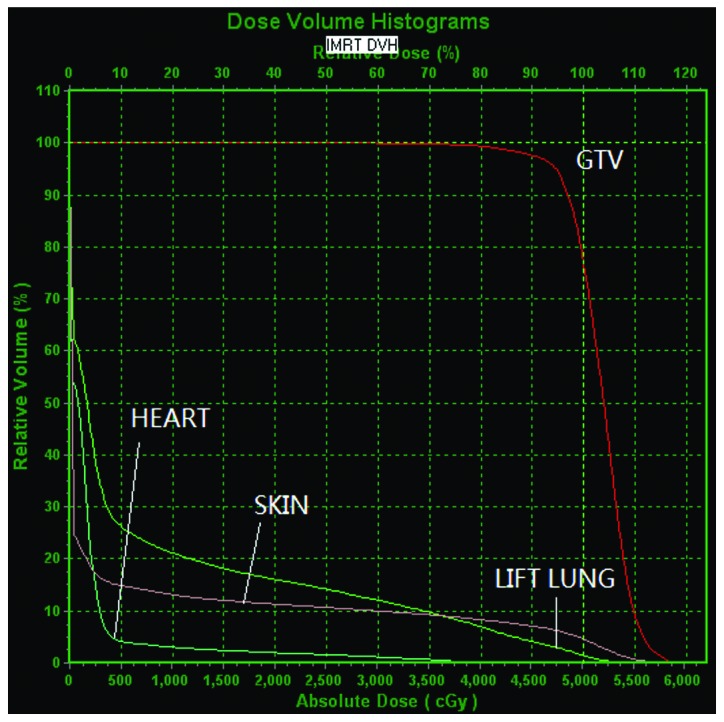
DVH of IMRT for a typical patient, showing results for the ipsilateral lung, heart and skin. DVH, dose-volume histogram; IMRT, intensity-modulated radiotherapy; GTV, gross tumor volume.
Figure 2.
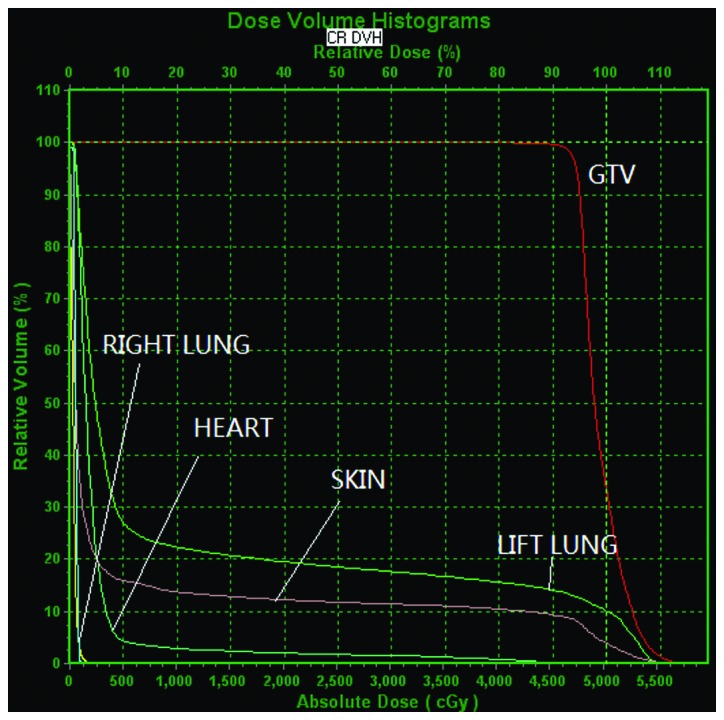
DVH of CR for a typical patient, showing results for the lungs, heart and skin. DVH, dose-volume histogram; CR, conventional radiotherapy; GTV, gross tumor volume.
Table I.
Comparison between the D95, Dmin, Dmax and Dmean of the PTV in IMRT and CR.
| Variables | D95 | Dmin, cGy | Dmax, cGy | Dmean, cGy |
|---|---|---|---|---|
| CR | 4518.3±60.4 | 3807.9±243.6 | 5832.2±61.4 | 5086.9±49.0 |
| IMRT | 4541.4±35.4 | 3868.4±248.3 | 5795.0±54.5 | 5075.8±47.3 |
| P-value | 0.009 | 0.031 | 0.016 | 0.326 |
D95, maximal dose at 95%; Dmin, minimum dose; Dmax, maximum dose; Dmean, mean dose; PTV, planning tumor volume; IMRT, intensity-modulated radiotherapy; CR, conventional radiotherapy.
Figure 3.
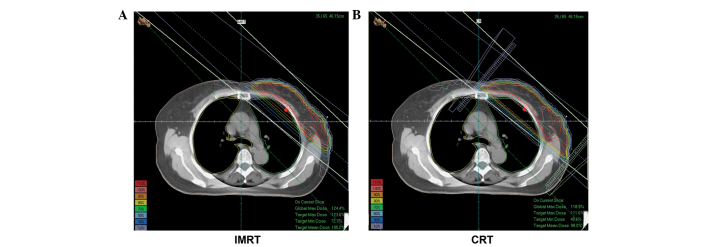
Isodose distributions from (A) intensity-modulated radiotherapy and (B) conventional radiotherapy for a typical patient.
Comparison between the dosimetric parameters of OARs
The dosimetric parameters of OARs, including V5, V10, V20 and V30 of the ipsilateral lung and V30, V40, V50 and Dmean of the heart are listed in Tables II and III, respectively. DVH plots for OARs of the two modalities are depicted in Figs 1. and 2. V5, V10, V20 and V30 of the ipsilateral lung were significantly reduced by 10.8, 8.4, 6.9 and 6.4%, (P=0.000, P=0.001, P=0.003 and P=0.002, respectively) in IMRT, as compared with the CR. Additionally, V30 of the heart was significantly reduced (P=0.046) and decreasing trends for V40 and V50 were observed in the IMRT. Collectively, the IMRT improved OAR protection by decreasing the irradiation dose and volume sparing the OARs, as compared with the CR.
Table II.
Comparison between the dosimetric parameters of the ipsilateral lung.
| Ipsilateral lung | ||||
|---|---|---|---|---|
|
|
||||
| Variables | V5, % | V10, % | V20, % | V30, % |
| CR | 38.3±0.8 | 31.8±0.8 | 27.7±0.9 | 24.9±1.0 |
| IMRT | 27.5±1.7 | 23.4±2.0 | 20.8±2.0 | 18.5±2.0 |
| P-value | 0.000 | 0.001 | 0.003 | 0.002 |
CR, conventional radiotherapy; IMRT, intensity-modulated radiotherapy. Vn the volume of the lung when the patient received a dose of nGy of radiation.
Table III.
Comparison between the dosimetric parameters of the heart in patients with left breast cancer.
| Heart | ||||
|---|---|---|---|---|
|
|
||||
| Variables | V30, % | V40, % | V50, % | Dmean, cGy |
| CR | 10.06±1.7 | 4.13±1.0 | 1.3±0.5 | 598.4±118.2 |
| IMRT | 5.3±1.4 | 1.9±0.5 | 0.0±0.0 | 348.3±91.6 |
| P-value | 0.046 | 0.095 | 0.076 | 0.004 |
Dmean, mean dose; CR, conventional radiotherapy; IMRT, intensity-modulated radiotherapy. Vn the volume of the lung when the patient received a dose of nGy of radiation.
Discussion
Over the past decade, there has been a rapid rise in the application of advanced radiation delivery technologies for the curative management of numerous types of solid cancer. Clinical irradiation patterns have shifted from conventional 2D therapy to a more developed 3D therapy based on CT (4,19). Furthermore, 3D conformal radiation, including IMRT, exhibits accurate information with regard to the radiation dose to the affected breast, regional nodes and adjacent normal tissues. Therefore, this approach may reduce morbidity and improve long-term cosmesis, while maintaining local tumor control (20–22). In the three published randomized trials of IMRT in breast cancer (2,23,24), the focus was on early-stage breast cancer treatment and, thus, the radiation target volume was only the breast. In these trials, IMRT only improved the radiation dose homogeneity, which was due to the elimination of significant hotspots in the breast that presented in wedge-based 2D plans (23,24). Improved radiation dosimetry is associated with improvements in acute radiation reactions, including skin dermatitis and overall breast cosmesis (12,25,26). IMRT may be beneficial for the treatment of node-positive breast cancer, particularly when the internal mammary nodal regions require treatment (17,27,28). At the Department of Tumor Radiotherapy, The First Affiliated Hospital of Anhui Medical University, CR is the routine technique for patients receiving breast-conserving surgery. Larger irradiated volumes of the ipsilateral lung and heart have been identified in CR compared with IMRT, as well as larger fields of rectangular shape, formed by jaws in the X and Y direction. However, intensity gradients in CR are only generated in a single direction (?).
The advantages of IMRT have been discussed in several studies; however, the results remain contradictory (17,19). Dogan et al (28) demonstrated the benefits of the IMRT plan as compared with a standard treatment plan using optimized beam weights and wedges. However, the treatment plans were not compared with the rectangular tangential fields commonly used in clinical practice. Additionally, it has been demonstrated that standard dose optimization, also known as two-step IMRT (21,27,29), does not generate the optimum intensity distributions, due to degradation during the segmentation process. Therefore, the clinical application of IMRT for breast cancer remains unknown.
In the current study, although no significant difference in Dmean was identified between IMRT and CR (P=0.326), IMRT acquired a significantly lower Dmax (P=0.015) and a higher Dmin (P=0.031), which is consistent with previous reports (6,28,30,31). This suggested that IMRT improves dosimetric homogeneity and uniformity without dosimetric hot and cold spots. Furthermore, compared with CR, IMRT decreased the OAR volumes receiving high doses and increased the volumes receiving low doses. Additionally, CR increased the volumes exposed to the ipsilateral lung and heart compared with the IMRT. Accordingly, in order to obtain clinically accepted plans, the IMRT plan requires clinical planners with advanced treatment skills to achieve different combinations of wedge angles, collimator angles and beam weights.
IMRT has been used to avoid late toxicity, including pneumonitis, lung fibrosis and coronary heart disease (15). However, the probability for radiation-induced secondary malignancies may increase when larger volumes of normal tissue are exposed to lower doses. In the outcomes presented in this study, IMRT yielded a smaller proportion of irradiated volume in high-dose areas when compared with CR, but a larger proportion in low-dose areas. This was likely to be due to increased leakage from more segments in IMRT. To date, only skin reactions from reverse IMRT have been reported (26).
With regard to set-up uncertainty during the treatment process, the boundary of the irradiation fields may be expanded far enough to ensure the PTV is completely included when using CR and IMRT techniques. However, set-up accuracy in IMRT must be the focus, which may be improved through breath gating and imaging guidance techniques. Notably, the lymph nodes were not included in the treatment volume in this study. In addition, with the limited sample size, clinically meaningful improvements with the use of IMRT require further, large randomized trials.
In conclusion, CR has exhibited satisfactory results in breast cancer patients treated with breast-conserving surgery in our department at The First Affiliated Hospital of Anhui Medical University. However, the present dosimetric analyses demonstrated that IMRT provides improved uniformity and coverage of target volumes and an associated reduction of dose delivery to critical organs when compared with CR. In addition, IMRT decreased the OAR volumes receiving higher doses and increased the volumes receiving lower doses. Clinical trials and long-term follow-up may be required to evaluate the clinical significance of the dosimetric characteristics associated with IMRT.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 3.Haviland JS, Yarnold JR, Bentzen SM. Hypofractionated radiotherapy for breast cancer. N Engl J Med. 2010;362:1843–1844. doi: 10.1056/NEJMc1002798. [DOI] [PubMed] [Google Scholar]
- 4.Haffty BG, Buchholz TA, McCormick B. Should intensity-modulated radiation therapy be the standard of care in the conservatively managed breast cancer patient? J Clin Oncol. 2008;26:2072–2074. doi: 10.1200/JCO.2007.15.9442. [DOI] [PubMed] [Google Scholar]
- 5.Zhou GX, Xu SP, Dai XK, et al. Clinical dosimetric study of three radiotherapy techniques for postoperative breast cancer: Helical Tomotherapy, IMRT, and 3D-CRT. Technol Cancer Res Treat. 2011;10:15–23. doi: 10.7785/tcrt.2012.500174. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F, Zheng M. Dosimetric evaluation of conventional radiotherapy, 3-D conformal radiotherapy and direct machine parameter optimisation intensity-modulated radiotherapy for breast cancer after conservative surgery. J Med Imaging Radiat Oncol. 2011;55:595–602. doi: 10.1111/j.1754-9485.2011.02313.x. [DOI] [PubMed] [Google Scholar]
- 7.Rudat V, Alaradi AA, Mohamed A, Ai-Yahya K, Altuwaijri S. Tangential beam IMRT versus tangential beam 3D-CRT of the chest wall in postmastectomy breast cancer patients: a dosimetric comparison. Radiat Oncol. 2011;6:26. doi: 10.1186/1748-717X-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ercan T, Iğdem S, Alço G, et al. Dosimetric comparison of field in field intensity-modulated radiotherapy technique with conformal radiotherapy techniques in breast cancer. Jpn J Radiol. 2010;28:283–289. doi: 10.1007/s11604-010-0423-3. [DOI] [PubMed] [Google Scholar]
- 9.Askoxylakis V, Jensen AD, Häfner MF, et al. Simultaneous integrated boost for adjuvant treatment of breast cancer - intensity modulated vs. conventional radiotherapy: the IMRT-MC2 trial. BMC Cancer. 2011;11:249. doi: 10.1186/1471-2407-11-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatnagar AK, Beriwal S, Heron DE, et al. Initial outcomes analysis for large multicenter integrated cancer network implementation of intensity modulated radiation therapy for breast cancer. Breast J. 2009;15:468–474. doi: 10.1111/j.1524-4741.2009.00761.x. [DOI] [PubMed] [Google Scholar]
- 11.McDonald MW, Godette KD, Butker EK, Davis LW, Johnstone PA. Long-term outcomes of IMRT for breast cancer: a single-institution cohort analysis. Int J Radiat Oncol Biol Phys. 2008;72:1031–1040. doi: 10.1016/j.ijrobp.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 12.Smith BD, Pan IW, Shih YC, et al. Adoption of intensity-modulated radiation therapy for breast cancer in the United States. J Nat Cancer Inst. 2011;103:798–809. doi: 10.1093/jnci/djr100. [DOI] [PubMed] [Google Scholar]
- 13.Selvaraj RN, Beriwal S, Pourarian RJ, et al. Clinical implementation of tangential field intensity modulated radiation therapy (IMRT) using sliding window technique and dosimetric comparison with 3D conformal therapy (3DCRT) in breast cancer. Med Dosim. 2007;32:299–304. doi: 10.1016/j.meddos.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Guerrero M, Li XA, Earl MA, Sarfaraz M, Kiggundu E. Simultaneous integrated boost for breast cancer using IMRT: a radiobiological and treatment planning study. Int J Radiat Oncol Biol Phys. 2004;59:1513–1522. doi: 10.1016/j.ijrobp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Tan W, Liu D, Xue C, et al. Anterior myocardial territory may replace the heart as organ at risk in intensity-modulated radiotherapy for left-sided breast cancer. Int J Radiat Oncol Biol Phys. 2012;82:1689–1697. doi: 10.1016/j.ijrobp.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Peulen H, Hanbeukers B, Boersma L, et al. Forward intensity-modulated radiotherapy planning in breast cancer to improve dose homogeneity: feasibility of class solutions. Int J Radiat Oncol Biol Phys. 2012;82:394–400. doi: 10.1016/j.ijrobp.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 17.White JR, Meyer JL. Intensity-modulated radiotherapy for breast cancer: advances in whole and partial breast treatment. Front Radiat Ther Oncol. 2011;43:292–314. doi: 10.1159/000322461. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 19.Yu JM, YWB, et al. Tumor Accurate Radiotherapy Treating Learning. 1st edition. Shandong Science and Technology Press; China: 2004. pp. 815–824. [Google Scholar]
- 20.Kachnic LA, Powell SN. IMRT for breast cancer - balancing outcomes, patient selection, and resource utilization. J Nat Cancer Inst. 2011;103:777–779. doi: 10.1093/jnci/djr136. [DOI] [PubMed] [Google Scholar]
- 21.Coles CE, Moody AM, Wilson CB, Burnet NG. Reduction of radiotherapy-induced late complications in early breast cancer: the role of intensity-modulated radiation therapy and partial breast irradiation. Part II - Radiotherapy strategies to reduce radiation-induced late effects. Clin Oncol (R Coll Radiol) 2005;17:98–110. doi: 10.1016/j.clon.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Vicini FA, Sharpe M, Kestin L, et al. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54:1336–1344. doi: 10.1016/S0360-3016(02)03746-X. [DOI] [PubMed] [Google Scholar]
- 23.Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7:467–471. doi: 10.1016/S1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- 24.Barnett GC, Wilkinson JS, Moody AM, et al. Randomized controlled trial of forward-planned intensity modulated radiotherapy for early breast cancer: interim results at 2 years. Int J Radiat Oncol Biol Phys. 2012;82:715–723. doi: 10.1016/j.ijrobp.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 25.Donovan E, Bleakley N, Denholm E, et al. Breast Technology Group. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol. 2007;82:254–264. doi: 10.1016/j.radonc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Freedman GM, Anderson PR, Li J, et al. Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer. Am J Clin Oncol. 2006;29:66–70. doi: 10.1097/01.coc.0000197661.09628.03. [DOI] [PubMed] [Google Scholar]
- 27.Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085–2092. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 28.Dogan N, Cuttino L, Lloyd R, Bump EA, Arthur DW. Optimized dose coverage of regional lymph nodes in breast cancer: the role of intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1238–1250. doi: 10.1016/j.ijrobp.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 29.Jagsi R, Moran J, Marsh R, Masi K, Griffith KA, Pierce LJ. Evaluation of four techniques using intensity-modulated radiation therapy for comprehensive locoregional irradiation of breast cancer. Int J Radiat Oncol Biol Phys. 2010;78:1594–1603. doi: 10.1016/j.ijrobp.2010.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krueger EA, Fraass BA, Pierce LJ. Clinical aspects of intensity-modulated radiotherapy in the treatment of breast cancer. Semin Radiat Oncol. 2002;12:250–259. doi: 10.1053/srao.2002.32468. [DOI] [PubMed] [Google Scholar]
- 31.Tan W, Wang X, Qiu D, et al. Dosimetric comparison of intensity-modulated radiotherapy plans, with or without anterior myocardial territory and left ventricle as organs at risk, in early-stage left-sided breast cancer patients. Int J Radiat Oncol Biol Phys. 2011;81:1544–1551. doi: 10.1016/j.ijrobp.2010.09.028. [DOI] [PubMed] [Google Scholar]


