Abstract
• Background and Aims Euphorbia boetica (Euphorbiaceae) is a functional andromonoecious species that shows both intra- and interfloral dichogamy, hermaphrodite cyathia being protogynous. Sexual dimorphism of the cyathia of E. boetica is examined according to their gender and arrangement on the inflorescence.
• Methods Data were obtained from two natural populations, where the distribution of male and hermaphrodite cyathia in the inflorescence was recorded. The size, pollen production and viability, and nectar secretion were measured in both types of cyathia.
• Key Results Most cyathia were male at the first levels of the inflorescence, then hermaphrodite cyathia predominated at the successive levels, although at the last levels the proportion of male cyathia increased. Male cyathia at basal positions lack ovaries, whereas those at distal positions showed vestigial ovaries. The size of the cyathia varied significantly depending on the level of the inflorescence where they were produced: those of the last levels were usually smaller. The hermaphrodites were significant bigger than males; however, these differences were due to the differential distribution of each cyathium type in the inflorescence. Male cyathia produced significantly more pollen and nectar than hermaphrodites.
• Conclusions In Euphorbia boetica, basal male cyathia could be explained by the presence of protogyny, and apical male cyathia seem to respond to a preemption of resources. A true dimorphism affecting primary sexual characters and related to gender function appears at lower levels of the inflorescence, whereas an apparent size dimorphism due to positional effects occurs at upper positions. Longevity and distribution of cyathia, and their pattern of nectar production, could improve both male and female fitness.
Keywords: Andromonoecy, protogyny, positional effects, viable pollen production, nectar secretion, Euphorbiaceae
INTRODUCTION
The temporal separation of anther dehiscence and stigmatic receptivity can occur within a hermaphrodite flower or between flowers in monoecious species, giving rise to intrafloral or interfloral dichogamy, respectively (Lloyd and Webb, 1986). Intrafloral dichogamy is thought to have evolved to reduce interference between the two main functions of a hermaphroditic flower: pollen export and pollen import (Wyatt, 1983; Lloyd and Webb, 1986; Bertin and Newman, 1993). Self-pollination could be considered a form of interference that may reduce female fitness by reducing the fertilization success of incoming outcross pollen and reduce male fitness by reducing the export of pollen to other individuals (Harder and Wilson, 1998). However, the form of dichogamy (protogyny and protandry) is likely to have different consequences for autogamy versus geitonogamy. Protandry, may be effective at reducing geitonogamy if flowers open from the bottom to the apex in the inflorescence and the visitors move upwards (Harder et al., 2000). Protogyny may be effective at reducing autogamy since it provides a period of stigma receptivity in the absence of self-pollen (Griffin et al., 2000). Protogyny may also reduce geitonogamy if the flowers on an inflorescence develop so that all open flowers are in either male or female phase at any given time (Stout, 1928). Thus, the consequences of dichogamy are markedly affected by inflorescence architecture (Harder and Barrett, 1996) and by the phenology of male and female phases.
In both intra- and interfloral dichogamy, each sexual function can be accompanied by a set of differential secondary sex characters favouring such function. Thus, the perianth of male flowers is often larger than that of female flowers (Bell, 1985; Delph, 1996). Likewise, in some hermaphrodite flowers dichogamy is accompanied by a certain dimorphism of the perianth: in the male phase the lobes of the corolla are completely extended, but in the female, they are curved back, decreasing the size of the corolla (Ortiz et al., 2000). This sexual dimorphism has been interpreted as a way of ensuring the success of the male function, which may be limited by the number of visits (Bateman, 1948; Charnov, 1982). A larger perianth would increase floral attractiveness and could lead to more visits from pollinators (Bell, 1985; Ashman and Staton, 1991). Differences in nectar secretion between sexual phases have also been found; in some cases the male phase secretes more nectar than the female (Devlin and Stephenson, 1985; Aizen and Basilio, 1998) but in others the reverse pattern has been found (Talavera et al., 1996).
When the sexual functions are separated in different flowers, however, such differences in secondary sexual characters might not be related to the improvement of the sexual functions, but rather may be simply due to plastic responses to resource pre-emption or to different position effects within the architecture of the inflorescence (Gibbs et al., 1999; Diggle, 2003; Diggle and Miller, 2004). There is considerable variation in investment in attractive traits (petals, nectar), biomass distribution, and reproductive potential between flowers within an inflorescence (Stephenson, 1981; Diggle, 1995). Distal flowers are often smaller and produce fewer or smaller ovules, seeds and fruits, but they may have higher pollen production (Herrera, 1991; Ashman and Baker, 1992; Diggle, 1995; Guitián and Navarro, 1996; Ashman and Hitchens, 2000). Recent evidence suggests that, when flowers of different sexual phenotypes are produced in distinct locations within the architecture of inflorescences, the effects of gender dimorphism can be confounded with architectural differences (Diggle and Miller, 2004).
In the genus Euphorbia, cyathia function as hermaphroditic protogynous flowers, although in fact they comprise 1-pistillate and 1-staminate floret within a cup-like involucre. The cyathia are arranged in pleiochasia, each pleiochasial branch forming several pleiochasia or dichasia which bloom sequentially (Weberling, 1986). Some species of Euphorbia are functionally andromonoecious, as they produce male and hermaphrodite cyathia (Gilbert, 1992; Kiflawi, 2000; Narbona et al., 2000, 2002). The male cyathia bloom first, because of their basal position on the inflorescence, originating interfloral dichogamy (Narbona et al., 2002). The simultaneous presence of functional andromonoecy and of intra- and interfloral dichogamy establishes secondary sexual differentiation at two levels: between cyathia of different gender, and between sexual phases of the hermaphrodite cyathia.
Secondary sexual differentiation in the andromonoecious Euphorbia boetica was investigated according to the gender of the cyathia and their arrangement on inflorescences, focusing, in particular, on the distribution of male and hermaphrodite cyathia on the inflorescence, morphological characters of the cyathia, longevity, pollen production and viability, and nectar secretion.
MATERIALS AND METHODS
Study species and study area
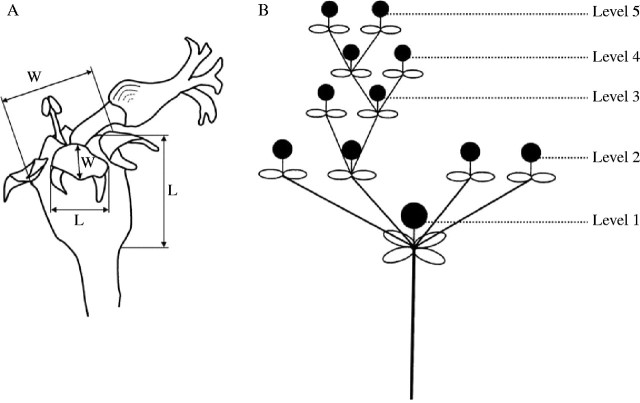
Euphorbia boetica Boiss. is a perennial spurge endemic to the southern Iberian Peninsula. Euphorbia boetica is functionally andromonoecious, and the staminate cyathia are situated predominantly at the first levels of the inflorescence (Narbona et al., 2000). The degree of branching in the inflorescence is high. Level 1 is assigned to the terminal cyathium (i.e. the first to be produced) in each inflorescence (Fig. 1).
Fig. 1.
Diagrammatic representation of the cyathium and the inflorescence of Euphorbia boetica. (A) Hermaphrodite cyathium showing the characteristics measured (length and width of the cyathium involucre and the nectary). (B) Inflorescence showing the flowering order of the cyathia (levels).
Euphorbia boetica was studied in two populations: Hinojos (Huelva province) and El Gandul (Seville province). The Hinojos population is located on a peneplane at 80–90 m a.s.l. and approx. 30 km from the sea; the vegetation consists of mixed woodland of Pinus pinea and Quercus suber, with a scrub layer comprising mainly Cistaceae, Lamiaceae and Leguminosae. The Gandul population is also located on a peneplane, at 45 m a.s.l. in an area of abandoned farmland without tree cover. The vegetation consists of scattered scrubs [Chamaerops humilis, Helichrysum stoechas, Asparagus aphyllus, Micromeria graeca, etc.] and herbs (mainly Poaceae).
Methods
The inflorescence development and the proportion of male and hermaphrodite cyathia were monitored in three branches from 11 or 12 plants of each population. Each plant was marked at the beginning of the flowering and visited every 7–10 d noting the number of male and hermaphrodite cyathia at each level of the inflorescence. The cyathia and their nectaries were measured with an electronic digital caliper in 9–11 plants of each population, noting the sexuality of the cyathia and their position on the inflorescence. The main axes (length and width) of the cyathium involucre and the nectary were measured (Fig. 1).
The duration of anthesis of the hermaphrodite and male cyathia was monitored in 76 marked cyathia on 14 plants at Hinojos. These cyathia were examined daily, noting their floral phase and, where applicable, the number of stamens exposed. The receptivity of the stigmata was determined in flowers at different stages of development using the hydrogen peroxide technique (Kearns and Inouye, 1993).
The daily cycle of pollen presentation was estimated at Hinojos in 40 cyathia of five plants that were examined every 2 h from 0800 h to 2000 h, noting the number of exposed stamens with pollen. In addition, 30 cyathia that had finished flowering were collected in each population and dissected to discover how many stamens had remained unexposed. A cyathium was considered to have finished flowering when no stamen had been exposed for 3 d.
For each population, the pollen production was estimated separately in the male and hermaphrodite cyathia from intermediate levels where both types of cyathia co-occur. The number of stamens was counted in 20–30 cyathia of each sex on ten plants. The pollen production per cyathium was estimated in five of these plants by counting the pollen on two stamens of 9–14 cyathia taken from each plant. Each stamen was dissected on a slide and all its pollen grains were counted under a microscope.
The pollen viability was analysed in 15 plants at El Gandul, considering separately the cyathia at different levels on the inflorescence of each plant. Pollen viability was also analysed separately in male and hermaphrodite cyathia from the intermediate levels of the inflorescence. Pollen viability was estimated by sowing the pollen on a solid nutritive medium in Petri dishes (Bar-Shalon and Mattson, 1977). For each plant, all the stamens available at the time of sowing were used. After sowing, the dishes were kept for 6 h at 22–24 °C. A prior check showed that a longer time did not increase the percentage of germination. Three samples (replicates) were taken from each dish, and the proportion of germinated pollen grains was determined as those whose pollen tube was longer than the diameter of the grain. All the pollen grains in each replicate were counted. The average number was 200.
The nectar production at Hinojos was measured in the hermaphrodite and male cyathia from the intermediate levels of 17 inflorescences (13 plants) bagged in situ for 24 h using plastic bags for excluding insect visits. The volume and concentration of nectar were used to estimate the weight of sugar produced per cyathium (Cruden and Hermann, 1983). Calculation of the mean values of nectar production excluded the relatively rare cyathia that did not present secretion. Activity of the nectaries through the day was monitored visually in 113 cyathia from the intermediate levels of eight plants at Hinojos. These cyathia were examined at regular intervals from 1000 h to 2000 h on sunny days in June and July, noting the number of nectaries presenting nectar on their surface.
Statistical analyses
Differences in the proportions of male and hermaphrodite cyathia between inflorescence levels were tested using the Kruskal–Wallis test (Zar, 1999) because data normalization by the usual transformation was not achieved; when differences were significant a Mann–Whitney test was carried out. Differences in the size of the cyathia and their nectaries according to the gender (male versus hermaphrodite) and according to the inflorescence level were analysed using MANOVAs test, including width and length as predictor variables.
The number of stamens and the total number of pollen grains of the male and hermaphrodite cyathia from the intermediate levels were compared using mixed-model ANOVAs, with ‘plant’ as random factor and ‘sex’ as fixed. Differences in pollen production per stamen between cyathia, sex and plants were analysed using mixed ANOVAs, in which the factor ‘plant’ was random, the factor ‘sex’ was fixed, and the factor ‘cyathium’ was nested in ‘plant’ and ‘sex’. Comparison of stamen number and pollen production between apical male cyathia and both hermaphrodite and male cyathia (from intermediate levels) were performed using the Mann–Whitney test because data normalization by the usual transformation was not achieved (Day and Quinn, 1989). Differences in pollen viability between different levels of the inflorescence were tested using a mixed-model ANOVA where the factor ‘plant’ was considered random and the factor ‘level’ was fixed. A similar analysis was performed to determine differences in pollen viability between cyathia of different sex from intermediate levels; in this case, the factor ‘plant’ was considered random and the factor ‘sex’ was fixed. The data were arcsine transformed.
The characteristics of the nectar produced by male and hermaphrodite cyathia from the intermediate levels in Euphorbia boetica were compared using mixed-model ANOVAs where the factor ‘plant’ was considered random and the factor ‘sex’ was fixed. Percentages of nectary activity through the day were compared using a χ2-test for contingency tables of percentages (Zar, 1999).
When the ANOVA/MANOVA showed significant differences, the means of groups were compared using the t-test with estimation of the separate variance (Welch test), as the variance of the groups was not equal (Day and Quinn, 1989). To control for the experiment-wise type I error produced by multiple comparisons, the sequential Bonfferroni test for fitting the significance level was applied (Rice, 1989).
RESULTS
Frequency of cyathia and dychogamy
In the El Gandul population the inflorescence of Euphorbia boetica developed up to seven or eight levels, while in the Hinojos population this number was lower, reaching up to four or five levels. In both populations, hermaphrodite and male cyathia were found; most cyathia were male at the first level of the inflorescence, then their proportion diminished significantly at the intermediate levels (H = 53·2, P < 0·0001, d.f. = 7; H = 24·7, P < 0·0001, d.f. = 3; in El Gandul and Hinojos, respectively), and increased slightly at the lasts (Fig. 2). In general, in male cyathia from the basal and central levels of the inflorescence, female flowers were totally absent. In contrast, male cyathia from the last levels were visibly smaller than male cyathia from the other levels, and usually bore a vestigial female flower, although they never produced seeds.
Fig. 2.
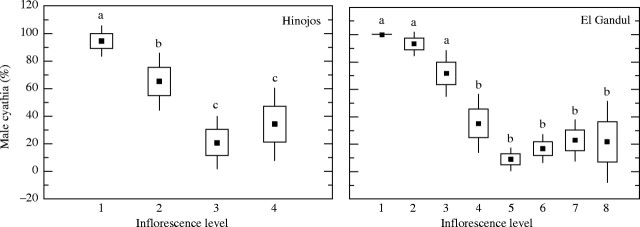
Mean proportions of male cyathia in each inflorescence level in the two populations studied. Boxes represent standard errors and the bars are 1·96 s.e. Different letters indicate significant differences at a < 0·05.
The hermaphrodite cyathia are protogynous, and the female flower is receptive for 3–8 d (mean = 4, n = 43); in the cyathia of the same level of the inflorescence, this phase had a variable duration. When receptive, the female flower remains erect, and later hangs between two of the nectaries. At the end of the female phase, the stamens issue from the cyathium involucre at a rate of 0–4 stamens daily (mean = 1·6, n = 129). Each stamen remains exposed 1 d, usually dropping in the night or following morning when pushed out by the new ones. The stamens emerged from the cyathium involucre at 1000 h in the morning, and were open from 1100 h to 1900 h, although little pollen remained after 1600 h. The male phase lasted 5–18 d (mean = 12·3, n = 35). In the male cyathia, the duration of anthesis was similar to that of the male phase of the hermaphrodites (5–19 d, mean = 12·0, n = 31). The stamen issue rate was also similar (0–4 stamens daily, mean = 1·8, n = 126). In both types of cyathium, all the stamens produced were usually exposed; only in 6 % of the hermaphrodites and 4 % of the males did one or two stamens remain inside the cyathium involucre.
Cyathia size
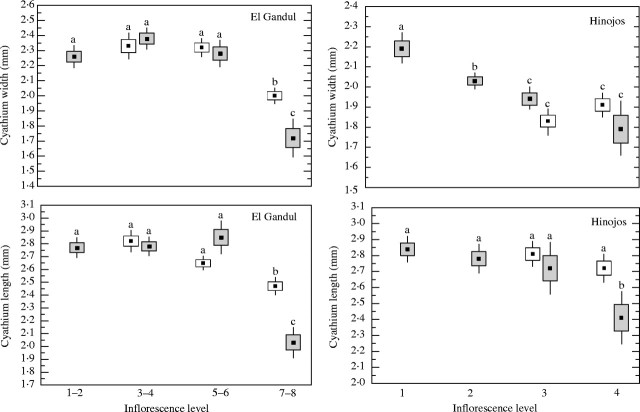
The size of the cyathia varied significantly depending on the level of the inflorescence where they were produced: in both populations, those of the last levels were usually smaller (Table 1 and Fig. 3). Similarly, in both populations the size of the nectaries was significantly different between levels (Table 1). Significant differences between sexes in the size of the cyathia were found in both populations, the hermaphroditic cyathia generally were larger (Table 1). However these differences are due to the differential distribution of each cyathium type in the inflorescence (Fig. 3); in fact, when male and hermaphrodite cyathia were measured at the same level of the inflorescence differences in size were not significant, excepting at the last levels where male cyathia were smaller (see Fig. 3).
Table 1.
MANOVA results comparing the size of cyathia and nectaries from male and hermaphrodite cyathia of different inflorescence level in two populations of Euphorbia boetica (Hinojos and El Gandul)
| Cyathium |
Nectary |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Multivariate test |
Univariate test for variable |
Multivariate test |
Univariate test for variable |
||||||||||||||||||||
| Population |
Factor |
Willlk's λ |
F |
d.f. |
P |
Cyathium width |
Cyathium length |
Willlk's λ |
F |
d.f. |
P |
Nectary width |
Nectary length |
||||||||||
| El Gandul | Level | 0·50 | 37·2 | 6, 548 | **** | **** | **** | 0·59 | 27·5 | 6, 550 | **** | **** | **** | ||||||||||
| Sexuality | 0·92 | 12·2 | 2, 274 | **** | *** | **** | 0·93 | 9·7 | 2, 275 | **** | n.s. | **** | |||||||||||
| Level × sexuality | 0·85 | 12·0 | 4, 548 | **** | *** | **** | 0·89 | 8·1 | 4, 550 | **** | **** | **** | |||||||||||
| Hinojos | Level | 0·68 | 13·2 | 6, 370 | **** | **** | **** | 0·81 | 6·9 | 6, 368 | **** | **** | *** | ||||||||||
| Sexuality | 0·93 | 7·2 | 2, 185 | *** | ** | *** | 0·91 | 8·7 | 2, 184 | *** | **** | ** | |||||||||||
| Level × sexuality | 0·98 | 2·3 | 2, 185 | n.s. | n.s. | * | 0·95 | 4·6 | 2, 184 | * | ** | n.s. | |||||||||||
P < 0·0001;
P < 0·001;
P < 0·01;
P < 0·05;
n.s., not significant.
Fig. 3.
Mean length and width of the male (shaded boxes) and hermaphrodite (open boxes) cyathia of Euphorbia boetica according with the level of the inflorescence in the two populations studied. Boxes represent standard errors and the bars are 1·96 s.e. Different letters indicate significant differences with Bonferroni adjusted a.
The number of nectaries per cyathium varied through the inflorescence: at El Gandul, the first-level cyathia presented between five and seven nectaries, and those of the remaining levels presented four; at Hinojos, most of the first-level cyathia had five nectaries, and at the remaining levels there were always four.
Pollen production and viability
In both populations, at the intermediate levels of the inflorescence, the stamen number in male cyathia was significantly higher than in hermaphrodites (Table 2). In the male cyathia from the last level, the stamen number ranged between 2 and 16 (mean ± s.e. = 9 ± 0·8, n = 19, seven plants) and was significantly lower than in the male and hermaphrodite cyathia from intermediate levels (U = 2·5, n1 = 19, n2 = 20, P < 0·0001 and U = 36·5, n1 = 19, n2 = 20, P < 0·0001). The number of pollen grains per stamen at El Gandul ranged between 705 and 1242, with a mean of 993. At the intermediate levels of the inflorescence, the stamens of the male and hermaphrodite cyathia produced a similar number of pollen grains (Table 2). At Hinojos, the number of pollen grains per stamen ranged between 566 and 1174, with a mean of 840. In this population, the male cyathia produced on average 100 pollen grains more per stamen than the hermaphrodites, but this difference was not significant (Table 2). At El Gandul the production of pollen per stamen was statistically higher than that at Hinojos (F1,90 = 25·75, P < 0·0001).
Table 2.
Number of stamens per cyathium, and pollen grains per stamen and cyathium in male and hermaphrodite cyathia from the same level of the inflorescence
| Stamens/cyathium |
Pollen/stamen |
Pollen/cyathium |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population |
Cyathium sexuality |
N |
n |
Mean ± s.e. |
Range |
N |
n |
Mean ± s.e. |
Range |
N |
n |
Mean ± s.e. |
Range |
|||||||||
| El Gandul | Male | 10 | 30 | 23 ± 0·6a | 16–29 | 5 | 28 | 1000 ± 26·2a | 705–1242 | 5 | 14 | 20951 ± 1159a | 13152–26959 | |||||||||
| Hermaphrodites | 10 | 30 | 20 ± 0·6b | 13–25 | 5 | 28 | 988 ± 22·3a | 761–1231 | 5 | 14 | 16638 ± 721b | 11908–20366 | ||||||||||
| Total | 10 | 60 | 21 ± 0·5 | 13–29 | 5 | 56 | 993 ± 17·0 | 705–1242 | 5 | 28 | 18676 ± 797 | 11908–26959 | ||||||||||
| Hinojos | Males | 10 | 20 | 21 ± 0·7a | 14–27 | 5 | 18 | 897 ± 30·1a | 726–1174 | 5 | 9 | 18883 ± 1373a | 12607–24636 | |||||||||
| Hermaphrodites | 10 | 20 | 17 ± 1·0b | 10–25 | 5 | 18 | 782 ± 41·6a | 566–1155 | 5 | 9 | 13718 ± 2018a | 7105–25425 | ||||||||||
| Total | 10 | 40 | 19 ± 0·7 | 10–27 | 5 | 36 | 840 ± 27·1 | 566–1174 | 5 | 18 | 16300 ± 1340 | 7105–25425 | ||||||||||
N = number of plants, n = number of samples.
In each population, means followed by different letters indicate significant differences (α < 0·05).
The mean production of pollen per cyathium was 18 676 grains at El Gandul and 16 300 at Hinojos (Table 2), and was statistically similar between populations (F1,44 = 2·63, P = 0·11). At the intermediate levels of the inflorescence, the number of pollen grains of the male cyathia was >25 % higher that that of the hermaphrodites, but the differences were significant only at El Gandul (Table 2). The male cyathia from the last level of the inflorescence produced only 314 ± 23 pollen grains per stamen (n = 27, seven plants). Moreover, the pollen from four of these cyathia on two plants was found to be inviable. The total number of pollen grains per male cyathia at the last level of the inflorescence ranged between 492 and 7952 (mean ± s.e. = 2946 ± 421), which was markedly lower than that of the male and hermaphrodite cyathia from the intermediate levels (U = 0, P < 0·0001, n1 = 9, n2 = 19; U = 2, P < 0·0001, n1 = 9, n2 = 9).
In Euphorbia boetica, pollen viability was significantly variable between plants (F12,94 = 4·15, P = 0·0056), ranging between 7·7 ± 1·9 % and 66·6 ± 4·4 %, with a mean of 31·8 ± 1·8 %. Pollen viability was 40·2 ± 3·4 % at the first levels of the inflorescence, 32·4 ± 2·2 % at the intermediate levels and 15·5 ± 2·2 % at the last levels, although the differences were not statistically significant (P > 0·05). At the intermediate levels of the inflorescence, the pollen viability of the male cyathia was slightly higher than that of the hermaphrodites (35·8 % against 28·9 %), but the differences were not significant (P > 0·05).
Nectar secretion
The pattern of nectar secretion through the day, measured as the proportion of active nectaries, was different in male and hermaphrodite cyathia (χ2 = 39·29, d.f. = 4, P < 0·0001). This is because in the morning more male cyathia than hermaphrodites secreted nectar (30 % against 13 %); however, from 1300 h, the proportion of male cyathia and hermaphrodites secreting nectar was similar. Some 30 % of cyathia of both types did not produce nectar at any time of the day; most of those that did secrete had all their nectaries active.
The volume of nectar accumulating in 24 h ranged between 0·19 and 2 μL per cyathium, with a mean of 0·61 ± 0·068 μL (n = 150). The male cyathia produced a mean of 0·74 ± 0·073 μL of nectar, slightly higher than that of the hermaphrodites in male phase and significantly higher than that of the hermaphrodites in female phase (Fig. 4). The mean concentration of sugar in the nectar was 5·8 ± 0·67 % (n = 150). The nectar of the hermaphrodite cyathia was slightly more concentrated than that of the males, but the differences were not significant (Fig. 4). The mean production of sugar per cyathium per day was 31·4 ± 4·67 µg. The amount of sugar produced by male cyathia and the hermaphrodites in male phase was significantly greater than that by the hermaphrodites in female phase (36·4 and 35·8 µg, respectively, against 22·1 µg; Fig. 4).
Fig. 4.
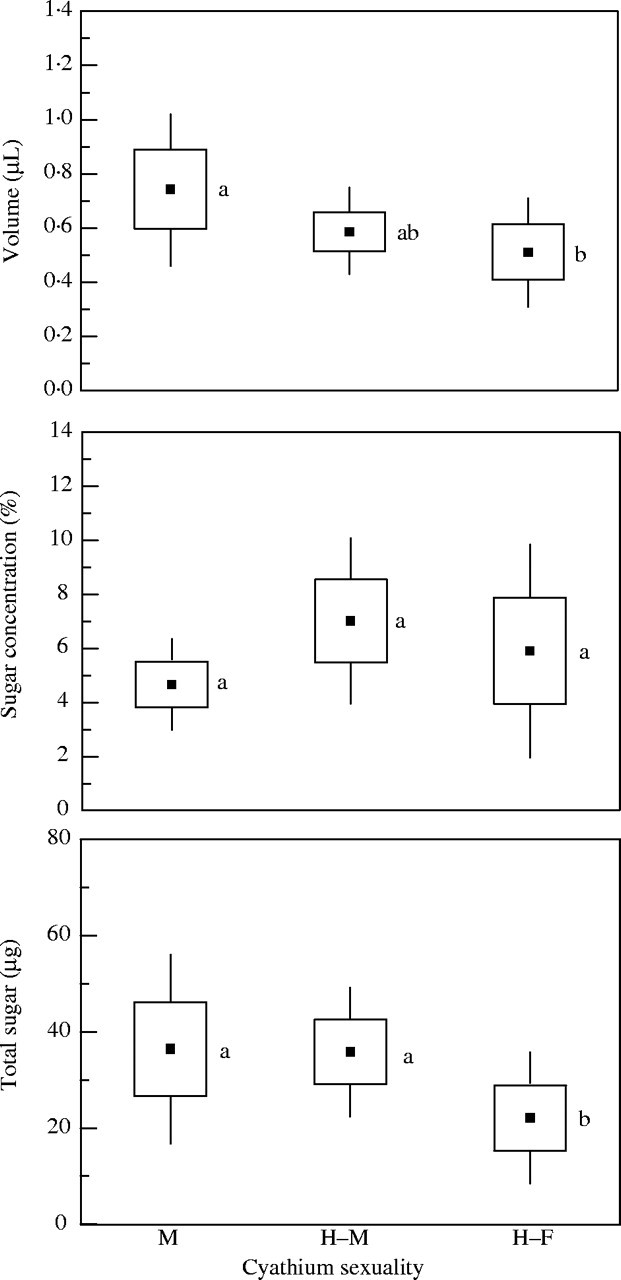
Volume, concentration and sugar weight of the nectar produced by male cyathia (M), male phase of hermaphrodite cyathia (H–M) and female phase of hermaphrodite cyathia (H–F) in Euphorbia boetica; sample sizes were 31, 73 and 46, respectively. Boxes represent standard errors and the bars are 1·96 s.e. Different letters indicate significant differences at a < 0·05.
DISCUSSION
Euphorbia boetica plants produced both male and hermaphrodite cyathia and both populations showed a similar pattern of cyathia distribution. The first levels of the inflorescence were almost exclusively male, then hermaphrodite cyathia predominated at the successive levels, although at the last level the proportion of male cyathia increased. Production of male cyathia of E. boetica seem to have two different origins, that is, the explanation for the production of basal male cyathia differs from that for distal male cyathia.
The complete loss of initiation of female flower in male cyathia from basal and central levels suggests that their maleness is determined from inception before flowering. Brunet and Charlesworth (1995) concluded that intrafloral dichogamy favours variation in sex allocation among sequentially blooming flowers: with protandry, early-blooming flowers should be relatively more female, and with protogyny, early-blooming flowers should be relatively more male. Cyathia of Euphorbia boetica are protogynous, so that cyathia that open early could experience a mating environment in which the ovule : pollen ratio is higher than that for later cyathia. In such case, ovules of basal cyathia would be unlikely to produce offspring, giving rise to variation in fitness contribution through female function among individual flowers of the inflorescence (Brunet and Charlesworth, 1995). This variation could lead to lower cyathia evolving to specialize as male. Thus, basal cyathia of Euphorbia boetica lack female flowers. This has been found in other Euphorbia species (Narbona et al., 2002) and in protogynous Apioideae species (Schlessman and Graceffa, 2002). Interestingly, the reverse pattern is found in protandrous species (Brunet, 1996).
By contrast, male cyathia at apical levels bore an aborted pistillate flower, stamen number was lower than in basal and intermedite cyathia, and their pollen production was very low. In fact, all the cyathia situated at the apical levels of the pleiochasium were smaller that those at other positions and, moreover, apical male cyathia were smaller than apical hermaphrodites. These facts suggest an exhaustion of the resources at the distal positions of the inflorescence; as a consequence, cyathia are small and the pistillate flower aborts in the smallest ones, perhaps because development of ovaries is more sensitive to nutrient status than development of stamens (Lloyd et al., 1980; Lloyd and Bawa, 1984). Thus, an apparent sexual dimorphism exists at the last levels of the inflorescence due to differential gender sensitivity to lack of resources.
Male and hermaphrodite cyathia at the central levels of the inflorescence were similar in size. However, in addition to absence or presence of ovaries, they showed a dimorphism in primary male reproductive characters. The males generally produced more stamens and pollen than did the hermaphrodites, but their pollen viability was not significantly different. In andromonoecious species, the male flowers often produce more (though usually not significantly more) pollen (Anderson, 1979; Dulberger et al., 1981; Solomon, 1986; Anderson and Symon, 1989); in some species, a greater pollen viability in the male flowers has also been found (Traveset, 1995). The dimorphism in reproductive characters in E. boetica could be interpreted as a mechanism enhancing the male function (Charnov, 1982).
In Euphorbia boetica, male function would be also improved by the higher rate of nectar secretion found in the male cyathia and in the male phase of the hermaphrodites. In nectariferous species with unisexual flowers or with dichogamy, the female presents only nectar as a floral reward, while the male also presents pollen, thereby possibly attracting more visits from pollinators (Ortiz et al., 1996). However, the pollinators of Euphorbia boetica exclusively seek nectar (E. Narbona, unpubl. res.) so that it is this higher rate of secretion which will most likely enhance the number of visits (Andersson, 1996; Delph, 1996; Ortiz et al., 2000). Furthermore, the greater duration of the male phase will increase the opportunity to receive visits, improving the efficiency of both floral phases; in general the male phase needs to be visited more than once for the dispersion of all the pollen, while the female can cover all its pollen needs with a single visit (Stanton et al., 1986). This is likely to be the case in Euphorbia, as the cyathium has only three ovules, so that a single visit could ensure female reproductive success. Lastly, the fact that some of the male cyathia begin to secrete nectar before the hermaphrodites could give rise to a directionality in pollinator behaviour (Pyke, 1978). These would go first to the male cyathia (which also produce more pollen) and then to the hermaphrodites, increasing the probability that, when they visit hermaphrodite cyathia in the female phase, they are transporting pollen.
The cyathia of E. boetica have great longevity (a mean of 16 d in hermaphrodites and 12 d in males). Theoretically, floral longevity can be prolonged when the levels of pollen reception and liberation are low, or when the cost of maintenance is low (Primack, 1985; Ashman and Schoen, 1994; Schoen and Ashman, 1995). Flowering cyathia are green, and thus photosynthetic, which could offset their cost of maintenance. Cyathial longevity would reinforce overlapping of flowering in more cyathia, increasing the floral display size, and offsetting the scant visual attractiveness of the individual cyathia. This scenario is consistent with the suggestion by Ishii and Sakai (2001) that the evolution of greater floral longevity may occur if larger display size benefits the plant.
In E. boetica, the cyathia open acropetally, and the display size is small when cyathia at basal positions open. In sequentially blooming plants, the display size of a plant can vary with time, and the frequency of pollinator visits per flower is greater when the display size is large (Ishii and Sakai, 2001). Thus, the amount of pollen grains removed per flower by pollinators could be greater in middle and upper flowers than in lower ones (Ishii and Sakai, 2002). In E. boetica the predicted decrease of male success when display size is small could be offset, because in lower positions male cyathia are predominant and these cyathia produce more nectar, which can increase the frequency of pollinator visits.
In conclusion, Euphorbia boetica is a functionally andromonoecious species with large male cyathia without ovaries situated predominantly at basal positions, and small male cyathia with vestigial ovaries in distal positions. The occurrence of basal male cyathia could be explained by the presence of protogyny in this species, and apical male cyathia seem to respond to a pre-emption of resources. Moreover, E. boetica exhibits a complex pattern of sexual polymorphism. On one hand, a true dimorphism affecting primary sexual characters and related to gender function appears at basal and central levels of the inflorescence. On the other hand, patterns of size variation are complex. At intermediate levels of the inflorescence, male and hermaphroditic cyathia are of equivalent size. At distal most positions, however, male cyathia are smaller than hermaphroditic cyathia. Lastly, longevity and distribution of cyathia, and their pattern of nectar production could improve both male and female fitness.
Acknowledgments
We are grateful to Drs P. K. Diggle and P. E. Gibbs for valuable suggestions and constructive criticism, which markedly improved the manuscript. This work was supported by grant of the Programa de Ayuda a los Grupos de Investigación (Junta de Andalucía).
LITERATURE CITED
- Aizen MA, Basilio A. 1998. Sex differential nectar secretion in protandrous Alstroemeria aurea (Alstroemeriaceae): is production altered by pollen removal and receipt? American Journal of Botany 85: 245–252. [PubMed] [Google Scholar]
- Anderson GJ. 1979. Dioecious Solanum of hermaphroditic origin is an example of a broad convergence. Nature 282: 836–838. [Google Scholar]
- Anderson GJ, Symon DE. 1989. Functional dioecy and andromonoecy in Solanum Evolution 43: 204–219. [DOI] [PubMed] [Google Scholar]
- Andersson S. 1996. Floral display and pollination success in Senecio jacobaea (Asteraceae): interactive effects of head and corymb size. American Journal of Botany 83: 71–75. [Google Scholar]
- Ashman TL, Baker I. 1992. Variation in floral sex allocation with time of season and currency. Ecology 73: 1237–1243. [Google Scholar]
- Ashman TL, Hitchens MS. 2000. Dissecting the causes of variation in intra-inflorescence allocation in a sexual polymorphic species Fragaria virginiana (Rosaceae). American Journal of Botany 87: 197–204. [PubMed] [Google Scholar]
- Ashman TL, Shoen DJ. 1994. How long should flowers live? Nature 371: 788–791. [Google Scholar]
- Ashman TL, Staton ML. 1991. Seasonal variation in pollination dynamics of sexually dimorphic Sidalcea oregana spp. spicata (Malvaceae). Ecology 72: 993–1003. [Google Scholar]
- Bar-Shalom D, Mattson O. 1977. Mode of hydration, an important factor in the germination of trinucleate pollen grains. Botanisk Tidsskrift 71: 245–251. [Google Scholar]
- Bateman AJ. 1948. Intrasexual selection in Drosophila Heredity 2: 349–369. [DOI] [PubMed] [Google Scholar]
- Bell G. 1985. On the function of flowers. Proceedings of the Royal Society of London. Series B. Biological Sciences 224: 223–265. [Google Scholar]
- Bertin RI, Newman CN. 1993. Dichogamy in angiosperms. The Botanical Review 59: 112–150. [Google Scholar]
- Brunet J. 1996. Male reproductive success and variation in fruit and seed set in Aquilegia caerulea (Ranunculaceae). Ecology 77: 2458–2471. [Google Scholar]
- Brunet J, Charlesworth D. 1995. Floral sex allocation in sequentially blooming plants. Evolution 49: 70–79. [DOI] [PubMed] [Google Scholar]
- Charnov EL. 1982.The theory of sex allocation. New Jersey: Princeton University Press. [Google Scholar]
- Cruden RW, Hermann SM. 1983. Studying nectar? Some observation on the art. In: Bentley B, Elias T, eds. The biology of nectaries. New York: Columbia University Press, 223–241. [Google Scholar]
- Day RW, Quinn GP. 1989. Comparisons of treatments after an analysis of variance in ecology. Ecological Monographs 59: 433–463. [Google Scholar]
- Delph LF. 1996. Flower size dimorphism in plants with unisexual flowers. In: Lloyd DG, Barrett SCH, eds. Floral Biology: studies on floral evolution in animal-pollinated plants. New York: Chapman and Hall, 217–237. [Google Scholar]
- Devlin B, Stephenson AG. 1985. Sex differential floral longevity, nectar secretion, and pollinator foraging in a protandrous species. American Journal of Botany 72: 303–310. [Google Scholar]
- Diggle PK. 1995. Architectural effects and the interpretation of patterns of fruit and seed development. Annual Review of Ecology and Systematics 26: 531–552. [Google Scholar]
- Diggle PK. 2003. Architectural effects on floral form and functions: a review. In: Stuessy, TF, Mayer V, Horandl E, eds. Deep morphology: towards a renaissance of morphology in plant systematics. Koenigstein: Koeltz Scientific Books, 63–80. [Google Scholar]
- Diggle PK, Miller JS. 2004. Architectural effects mimic floral sexual dimorphism in Solanum (Solanaceae). American Journal of Botany 91: 2030–2040. [DOI] [PubMed] [Google Scholar]
- Dulberger R, Levy A, Palevitch D. 1981. Andromonoecy in Solanum marginatum Botanical Gazette 142: 259–266. [Google Scholar]
- Gibbs PE, Lewis GP, Lughadha EN. 1999. Fruit-set induced changes in the sex of flowers in Caesalpinia calycina (Leguminosae). Plant Biology 1: 665–669. [Google Scholar]
- Gilbert MG. 1992. Notes on Euphorbia subgenus Euphorbia in Ethiopia. Collectanea Botanica 21: 67–77. [Google Scholar]
- Griffin SR, Mavraganis K, Eckert CG. 2000. Experimental analysis of protogyny in Aquilegia canadiensis (Ranunculaceae). American Journal of Botany 87: 1246–1256. [PubMed] [Google Scholar]
- Guitián J, Navarro L. 1996. Allocation of reproductive resources within the inflorescences of Petrocoptis grandiflora (Caryophyllaceae). Canadian Journal of Botany 74: 1482–1486. [Google Scholar]
- Harder LD, Barret SCH. 1996. Pollen dispersal and mating patterns in animal-pollinated plants. In: Lloyd DG, Barrett SCH, eds. Floral biology: studies on floral evolution in animal-pollinated plants. New York: Chapman and Hall, 140–190. [Google Scholar]
- Harder LD, Wilson WG. 1998. A clarification of pollen discounting and its joint effects with inbreeding depression on mating-system evolution. American Naturalist 152: 684–684. [DOI] [PubMed] [Google Scholar]
- Harder LD, Barret SCH, Cole WW. 2000. The mating consequences of sexual segregation within inflorescences of flowering plants. Proceedings of the Royal Society of London. Series B. Biological Sciences 267: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera J. 1991. Allocation of reproductive resources within and among inflorescences of Lavandula stoechas (Lamiaceae). American Journal of Botany 78: 789–794. [Google Scholar]
- Ishii HS, Sakai S. 2001. Effects of display size and positions on individual floral longevities in racemes of Narthecium asiaticum (Liliaceae). Functional Ecology 15: 396–405. [Google Scholar]
- Ishii HS, Sakai S. 2002. Temporal variation in floral display size and individual floral sex allocation in racemes of Narthecium asiaticum (Liliaceae). American Journal of Botany 89: 441–446. [DOI] [PubMed] [Google Scholar]
- Kearns CA, Inouye DW. 1993.Techniques for pollination biologists. Niwot: University Press of Colorado. [Google Scholar]
- Kiflawi M. 2000. Developmental instability and within-population variation in sex expression by andromonoecious Euphorbia xanti Oikos 89: 107–114. [Google Scholar]
- Lloyd DG, Bawa, KS. 1984. Modification of the gender of seed plants in varying conditions. Evolutionary Biology 17: 255–338. [Google Scholar]
- Lloyd DG, Webb CJ. 1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperm. I. Dichogamy. New Zealand Journal of Botany 24: 135–162. [Google Scholar]
- Lloyd DG, Webb CJ, Primack RB. 1980. Sexual strategies in plants. II. Data on the temporal variation of maternal investment. New Phytologist 86: 81–92. [Google Scholar]
- Narbona E, Ortiz PL, Arista M. 2000. Ciatios masculinos en dos especies perennes de Euphorbia (Euphorbiaceae): E. boetica Boiss. y E. nicaeensis All. Anales del Jardín Botánico de Madrid 58: 183. [Google Scholar]
- Narbona E, Ortiz PL, Arista M. 2002. Functional andromonoecy in Euphorbia (Euphorbiaceae). Annals of Botany 89: 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz PL, Arista M, Talavera S. 1996. Producción de néctar y frecuencia de polinizadores en Ceratonia siliqua L. (Caesalpiniaceae). Anales del Jardín Botánico de Madrid 54: 540–546. [Google Scholar]
- Ortiz PL, Arista M, Talavera S. 2000. Pollination and breeding system of Putoria calabrica (Rubiaceae), a Mediterranean dwarf shrub. Plant Biology 2: 325–330. [Google Scholar]
- Primack RB. 1985. Longevity of individual flowers. Annual Review of Ecology and Systematics 16: 15–37. [Google Scholar]
- Pyke GH. 1978. Optimal foraging in bumblebees and coevolution with their plants. Oecologia 36: 281–293. [DOI] [PubMed] [Google Scholar]
- Rice WR. 1989. Analyzing tables of statistical tests. Evolution 43: 223–225. [DOI] [PubMed] [Google Scholar]
- Schlessman MA, Graceffa LM. 2002. Protogyny, pollination, and sex expression of andromonecious Pseudocymopterus montanus (Apiaceae, Apioideae). International Journal of Plant Sciences 163: 409–417. [Google Scholar]
- Shoen DJ, Ashman TL. 1995. The evolution of floral longevity: resource allocation to maintenance versus construction of repeated parts in modular organisms. Evolution 49: 131–139. [DOI] [PubMed] [Google Scholar]
- Solomon BP. 1986. Sexual allocation and andromonoecy: resource investment in male and hermaphrodite flowers of Solanum carolinense (Solanaceae). American Journal of Botany 73: 1215–1221. [Google Scholar]
- Stanton ML, Snow AA, Handel SN. 1986. Floral evolution: attractiveness to pollinators increases male fitness. Science 232: 1625–1627. [DOI] [PubMed] [Google Scholar]
- Stephenson AG. 1981. Flower and fruit abortion: proximate causes and ultimate functions. Annual Review of Ecology and Systematics 12: 253–279. [Google Scholar]
- Stout AB. 1928. Dichogamy in flowering plants. Bulletin of the Torrey Botanical Club 55: 141–141. [Google Scholar]
- Talavera S, Arista M, Salgueiro FJ. 1996. Population size, pollination and breeding system of Silene stockenii Chater (Caryophyllaceae), an annual gynodioecious species of southern Spain. Botanica Acta 109: 333–339. [Google Scholar]
- Traveset A. 1995. Reproductive ecology of Cneorun tricoccum L. (Cneoraceae) in the Balearic Islands. Botanical Journal of the Linnean Society 117: 221–232. [Google Scholar]
- Weberling F. 1986.Morphology of flowers and inflorescences. Cambridge: Cambridge University Press. [Google Scholar]
- Wyatt R. 1983. Pollinator–plant interactions and the evolution of breeding systems. In: Real L, ed. Pollination biology. Orlando: Academic Press, 51–95. [Google Scholar]
- Zar JH. 1999.Bioestatistical análisis. Prentice-Hall: New Jersey. [Google Scholar]