Abstract
• Background and Aims Gossypium is an economically important, globally distributed taxon comprising more than 50 species. DNA content estimates from about half of the species indicate over a 3-fold variation exists. However, the nine DNA content estimates for G. hirsutum reveal over a 2-fold difference for this species alone. Recent reports have shown that several plant compounds can bias DNA content estimates obtained by commonly used methods. The purpose of this research was to examine the standardization procedures used for DNA content determinations with flow cytometry as applied to Gossypium, and generate revised DNA content estimates for all available Gossypium species using best-standard practices.
• Methods Flow cytometry was used to measure fluorescence of isolated Gossypium nuclei stained with propidium iodide. Fluorescence values were converted to DNA content estimates based on the nuclear fluorescence of standard genotypes of barley, corn and rice. Various combinations of nuclei preparations relative to the standards were evaluated for their influence on the estimates.
• Key Results Both external standardization and internal standardization with Oryza sativa ‘IR36’ yielded statistically similar DNA content estimates for Gossypium. Internal standardization with Hordeum vulgare ‘Sultan’ resulted in a high estimate of DNA content. Nuclear DNA content estimates were generated for 37 Gossypium species using external standardization. Estimates of ancestral genome sizes reveal that both increases and decreases in nuclear DNA content have occurred. Variation in intraspecific and intragenomic DNA content was low, and the allopolyploid AD-genome size was nearly the additive of its progenitor genomes.
• Conclusions Due to unknown factors, internal standardization with H. vulgare ‘Sultan’ may not be appropriate for DNA content determinations of Gossypium. The current DNA content estimates support accepted cytogenetic divisions of the genus. Gossypium is a genus that exhibits genome constancy both through speciation within genomic groups and allopolyploidization.
Keywords: DNA content, Gossypium, flow cytometry, standardization, genome conservation, cytosolic interference, propidium iodide
INTRODUCTION
Gossypium comprises approx. 50 species (Fryxell, 1992) native to arid and semi-arid regions of the Americas, Asia, Africa and Australia. These species are divided into genomic groups, eight diploid (n = 13; A–G and K) and 1 tetraploid (n = 26; AD), based on chromosome size and homoeologous chromosome pairing in interspecific hybrids (Endrizzi et al., 1985; Stewart, 1995). Ambiguity in the exact number of species in the genus arises chiefly from the poorly described E-genome species native to Somalia and surrounding regions, and the continuing discovery of new Gossypium species in other parts of the world. In terms of economics, the polyploid AD genome is by far the most important as >90 % of the world's cotton fibre is produced by G. hirsutum and G. barbadense, two of the tetraploid species. The genus is unique in that four species, the two above plus two diploid species, G. herbaceum and G. arboreum, were independently domesticated for fibre production in different parts of the world (Wendel and Cronn, 2003). In the US and most other cotton-producing countries, cotton is grown as an annual crop, although its natural growth habit is perennial in nature. Many of the challenges faced by cotton breeders have roots in this paradox.
At present, estimates of nuclear DNA content have been reported for 24 Gossypium species representing eight of the nine genomic groups (Edwards et al., 1974; Kadir, 1976; Walbot and Dure, 1976; Edwards and Mirza, 1979; Bennett et al., 1982; Michaelson et al., 1991; Gomez et al., 1993; Bennett et al., 1997; Wendel et al., 2002). Edwards et al. (1974) and Kadir (1976) examined DNA content on a genus-wide scale, while most of the other authors reported values only for G. hirsutum. Reports of DNA content estimates for G. hirsutum using various techniques (i.e. flow cytometry, Feulgen microspectrophotometry, reassociation kinetics) cover a 25-year period. Variability in estimates among these reports makes it difficult to determine which values are most accurate. For example, estimates of the 2C nuclear DNA content of G. hirsutum range from 3·2 pg (Walbot and Dure, 1976) to 6·45 pg (Bennett et al., 1982), a 2-fold difference for the same species. Variation in intraspecific DNA content of this magnitude has been reported in other species (e.g. Helianthus annus; Sims and Price, 1985), but recent studies (Price et al., 2000; Noroit et al., 2000) cast doubt on the validity of these conclusions due to the presence of compounds that affect the fluorescence of propidium iodide, a dye commonly used to quantify DNA content. Here, an attempt is made to resolve the ambiguities concerning the DNA content of G. hirsutum through an evaluation of standardization procedures for determining DNA content by flow cytometry. Based on the standardized method, the 2C nuclear DNA contents of 37 Gossypium species are reported.
MATERIALS AND METHODS
Plant materials
Thirty-seven Gossypium species (Table 1) representing all the recognized genome divisions within the genus were included in the assessments. Most Gossypium seeds were from the germplasm collection maintained by the Cotton Biotechnology and Germplasm Laboratory at the University of Arkansas. When possible, original seeds collected from wild populations were analysed, but for old and otherwise nonviable seed, seed increase stocks were used. Seeds of Gossypium species not on hand and of Oryza sativa ‘IR36’, Zea mays ‘W64A’ and Hordeum vulgare ‘Sultan’, recommended standard reference species for estimating DNA content (Price and Johnston, 1996), were obtained from the appropriate USDA germplasm collections (GRIN: http://www.ars-grin.gov/npgs/). Each Gossypium species or accession is identified by a plant identification number, if available, otherwise by field collection number or accession number (Table 1).
Table 1.
DNA content of 37 Gossypium species determined by flow cytometric analysis of propidium-iodide-stained nuclei using O. sativa ‘IR36’, Z. mays ‘W64’, H. vulgare ‘Sultan’ as external reference standards
| Mean DNA content |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome |
Species |
|||||||||||
| Taxon |
ID |
pg 2C |
Mbpb 1C |
pg (±s.e.) 2C |
Mbpb 1C |
n |
||||||
| Karpas (AD-genome) | 4·91 | 2401 | 70 | |||||||||
| G. hirsutum L. | TM-1 | 4·96 (0·09) | 2425 | 11 | ||||||||
| G. hirsutum ‘Tamcot CAMD-E’ | PI529633 | 4·80 (0·05) | 2347 | 4 | ||||||||
| G. hirsutum | PI631052 | 4·82 (0·04) | 2357 | 5 | ||||||||
| G. hirsutum | PI631019 | 4·82 (0·02) | 2357 | 5 | ||||||||
| G. hirsutum ‘Acala Maxxa’ | PI540885 | 5·08 (0·04) | 2484 | 11 | ||||||||
| G. hirsutum ‘DP491’ | PI618609 | 5·09 (0·03) | 2489 | 18 | ||||||||
| G. barbadense L. | 3–79 | 5·01 (0·07) | 2450 | 4 | ||||||||
| G. tomentosum Nutt. ex Seem. | unknown | 4·87 (0·06) | 2381 | 4 | ||||||||
| G. mustelinum Miers ex G. Watt. | AD4-9 | 4·85 (0·08) | 2372 | 4 | ||||||||
| G. darwinii G. Watt. | AD5-14 | 4·83 (0·11) | 2362 | 4 | ||||||||
| Gossypium | ||||||||||||
| Gossypium | ||||||||||||
| Gossypium (A-genome) | 3·47 | 1697 | 16 | |||||||||
| G. herbaceum ssp. africanum J. Hutch. Ex S. C. Harland. | wild collection | 3·41 (0·06) | 1667 | 5 | ||||||||
| G. arboreum L. | PI529755 | 3·57 (0·01) | 1746 | 2 | ||||||||
| G. arboreum | PI529747 | 3·54 (0·01) | 1731 | 2 | ||||||||
| G. arboreum | PI129723 | 3·45 (0·03) | 1687 | 4 | ||||||||
| G. arboreum | PI615703 | 3·43 (0·06) | 1677 | 6 | ||||||||
| G. arboreum | PI152088 | 3·5 | 1712 | 1 | ||||||||
| Anomala (B-genome) | 2·76 | 1350 | 8 | |||||||||
| G. anomalum Wawra | unknown | 2·78 (0·07) | 1359 | 4 | ||||||||
| G. capits-virdis Mauer | PI530747 | 2·75 (0·03) | 1345 | 4 | ||||||||
| Pseudopambak (E-genome) | 3·19 | 1560 | 9 | |||||||||
| G. stocksii Mast. | PI530976 | 3·13 (0·01) | 1531 | 2 | ||||||||
| G. somalense (Gürke) J.B. Hutch. | PI530981 | 3·06 (0·09) | 1496 | 3 | ||||||||
| G. areysianum Deflers. | PI530982 | 3·40 (0·05) | 1663 | 4 | ||||||||
| Longiloba (F-genome) | 2·68 | 1311 | 4 | |||||||||
| G. longicalyx J.B. Hutch. & B.J.S. Lee | unknown | 2·68 (0·04) | 1311 | 4 | ||||||||
| Houzingenia (D-genome) | 1·81 | 885 | 44 | |||||||||
| Houzingenia | ||||||||||||
| Houzingenia | ||||||||||||
| G. thurberi Tod. | 1·72 (0·02) | 841 | 3 | |||||||||
| G. trilobum (DC.) Skovst. | unknown | 1·74 (0·05) | 851 | 4 | ||||||||
| Integrifolia | ||||||||||||
| G. davidsonii Kellogg. | unknown | 1·86 (0·04) | 910 | 7 | ||||||||
| G. klotzschianum Andersson | unknown | 1·80 (0·04) | 880 | 4 | ||||||||
| Caducibracteolata | ||||||||||||
| G. turneri Fryxell | unknown | 1·86 | 910 | 1 | ||||||||
| G. armourianum Kearney | PI530801 | 1·75 (0·02) | 856 | 4 | ||||||||
| G. harknessii Brandegee | unknown | 1·86 | 910 | 1 | ||||||||
| Erioxylum | ||||||||||||
| Erioxylum | ||||||||||||
| G. laxum L. L. Phillips | unknown | 1·91 (0·02) | 934 | 4 | ||||||||
| G. lobatum Gentry | US-101 | 1·91 (0·07) | 934 | 2 | ||||||||
| G. aridum (Rose & Standl.) Skovst. | PI530893 | 1·88 (0·09) | 919 | 4 | ||||||||
| Selera | ||||||||||||
| G. gossypioides (Ulbr.) Standl. | PI530954 | 1·72 (0·13) | 841 | 4 | ||||||||
| Austroamericana | ||||||||||||
| G. raimondii Ulbr. | unknown | 1·80 (0·04) | 880 | 5 | ||||||||
| Sturtia | 4·05 | 1980 | ||||||||||
| Sturtia (C-genome) | 8 | |||||||||||
| G. robinsonii F. Muell. | SC-15 | 3·99 (0·03) | 1951 | 4 | ||||||||
| G. sturtianum J.H. Willis. | PI464863 | 4·12 (0·07) | 2015 | 4 | ||||||||
| Hibiscoide (G-genome) | 3·65 | 1785 | 10 | |||||||||
| G. bickii Prokh. | PI464843 | 3·59 (0·01) | 1756 | 2 | ||||||||
| G. australe F. Muell. | PI464839 | 3·75 (0·16) | 1834 | 4 | ||||||||
| G. nelsonii Fryxell. | PI499782 | 3·59 (0·08) | 1756 | 4 | ||||||||
| Grandicalyx (K-genome) | 5·26 | 2572 | 26 | |||||||||
| pG. rotundifolium Fryxell et al. | PI413167 | 5·01 | 2450 | 1 | ||||||||
| pG. rotundifolium Fryxell et al. | PI478762 | 5·07 (0·20) | 2479 | 2 | ||||||||
| pG. exiguum Fryxell et al. | NWA-42 | 5·03 (0·06) | 2460 | 3 | ||||||||
| aG. pilosum Fryxell | PI499804 | 5·10 (0·03) | 2494 | 3 | ||||||||
| eG. cunninghamii Tod. | PI499776 | 5·11 (0·08) | 2499 | 4 | ||||||||
| eG. enthyle Fryxell et al. | PI499809 | 5·26 (0·06) | 2572 | 3 | ||||||||
| aG. marchantii Fryxell et al. | NWA-6 | 5·35 (0·12) | 2616 | 4 | ||||||||
| eG. nobile Fryxell et al. | PI499806 | 5·68 (0·15) | 2778 | 2 | ||||||||
Plant type: p, prostrate; a, ascending; e, erect.
Mbp (1C) = ([978x pg (2C)]/2) (Dolezel et al., 2003).
Isolation and staining of nuclei
Seeds were germinated in the dark on 0·7 % phytoagar (Sigma, St Louis, MO, USA) in sealed Petri plates at 28 °C. Seeds with low germination were surface sterilized (20 % commercial bleach, 1 drop Tween 20, for 20 min) and placed on media with supplemental nutrients (SGM; Stewart and Hsu, 1977), 0·3 % sucrose, and 3·5 mm MgCl2 (Mishra et al., 2003). After 5–7 d, nuclei were isolated from approx. 100 mg of etiolated hypocotyl and cotyledon tissue (equal parts) as described by Price and Johnston (1996) with minor modifications. The tissue was chopped with a new double-edge razor in 2 mL of cold chopping buffer (Galbraith et al., 1983), filtered through Miracloth™ and then through a 20-µm nylon mesh filter (Millipore, Billerica, MA, USA), and placed at 4 °C. Once all samples were chopped and filtered, nuclei were pelleted by centrifugation at 228 g for 5 min at 4 °C. The nuclear pellet was resuspended in 300 μL of chopping buffer plus 10 µg mL−1 ribonuclease A (Sigma) and 100 ppm propidium iodide (PI) (Sigma). Samples were analysed by flow cytometry <2 h after preparation.
Evaluation of standardization procedures
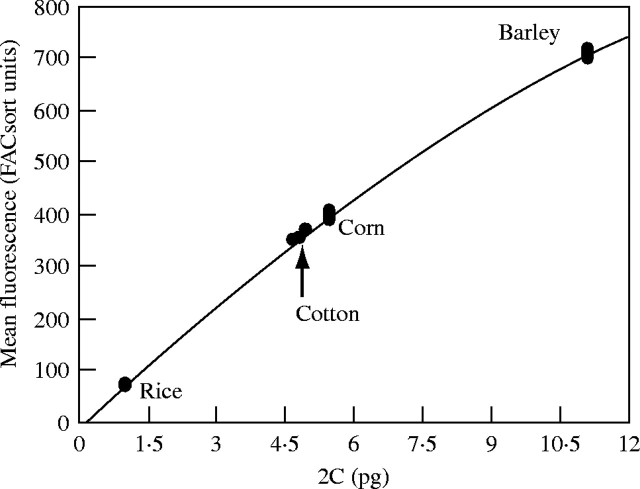
Etiolated hypocotyl and cotyledon tissue from G. hirsutum ‘DP 491’ was processed separately with two internal standards and alone to evaluate which standardization method was most reliable. Cotton tissue was divided into three 50-mg sub-samples. Prior to chopping and nuclei isolation, two of the sub-samples were combined separately with a 50-mg sub-sample of barley or 35-mg sub-sample of rice. The mixtures were chopped, incubated 1 h at 4 °C, and analysed for DNA content using the respective internal standard. The remaining cotton sub-sample and reference species sub-samples were chopped alone, incubated 1 h at 4 °C, and analysed for DNA content by reference to a standard curve generated from the external standard reference species (rice, corn and barley; Fig. 1).
Fig. 1.
Example of standard curve used to calculate Gossypium nuclear DNA content values (n = 8 for all species). In all instances, the relationship between PI fluorescence and DNA content was better defined by a polynomial (e.g. this curve y = −1·9131x2 + 86·157x − 10·917, r2 = 0·995). The arrow highlights a set of fluorescence measurements for G. hirsutum ‘DP491’ applied to the regression equation. Cotton nuclei fluorescence is lower than that of corn (2C = 5·47 pg) and barley (2C = 11·12 pg) but higher than that of rice (2C = 1·01 pg), indicative of tetraploid cotton's genome size (2C = 4·93).
Additionally, a cytoplasmic-switch experiment was performed to determine which species, if any, contributed compounds that altered PI fluorescence. Cotton, barley and rice were chopped alone, divided into sub-samples as described above, and nuclei were isolated by centrifugation. The cytoplasmic fractions of all the species were recovered, and the nuclei of each species suspended in the cytoplasm of another and incubated for 1 h at 4 °C. As a control, a sub-sample of nuclei of each species was incubated in buffer only. PI-induced fluorescence was then measured via flow cytometry, and DNA content was calculated from the pseudo-internal or external standards.
2C DNA content measurement
PI-stained nuclei were analysed for DNA content with a FACsort flow cytometer (Becton Dickinson Immunocytometry System, San Jose, CA, USA). The three standard species recommended by Price and Johnston (1996), Oryza sativa ‘IR36’ (2C = 1·01 pg), Zea mays ‘W64A’ (2C = 5·47 pg) and Hordeum vulgare ‘Sultan’ (2C = 11·12 pg), were used to correlate fluorescence values to DNA content. Nuclei prepared from these three species were included with each group of test samples. The mean fluorescence of the G0/G1 peak for each standard was determined after analysis of the data collected by CellQuest software (Becton Dickinson Immunocytometry System, San Jose, CA, USA). These values were plotted, and a regression equation was calculated each day for use in estimation of the DNA content of the experimental samples (Fig. 1). Two samples of each standard were measured in random order during analysis as a control for instrument drift.
Statistical analyses of data
In all experiments, the fluorescence of at least 1000 G1-phase nuclei was measured. For the standardization experiments, eight independent replicates, measured in two groups of four on different days, were analysed for each processing method. Four replicates of most Gossypium species were analysed to obtain an estimation of their DNA content; however, for a few species with limited seed availability, less than four replicates were analysed. Mean 2C DNA-content values were generated as described above, and data are reported as mean 2C DNA mass in picograms (±s.e.). Means were separated with the Student's t-test (α = 0·05) (JMP 5·0·1, Academic Version; SAS Institute). Ancestral genome sizes for seven diploid genomes (A–G) were estimated with the phylogenetic least squares method in Compare version 4·4 (Martins, 2003) based on phylogenetic positions derived previously (Cronn et al., 2002).
RESULTS
Effect of tissue source on preparation of nuclei
Variation in estimated DNA content of nuclei due to developmental stage and tissue type from which the nuclei were obtained was determined for G. hirsutum ‘DP 491’ using external standards. The DNA content for etiolated hypocotyl/cotyledon tissue was not significantly different from that of mature leaves (data not shown). The use of etiolated tissue for determination of DNA content in this study was advantageous for many of the Gossypium species, as it was difficult to obtain clean preparations of nuclei from mature leaf tissue.
Effect of various internal/external standard combinations
When G. hirsutum ‘DP491’ tissue was co-processed with the barley reference, a non-significant 0·5 % decrease in the mean fluorescence of the barley G1 peak was observed; however, for cotton a statistically significant (α = 0·05) 7·5 % increase in the mean fluorescence of the G1 peak was observed (Fig. 2). When cotton tissue was co-processed with rice the mean fluorescence of the G1 peaks of these two species increased 0·8 % and 10·8 %, respectively. The difference in PI fluorescence resulted in estimated DNA content values for cotton of 4·97 pg (±0·07; rice co-processed), 5·13 pg (±0·04; external standard curve) or 5·88 pg (±0·009; barley co-processed).
Fig. 2.
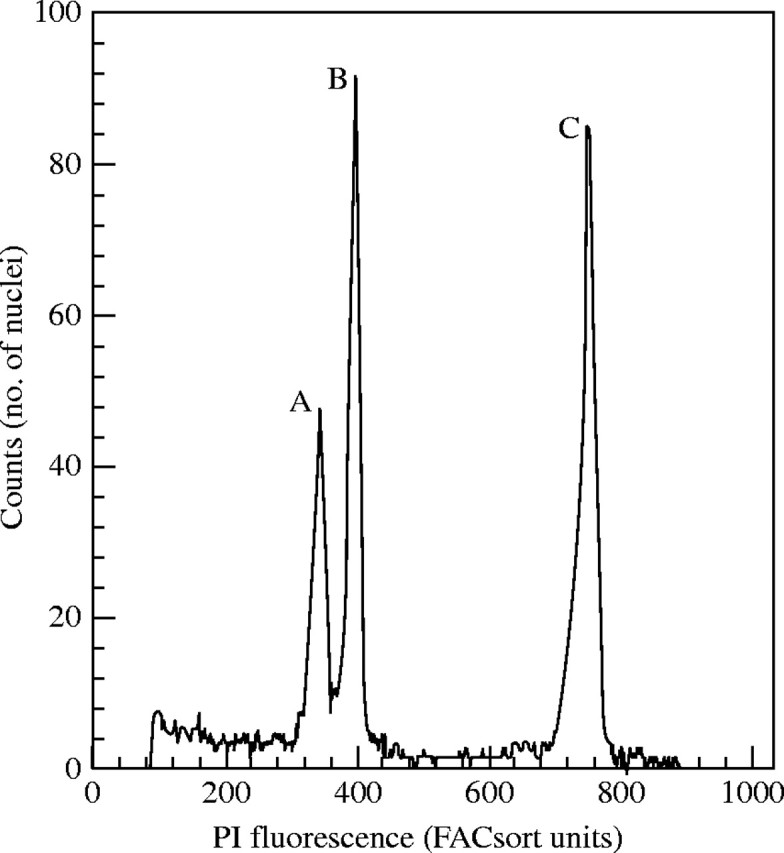
Compounds from H. vulgare ‘Sultan’ enhance the PI fluorescence G. hirsutum ‘DP491’. Peak A: fluorescence peak of cotton processed alone. Peak B and C: fluorescence peaks of co-processed cotton and barley, respectively. Co-processed sample was mixed with the independently processed cotton sample after separate measurements to illustrate the effect.
When the plant tissues were processed alone and then mixed with the cytoplasmic fractions of another species, a different effect was observed for the mean fluorescence of the barley G1 nuclei. The mean fluorescence of the G1-peak of cotton was increased 5·25 % by barley cytoplasm, whereas, the mean fluorescence of the G1 peak for barley increased 2·13 % when incubated with cotton cytoplasm. Both values were statistically significant (α = 0·05). The fluorescence values of the cotton/rice cytoplasm-switch combinations increased 1·4 % for cotton and 4·5 % for rice. The DNA content of cotton calculated from pseudo-internal or external standards was 4·93 pg (±0·05; rice cytoplasm), 5·05 pg (±0·03; external standard curve) and 5·79 pg (±0·06; barley cytoplasm).
Comparison of the DNA content values from both experiments revealed no significant differences when tissues were co-processed or cytoplasmic fractions were switched. However, in both experiments significant differences occurred that were related to the processing method. The mean 2C DNA content of cotton processed alone (external standardization) was 5·09 pg (±0·03). Cotton co-processed with rice as an internal standard had a mean 2C DNA content of 4·97 pg (±0·06), which was not significantly different from cotton processed alone. The remainder of the DNA values differed significantly from cotton processed alone, with barley/cotton combinations being the most extreme in this regard. The average values of these data are presented in Table 2.
Table 2.
DNA content of G. hirsutum ‘DP491’ estimated by external standards following incubation with the cytoplasm of the reference standards (cyto) or by co-processing with internal standards (coproc)
| Standard |
2C DNA content in pg (±s.e.)* |
|---|---|
| Rice (cyto) | 4·93 (0·05)a |
| Rice (coproc) | 4·97 (0·07)ab |
| Cotton ‘DP491’ (control) | 5·09 (0·05)b |
| Barley (cyto) | 5·79 (0·06)c |
| Barley (coproc) | 5·88 (0·009)c |
Values followed by different letters are significantly different(α = 0·05).
2C DNA content
The nuclear DNA contents of 37 Gossypium species in nine genome groups are listed in Table 1. The K genome is the largest genome in the genus with a mean 2C DNA content of 5·26 pg for the nine species measured in this study. The smallest genome in the genus is the D genome with a mean 2C DNA content of 1·81 pg. Means comparison (α = 0·05) yielded significant differences among all of the genomic groups, with the exception of the B (2C = 2·76 pg) and the F (2C = 2·68 pg) genomes.
To assess the fate of nuclear DNA in the AD genome following polyploidization, five replicate measurements were made of the DNA contents of the species thought to be most closely related to the progenitors of the AD genome, namely G. herbaceum spp. africanum (A genome) and G. raimondii (D genome) (Wendel and Cronn, 2002). The mean estimated values for the 2C DNA contents of the two species are 3·41 pg and 1·80 pg, respectively.
The DNA content for multiple accessions of both G. hirsutum and G. arboreum were also determined (Table 1). Intraspecific variation in DNA content was low for both species. A 6·04 % difference in DNA content between the smallest and the largest accession values was observed within the six accessions of G. hirsutum (4·92 pg ± 0·05) measured. The variation in DNA content among five accessions of G. arboreum (3·50 pg ± 0·04) was 4·08 %.
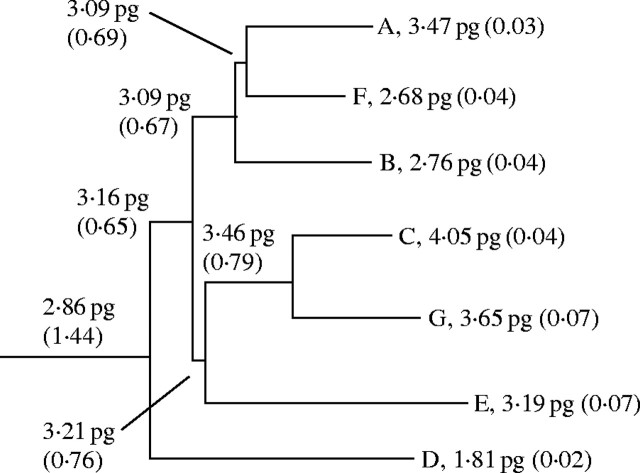
Ancestral DNA content estimates (±s.e.) derived from measured genome sizes are reported in Fig. 3. The DNA content of the hypothetical basal member of the genus was estimated at 2·86 pg (±1·44). Similar to trends observed in the larger cotton tribe (Wendel et al., 2002), both increasing and decreasing DNA content values were predicted along the lineages leading to extant genomes.
Fig. 3.
Ancestral genome sizes (±s.e.) predicted with the PGLS method based on phylogeny developed by Cronn et al. (2002). Evidence of bidirectional genome size change is evident within Gossypium. Note that the K genome, sister to the C/G genome clade, was not included in Wendel et al. (2002), hence had to be omitted from the calculation.
DISCUSSION
Changes in PI fluorescence
Gossipium hirsutum ‘DP491’, H. vulgare ‘Sultan’ and O. sativa ‘IR36’ appear to contain compounds that enhance PI fluorescence to differing degrees. Similar PI fluorescence anomalies were observed in Coffea sp. (Noriot et al., 2000) and Helianthus annus (Price et al., 2000) using flow cytometry for DNA content determinations. In coffee, Noriot et al. (2003) showed that caffeine enhances PI fluorescence, while phenolics like chlorogenic acid (CGA) reduce PI fluorescence. Gossypium spp. are rich in phenolic compounds, thus an inhibitory affect on the PI fluorescence of the internal standards was expected. Initial experiments with barley/cotton combinations gave results consistent with inhibitory effects, i.e. a reduction in barley fluorescence and an increase in cotton fluorescence. The rice/cotton combination, however, did not respond as if an inhibitor was present, in that rice fluorescence increased 10·8 % when co-processed with cotton. To further investigate these observations, the cytoplasmic fractions of the species involved were exchanged and it was found that cotton and barley cytoplasmic fractions both enhanced the fluorescence of PI-stained nuclei. Apparently, when cotton and barley are co-processed the stimulatory effect of cotton on barley fluorescence is mitigated, perhaps similar to the caffeine/CGA interaction reported by Noriot et al. (2003). However, these PI fluorescence anomalies could not explain why DNA content determinations from the barley/cotton combination were nearly 0·75 pg larger than those derived from rice/cotton combinations or external standardization (Table 2).
Barley and DNA content determinations for Gossypium
To investigate further the discrepancies between published DNA content values and the present results, nine determinations for G. hirsutum derived via Feulgen microspectrophotometry or flow cytometry were examined (Table 3). The mean 2C DNA content of these values is 5·64 pg. Of the three standardization methods tested, only when barley was used as an internal standard were values obtained (average of 5·88 pg) that were close to the mean figure. From the present results, the 2C DNA content of cotton based on rice as an internal standard, or based on an external three-species standard curve, was 4·97 pg and 5·09 pg, respectively. When the KEW database (http://www.rbgkew.org.uk/cval/database1.html) values are parsed by method of standardization, the six values derived with barley as an internal standard have a mean 2C DNA content of 5·98 pg, in close agreement to the present value from internal barley standardization. However, corn (5·47 pg 2C) was used as an external standard in this study, and G. hirsutum fluorescence values were always lower than that of corn, indicating that cotton had a smaller genome size (Fig. 1). The presence of a PI fluorescence inhibitor in cotton could account for this discrepancy, but the data indicate that compounds in cotton enhance, rather than inhibit, PI fluorescence. Additionally, Arumuganathan and Earle (1991) reported a DNA content value of 4·9 pg for G. hirsutum using chicken red blood cells (CRBC) as an internal standard, a value that closely agrees with the authors' values based on rice-internal and -external standardization. In the same study (Arumuganathan and Earle, 1991), it was also reported that the DNA content of G. hirsutum is 4·4 pg. Although not indicated in the study, this discrepancy may be explained by varietal differences, which is consistent, in relative terms, to the intraspecific variation reported by Gomez et al. (1993). Based on available data, it is concluded that estimation of DNA content based on rice as an internal standard, or on an external standard curve derived from three reference species, is more accurate than barley internal standardization alone. Due to unknown factors, internal standardization with H. vulgare ‘Sultan’ may not be appropriate for DNA content determination in Gossypium spp.
Table 3.
Previously reported DNA content values for G. hirsutum
| Reference |
Method† |
2C (pg) |
Standard |
|---|---|---|---|
| Bennett et al. (1982) | Fe | 6·45 | H. vulgare ‘Sultan’ |
| Kadir (1976) | Fe | 6·20 | H. vulgare ‘Sultan’* |
| Michaelson et al. (1991) | Fe | 6·05 | Zea mays ‘Va35’ |
| Gomez et al. (1993) | FC | 6·05 | H. vulgare ‘Sultan’ |
| Edwards et al. (1974) | Fe | 6·05 | H. vulgare ‘Sultan’* |
| Michaelson et al. (1991) | FC | 5·60 | H. vulgare ‘Sultan’ |
| Gomez et al. (1993) | FC | 5·10 | H. vulgare ‘Sultan’ |
| Arumuganathan and Earle (1991) | FC | 4·90 | CRBC |
| Arumuganathan and Earle (1991) | FC | 4·40 | CRBC |
CRBC, chicken red blood cells.
Original publication reported DNA content in arbitrary fluorescence units. Bennett et al. (1982) converted these values to pg using H. vulgare ‘Sultan’ as an internal standard.
Fe, Feulgen microspectrophotometry; FC, Flow cytometry.
External standardization
Rice as an internal standard or external standardization yielded statistically similar DNA content determinations (Table 2), but the mean values differed by approx. 0·1 pg. It was then necessary for us to make a judgment as to which values were most accurate. It should be emphasized that, in the authors' view, either method would be sufficiently accurate, but in the present study external standardization for the DNA content determinations were used. Price et al. (2000) and others (Noriot et al., 2003) have strongly recommended the use of internal standardization for determinations of DNA content, especially when compounds that alter PI fluorescence are present. The underlying assumption is that these compounds affect host nuclei fluorescence and, therefore, would skew determinations if standard-nuclei fluorescence were not also altered by the same compounds. While it is not possible to directly test this assumption, two observations suggest this assumption is not entirely true. First, in terms of direction of the effect, the cotton/rice combinations affected fluorescence differently (i.e. both increase) than that of the cotton/barley combinations (i.e. cotton increased, barley slightly decreased). This observation establishes that variation among species exists in their response to co-processing, and the fluorescence response of a species to the compounds is not an absolute. Secondly, it was established that G. hirsutum ‘DP491’ contains compounds that enhance PI fluorescence. It is reasonable to assume that there is variation for these compounds among the 37 Gossypium spp. in this study. Therefore, if these compounds affect host nuclei fluorescence in a 1 : 1 manner with respect to internal standard nuclei, one would expect great variation in estimated DNA content in the genomic groupings within Gossypium when using external standardization. In the present study, such variation was not observed, as the variation among the species within a genome group and among accessions within a species was similar. To further complicate the matter of internal standardization, mixing barley nuclei and cotton cytoplasm changes the direction of the effect, both in relative and absolute terms, which cotton has on the PI fluorescence of barley nuclei, further supporting the idea that the compounds that effect PI fluorescence can interact (Noriot et al., 2003). These observations lead the authors to believe that external standardization is preferable until the issues with internal standardization of Gossypium spp. are fully understood. It should be noted that these statements are only applicable to the species/buffer combination used in this study. Additionally, had rice been used as an internal standard, the DNA content estimations would be similar to those reported for external standardization.
2C DNA content
The estimated values of DNA content within Gossypium strongly support the previous cytogenetic divisions proposed for the genus (Endrizzi et al., 1985). A 3·3-fold difference in DNA content was observed between G. thurberi (D genome, 1·72 pg ± 0·02 s.e.) and G. nobile (K genome, 5·68 pg ± 0·15 s.e.), the smallest and largest DNA values for species within the genus, respectively. This range of DNA content variation is consistent with the ranges reported for other angiosperms (Bennett et al., 1982). However, within species and genomic groups surprising consistency was observed. The mean percentage variation within species (5·06 %) was similar to the mean variation (6·02 %) seen within the nine genomic groups. These variations may reflect changes in the portions of the genome composed of repetitive DNA or changes in the single-copy fraction of the genome. In either case, within species or genomic groups of Gossypium, DNA content is largely static. This observation suggests that speciation within the genomic groups of the genus is not accompanied by large changes in genome size, and a relatively small portion of the genome, then, must govern the major phenotypic differences observed within and between species in a genomic group.
Similar intraspecific genome consistency has been reported in Allium cepa (Bennett et al., 2000), H. vulgare, Vicia faba (Bennett and Smith, 1976), Pisum sativum (Greilhuber and Ebert, 1994) and Glycine max (Greilhuber and Obermayer, 1997). Gossypium, thus, is another in the group of angiosperms that tend to maintain its nuclear DNA content. Bennett et al. (2000) suggested that genetic mechanisms must exist for such genome constancy to be maintained. Based on the data for Gossypium, this hypothesis is clearly applicable not only within species but also within genomic groups. Furthermore, the inactivation of such a mechanism to control genome size in Gossypium would be a rare event in that it could have occurred only four times during the evolution of the genus, resulting in four major phylogenetic lineages (reviewed in Wendel and Cronn, 2002). As discussed below, allopolyploidization has acted within the genus to change size, but the mechanisms that produced the major lineages in the genus are independent of the ‘genomic shock’ (McClintock, 1984) often associated with allopolyploidization. Environmental factors, however, may have contributed to these divergences, as these lineages developed unique features that enabled survival in conditions that range from arid to mesic.
The AD genome is thought to have formed via a single hybridization/polyploidization event between ancestral A- and D-genome species some 1·5 million years ago (Senchina et al., 2003). The sum of the mean 2C DNA contents of G. herbaceum ssp. africanum (3·41 pg) and G. raimondii (1·80 pg), the species thought to be most representative of the taxa involved in the polyploidization event, was not additive to the mean estimate reported for the AD genome, 5·21 pg vs. 4·91 pg. DNA loss between 5 % and 6 % appears to have occurred during the evolution of the tetraploid species. Previous reports stated that the AD genome exhibited complete additivity with respect to its progenitor genomes (Wendel et al., 2002). Ambiguity concerning the DNA content of G. hirsutum may have led to this belief, but the present results indicate that an amended view of near complete additivity is more appropriate. However, caution should be used when interpreting these data, as the species chosen as representative of the original progenitors may themselves differ by that amount from the true progenitors, or have lost or gained DNA since the initial hybridization event. Furthermore, simple intraspecific DNA content could account for this difference, as a 5–6 % variation in DNA content is common within the genus. Because of the impossibility of examining the taxa involved in the hybridization event, no absolute conclusion can be reached, but it is clear that allopolyploidization did not greatly affect genome size within the AD genome. Similar conservation of DNA content (1–5 % loss) following polyploidization has been reported for Hordeum polyploids (Jakob et al., 2004).
Gossipium gossypioides is a cotton species with a complex evolutionary history, with evidence of both nuclear and cytoplasmic introgressions from African (A-, F-, B- or E-genome) and D-genome species, respectively (Cronn et al., 2003). In light of the observation of genome constancy during allopolyploidization within Gossypium, it is in contrast to note that the nuclear DNA content of G. gossypioides (1·72 pg) is not additive to a putative D-genome × African-genome hybrid (approx. 2·4 pg). Eight low-copy nuclear genes resolved G. gossypioides as the basal member of the D genome (Cronn et al., 2003). The observation would support the hypothesis that the D-genome × African-genome hybridization occurred before the divergence of the D genome, and thus this event and the resulting loss of nuclear DNA ‘set’ the genome size for the taxon. However, different data sets (ITS, cpDNA) place G. gossypioides in different phylogenetic positions within the phylogenetic clades, further complicating the matter. More work is needed to fully understand the relationship between the nuclear DNA content of G. gossypioides and the cryptic African-genome hybridization event.
The largest variation (13·1 %) in DNA content in Gossypium was observed within the K genome. Interestingly, the values tend to correspond to plant growth habit. Prostrate and ascending plant types have a lower DNA content, while the erect plant types have a higher DNA content (Table 1). The results are supported by the phylogenetic separation of prostrate and erect plant types within the K genome (Seelanan et al., 1999). Additionally, the D genome has over 11 % variation in DNA content that is reflected to some degree by the six taxonomic subsections comprising the genomic group. With even higher variation in DNA content, perhaps the K genome is deserving of additional attention to its phylogeny. The previously reported (Wendel and Cronn, 2002) estimate of 7·0 pg for the 2C K-genome mass was based on comparative chromosome observations (J. McD. Stewart, University of Arkansas, unpubl. res.) of K- and AD-genome species based on an assumed value of approx. 6 pg for the AD genome and is considerably higher than the 5·26 pg (2C) we obtained by direct measurement.
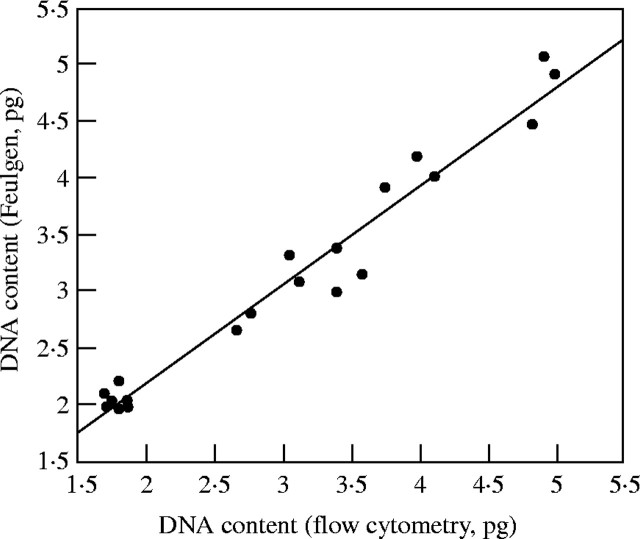
DNA content values for 20 Gossypium species measured by Feulgen microspectrophotometry (Kadir, 1976) were compared with those reported in this study (Fig. 4). It should be noted that Kadir reported his results as nuclear fluorescence values relative to values obtained from a standard G. arboreum. Bennett et al. (1982) converted the G. arboreum fluorescence values reported by Kadir (1976) into picograms using H. vulgare ‘Sultan’ as an internal standard, and then back-calculated picogram values for the remainder of the species values. The resulting DNA content values are greater, by an average of 27 %, than those reported in this study for the same species. When the values from the study of Kadir (1976) were recalculated using the mean DNA content measured for G. arboreum in the present study (3·5 pg; see Table 1), the species DNA content values based on Feulgen microspectrophotometry agree closely with those reported in the present study derived from flow cytometry.
Fig. 4.
Comparison of 2C DNA content values for 20 Gossypium species determined with Feulgen microspectrophotometry (Kadir, 1976) to values reported in this study. Bennett et al. (1982) converted the arbitrary fluorescence units reported by Kadir (1976) into picograms using H. vulgare ‘Sultan’ as an internal standard. The revised values shown in this figure were derived from arbitrary fluorescence units converted into picograms using the G. arboreum DNA content reported in this study (2C = 3·5 pg). y = 0·4462367 + 0·8759436x (r2 = 0·961).
It was concluded that external standardization produced the most reliable DNA content estimates for Gossypium species. Internal standardization with O. sativa ‘IR36’ may also yield acceptable results. Hordeum vulgare ‘Sultan’, however, appeared to be unsuitable as an internal standard for Gossypium as the DNA content estimates produced by this method were high. The DNA content values for the 37 Gossypium species provide additional support for previous cytogenetic divisions of the genus (Endrizzi et al., 1985) and closely agree with other reports (Kadir, 1976) of DNA content derived by Feulgen microspectrophotometry. The lack of significant intraspecific and intragenomic DNA content variation indicates that considerable speciation not accompanied by mass changes in DNA content has occurred within Gossypium. As such, Gossypium is a useful model for the study of genome constancy.
Acknowledgments
We thank Dr Gisela Erf for assistance and training on the use of the flow cytometer. This research was funded in part by a grant from Cotton Inc., which is gratefully acknowledged.
LITERATURE CITED
- Arumuganathan K, Earle ED. 1991. Estimation of nuclear DNA content of plants by flow cytometry. Plant Molecular Biology Reporter 9: 229–233. [Google Scholar]
- Bennett MD, Cox AV, Leitch IJ. 1997. Angiosperm DNA c-values database, http://www.rbgkew.org.uk/cval/database1.html. 25 June 2004. [Google Scholar]
- Bennett MD, Johnston S, Hodnett GL, Price HJ. 2000.Allium cepa L. cultivars from four continents compared by flow cytometry show nuclear DNA constancy. Annals of Botany 85: 351–357. [Google Scholar]
- Bennett MD, Smith JB. 1976. Nuclear DNA amounts in angiosperms. Philosophical Transactions of the Royal Society of London. Series B 274: 227–274. [DOI] [PubMed] [Google Scholar]
- Bennett MD, Smith JB, Heslop-Harrison JS. 1982. Nuclear DNA amounts in angiosperms. Proceedings of the Royal Society of London. Series B 216: 179–199. [Google Scholar]
- Cronn RC, Small RL, Haselkorn T, Wendel JF. 2002. Rapid diversification of the cotton genus (Gossypium: Malvaceae) revealed by analysis of sixteen nuclear and chloroplast genes. American Journal of Botany 89: 707–725. [DOI] [PubMed] [Google Scholar]
- Cronn RC, Small RL, Haselkorn T, Wendel JF. 2003. Cryptic repeated genomic recombination during speciation in Gossypium gossypioides Evolution 57: 2475–2489. [DOI] [PubMed] [Google Scholar]
- Dolezel J, Bartos J, Voglmayr H, Greilhuber J. 2003. Nuclear DNA content and genome size of trout and human. Cytometry 51A: 127–128. [DOI] [PubMed] [Google Scholar]
- Edwards GA, Endrizzi JE, Stein R. 1974. Genomic DNA content and chromosome organization in Gossypium Chromosoma 47: 309–326. [Google Scholar]
- Edwards GA, Mirza MA. 1979. Genomes of the Australian wild species of cotton. II. The designation of a new G genome for Gossypium bickii Canadian Journal of Genetics and Cytology 21: 367–372. [Google Scholar]
- Endrizzi JE, Turcotte EL, Kohel RJ. 1985. Qualitative genetics, cytology, and cytogenetics. In: Kohel RJ, Lewis CF, eds. Cotton. ASA Monograph 24. Madison, WI: American Society of Agronomy. [Google Scholar]
- Fryxell PA. 1992. A revised taxonomic interpretation of Gossypium L. (Malvaceae). Rheedea 2: 108–165. [Google Scholar]
- Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E. 1983. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220: 1049–1051. [DOI] [PubMed] [Google Scholar]
- Gomez MJ, Johnston JS, Price HJ. 1993. Nuclear 2C DNA content of Gossypium hirsutum accessions determined by flow cytometry. Biologisches Zentralblatt 112: 351–358. [Google Scholar]
- Greilhuber J, Ebert I. 1994. Genome size variation in Pisum sativum Genome 37: 646–655. [DOI] [PubMed] [Google Scholar]
- Greilhuber J, Obermayer R. 1997. Genome size and maturity group in Glycine max (soybean). Heredity 78: 547–551. [Google Scholar]
- Jakob SS, Meister A, Blattner FR. 2004. The considerable genome size variation of Hordeum species (Poaceae) is linked to phylogeny, life form, ecology, and speciation rates. Molecular Biology and Evolution 21: 860–869. [DOI] [PubMed] [Google Scholar]
- Kadir ZBA. 1976. DNA evolution in the genus Gossypium Chromosoma 56: 85–94. [Google Scholar]
- McClintock B. 1984. The significance of responses of the genome to challenge. Science 226: 792–801. [DOI] [PubMed] [Google Scholar]
- Martins EP. 2003. COMPARE: Computer programs for the statistical analysis of comparative data. Version 4·4. http://compare.bio.indiana.edu/. 9 July 2004. [Google Scholar]
- Michaelson MJ, Price HJ, Ellison JR, Johnston JS. 1991. Comparison of plant DNA contents determined by Feulgen microspectrophotometry and laser flow cytometry. American Journal of Botany 78: 183–188. [Google Scholar]
- Mishra R, Wang HY, Yadav N, Wilkins TA. 2003. Development of highly regenerable elite Acala cotton (Gossypium hirsutum L.)—a step towards genotype-independent regeneration. Plant Cell, Tissue and Organ Culture 73: 21–35. [Google Scholar]
- Noirot M, Barre P, Duperray C, Louarn J, Hamon S. 2003. Effects of caffeine and chlorogenic acid on propidium iodide accessibility to DNA: consequences on genome size evaluation in coffee tree. Annals of Botany 92: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noirot M, Barre P, Louarn J, Duperray C, Hamon S. 2000. Nucleus–cytosol interactions—a source of stoichiometric error in flow cytometric estimation of nuclear DNA content in plants. Annals of Botany 86: 309–316. [Google Scholar]
- Price HJ, Hodnett G, Johnston JS. 2000. Sunflower (Helianthus annuus) leaves contain compounds that reduce nuclear propidium iodide fluorescence. Annals of Botany 86: 929–934. [Google Scholar]
- Price HJ, Johnston JS. 1996. Analysis of plant DNA content by Fuelgen micro-spectrophotometry and flow cytometry. In: Jauhur P, ed. Methods of genome analysis in plants. Boca Raton, FL: CRC Press, 115–132. [Google Scholar]
- Seelanan T, Brubaker CL, Stewart JM, Craven LA, Wendel, JF. 1999. Molecular systematics of Australian Gossypium section Grandicalyx (Malvaceae). Systematic Botany 24: 183–208. [Google Scholar]
- Senchina DS, Alvarez I, Cronn RC, Liu B, Rong J, Noyes RD, Paterson AH, Wing RA, Wilkins TA, Wendel JF. 2003. Rate variation among nuclear genes and the age of polyploidy in Gossypium Molecular Biology and Evolution 20: 633–643. [DOI] [PubMed] [Google Scholar]
- Sims LE, Price HJ. 1985. Nuclear DNA content variation in Helianthus (Asteraceae). American Journal of Botany 72: 1213–1219. [Google Scholar]
- Stewart JM. 1995. Potential for crop improvement with exotic germplasm and genetic engineering. In: Constable GA, Forrester NW, eds. Challenging the future: Proceedings of the World Cotton Research. CSIRO, 313–327. [Google Scholar]
- Stewart JM, Hsu CL. 1977.In ovulo embryo culture and seedling development of cotton (Gossypium hirsutum L.). Planta 137: 113–117. [DOI] [PubMed] [Google Scholar]
- Walbot V, Dure LS. 1976. Developmental biochemistry of cotton seed embryogenesis and germination. VII. Characterization of the cotton genome. Journal of Molecular Biology 101: 503–536. [DOI] [PubMed] [Google Scholar]
- Wendel JF, Cronn R. 2002. Polyploidy and the evolutionary history of cotton. Advances in Agronomy 78: 139–186. [Google Scholar]
- Wendel JF, Cronn RC, Johnston JS, Price HJ. 2002. Feast and famine in plant genomes. Genetica 115: 37–47. [DOI] [PubMed] [Google Scholar]