Abstract
• Background and Aims Roridula plants capture insects but have no digestive enzymes. It has been hypothesized that Roridula leaves absorb nitrogen from the faeces of obligately associated, carnivorous hemipterans. But rapid movement across the leaf surfaces of most plant leaves is prevented by the presence of an impermeable cuticle. However, in carnivorous plants, cuticular gaps or pores in digestive/absorptive cells allow rapid movement across the leaf surface. Recently, it was suggested that the hemipteran–plant interaction constituted a new pathway for plant carnivory. Here, a further adaptation to this pathway is described by demonstrating how Roridula plants probably absorb hemipteran faeces rapidly through their leaf cuticles.
• Methods The dye neutral red was used to document the rapidity of foliar absorption and TEM to determine the nature of cuticular discontinuities in the leaf of Roridula.
• Key Results Aqueous compounds diffuse rapidly across the cuticle of Roridula's leaves but not across the cuticles of co-occurring, non-carnivorous plant leaves. Furthermore, immature Roridula leaves were unable to absorb neutral red whereas mature leaves could. Using TEM, cuticular gaps and pores similar to those in other carnivorous plants were found in the epidermal cells of mature Roridula leaves.
• Conclusions The leaf cuticle of Roridula is very thin (0–120 nm) and cell wall elements project close to the leaf surface, possibly enhancing foliar absorption. In addition to these, cuticular gaps were frequently seen and probably perfom a function similar to those found in other carnivorous plants: namely the absorption of aqueous compounds. The cuticular gaps of Roridula are probably an adaptation to plant carnivory, supporting the newly described pathway.
Keywords: Foliar absorption, faeces, plant carnivory
INTRODUCTION
Roridula plants catch large numbers of insects using very sticky traps that are superficially similar to those found in Drosera but there are no digestive enzymes. This has lead to the exclusion of Roridula from the list of recognized carnivorous plants (see Marloth, 1925; Lloyd, 1934; Juniper et al., 1989), although Anderson and Midgley (2003) have argued otherwise. The plants are able to assimilate insect nitrogen (Ellis and Midgley, 1996; Anderson and Midgley, 2002, 2003) despite the fact that they have no digestive enzymes (Marloth, 1910; Lloyd, 1934; Ellis and Midgley, 1996). Ellis and Midgley (1996) suggest that indirect carnivory after leaf fall or by fungi/bacteria is unlikely. The primary reason for this is that a carnivorous hemipteran lives obligately in very close association with Roridula (Dolling and Palmer, 1991) and consumes prey within hours of capture, leaving behind only the exoskeleton. They suggest that Roridula absorbs nitrogen from the liquid hemipteran faeces directly through the cuticle. In support of this, nitrogen from labelled flies was absorbed extremely rapidly (within 72 h), making leaf fall and fungal contributions unlikely. In addition, labelled nitrogen was only incorporated into plant tissue when hemipterans were present, but not when hemipterans were excluded from plants (Ellis and Midgley, 1996). However, it remains to be determined whether Roridula has cuticular adaptations to make such rapid nutrient absorption possible.
The cuticle is the aerial layer covering all terrestrial, higher plants (Viougeas et al., 1995) and it is characterized by hydrophobic properties that have a protective function (Schönherr, 1982). A layer of epicuticular wax covers the leaves of all terrestrial plants and this layer can be amorphous to crystalline (Baker, 1982), dense or diffuse. Below this is the cuticle, which can be divided into two layers. The upper layer is the cuticularized layer, which consists entirely of lipid material, with no cellulose or cell wall material (Martin and Juniper, 1970). Below this is a cutinized layer (Martin and Juniper, 1970), which may contain permeable cell wall elements (Lyshede, 1978; Juniper et al., 1989). The cell wall, which is composed of polysaccharides and small amounts of protein, lies below the last cuticle layer. The presence of the cuticle effectively forms a transport barrier into and out of the leaf for hydrophilic substances (Price, 1982) and this may pose a problem for the glandular cells (e.g. those in carnivorous plants), which specialize in absorption or digestion. The conflicting roles of epidermal cells are highlighted in carnivorous plants whose digestive and absorptive cells must be highly permeable but still provide a barrier against water loss (Schönherr and Schmidt, 1979) and pathogens (Tulloch, 1976). The cuticle of digestive/absorptive structures is often very thin and this is thought to aid rapid movement across this layer (Heslop-Harrison, 1976; Robins, 1976). However, there are situations in which thicker cuticles are more permeable than thinner ones, which suggests that other factors may also influence transport across the cuticle (Norris, 1974; Price, 1982). Evidence suggests that the cuticle is not always a homogeneous lipid layer. For example, carbohydrate fibres may extend into the cuticle from the cell wall and middle lamellae (Norris and Bukovac, 1968) and these fibres may provide hydrophilic pathways, which extend from the cell walls to the proximity of the cuticle surface (Hoch, 1979). Cuticular discontinuities in carnivorous plants have also been identified in the form of gaps, pores and more ill-defined discontinuities. Using SEM, large pores have been identified in the heads of Drosera tentacles (Williams and Pickard, 1969, 1974). These are sections where both the cuticularized layer and cutinized layer are absent (Juniper et al., 1989) and the cell wall is exposed to the exterior (Ragetli et al., 1972; Joel and Juniper, 1982). In other carnivorous plants, pores are hard to observe using SEM because extensions of the cell wall project to the surface of the leaf (Juniper et al., 1989). These gaps are invisible under SEM because they are filled by permeable wall material and are referred to as cuticular gaps (Joel and Juniper, 1982). The cuticularized layer in these cells is often thin or absent (Joel and Juniper, 1982). Joel and Juniper (1982) suggest several ways in which cuticular gaps may form. These include the selective deposition and breakdown of cutin. But the mechanism in Drosopyllum is thought to be the tearing of the cutin layer through cell wall extension, which requires no special control mechanisms and can be due to normal cell growth (Joel and Juniper, 1982). Joel and Juniper (1982) found that rapid uptake of a water-soluble dye (neutral red) is a reliable indicator of cuticular gaps and that there is a correlation between stainability and the presence of cuticular gaps. They also showed that immature Drosophyllum leaves could not absorb the dye neutral red because a lack of cell wall extension had allowed the cuticle to maintain its integrity. In contrast, mature leaves absorbed the dye due to tears in their cuticles caused by cellular extensions.
In this study, neutral red is used as an indicator of cuticular gaps in the plant Roridula to determine whether dissolved compounds lying on the leaf surface can be rapidly absorbed into the leaves of Roridula. TEM is also used to obtain direct evidence of cuticular gaps. The presence of cuticular gaps and the rapid uptake of neutral red should support the hypothesis of Ellis and Midgley (1996) who suggest that Roridula can absorb nitrogenous compounds from hemipteran faeces on their leaf surfaces. It would support the hypothesis that a mutualism exists between the carnivorous hemipteran Pameridea and the insect-trapping plant Roridula. Finally, the presence of cuticular gaps may be regarded as yet another adaptation to carnivory, sealing the debate on whether Roridula should be considered a carnivorous plant or not (see Anderson and Midgley, 2003; cf. Marloth, 1925; Lloyd, 1934; Juniper et al., 1989).
MATERIALS AND METHODS
The leaves of R. dentata and R. gorgonias, collected from the Vogelgat Nature Reserve (34°23′S, 19°19′E), were partially submerged in a 1 % solution of neutral red for 2 min (see Juniper and Joel, 1982). Both mature leaves and leaves in bud were treated in this manner (n = 5 per species). In addition, the following three species of co-occurring non-carnivorous plants were treated in the same manner: Saltera sarcocolla, Nivenia stokoei and Tetraria thermalis. After 2 min of submersion, the leaves were removed from the solution and rinsed. Transverse sections were made from neutral red absorbent leaves and these were examined under a light microscope with no additional staining.
Leaf material was examined with a TEM using the methods of Joel and Juniper (1982). Leaves were fixed in 3 % glutaraldehyde (in caccodylate buffer pH 7·2, 0·01 m) for 5 h. The material was then post-fixed in 1 % OsO4 (in the same buffer) for 2 h, dehydrated in an ethanol series, stained in uranyl acetate and embedded in Spurr's resin (Spurr, 1969). The material was sectioned using a Reichert ultracut S microtome and the sections were stained in lead citrate (Reynolds, 1963). The material was photographed in a Zeiss EM109 electron microscope. Both mature and immature leaf material was examined from both Roridula species and all non-carnivorous plants. Results of Roridula and a single non-carnivorous plant (Saltera) are given here.
RESULTS
The mature leaves of both R. dentata and R. gorgonias are permeable to neutral red following 2 min of immersion and the results were the same for both species (results are only shown for R. dentata in this text). The leaves show a distinct discontinuity when they were immersed in neutral red on one side (left hand side; Fig. 1A) and had no contact with neutral red (right hand side; Fig. 1A). In contrast, the immature leaves of Roridula and all leaves of co-occurring non-carnivorous plants are impermeable, suggesting a lack of cuticular discontinuities. The dye was taken up almost uniformly by the entire leaf surface of mature Roridula, barring some small patches of cells (Fig. 1B). Transverse sections of Roridula leaves also indicate that dye was taken up by the majority of epidermal cells (Fig. 1C and D). Mesophyll cells adjacent to the epidermis and sclerenchyma surrounding the vascular bundles both showed that they had taken up large quantities of neutral red (Fig. 1C and D). TEM indicates that that the epicuticular wax layer is extremely thin (0–120 nm) and diffuse with many gaps that make it porous; it also seems very loosely attached to the underlying layers (Fig. 1F–H and J). In contrast, the wax layer on non-carnivorous plants was comparatively thick (300–400 nm) and continuous (Fig. 1E). Similarly the cuticle of Roridula is extremely thin (0–120 nm) in comparison to non-carnivorous plants such as Saltera (Fig. 1E vs. F–K) that has a cuticle 2400–4000 nm thick. The epidermis of R. dentata and R. gorgonias leaves (mature and immature) both commonly showed invaginations of the cell wall (Fig. 1H) and these extended close to the leaf surface, although they seldom broke through the cuticle (Fig. 1G). Holes, tears and pores were frequently observed in the cuticles of mature Roridula epidermal cells that allowed permeable cell walls to be in contact with the outside (Fig. 1F and I–K). The pore in Fig. 1K seems to have a plug of either cuticular wax or other external detritus and hence it is uncertain whether it would be permeable to liquid compounds.
Fig. 1.
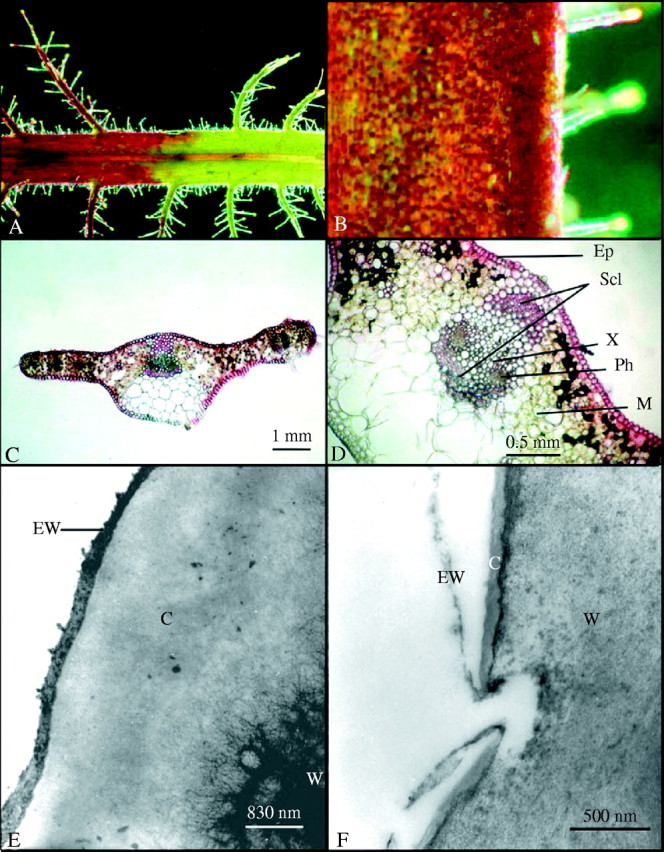
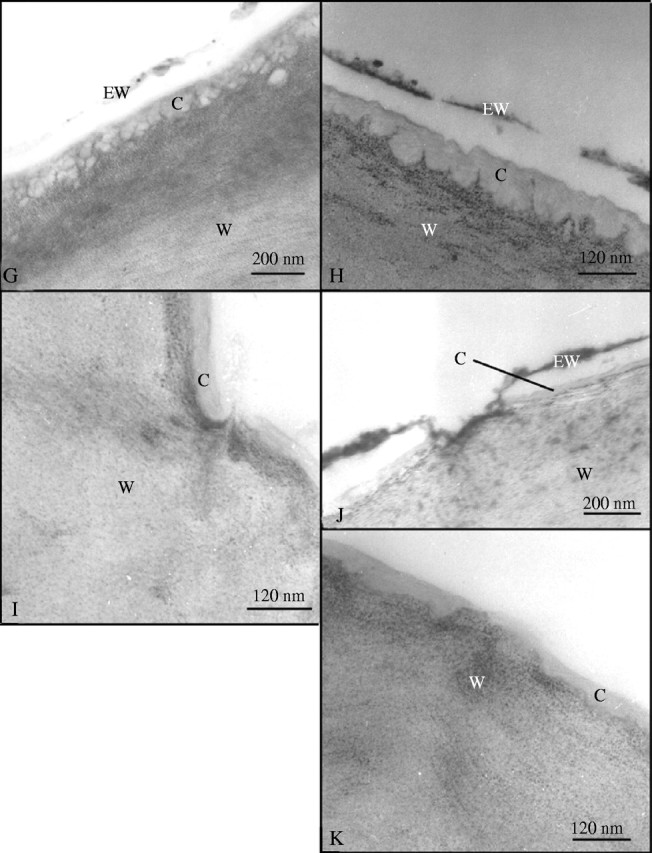
(A) Roridula dentata leaf after being immersed in neutral red for 2 min. (B) Patchwork of absorptive and non-absorptive cells on the leaf of R. dentata. (C) Transverse section of a R. dentata leaf showing that most epidermal cells are capable of absorption. (D) Transverse section of a R. dentata leaf showing that neutral red rapidly reaches scherenchyma and mesophyll. (E) TEM of Saltera epidermal cell. (F) TEM of mature R. dentata epidermal cell showing cuticular pore. (G) TEM of mature R. dentata epidermal cell showing reticulated wall elements reaching to the cell surface. (H) TEM of mature R. dentata epidermal cell showing cell wall elements extending towards the leaf surface. (I) TEM of mature R. dentata epidermal cell showing cuticular gap with wall elements extending to the leaf surface. (J) TEM of mature R. dentata epidermal cell showing a cuticular pore with a discontinuous cuticle. (K) TEM of mature R. dentata epidermal cell showing cuticular gap. C, Cuticle; Ep, epidermis; EW, epicuticular wax; Ph, phloem; M, mesophyll; Scl, sclerenchyma; W, cell wall; X, xylem.
DISCUSSION
The cell walls of Roridula leaves are often highly invaginated and reticulated in places, and this is likely to create aqueous pathways between the inside and outside of the leaf. These invaginations are found in both old and young leaves and seldom reach the leaf surface. Since young leaves are not as permeable as older leaves, the primary cause of high cuticular permeability in Roridula leaves is most likely the presence of cuticular gaps and pores. Kerstiens (1996) suggests that the existence of wide pores or cracks in the cuticle could increase cuticular permeability, even if they occupy a very small proportion of the leaf surface area. Joel and Juniper (1982) postulate that tearing can take place with normal cell growth.
The extensive staining by neutral red in Roridula suggests that the majority of epidermal cells are highly absorptive and that the entire epidermis has an absorptive function. This may be similar to the bottom zone of the pitcher plant, Sarracenia, where the entire inner leaf surface seems to be highly absorptive (Joel and Heide-Jørgensen, 1985). In contrast, only a few specialized digestive cells in Drosophyllum have cuticular gaps and are capable of rapid neutral red absorption (Joel and Juniper, 1982). In the light of the spectacular neutral red absorption, the foliar absorption of nitrogen by Roridula is likely to be particularly efficient, although this must come at a cost of being highly water sensitive. However, problems of desiccation may be minimized by the waterlogged nature of the soils and the hairiness of the leaves, which may alter the humidity of the leaf microclimate.
These results lend credence to the results of Ellis and Midgley (1996) who show that nitrogen from trapped flies is very rapidly absorbed by Roridula plants. Their results show that substantial amounts of fly nitrogen are incorporated into plant leaves after only 72 h of capture. They postulate that such rapid nitrogen incorporation can only take place if digestion is immediate. The continuous absorptive surface in Roridula contrasts to the specificity of absorption in Drosophyllum that only absorbs neutral red into a comparatively small number of specialized digestive cells (see Joel and Juniper, 1982). The presence of cuticular gaps and pores over the entire epidermis provides the necessary absorptive structure for the rapid absorption of nitrogenous compounds on the leaf surface. These are the first data to suggest that Roridula has cuticular gaps similar to other carnivorous plants that would make the rapid nitrogen uptake postulated by Ellis and Midgley (1996) possible.
Lastly, I hypothesize that cuticular gaps in Roridula plants are another primary adaptation to carnivory, suggesting that Roridula may be regarded as a carnivorous plant. Givnish (1989) defines carnivorous plants as those that have primary adaptations for prey attraction, prey capture and absorption. Many carnivorous plants only satisfy two of these criteria. For example, Darlingtonia, Heliamphora and Sarracenia purpurea do not produce digestive enzymes and are thought to digest prey with the aid of both micro- and macro-organisms. Catopsis is another carnivorous plant that passively traps prey and does not attract prey items or digest them. Anderson and Midgley (2003) regard the hemipterans living on Roridula as highly specific, obligate mutualists that act as functional digestive glands. The carnivorous status of this plant should be reinstated because Roridula traps large amounts of prey, it has the means (through obligate mutualism) to digest this prey and it has now been shown to have unusual adaptations to facilitate rapid absorption of digested prey.
Supplementary Material
Acknowledgments
I would like to thank Professor D. M. Joel for his interest in this project and for his help in interpreting micrographs and his ideas on cuticular permeability. Mohammed Jaffer's help with the electron microscopy was invaluable and Nicci Berg gave me the original idea for this section. I also thank two anonymous reviewers for their helpful comments.
LITERATURE CITED
- Anderson B, Midgley JJ. 2002. It takes two to tango but three is a tangle: mutualists and cheaters on the carnivorous plant Roridula Oecologia 132: 369–373. [DOI] [PubMed] [Google Scholar]
- Anderson B, Midgley JJ. 2003. Digestive mutualism, an alternate pathway in plant carnivory. Oikos 102: 221–224 [Google Scholar]
- Baker EA. 1982. Chemistry and morphology of plant epicuticular waxes. In: Cutler DF, Alvin KL, Price CE, eds. The plant cuticle. London: Academic Press, 45–85. [Google Scholar]
- Dolling WR, Palmer JM. 1991.Pameridea (Hemiptera: Miridae): predaceous bugs specific to a highly viscid plant genus Roridula Systematic Entomology 16: 319–328. [Google Scholar]
- Ellis AG, Midgley JJ. 1996. A new plant–animal mutualism involving a plant with sticky leaves and a resident hemipteran. Oecologia 106: 478–481. [DOI] [PubMed] [Google Scholar]
- Givnish TJ. 1989. Ecology and evolution of carnivorous plants. In: Abrahamson WG, ed. Plant–animal interactions. New York: McGraw-Hill, 243–290. [Google Scholar]
- Heslop-Harisson Y. 1976. Enzyme secretion and digest uptake in carnivorous plants. In: Sunderland NE, ed. Perspectives in experimental biology. Oxford: Pergamon Press, 463–476 [Google Scholar]
- Hoch HC. 1979. Penetration of chemicals into the Malus leaf cuticle. An ultrastructural analysis. Planta 147: 186–195. [DOI] [PubMed] [Google Scholar]
- Joel DM, Heide-Jørgensen HS. 1985. Ultrastructure and development of the pitcher epithelium of Sarracenia. Israel Journal of Botany 34: 331–349. [Google Scholar]
- Joel DM, Juniper BE. 1982. Cuticular gaps in carnivorous plant glands. In: Cutler DF, Alvin KL, Price CE, eds. The plant cuticle. London: Academic Press, 121–130. [Google Scholar]
- Juniper BE, Robins RJ, Joel DM. 1989.The carnivorous plants. London: Academic Press. [Google Scholar]
- Kerstiens G. 1996. Cuticular water permeability and its physiological significance. Journal of Experimental Botany 47: 1813–1832. [Google Scholar]
- Lloyd FE. 1934. Is Roridula a carnivorous plant? Canadian Journal of Research 10: 780–786. [Google Scholar]
- Lyshede OB. 1978. Studies on outer epidermal cell walls with microchannels in xerophytic species. New Phytologist 80: 421–426. [Google Scholar]
- Marloth R. 1910. Further observations on the biology of Roridula Transactions of the Royal Society of South Africa 2: 59–61. [Google Scholar]
- Marloth R. 1925.The flora of South Africa, Vol. 2. Cape Town: Darther Brothers. [Google Scholar]
- Martin JT, Juniper BE. 1970.The Cuticles of Plants. London: Edward Arnold. [Google Scholar]
- Norris RF. 1974. Penetration of 2,4-D in relation to cuticle thickness. American Journal of Botany 61: 74–79. [Google Scholar]
- Norris RF, Bukovac MJ. 1968. Structure of the pear leaf cuticle with special reference to cuticular penetration. American Journal of Botany 55: 975–983. [Google Scholar]
- Price CE. 1982. A review of the factors influencing the penetration of pesticides through plant leaves. In: Cutler DF, Alvin KL, Price CE, eds. The plant cuticle. London: Academic Press. [Google Scholar]
- Ragetli HW, Weintraub LOE. 1972. Characteristics of Drosera tentacles. I. Anatomical and cytological details. Canadian Journal of Botany 50: 159–168. [Google Scholar]
- Reynolds ES. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. Journal of Cell Biology 17: 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins RJ. 1976. The nature of the stimuli causing digestive juice secretion in Dionaea muscipula Ellis (Venus's flytrap). Planta 128: 263–265. [DOI] [PubMed] [Google Scholar]
- Schönherr J. 1982. Resistance of plant surfaces to water loss: transport properties of cutin, suberin and associated lipids. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, eds. Encyclopedia of plant physiology, New series, Vol. 12B. Berlin: Springer, 153–179. [Google Scholar]
- Schönherr J, Schmidt HW. 1979. Water permeability of plant cuticles: dependence of permeability coefficients of cuticular transpiration on vapour pressure saturation deficit. Planta 144: 391–400. [DOI] [PubMed] [Google Scholar]
- Spurr AR. 1969. A low viscosity resin embedding medium for electron microscopy. Journal of Ultrastructural Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- Tulloch AP. 1976. Chemistry of waxes of higher plants. In: Kolattukudy PE, ed. Chemistry and biochemistry of natural waxes. Amsterdam: Elsevier, 235–287. [Google Scholar]
- Viougeas MA, Rohr R, Chamel A. 1995. Structural changes and permeability of ivy (Hedera helix L.) leaf cuticles in relation to leaf development and after selective chemical treatments. New Phytologist 130: 337–348. [Google Scholar]
- Williams SE, Pickard BG. 1969. Secretion, absorption and cuticular structure in Drosera tentacles. Plant Physiology 44: 5. [Google Scholar]
- Williams SE, Pickard BG. 1974. Connections and barriers between cells of Drosera tentacles in relation to their electrophysiology. Planta 116: 1–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


