Abstract
• Background and Aims The genus Equisetum is cytologically uniform, having a base chromosome number of x = 108. All previously known species and hybrids that have been counted represent diploids with a sporophytic chromosome number of 2n = 216. Biosystematic studies on Equisetum subgenus Hippochaete revealed evidence that triploids occur in nature. The objective of this study was to confirm that triploid plants exist in the natural environment.
• Methods Flow cytometry was used to establish nuclear DNA values and cytological investigations of meiosis were carried out to obtain information on chromosome number and pairing behaviour.
• Key Results Triploidy exists in three morphologically different hybrid taxa. Two of these are morphologically intermediate between a primary diploid hybrid and a parent, while the third apparently combines genomes from all three Central European Hippochaete species. Nuclear 1C DNA values for the four European Hippochaete species range from 21·4–31·6 pg. For the hybrids, the 1C DNA values not only occupy the same range as the species, but their total DNA amounts agree closely with values predicted by adding the 1C DNA values of each parental genome. Chromosome counts confirm diploidy in the species E. hyemale and E. variegatum and in the hybrid E. ×trachyodon (= E. hyemale × E. variegatum). For the triploids (2n ≈ 324), cytological information is presented for the first time.
• Conclusions Triploid taxa may have originated by backcrossing or by crossing of a diploid hybrid with an unrelated diploid species. As tetraploid plants are unknown, these crossings probably involve diploid gametophytes that developed from unreduced diplospores. By repeated crossing events or backcrossing, reticulate evolution patterns arise that are similar to those known for a number of ferns and fern allies.
Keywords: Equisetum, subgenus Hippochaete, flow cytometry, nuclear DNA content, triploidy, chromosome numbers, hybridization, reticulate evolution
INTRODUCTION
The Equisetopsida are primitive spore-producing vascular plants, and some of their members are among the oldest land plants known; their earliest fossil remains are from the Upper Devonian (Bateman, 1991). The arborescent giant horsetails (Calamitaceae and Archaeocalamitaceae) reached heights of 18 m or more and were major components of the Carboniferous swamplands; besides lycopod trees (Lepidodendrales), they were important contributors to coal formation. Like the lycopod trees, these woody calamites scarcely survived the ‘Age of Coal’, and by the mid-Permian they were extinct (Bateman, 1991).
The only lineage that has survived is the horsetails (Equisetum), which are herbaceous and share characters with their extinct progenitors such as articulate stems with microphylls arranged in whorls. Recent phylogenetic studies, using both combined analyses of DNA sequences from multiple loci and morphological characters, suggest that the horsetails together with the ferns (including the whisk ferns, Psilotopsida) form a clade representing one of the three major lineages of vascular plants (Pryer et al., 2001).
Equisetum is believed to have diverged in the Tertiary from an older genus, Equisetites, which is of Paleozoic (mid-Permian or possibly Carboniferous) origin (Stewart and Rothwell, 1993; Des Marais et al., 2003). Some fossil forms of Equisetites are indistinguishable from extant horsetails, and thus Equisetum might be regarded as the oldest surviving genus of vascular plants in the world (Guillon, 2004).
While in Milde's (1867) basic and (in its morphological details still unsurpassed) work Monographia Equisetorum the boundaries were not consistently drawn between species, taxa meriting solely infraspecific rank, and hybrids, Hauke (1963, 1978) created a more modern treatment of horsetail taxonomy. He critically revised the great number of Equisetum taxa and reduced them to no more than 15 species. These show a nearly worldwide distribution with the exception of Australia, New Zealand and Antarctica. Hauke's circumscription of species is still generally accepted. Of the 15 Equisetum species, 12 have been cytologically checked and found to be uniform, with a base chromosome number of x = 108 (Manton, 1950; Ninan, 1955; Mehra and Bir, 1959; Bir, 1960; Löve and Löve, 1961; Packer and McPherson, 1974; Löve et al., 1977; Freeman and Brooks, 1988; Obermayer et al., 2002). This is an unusually high number, even within the pteridophytes; diploid sporophytes have 216 chromosomes. While most Equisetum species have been studied cytologically, chromosome counts are very scarce for hybrids. Hybridization is especially frequent within the subgenus Hippochaete, where seven hybrids are known, three of which occur in Europe. The only hybrid for which detailed cytological information is available is E. ×trachyodon (= E. hyemale × E. variegatum). It was studied by Manton (1950), who found a complete failure of chromosome pairing during meiosis, but refrained from indicating the number of chromosomes she saw. It was Bir (1960), who reported the number to be 2n = 216. Other hybrids (E. ×ferrissii [listed under the species name E. laevigatum], E. ×litorale, E. ×moorei) were studied, but no countable stages were obtained (Manton, 1950; Hauke, 1958).
Nuclear DNA C-values are available for eight of the 15 Equisetum species (determined by flow cytometry). They are distinctly different between the two subgenera Equisetum and Hippochaete (Obermayer et al., 2002) with ranges from 1C = 12·5–14·2 pg (subgenus Equisetum) and 21·3–30·4 pg (subgenus Hippochaete). To our knowledge, no hybrids have been studied, and no indications for a sporophytic ploidy level other than diploid have been obtained so far.
During recent biosystematic studies on European Equisetum species and hybrids, we repeatedly found plants of subgenus Hippochaete in which morphological characters suggested that they represent triploid backcrosses between a primary diploid hybrid and one of the diploid ancestors. To test whether such triploid hybrids exist, flow cytometry was used. This method not only allows the determination of ploidy level, but also gives estimates of the nuclear DNA content. Furthermore, meiosis of spore mother cells was analysed to obtain direct evidence for triploidy.
MATERIALS AND METHODS
For the analyses, fresh plant material (shoots or rhizomes) was collected and either used on the same day or kept in a refrigerator for no longer than 3 d. All four European species of subgenus Hippochaete (E. hyemale, E. ramosissimum, E. scirpoides, E. variegatum) and three diploid hybrids (E. ×meridionale, E. ×moorei, E. ×trachyodon) were investigated. Two or three different geographical origins were selected for each species and each diploid hybrid (Table 1). For the triploids, samples from various localities were analysed. All plants are now in cultivation in the private garden of one of the authors (M.L.). Vouchers for each taxon and geographic origin are deposited in the herbarium of Bochum (BOC).
Table 1.
Origin of Equisetum species and hybrids used for flow cytometry analyses
| Identification |
Species |
Locality |
|---|---|---|
| ML 28 | E. hyemale | Furlbachtal, Senne, NRW, D |
| ML 29 | E. hyemale | Pilsholz, Hamm, NRW, D |
| ML 70 | E. hyemale | Eggenstein-Leopoldshafen, BW, D |
| ML 33 | E. ramosissimum | Altpoderschau, TH, D |
| ML 35 | E. ramosissimum | Cagnes-sur-Mer, Dépt Alpes-Maritimes, F |
| ML 91 | E. ramosissimum | Port-Vendres, Dépt Pyrénées-Orientales, F |
| ML 41 | E. scirpoides | Abisko, S |
| ML 66 | E. scirpoides | Gudbrandsdalen, Oppland, N |
| ML 36 | E. variegatum | Bad Reichenhall, BAY, D |
| ML 63 | E. variegatum | Gudbrandsdalen, Oppland, N |
| SP 72/93 | E. variegatum | Chur, Graubünden, CH |
| ML 51 | E. ×moorei | Mönchenwerth, Düsseldorf, NRW, D |
| ML 52 | E. ×moorei | Rheidter Werth, Bonn, NRW, D |
| ML 55 | E. ×trachyodon | Dahlhunden, Dépt Bas-Rhin, F |
| ML 56 | E. ×trachyodon | Dahlhunden, Dépt Bas-Rhin, F |
| ML 73 | E. ×trachyodon | Isle of Harris, Scotland, GB |
| ML 44 | E. ×meridionale | Algund, Südtirol, I |
| ML 45 | E. ×meridionale | Altpoderschau, TH, D |
| ML 57 | E. ×alsaticum (triploid) | Sponeck, BW, D |
| ML 58 | E. ×alsaticum (triploid) | Dahlhunden, Dépt Bas-Rhin, F |
| ML 75 | E. ×alsaticum (triploid) | Burkheim, BW, D |
| ML 78 | E. ×alsaticum (triploid) | Oberwört, Dépt Bas-Rhin, F |
| ML 86 | E. ×alsaticum (triploid) | Plittersdorf, BW, D |
| ML 100 | E. ×alsaticum (triploid) | Au a. Rhein, BW, D |
| ML 101 | E. ×alsaticum (triploid) | Kastenwört, BW, D |
| ML 103 | E. ×alsaticum (triploid) | Ottenheim, BW, D |
| ML 105 | E. ×alsaticum (triploid) | Rust, BW, D |
| ML 106 | E. ×alsaticum (triploid) | Whyl/Weisweil, BW, D |
| ML 107 | E. ×alsaticum (triploid) | Breisach, BW, D |
| ML 53 | E. ×moorei (triploid) | Bois de Sommerley, Dépt Bas-Rhin, F |
| ML 84 | E. ×moorei (triploid) | Ketscher Rheininsel, BW, D |
| ML 85 | E. ×moorei (triploid) | Plittersdorf, BW, D |
| ML V60 | E. ×moorei (triploid) | Greffern, BW, D |
| ML 77 | E. trachyodon (triploid) | Au a. Rhein, BW, D |
Abbreviations used: BAV, Bavaria; BW, Baden-Württemberg; CH, Switzerland; D, Germany; F, France (with Département given); GB, Great Britain; I, Italy; N, Norway; NRW, North Rhine-Westphalia; PL, Poland; S, Sweden; TH, Thuringia. For assumed origin of diploid and triploid hybrids, see Fig. 2.
A small stem or rhizome piece (about 0·5 cm long) was chopped up with a new razor blade in 0·3 mL buffer solution (nuclear extraction buffer; solution A, Partec GmbH, Münster) for 30–60 s, incubated for 10–15 min, filtered through a 50 µm mesh nylon tissue, and processed in a staining buffer (solution B, Partec GmbH, Münster) containing RNAase and propidium iodide (PI) for 30 min. The calibration standard was Allium sativum ‘Ailsa Craig’ (4C = 67·00 pg; Obermayer et al., 2002).
Samples were analysed using a Partec PA II flow cytometer (Münster, Germany), equipped with a 20 mV argon gas laser, a quartz–air objective and a high-quality red-sensitive photo-multiplier. For each sample (i.e. each geographical origin), two or three preparations were made. The G1 fluorescence peaks of the Equisetum sample and the Allium standard were used to calculate the peak ratio (mean channel number of Equisetum sample/mean channel number of standard). This was multiplied by 33·5 (the 2C-value of the Allium standard) to yield the absolute 2C-value (pg) of the Equisetum sample.
For chromosome counts, young cones were fixed in a mixture of acetic acid and ethanol (1 : 3). Immature sporangia were used to yield squash preparations following the method of Manton (1950, p. 293), with slight modifications according to Van den heede (2003) and H. Rasbach (unpubl.).
RESULTS AND DISCUSSION
Nuclear DNA C-values of the species
A considerable variation (1·5-fold) of nuclear DNA C-values occurs in the species of subgen. Hippochaete (Table 2) with E. scirpoides showing the smallest 1C value (mean 21·4 pg) and E. variegatum the largest (mean 31·6 pg). The values reported here and those published by Obermayer et al. (2002) agree closely in E. scirpoides (Table 3). In E. ramosissimum and E. variegatum, the C-value estimates differ by approx. 2 pg. Differences in methodology may account for this, but an intraspecific variation of genome size cannot be excluded. In E. variegatum, there was a significant difference (1·5 pg) between plants from Germany and Norway, and the latter have a 1C-value 2·2 pg larger than the material studied by Obermayer et al. (2002), who did not indicate the geographical origin of their plants. In the case of E. ramosissimum, intraspecific variation may also be involved; the European material (subsp. ramosissimum) is very homogeneous and contrasts significantly with the Asiatic subsp. debile. In flowering plants, intraspecific genome size variation clearly exists, but seems to be less frequent than previously assumed (Greilhuber, 1998; Ellul et al., 2002; Emshwiller, 2002). For pteridophytes, data are too scant to allow for a realistic judgement on the occurrence and frequency of such a variation.
Table 2.
Nuclear DNA content (±s.e.) for the diploid taxa studied; for abbreviations, seeTable 1
| Taxon |
Identification |
Origin |
2C (pg) |
1C (pg) |
||||
|---|---|---|---|---|---|---|---|---|
| Species | ||||||||
| E. hyemale | ML 28 | Senne, NRW, D | 52·5 ± 0·71 | 26·3 ± 0·36 | ||||
| E. hyemale | ML 29 | Pilsholz, Hamm, NRW, D | 53·3 ± 0·00 | 26·7 ± 0·00 | ||||
| E. hyemale | ML 70 | Eggenstein-Leopoldshafen, BW, D | 52·4 ± 0·28 | 26·2 ± 0·14 | ||||
| Mean | 52·7 ± 0·54 | 26·3 ± 0·27 | ||||||
| E. ramosissimum | ML 33 | Altpoderschau, TH, D | 56·4 ± 0·06 | 28·2 ± 0·03 | ||||
| E. ramosissimum | ML 35 | Cagnes-sur-Mer, Dépt Alpes-Maritimes, F | 56·1 ± 0·56 | 28·1 ± 0·28 | ||||
| E. ramosissimum | ML 91 | Port-Vendres, Dépt Pyrénées-Orientales, F | 56·5 ± 0·05 | 28·3 ± 0·03 | ||||
| Mean | 56·3 ± 0·36 | 28·2 ± 0·18 | ||||||
| E. scirpoides | ML 41 | Abisko, S | 43·4 ± 0·26 | 21·7 ± 0·13 | ||||
| E. scirpoides | ML 66 | Gudbrandsdalen, Oppland, N | 42·2 ± 0·05 | 21·1 ± 0·03 | ||||
| Mean | 42·8 ± 0·62 | 21·4 ± 0·31 | ||||||
| E. variegatum | ML 36 | Bad Reichenhall, BAV, D | 62·2 ± 0·70 | 31·1 ± 0·35 | ||||
| E. variegatum | ML 63 | Gudbrandsdalen, Oppland, N | 65·1 ± 0·27 | 32·6 ± 0·14 | ||||
| E. variegatum | SP 72/93 | Chur, Graubünden, CH | 62·6 ± 0·19 | 31·3 ± 0·10 | ||||
| Mean | 63·3 ± 1·51 | 31·6 ± 0·75 | ||||||
| Hybrids | ||||||||
| E. ×meridionale | ML 44 | Algund, Südtirol, I | 61·2 ± 0·61 | 30·6 ± 0·31 | ||||
| E. ×meridionale | ML 45 | Altpoderschau, TH, D | 60·7 ± 0·04 | 30·9 ± 0·02 | ||||
| Mean | 60·9 ± 0·50 | 30·5 ± 0·25 | ||||||
| E. ×moorei | ML 51 | Düsseldorf, NRW, D | 53·9 ± 0·56 | 27·0 ± 0·28 | ||||
| E. ×moorei | ML 52 | Bonn, NRW, D | 54·0 ± 0·34 | 27·0 ± 0·17 | ||||
| Mean | 54·0 ± 0·46 | 27·0 ± 0·23 | ||||||
| E. ×trachyodon | ML 55 | Dahlhunden, Dépt Bas-Rhin, F | 59·0 ± 0·68 | 29·5 ± 0·34 | ||||
| E. ×trachyodon | ML 56 | Dahlhunden, Dépt Bas-Rhin, F | 57·8 ± 0·71 | 28·9 ± 0·36 | ||||
| E. ×trachyodon | ML 73 | Isle of Harris, Scotland, GB | 59·7 ± 0·40 | 29·9 ± 0·20 | ||||
| Mean | 58·9 ± 0·62 | 29·5 ± 0·31 | ||||||
Table 3.
DNA 1C-values (pg, ±s.e.) for the species of Equisetum subgen. Hippochaete; data from Obermayer et al. (2002) and from the present work; for abbreviations, see Table 1
| Species |
Present work |
Obermayer et al. |
|---|---|---|
| E. giganteum | 26·1 ± 0·25 | |
| E. hyemale | 26·3 ± 0·36 (D) | |
| 26·7 ± 0·00 (D) | ||
| 26·2 ± 0·14 (D) | ||
| E. myriochaetum | 25·7 ± 0·20 | |
| E. ramosissimum* | ||
| subsp. ramosissimum | 28·2 ± 0·03 (D) | |
| 28·1 ± 0·28 (F) | ||
| 28·3 ± 0·03 (F) | ||
| subsp. debile | 26·2 ± 0·12 | |
| E. scirpoides | 21·7 ± 0·13 (S) | 21·3 ± 0·02 |
| 21·1 ± 0·03 (N) | ||
| E. variegatum | 31·1 ± 0·35 (D) | 30·4 ± 0·10 |
| 31·3 ± 0·10 (CH) | ||
| 32·6 ± 0·14 (N) |
As indicated, this species comprises two subspecies, subsp. ramosissimum (which is the one occurring in Europe) and subsp. debile (mainly in Eastern Asia; sometimes recognized as a separate species, as by Obermayer et al., 2002); many authors include subsp. debile in the nominate subspecies.
For E. hyemale, we report a DNA C-value for the first time. It is similar to that of the other species of Equisetum (except the two extremes, E. scirpoides and E. variegatum); this brings the total number of species of subgen. Hippochaete studied to six out of seven (the North American E. laevigatum is still lacking). The dissimilarity between E. scirpoides and E. variegatum (see above) is rather unexpected, as these species have been considered to be closely related, constituting the only two species of subsection Homocormia of section Hippochaete according to Hauke (1963). They are similar in their gross morphology and their general distribution pattern. Recent studies based on chloroplast DNA sequence data show, however, that E. scirpoides is sister to a clade formed by the other species of subgenus Hippochaete (Des Marais et al., 2003; Guillon, 2004).
Nuclear DNA C-values in diploid hybrids
The hybrids studied were initially identified by their intermediate morphology and their aborted spores, the latter being a reliable character for tracing hybrid origin. Those plants that showed the intermediate morphology typical of primary diploid hybrids (E. ×meridionale, E. ×moorei, and E. ×trachyodon) yielded 1C DNA values ranging from 27·0 pg (E. ×moorei) to 30·5 pg (E. ×meridionale), thus being close to the (diploid) species (Tables 2 and 5).
Table 5.
Nuclear DNA content for the species and hybrids of Equisetum subgen. Hippochaete studied. For the hybrids, a comparison is made between the values measured and those predicted on the basis of the parental genome sizes; the assumed hybrid origin is indicated by genome formulae. Values in parentheses are the range obtained in plants from different geographical origins
| Values measured |
Values predicted |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxon and genome formula |
Mean 1C (pg) |
Mean 2C (pg) |
Mean 3C (pg): triploid hybrids |
2C (pg): diploid hybrids |
3C (pg): triploid hybrids |
Difference: measured — predicted (pg) |
||||||
| Species | ||||||||||||
| E. hyemale HH | 26·3 (26·2–26·7) | 52·7 (52·4–53·3) | ||||||||||
| E. ramosissimum RR | 28·2 (28·1–28·3) | 56·3 (56·1–56·5) | ||||||||||
| E. variegatum VV | 31·6 (31·1–32·6) | 63·3 (62·2–65·1) | ||||||||||
| Diploid hybrids | ||||||||||||
| E. ×meridionale RV | 60·9 (60·7–61·2) | 59·8 (59·2–60·9) | +1·1 | |||||||||
| E. ×moorei HR | 54·0 (53·9–54·0) | 54·5 (54·3–55·0) | −0·5 | |||||||||
| E. ×trachyodon HV | 58·9 (57·8–59·7) | 57·9 (57·3–59·3) | +1·0 | |||||||||
| Triploid hybrids | ||||||||||||
| E. ×alsaticum HHV | 83·5 (81·1–84·0) | 84·2 (83·5–86·0) | −0·7 | |||||||||
| E. ×moorei HHR | 79·3 (78·7–80·1) | 80·8 (80·5–81·5) | −1·5 | |||||||||
| E. ×trachyodon HRV | 83·8 | 86·1 (85·4–87·6) | −2·3 | |||||||||
The C-values presented here are the first ever published for Equisetum hybrids. Although plants from different localities were studied, their genome sizes are remarkably uniform, even when comparing plants from Scotland and the European continent, as in E. ×trachyodon (Table 2). The three diploid hybrids can be distinguished by their C-values, although they are similar in E. ×meridionale and E. ×trachyodon (Tables 2 and 5).
The 2C-values of the diploid hybrids can be predicted by adding the 1C-values of their putative parents. Measured and predicted values agree fairly well, particularly considering that there is geographic variation included in the sampling (Table 5).
Nuclear DNA C-values in triploid hybrids
For those plants with morphology suggesting a backcross of a primary hybrid with a parent, DNA amounts were obtained that were considerably larger than in the diploid taxa (Tables 4 and 5). As some of these were checked cytologically and found to be triploid (see below), their C-values are given as 3C-values, and their 1C-value was obtained by dividing by 3. The standard deviation in some triploids was higher than in the diploid taxa. In the two triploids, where plants from several localities were analysed, the means are quite homogeneous and no significant variation occurs between geographic regions. Measured and predicted values also agree fairly well in the triploids, with the exception of the new hybrid (E. ×trachyodon HRV), which obviously incorporates three different parental genomes.
Table 4.
Nuclear DNA content (±s.e.) for the triploid hybrids; for abbreviations, seeTable 1
| Taxon |
Identification |
Locality |
3C (pg) |
1C (pg) |
|---|---|---|---|---|
| E. ×alsaticum | ML 57 | Sponeck, BW, D | 81·1 | 27·0 |
| E. ×alsaticum | ML 58 | Dahlhunden, Dépt Bas-Rhin, F | 81·1 | 27·0 |
| E. ×alsaticum | ML 75 | Burkheim, BW, D | 83·5 ± 1·47 | 28·3 ± 0·24 |
| E. ×alsaticum | ML 78 | Oberwört, Dépt Bas-Rhin, F | 84·0 ± 1·35 | 27·8 ± 0·45 |
| E. ×alsaticum | ML 86 | Plittersdorf, BW, D | 83·4 | 27·8 |
| E. ×alsaticum | ML 100 | Au a. Rhein, BW, D | 83·6 ± 1·00 | 27·9 ± 0·33 |
| E. ×alsaticum | ML 101 | Kastenwört, BW, D | 84·0 ± 1·35 | 28·0 ± 0·45 |
| E. ×alsaticum | ML 103 | Ottenheim, BW, D | 83·7 ± 0·08 | 27·9 ± 0·03 |
| E. ×alsaticum | ML 105 | Rust, BW, D | 83·7 ± 0·05 | 27·9 ± 0·02 |
| E. ×alsaticum | ML 106 | Wyhl/Weisweil, BW, D | 84·0 ± 1·05 | 28·0 ± 0·35 |
| E. ×alsaticum | ML 107 | Breisach, BW, D | 82·8 ± 0·86 | 27·6 ± 0·29 |
| Mean | 83·5 ± 1·18 | 27·8 ± 0·39 | ||
| E. ×moorei | ML 53 | Bois de Sommerley, Dépt Bas-Rhin, F | 78·7 ± 1·00 | 26·4 ± 0·33 |
| E. ×moorei | ML 84 | Ketscher Rheininsel, BW, D | 79·5 ± 2·19 | 26·5 ± 0·73 |
| E. ×moorei | ML 85 | Plittersdorf, BW, D | 80·1 ± 1·31 | 26·7 ± 0·44 |
| E. ×moorei | ML V60 | Greffern, BW, D | 79·0 ± 0·03 | 26·3 ± 0·01 |
| Mean | 79·3 ± 1·98 | 26·4 ± 0·66 | ||
| E. ×trachyodon | ML 77 | Au a. Rhein, BW, D | 83·8 ± 1·38 | 27·9 ± 0·46 |
Chromosome numbers in species and hybrids
For E. hyemale and E. variegatum countable preparations were obtained, which showed the expected number of 108 bivalents (Fig. 1, Table 6). In the diploid hybrids, chromosome pairing during meiosis failed almost completely, and many univalents were found; their number could only be determined in the case of E. ×trachyodon. In the triploids with the assumed genome formulae HHV and HHR, a considerable number of uni- and bivalents occurred. For two origins of E. ×alsaticum (HHV) counts were obtained that yielded varying numbers of paired and unpaired chromosomes (approx. 0–3III + approx. 92–115II + approx. 94–131I), giving a total of approx. 324.
Fig. 1.
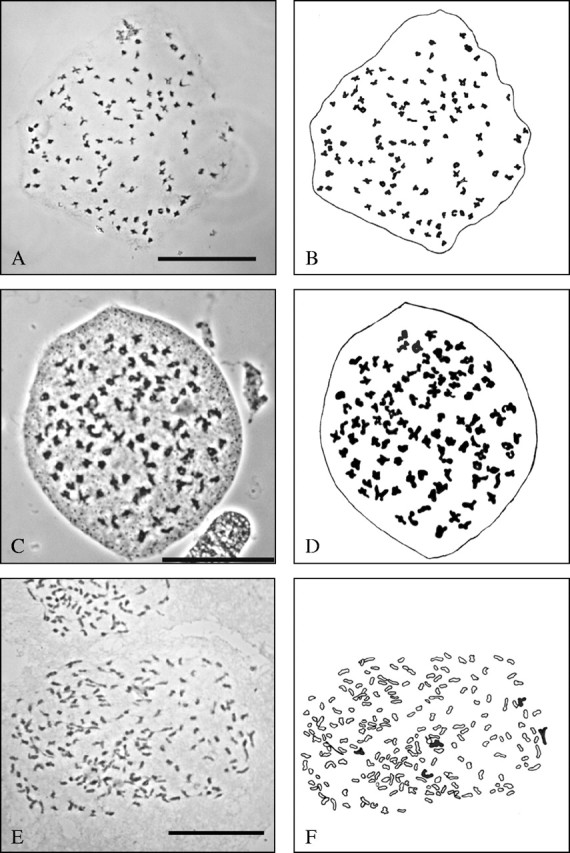
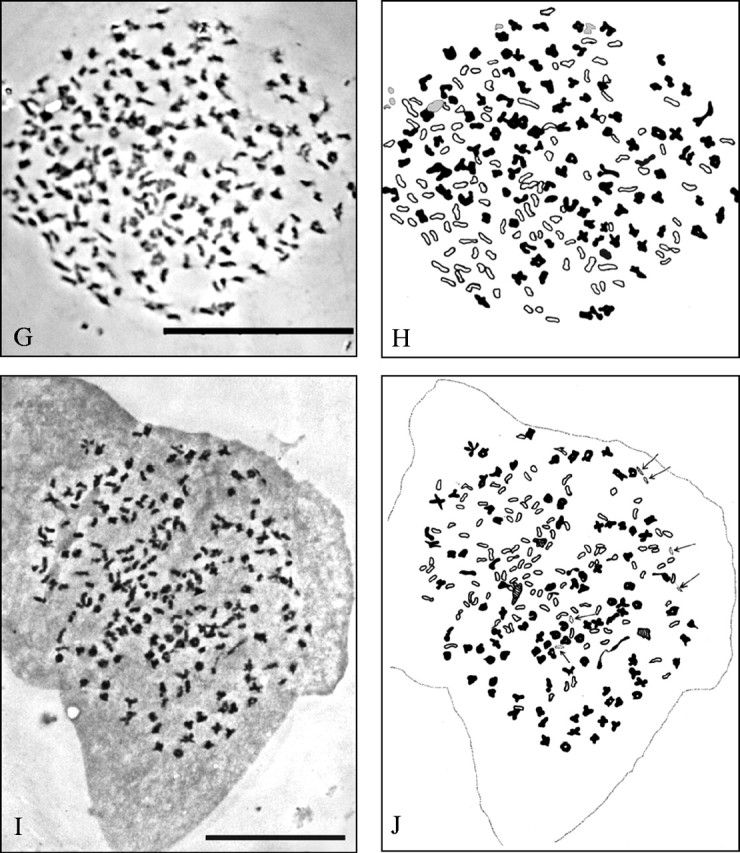
Meiotic pairing behaviour in species and hybrids of Equisetum subgenus Hippochaete; photograph and explanatory diagram for E. hyemale (A, B; ML 29), E. variegatum (C, D; SP 72/93), E. ×trachyodon (E, F; ML 56), and two origins of E. ×alsaticum (G, H; ML 57 and I, J; ML 75). Univalents outlined, bi- and trivalents black; scale bar = 50 μm. Interpretation of the pairing behaviour: 108II in E. hyemale and E. variegatum; approx. 206I + approx. 5II in E. ×trachyodon; approx. 111I + approx. 105II + 1III in E. ×alsaticum (ML 57); approx. 115I + approx. 100II + approx. 3III (and additionally six separate small particles, see arrows; preparation, photograph and interpretation by H. Rasbach) in E. ×alsaticum (ML 75).
Table 6.
Chromosome numbers and meiotic pairing behaviour in the species and hybrids of Equisetum subgen. Hippochaete studied
| Taxon and genome formula |
2n |
Meiotic pairing behaviour |
||
|---|---|---|---|---|
| Species | ||||
| E. hyemale HH | 216* | 108II | ||
| E. ramosissimum RR | – | – | ||
| E. variegatum VV | 216* | 108II | ||
| Diploid hybrids | ||||
| E. ×meridionale RV | – | – | ||
| E. ×moorei HR | n.c. | Mainly univalents | ||
| E. ×trachyodon HV | approx. 216* | approx. 5II + approx. 206I | ||
| Triploid hybrids | ||||
| E. ×alsaticum HHV | approx. 324* | approx. 0–3III + approx. 92–115II + approx. 94–131I | ||
| E. ×moorei HHR | n.c. | Bi- and univalents | ||
| E. ×trachyodon HRV | n.c. | Mainly univalents | ||
shown in Fig. 1; n.c. = not countable; – indicates that no meiotic stages were obtained.
There is one report of an even higher number: in their compilation of chromosome numbers for ferns and fern allies, Löve et al. (1977) indicated a number of 2n = 432 for Equisetum (×) trachyodon and referred to a paper by Bir (1960) on chromosome numbers of Equisetum species from the Netherlands. However, the figures in the original paper show this number to be a misinterpretation. Equisetum ×trachyodon is a hybrid displaying irregular meiosis. Due to the failure of chromosome pairing, univalents are formed instead of bivalents. Bir (1960) published a drawing of a spore mother cell showing complete failure of pairing, thus exhibiting 216 univalents. These univalents may have been counted as bivalents by Löve et al. (1977), resulting in the false number of 2n = 432; Bir (1960) explicitly stated the correct number (2n = 216).
According to Hauke (1990), only three of the 15 species have never been counted. These are E. bogotense, E. giganteum and E. myriochaetum. Data obtained from flow cytometry by Obermayer et al. (2002) and ourselves (unpublished results) suggest that the latter two species are also diploids.
Parentage of the triploid hybrids
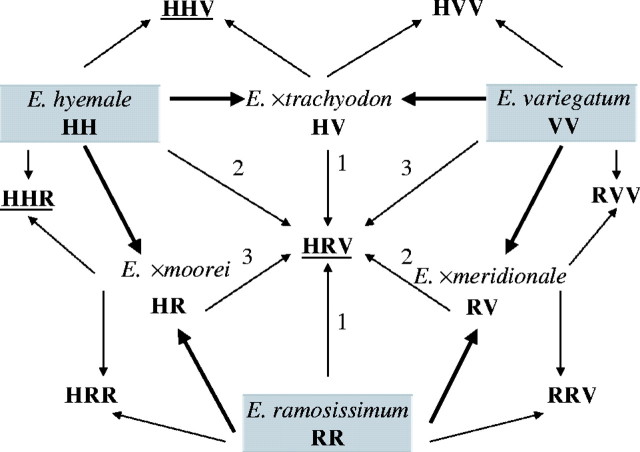
As discussed above, E. ×alsaticum is a triploid hybrid. Macro- and micromorphological characters as well as chorological evidence suggest that these plants are backcrosses between E. hyemale and E. ×trachyodon with the genome formula HHV (Fig. 2). The two genomes derived from E. hyemale account for the plant's close similarity to this species. There are no macro- or micromorphological characters detectable that would suggest E. ramosissimum to be involved.
Fig. 2.
Hybridization scheme showing origin of the known three diploid and all seven theoretically possible triploid hybrids within subgen. Hippochaete in Central Europe. Diploid species are marked grey, the origin of diploid hybrids is marked by solid arrows. For the triploid genotype HRV all three possible origins are indicated; genotypes of triploid hybrids presumably occurring in nature are underlined.
Like E. ×alsaticum, the triploid E. ×moorei is morphologically very close to E. hyemale, but shares certain characters with E. ramosissimum such as cross-bands of silica covering the ridges. No morphological traits are present that would indicate a parentage of E. variegatum. There can be little doubt that the genome formula HHR applies to this plant.
The third triploid is the most unusual, inasmuch as it combines the genomes of all three Central European diploid species (HRV). Previously, it was mistaken for E. ×trachyodon, as the existence of triploid crosses was not known or considered. This is illustrated by the photograph shown by Philippi (1993) picturing plants that were named E. ×trachyodon. These, however, belong to the triploid hybrid HRV, as the photograph was taken at the same locality where the triploid hybrid occurs (H. and K. Rasbach, pers. comm.). It is similar in its overall morphology to E. ×trachyodon, but shares certain features of phenology (only the lower half of the stem persists during winter) and micromorphology (silica cross-bands) with E. ramosissimum. Whereas the other triploids represent backcrosses, the formation of the HRV genotype involves a diploid hybrid that crosses with an unrelated diploid species. It is obvious from Fig. 2 that this triploid could be achieved by three different hybridization events, namely E. ×trachyodon (HV) × E. ramosissimum (RR), E. ×moorei (HR) × E. variegatum (VV), and E. ×meridionale (RV) × E. hyemale (HH). As all three diploid hybrids and species occur within the range of the triploids (upper Rhine valley), no decision can be made about how it evolved, and multiple origin involving more than one hybridization mode and several crossing events are not precluded.
How could triploids have formed in nature?
Generally, triploidy results from a cross between a tetraploid and diploid species. Backcrossing involving allotetraploids and their diploid progenitors is a common feature in many fern genera, like Asplenium, Dryopteris and Polystichum (see Kramer, 1984). Neither allo- nor autotetraploids are, however, known in the genus Equisetum. As chromosome counts are scarce for this group, and flow cytometry data were obtained from only a limited number of plants, the existence of such polyploids cannot be ruled out. In the field, such plants would not be recognizable, as their morphology is likely to be very close to the corresponding diploids.
Another route yielding triploids involves the formation of unreduced diplospores (through incomplete meiotic divisions or by somatic polyploidization occuring in the cone), which produce diploid gametophytes. These would then cross with a haploid gametophyte to yield a triploid sporophyte. Such diplospores may be produced by a diploid species (like E. hyemale, HH) or by a diploid hybrid (like E. ×trachyodon, HV). In the first case, a normal haploid gametophyte from E. variegatum (V) is required for obtaining the triploid hybrid HHV; the second case requires a normal haploid gametophyte from E. hyemale (H). Thus, the same triploid hybrid combination could arise by two different crossing events. The significance of diplospores for the formation of triploids has been shown by Schneller and Rasbach (1984) and Rasbach et al. (1991) for Athyrium, where several triploid taxa exist, and, as in Equisetum, no tetraploids are known.
Diplospores are recognizable due to their size, being larger than in the reduced meiospores, and, in the case of hybrids, by their normal shape. Equisetum hybrids produce aborted spores that are non-green and irregularly shaped, but also a small amount of globose, green spores. These obviously represent diplospores, and are regularly observed in all hybrids within subgenus Hippochaete. They were documented for E. ×meridionale (Hrouda and Krahulec, 1982; Krahulec et al., 1996), E. ×moorei (Dubois-Tylski and Girerd, 1986; Krahulec et al., 1996) and E. ×trachyodon (Page and Barker, 1985). According to Dubois-Tylski and Girerd (1986) diplospores in E. ×moorei have a diameter ranging from 80–140 µm, whereas normal meiospores in species like E. hyemale and E. ramosissimum fall within a range of 40–60 µm (Duckett, 1970; Hauke, 1978).
Germination experiments were performed with diplospores (of E. ×meridionale) by Hrouda and Krahulec (1982), but their attempts were not successful. Dubois-Tylski and Girerd (1986) obtained a gametophyte of E. ×moorei, which they kept alive for at least 2 months, but which formed no gametangia. Krahulec et al. (1996) were successful in growing gametophytes of E. ×meridionale and E. ×moorei. They suggested that (diploid) Hippochaete hybrids might produce gametophytes that would enable crosses between hybrids as well as backcrosses with their parents. They also noted that such a reticulate evolutionary pattern could well be the reason for the difficulties in delimiting species in this subgenus.
Triploidy in other pteridophytes
Polyploidy is increasingly being recognized as an important evolutionary force (Soltis and Soltis, 1999). It is far rarer in animals (although hundreds of examples are known) than in plants, where a frequency between 30 and 80 % has been estimated (Otto and Whitton, 2000). The pteridophytes are well known for having a high degree of polyploidy, and a frequency as high as 99·7 % has been calculated for the ferns (Otto and Whitton, 2000) assuming that taxa with a base number larger than 14 are ancient polyploids (‘paleopolyploids’). In pteridophytes, triploid formation by hybridization is widespread in genera that comprise diploid and tetraploid cytotypes, such as Adiantum, Asplenium, Dryopteris, Isoëtes, Polystichum and Pteris (Lovis, 1977; Walker, 1979; Kramer, 1984; Flora of North America Editorial Committee, 1993; Britton and Brunton, 1995). These hybrids usually represent allotriploids; examples of autotriploids have been found in Cystopteris, Isoëtes and Pteridium (Haufler et al., 1985; Rumsey et al., 1993; Sheffield et al., 1993).
In contrast to even-ploid plants, which are usually fertile, triploid cytotypes have been regarded as an evolutionary dead-end, as they have a much reduced fertility due to problems of chromosomal pairing during meiosis (Otto and Whitton, 2000). This reproductive incompetence can, however, be overcome through modifications of the normal sexual life cycle. Such events include agamospory (the chromosome number remains the same in both generations by means of diplospory and apogamy; see e.g. Wagner and Wagner, 1980; Schneller et al., 1998; Chang et al., 2003; Ishikawa et al., 2003; Park and Kato, 2003), segregation yielding a diploid parent (hypothesized for Polystichum; Pinter, 1995), and vegetative reproduction (as in triploid Pteridium aquilinum; Sheffield et al., 1993).
All horsetails, and those of the subgenus Hippochaete in particular, are known to reproduce readily by fragmentation, transport and propagation of rhizomes or aerial stems (Duval-Jouve, 1864; Milde, 1867; Schaffner, 1931; Praeger, 1934; Hauke, 1958, 1963; Bennert, 1999; Lubienski et al., 2004). This would explain the presence or abundance of plants of hybrid origin, even in the absence of one or both parents (Hauke, 1979; Page and Barker, 1985; Bennert and Böcker, 1991; Page, 1997).
Acknowledgments
The authors thank Mrs Helga Rasbach, Glottertal, for the cytological investigation and chromosome counts of one of the triploid hybrids (ML 75) and Mrs Ilse Wessel, Bochum, for her skilful help with part of the laboratory work. Thanks are also due to Mr S. Jeβen, Chemnitz, who supplied us with plants of Equisetum scirpoides and E. ×meridionale, to Wolfgang Jäger, Wülfrath, who gave detailed information on the type locality of Hippochaete alsatica, and to Dr Liz Sheffield, Manchester, for helpful comments on the manuscript.
LITERATURE CITED
- Bateman RM. 1991. Palaeobiological and phylogenetic implications of anatomically-preserved Archaeocalamites from the Dinantian of Oxroad Bay and Loch Humphrey Burn, southern Scotland. Palaeontographica B 223: 1–59. [Google Scholar]
- Bennert HW. 1999.Die seltenen und gefährdeten Farnpflanzen Deutschlands. Münster: Landwirtschaftsverlag. [Google Scholar]
- Bennert HW, Böcker R. 1991. Zur Verbreitung von Equisetum subgen. Hippochaete (Equisetaceae, Pteridophyta) in Berlin. Verhandlungen des Botanischen Vereins von Berlin und Brandenburg 124: 13–29. [Google Scholar]
- Bir SS. 1960. Chromosome numbers of some Equisetum species from the Netherlands. Acta Botanica Neerlandica 9: 224–234. [Google Scholar]
- Britton DM, Brunton DF. 1995.Isoetes ×marensis, a new interspecific hybrid from western Canada. Canadian Journal of Botany 73: 1345–1353. [Google Scholar]
- Chang H-M, Chiou W-L, Wang J-C. 2003. Supplemenets to the pteridophytes in Taiwan (I): Dryopteris decipiens (Hook.) Kuntze (Dryopteridaceae). Taiwania 48: 197–202. [Google Scholar]
- Des Marais DL, Smith AR, Britton DM, Pryer KM. 2003. Phylogenetic relationships and evolution of extant horsetails, Equisetum, based on chloroplast DNA sequence data (rbcl and trnL-F). International Journal of Plant Science 164: 737–751. [Google Scholar]
- Dubois-Tylski T, Girerd B. 1986. Étude comparative de quelques Equisetum du sous-genre Hippochaete Bulletin de la société botanique de France 2: 125–135. [Google Scholar]
- Duckett JG. 1970. Spore size in Equisetum New Phytologist 69: 333–346. [Google Scholar]
- Duval-Jouve J. 1864.Histoire naturelle des Equisetum de France. Paris: J.B. Baillière et fils. [Google Scholar]
- Ellul P, Boscaiu M, Vicente O, Moreno V, Rossello JA. 2002. Intra- and interspecific variation in DNA content in Cistus (Cistaceae). Annals of Botany 90: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emshwiller E. 2002. Ploidy levels among species in the ‘Oxalis tuberosa Alliance’ as inferred by flow cytometry. Annals of Botany 89: 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora of North America Editorial Committee. 1993.Flora of North America north of Mexico. Vol. 2, Pteridophytes and Gymnosperms. Oxford: Oxford University Press. [Google Scholar]
- Freeman CC, Brooks RE. 1988. Documented plant chromosome numbers 1988: 1. Chromosome counts for North American plants – I. Sida 13: 241–250. [Google Scholar]
- Greilhuber J. 1998. Intraspecific variation in genome size: A critical reassessment. Annals of Botany 82 (Supplement A): 27–35. [Google Scholar]
- Guillon J-M. 2004. Phylogeny of horsetails (Equisetum) based on the chloroplast rps4 gene and adjacent noncoding sequences. Systematic Botany 29: 251–259. [Google Scholar]
- Haufler CH, Windham MD, Britton DM, Robinson SJ. 1985. Triploidy and its evolutionary significance in Cystopteris protrusa Canadian Journal of Botany 63: 1855–1863. [Google Scholar]
- Hauke RL. 1958. Is Equisetum laevigatum a hybrid? American Fern Journal 48: 68–72. [Google Scholar]
- Hauke RL. 1963. A taxonomic monograph of the genus Equisetum subgenus Hippochaete Beihefte zur Nova Hedwigia 8: 1–123. [Google Scholar]
- Hauke RL. 1978. A taxonomic monograph of Equisetum subgenus Equisetum Nova Hedwigia 30: 385–455. [Google Scholar]
- Hauke RL. 1979.Equisetum ramosissimum in North America. American Fern Journal 69: 1–5. [Google Scholar]
- Hauke RL. 1990.Equisetaceae In: Kubitzki K, ed. The families and genera of vascular plants Vol. I. Pteridophyta and Gymnosperms. Berlin, Heidelberg, New York, London, Paris, Tokyo, Hong Kong, Barcelona: Springer, 46–48. [Google Scholar]
- Hrouda L, Krahulec F. 1982. Taxonomická a ekologická analýza společného výskytu druhu rodu Hippochaete (Equisetaceae) a jejich křížencu. [Taxonomic and ecological analysis of the occurrence of Hippochaete species and hybrids (Equisetaceae)]. Preslia 54: 19–43. [Google Scholar]
- Ishikawa H, Ito M, Watano Y, Kurita S. 2003. Electrophoretic evidence for homoeologous chromsome pairing in the apogamous fern species Dryopteris nipponenesis (Dryopteridaceae). Journal of Plant Research 116: 165–167. [DOI] [PubMed] [Google Scholar]
- Krahulec F, Hrouda L, Kovářová M. 1996. Production of gametophytes by Hippochaete (Equisetaceae) hybrids. Preslia 67: 213–218. [Google Scholar]
- Kramer KU. 1984.Illustrierte Flora von Mitteleuropa. Band I, Teil 1, Pteridophyta, 3rd edn. Berlin, Hamburg: Parey. [Google Scholar]
- Löve Á, Löve D. 1961. Some chromosome numbers of Icelandic ferns and fern-allies. American Fern Journal 51: 127–128. [Google Scholar]
- Löve Á, Löve D, Pichi Sermolli REG. 1977.Cytotaxonomical atlas of the Pteridophyta. Vaduz: J. Cramer. [Google Scholar]
- Lovis JD. 1977. Evolutionary patterns and processes in ferns. Advances in Botanical Research 4: 229–415. [Google Scholar]
- Lubienski M, Bennert HW, Jessen S. 2004.Equisetum ×font-queri Rothm. (= E. palustre L. × E. telmateia Ehrh., Equisetaceae, Pteridophyta) seit mehr als 150 Jahren auf Rügen. Tuexenia 24: 329–337. [Google Scholar]
- Manton I. 1950.Problems of cytology and evolution in the Pteridophyta. Cambridge: University Press. [Google Scholar]
- Mehra PN, Bir SS. 1959. A note on chromosome numbers in some Indian species of Equisetum American Fern Journal 49: 86–92. [Google Scholar]
- Milde J. 1867. Monographia Equisetorum. Verhandlungen der Kaiserlichen Leopoldino-Carolinischen deutschen Akademie der Naturforscher 32: 1–605. [Google Scholar]
- Ninan CA. 1955. Cytology of Equisetum debile Roxb. Journal of Indian Botanical Society 34: 112–114. [Google Scholar]
- Obermayer R, Leitch IJ, Hanson L, Bennett MD. 2002. Nuclear DNA C-values in 30 species double the familial representation in Pteridophytes. Annals of Botany 90: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto SP, Whitton J. 2000. Polyploid incidence and evolution. Annual Review of Genetics 34: 401–437. [DOI] [PubMed] [Google Scholar]
- Packer JG, McPherson GD. 1974. Chromosome numbers in some vascular plants from Alaska. Canadian Journal of Botany 52: 1095–1099. [Google Scholar]
- Page CN. 1997.The ferns of Britain and Ireland. 2nd edn. Cambridge: University Press. [Google Scholar]
- Page CN, Barker M. 1985. Ecology and geography of hybridization in British and Irish horsetails. Proceedings of the Royal Society of Edinburgh. Section B (Biological Sciences) 86: 265–272. [Google Scholar]
- Park C-H, Kato M. 2003. Apomixis in the interspecific triploid hybrid fern Cornopteris christenseniana (Woodsiaceae). Journal of Plant Research 116: 93–103. [DOI] [PubMed] [Google Scholar]
- Philippi G. 1993. Equisetaceae. In: Sebald O, Seybold S, Philippi G, Hrsg. 1993. Die Farn- und Blütenpflanzen Baden-Württembergs. Band 1. Allgemeiner Teil — Spezieller Teil (Pteridophyta, Spermatophyta) — Lycopodiaceae bis Plumbaginaceae. 2. Auflage.—Stuttgart: Ulmer, 78–92. [Google Scholar]
- Pinter I. 1995. Progeny studies of the fern hybrid Polystichum ×bicknellii (Dryopteridaceae: Pteridophyta). Fern Gazette 15: 25–40. [Google Scholar]
- Praeger RL. 1934. Propagation from aerial shoots in Equisetum Journal of Botany, British and Foreign 72: 175–176. [Google Scholar]
- Pryer KM, Schneider H, Smith AR, Cranfill R, Wolf PG, Hunt JS, Sipes SD. 2001. Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature 409: 618–622. [DOI] [PubMed] [Google Scholar]
- Rasbach H, Reichstein T, Schneller JJ. 1991. Hybrids and polyploidy in the genus Athyrium (Pteridophyta) in Europe. 2. Origin and description of two triploid hybrids and synthesis of allotetraploids. Botanica Helvetica 101: 209–225. [Google Scholar]
- Rumsey FJ, Thompson P, Sheffield E. 1993. Triploid Isoetes echinospora (Isoetaceae: Pteridophyta) in northern England. Fern Gazette 14: 215–221. [Google Scholar]
- Schaffner JH. 1931. Propagation of Equisetum from sterile aerial shoots. Bulletin of the Torrey Botanical Club 58: 531–535. [Google Scholar]
- Schneller JJ, Rasbach H. 1984. Hybrids and polyploidy in the genus Athyrium (Pteridophyta) in Europe. Botanica Helvetica 94: 81–99. [Google Scholar]
- Schneller J, Holderegger R, Gugerli F, Eichenberger K, Lutz E. 1998. Patterns of genetic variation detected by RAPDs suggest a single origin with subsequent mutations and long-distance dispersal in the apomictic fern Dryopteris remota (Dryopteridaceae). American Journal of Botany 85: 1038–1042. [PubMed] [Google Scholar]
- Sheffield E, Wolf PG, Rumsey FJ, Robson DJ, Ranker TA, Challinor SM. 1993. Spatial distribution and reproductive behaviour of a triploid bracken (Pteridium aquilinum) clone in Britain. Annals of Botany 72: 231–237. [Google Scholar]
- Soltis DE, Soltis PS. 1999. Polyploidy: recurrent formation and genome evolution. Trends in Ecology and Evolution 14: 348–352. [DOI] [PubMed] [Google Scholar]
- Stewart WN, Rothwell GW. 1993.Paleobotany and the evolution of plants. Cambridge: Cambridge University Press. [Google Scholar]
- Van den heede C. 2003.A biosystematic study of Asplenium subgenus Ceterach (Aspleniaceae, Pteridophyta) based on cytology, morphology, anatomy, isoenzyme analysis, and DNA sequencing. PhD Thesis, University of Ghent, Belgium. [Google Scholar]
- Wagner WH, Wagner FS. 1980. Polyploidy in pteridophytes. Basic Life Science 13: 199–214. [DOI] [PubMed] [Google Scholar]
- Walker TG. 1979. The cytogenetics of ferns. In: Dyer AF, ed. The experimental biology of ferns. London, New York, San Francisco: Academic Press, 87–132. [Google Scholar]