Abstract
• Background and Aims The desert legume genus Ammopiptanthus comprises two currently endangered species, A. mongolicus and A. nanus. Genetic variability and genetic differentiation between the two species and within each species were examined.
• Methods Inter-simple sequence repeat (ISSR) marker data were obtained and analysed with respect to genetic diversity, structure and gene flow.
• Key Results Despite the morphological similarity between A. mongolicus and A. nanus, the two species are genetically distinct from each other, indicated by 63 % species-specific bands. Low genetic variability was detected for both population level (Shannon indices of diversity Hpop = 0·106, percentage of polymorphic loci P = 18·55 % for A. mongolicus; Hpop = 0·070, P = 12·24 % for A. nanus) and species level (Hsp = 0·1832, P = 39·39 % for A. mongolicus; Hsp = 0·1026, P = 25·89 % for A. nanus). Moderate genetic differentiation was found based on different measures (AMOVA ΦST and Hickory θB) in both A. mongolicus (0·3743–0·3744) and A. nanus (0·2162–0·2369).
• Conclusions The significant genetic difference between the two species might be due to a possible vicariant evolutionary event from a single common ancestor through the fragmentation of their common ancestor's range. Conservation strategies for these two endangered species are proposed.
Keywords: Ammopiptanthus mongolicus, Ammopiptanthus nanus, desert, endangered plants, genetic diversity, ISSR
INTRODUCTION
Conservation of the genetic resources of endemic desert plants is crucial to worldwide efforts to combat desertification, to prevent further degradation of the fragile ecosystems in arid and semi-arid regions and to sustain biodiversity in deserts. Desert plants play a key role, as the primary producers, in maintaining these ecosystems. Desert ecosystems currently cover about 35 % of the Earth's land surface (Helldén, 1991) and they are expanding. This desertification and ongoing deterioration in arid and semi-arid regions worldwide has recently focused attention amongst the international community on the urgent need to protect the environment of the desert regions (FAO, 1997). The area of desert land in China amounts to approximately 2·080 million km2. As an adaptation to their dry and extremely cold environments, most desert plants in China have small, deciduous leaves. Ammopiptanthus Cheng f. is the only genus of evergreen broadleaf shrubs in the north-western desert of China. This genus belongs to the Leguminosae, a family consisting of about 690 genera worldwide, and members of the genus have been considered to be some of the most unique plants, and keystone components, of the region's flora.
Ammopiptanthus comprises two diploid species with high morphological similarity: A. mongolicus (Maxim.) Cheng f. and A. nanus (M. Pop.) Cheng f. (Cheng, 1959; Pan and Huang, 1993). They can be distinguished from each other by the shape of their leaves (trifoliate in A. mongolicus compared with simple leaves in A. nanus). Both species are narrowly distributed; A. mongolicus is endemic to the south Gobi desert (Liu et al., 1995; Liu, 1998), and A. nanus is restricted to the borders between China and Kyrgyzstan, growing in a narrow altitudinal strip between 1800 and 2800 m.
The evergreen broadleaf habit of Ammopiptanthus has been viewed as an ancestral trait that identifies it as a Tertiary relict taxon (Liu et al., 1995). During the interval spanning the early Paleocene to mid-Eocene (65–45 Ma; Willis and McElwain, 2002) in the early Tertiary period, the vegetation in north-western China was dominated by evergreen and/or deciduous broadleaf forest (Geng et al., 2001), according to fossil evidence. When subsequent changes made the climate colder and drier from the early Miocene (24–16 Ma) in central Asia (Guo et al., 2002), the forest was gradually replaced by steppe and then by desert (Yan et al., 2000). Ammopiptanthus is a relict survivor of the evergreen broadleaf forest of this region from the Tertiary period.
The two species are dominant in the local vegetation (Pan et al., 1992; Liu et al., 1995). Their habitats are stony and/or sandy deserts where the annual precipitation ranges from 100–160 mm. Plants flower profusely in spring (from early April to late May) with 12–16 and 10–14 flowers on each inflorescence of A. mongolicus and A. nanus, respectively (Yin and Wang, 1993). Both species are insect-pollinated. Their heavy seeds are dispersed by gravity within a short distance of the parent plant. Natural regeneration of both species is limited because of low seed germination rates in the harsh environment (Pan et al., 1992; Liu, 1998). Few young plants can be found in the wild. There has been no record of accurate data on the specific distribution range and population size for both species. A continuous distribution and large population size were suspected (Liu, 1995). However, in the past two decades, Ammopiptanthus have been subject to rapid demographic decline, mainly due to increasing anthropogenic pressures in their natural range (e.g. cutting for fuel wood and pollution). The estimated sizes of the extant populations range from 100 to more than 2000 individuals for both species (Table 1). The two species have been categorized as ‘endangered’ and given protected status in China (Fu, 1989). Because of the high academic interest in them, and their ecological usefulness in combating further desertification, many studies have been carried out on their anatomy (Liu and Qiu, 1982), drought-resistance mechanisms (Xu et al., 2002) and community structure (Liu et al., 1995).
Table 1.
Populations studied including estimated total population sizes (N) and sample sizes (Ns)
| Species |
Population |
Population code |
Locality |
Latitude (N) |
Longitude (E) |
Altitude (m) |
Ns |
N |
|---|---|---|---|---|---|---|---|---|
| A. mongolicus | West Ningxia | |||||||
| Shapotou | 1 | Zhongwei | 37°28′ | 104°58′ | 1300 | 23 | 100 | |
| East Ningxia | ||||||||
| Rujigou (1) | 2 | Pingluo | 39°03′ | 106°07′ | 1930 | 23 | 500 | |
| Rujigou (2) | 3 | Pingluo | 39°05′ | 106°09′ | 1920 | 23 | 100 | |
| Inner Mongolia | ||||||||
| Qianlishan | 4 | Wuhai | 39°50′ | 106°50′ | 1170 | 23 | >2000 | |
| Xindi | 5 | Wuhai | 39°52′ | 106°46′ | 1090 | 19 | 500 | |
| Yikebulage | 6 | Otog Qi | 40°05′ | 106°49′ | 1070 | 23 | 500 | |
| Taositu | 7 | Otog Qi | 40°09′ | 106°54′ | 1070 | 23 | 500 | |
| Muoshigou | 8 | Hangjin Qi | 40°07′ | 107°04′ | 1380 | 23 | 100 | |
| Balagong | 9 | Hangjin Qi | 40°16′ | 107°03′ | 1100 | 23 | 500 | |
| Dengkou (1) | 10 | Dengkou | 40°25′ | 106°43′ | 1050 | 23 | 100 | |
| Dengkou (2) | 11 | Dengkou | 40°25′ | 106°45′ | 1040 | 23 | >1000 | |
| A. nanus | Biaoertuokuoyi | 1 | Wuqia | 39°30′ | 74°51′ | 2700 | 24 | >2000 |
| Bacundaban | 2 | 39°39′ | 75°01′ | 2120 | 23 | 200 | ||
| Ohsalur | 3 | 39°40′ | 74°45′ | 2250 | 22 | 100 | ||
| Xiaoerbulake | 4 | 39°42′ | 75°01′ | 2200 | 30 | >2000 | ||
| Kangsu | 5 | 39°42′ | 75°04′ | 2170 | 22 | 100 | ||
| Baykurt | 6 | 39°50′ | 75°35′ | 2100 | 23 | 200 | ||
| Tielieke | 7 | do | 39°57′ | 75°39′ | 2300 | 24 | 200 |
Despite the general awareness of the importance of the Ammopiptanthus species for fixing moving sands and delaying further desertification, little is known about the distribution of genetic variation across their geographical ranges. Data related to genetic diversity within and between populations are essential for formulating appropriate management strategies for the conservation of rare and endangered species. Several aspects of conservation biology, such as the loss of genetic diversity in conservation programs and the restoration of threatened populations, can only be addressed by detailed population genetic studies (Hamrick and Godt, 1996). Compared with widespread and abundant species, endemic and rare taxa often contain significantly less genetic variability (Gitzendanner and Soltis, 2000). The loss of genetic variability may render populations more vulnerable to extinction in cases of habitat perturbation, reproductive bottlenecks, etc. (Barrett and Kohn, 1991), and such losses would be expected to increase the risk of local extinction in these taxa. In order to help formulate rational strategies to preserve genetic diversity within Ammopiptanthus, the levels and patterns of genetic variation in 18 populations of the genus were documented in the study reported here by analysing inter-simple sequence repeats (ISSRs).
ISSR PCR uses a single primer composed of a di- or trinucleotide simple sequence repeat [e.g., (CA)8, (AGC)6] with or without a 5′- or 3′-anchoring sequence of 1–3 nucleotides. ISSR primers target simple sequence repeats (microsatellites) that are abundant and dispersed throughout the genome, and reveal data that reflect the length variation between adjacent microsatellites. This technique has provided a powerful tool for the investigation of genetic variation within species (Wolfe and Liston, 1998). Recent ISSR studies of natural populations have demonstrated the hypervariable nature of these markers and their potential use for population-level studies (Esselman et al., 1999; Culley and Wolfe, 2001). Limitations of the ISSR technique, as is the case for Random Amplification of Polymorphic DNA (RAPD; Williams et al., 1990), are that bands are scored as dominant markers and that genetic diversity estimates are based on diallelic characters.
This investigation had three main purposes. Firstly, to estimate genetic differentiation between A. mongolicus and A. nanus. Secondly, to assess population genetic diversity and structures in A. mongolicus and A. nanus in order to obtain basic information for the development of conservation strategies. And thirdly, to contribute to our understanding of the effects of desertification on the genetic diversity of relict species.
MATERIALS AND METHODS
Plant material
A total of 251 individuals of Ammopiptanthus mongolicus representing 11 populations were sampled from three geographically isolated regions near the centre of its distribution: East Ningxia (two populations), West Ningxia (one population) and Inner Mongolia (eight populations). This sampling covers most of the extant A. mongolicus populations; however, the populations from Ala-shan region were not available for this study. One hundred and sixty-eighty individuals representing seven populations of A. nanus were sampled from Wuqia of Xinjiang Uygur Autonomous Region, the only county hosting A. nanus in China (Fig. 1; Table 1). This sampling scheme includes almost all the extant A. nanus populations known from China. Nineteen to 30 individuals were randomly collected from each population, regardless of their size and age. Young leaves were collected and dried in silica gel until DNA extraction.
Fig. 1.
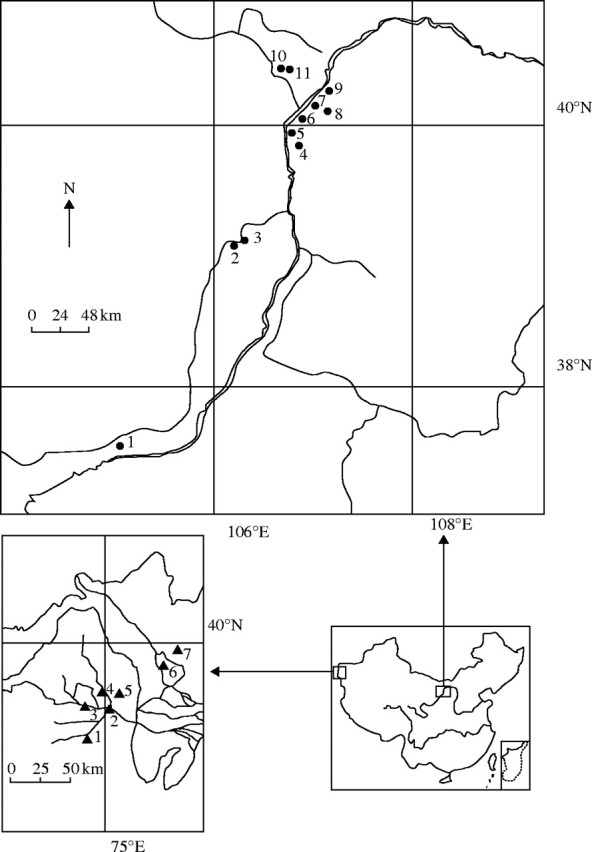
Locations of sampled populations of Ammopiptanthus mongolicus (upper map) and A. nanus (bottom left) in China. Population codes correspond to those given in Table 1.
DNA extraction and PCR amplification
Genomic DNA was extracted from approximately 0·5 g of leaf tissue using a modified CTAB method (Doyle and Doyle, 1987). The quality and concentration of the DNA were confirmed by electrophoresis on 1 % agarose gels with λ DNA markers. Nuclear DNA was amplified by PCR using ISSR primers from the University of British Columbia primer set 9 (Biotechnology Laboratory, University of British Columbia, primer set # 9: http://www.biotech.ubc.ca/services/naps/primers/Primers.pdf). Following an initial screen of 100 primers, 11 primers (UBC # 808, 809, 811, 813, 834, 840, 842, 880, 881, 886 and 888) that yielded maximum numbers of reliable and reproducible polymorphisms were then selected to analyse the populations. PCR amplifications were carried out in a total volume of 20 mL consisting of 20 ng of template DNA, 10 mm Tris–HCl (pH 9·0), 50 mm KCl, 0·1 % Triton X-100, 2·5 mm MgCl2, 0·1 mm dNTPs, 2 % formamide, 0·2 mm primer, 1·5 units of Taq polymerase and double-distilled water. Initial denaturation was at 94 °C for 5 min, followed by 45 cycles of 30 s at 94 °C, 1 min at 50–54 °C (depending on different primers), 2 min at 72 °C, and a final 7-min extension at 72 °C. PCR reactions were carried out in a PTC-200 thermal cycler (MJ Research, USA). PCR products were separated by gel electrophoresis on 2·0 % agarose gels in 0·5× TBE buffer and visualized using ethidium bromide staining (0·1 mg mL−1). Negative controls (no template DNA) were also included in each PCR. To ensure ISSR reproducibility, most PCR reactions were repeated twice. DNA fragments were visualized by LabWorks Version 3.0 image analysis software for gel documentation (UVP, Upland, CA 91786, USA).
Data analysis
Only bands that could be unambiguously scored across all the sampled populations were used in this study. ISSR profiles were scored for each individual as discrete characters (presence or absence of the amplified products). Genetic diversity was measured by the percentage of polymorphic bands (P), which was calculated by dividing the number of polymorphic bands at population and species levels by the total number of bands surveyed. Shannon indices of diversity, namely both the total diversity (Hsp) and the intra-population diversity (Hpop), were also calculated using the computer program POPGENE 1.31 (Yeh et al., 1999). The non-parametric Analysis of Molecular Variance (AMOVA) program v. 1.55 (Excoffier et al., 1992) was used to describe the genetic structure between populations. The significance of this F-statistic analogue was tested by 1000 random permutations.
In order to overcome potential problems caused by the dominance of ISSR markers, and to obtain an accurate estimate of FST, a Bayesian program, Hickory (0·8) (Holsinger et al., 2002), was also used to estimate parameters related to genetic structure (θB). The Bayesian method does not assume that genotypes are in Hardy–Weinberg proportions within populations, and it does not treat multilocus ISSR phenotypes as haplotypes. It takes full advantage of the information provided by dominant markers, allowing us to incorporate uncertainty about the magnitude of the within-population inbreeding coefficient into estimates of FST (Holsinger et al., 2002; Holsinger and Wallace, 2004). We used default values for burn-in (50 000), sampling (250 000) and thinning (50). The ‘f-free’ analysis option in Hickory was used because it avoids any potential bias that could be created by unreasonable estimates of the FIS analogue, f.
Gene flow was estimated indirectly using the formula: Nm = 0·25(1 − FST)/FST, where θB is used for the estimator of FST. In order to test for a correlation between pair-wise genetic distances (ΦST) and geographical distances (in km) between populations, a Mantel test was performed using Tools for Population Genetic Analysis (TFPGA; Miller, 1997) (computing 999 permutations).
RESULTS
The eleven primers produced 154 bands in the two species studied, among them 99 bands from A. mongolicus and 112 from A. nanus. The comparison of banding patterns between A. mongolicus and A. nanus indicated that 63 % of the bands were unique to each species. Forty-two bands were present only in A. mongolicus, whereas 55 bands were specific to A. nanus.
Ammopiptanthus mongolicus
In A. mongolicus, 39 of the 99 clear and reproducible bands (39·39 %) were polymorphic in at least one population. The average percentage of polymorphic loci (P) across populations was 18·55 %. The average Shannon's indices were 0·106 at the population level (Hpop) and 0·1832 at the species level (Hsp) (Tables 2 and 3).
Table 2.
Parameters of genetic variability
| Population |
Hpop |
P (%) |
||
|---|---|---|---|---|
| A. mongolicus | ||||
| Shapotou | 0·123 (±0·243) | 22·22 | ||
| Rujigou (1) | 0·112 (±0·235) | 19·19 | ||
| Rujigou (2) | 0·117 (±0·246) | 19·19 | ||
| Qianlishan | 0·123 (±0·248) | 20·20 | ||
| Xindi | 0·110 (±0·237) | 18·18 | ||
| Yikebulage | 0·091 (±0·216) | 16·16 | ||
| Taositu | 0·110 (±0·237) | 18·18 | ||
| Muoshigou | 0·087 (±0·215) | 15·15 | ||
| Balagong | 0·081 (±0·199) | 15·15 | ||
| Dengkou (1) | 0·104 (±0·214) | 21·21 | ||
| Dengkou (2) | 0·110 (±0·238) | 19·19 | ||
| Mean | 0·106 (±0·014) | 18·55 (±2·32) | ||
| A. nanus | ||||
| Biaoertuokuoyi | 0·113 (±0·239) | 18·75 | ||
| Bacundaban | 0·041 (±0·152) | 7·14 | ||
| Ohsalur | 0·050 (±0·172) | 8·04 | ||
| Xiaoerbulake | 0·063 (±0·179) | 11·61 | ||
| Kangsu | 0·061 (±0·179) | 10·71 | ||
| Baykurt | 0·058 (±0·178) | 9·82 | ||
| Tielieke | 0·106 (±0·229) | 18·75 | ||
| Mean | 0·070 (±0·028) | 12·12 (±4·78) | ||
Hpop, Shannon's index of gene diversity; P, percentage of polymorphic loci. Standard deviations are shown in parentheses.
Table 3.
Summary of genetic variability and partitioning of diversity
|
A. mongolicus |
A. nanus |
|
|---|---|---|
| P | 39·39 % | 25·89 % |
| HSP | 0·1832 | 0·1026 |
| ΦST | 37·43 % | 21·62 % |
| θB | 0·3744 | 0·2369 |
| Gene flow (Nm) | 0·418 | 0·805 |
P, percentage of polymorphic loci; Hsp, Shannon index of gene diversity at the species level; ΦST, genetic differentiation between populations estimated by using AMOVA; θB: genetic differentiation between populations estimated by using Hickory analysis; Nm, estimated gene flow.
Results of the Hickory analysis gave a θB value of 0·3744 (Table 3). The Nm estimate of 0·418 suggests that genetic exchange between populations is limited. The AMOVA analysis provided additional evidence for the genetic structure indicated by these parameters. Highly significant (P < 0·001) genetic differences were detected between regions, between populations (within regions), and between individuals (within both populations and regions) (Table 4). Of the total molecular variance, 24·93 % was attributable to regional divergence, 20·68 % to population differences within regions, and 54·39 % to individual differences within populations. When the total variance was partitioned without considering the regional distribution of the populations, 37·43 % was attributable to populations (ΦST) and 62·57 % to individual differences within populations. Likewise, analysis of the regional distribution of the variance suggested that 32·44 % of the total variance was due to diversity between regions and 67·56 % to individual differences within regions (Table 4). Within Inner Mongolia alone, 28·4 % was attributable to populations (ΦST).
Table 4.
Analysis of molecular variance (AMOVA) for 251 individuals in 11 populations of A. mongolicus and 168 individuals in seven populations of A. nanus
| Species |
Source of variation |
d.f. |
Sum of squares |
Mean squares |
Variance components |
% total variance |
P-value |
|---|---|---|---|---|---|---|---|
| A. mongolicus | Nested analysis | ||||||
| Among regions | 2 | 213·66 | 106·83 | 1·42 | 24·93 | <0·001 | |
| Among popns within region | 8 | 452·22 | 29·82 | 1·17 | 20·68 | <0·001 | |
| Within popns | 240 | 741·10 | 3·09 | 3·09 | 54·89 | <0·001 | |
| Analysis among popns | |||||||
| Among popns | 10 | 452·22 | 45·22 | 1·85 | 37·43 | <0·001 | |
| Within popns | 240 | 741·10 | 3·089 | 3·09 | 62·57 | <0·001 | |
| Analysis among regions | |||||||
| Among regions | 2 | 213·66 | 106·83 | 1·89 | 32·44 | <0·001 | |
| Within regions | 248 | 979·66 | 3·95 | 3·95 | 67·56 | <0·001 | |
| Analysis among popns within Inner Mongolia region | |||||||
| Among popns | 7 | 206·09 | 29·44 | 1·18 | 28·4 | <0·001 | |
| Within popns | 172 | 511·32 | 2·97 | 2·97 | 71·6 | <0·001 | |
| A. nanus | Among popns | 6 | 71·047 | 11·841 | 0·429 | 21·62 | <0·001 |
| Within popns | 161 | 250·573 | 1·556 | 1·556 | 78·38 | <0·001 |
P-values are the probabilities of having a more extreme variance component than the observed values by chance alone. Probabilities were calculated by 1000 random permutations of individuals across populations.
The Mantel test shows that there is a positive correlation between geographical distance and genetic distance in A. mongolicus (r = 0·6478, P = 0·001). The strong genetic differentiation in A. mongolicus suggests that the three regions examined are isolated and gene flow between the three regions is limited.
Ammopiptanthus nanus
Twenty-nine of the 112 clear and reproducible bands (25·89 %) were polymorphic in at least one population. The average percentage of polymorphic loci (P) across populations was 12·12 %. The Shannon's indices were 0·070 at the population level (Hpop) and 0·1026 at the species level (Hsp) (Tables 2, 3). Thus, A. nanus showed lower levels of genetic diversity than A. mongolicus.
The results of the Hickory analysis gave a θB value of 0·2369 (Table 3). AMOVA showed that approximately 21·62 % of the genetic variation was found between populations (ΦST). The gene flow (Nm) between populations was 0·805 (Table 3). The Mantel test detected no geographical tendency in the distribution of the genetic distance in A. nanus (r = 0·1544, P = 0·3020).
DISCUSSION
Genetic differentiation between A. mongolicus and A. nanus
Fifty-seven of the 154 bands were shared by the two studied species, indicating a common evolutionary history or homoplasy, while the remaining 97 bands reflect divergence between species. If one species was derived from the other, producing a progenitor–derivative pair, and this was associated with a reduction in effective population size, we would expect to observe only a subset of alleles in the derived species (Gemmill et al., 1998). The fact that A. mongolicus and A. nanus harbour 42 and 55 species-specific bands, respectively, does not support the hypothesis of a progenitor–derivative relationship between these two species. An alternative hypothesis is that differentiation of the two species of Ammopiptanthus might be due to a possible vicariance evolutionary event from a single common ancestor through the fragmentation of its natural distribution range. About 63 % of bands (97 of 154) that are specific to one of the two species strongly suggest that these two morphologically similar species are genetically very distinct.
The geographical barrier between these two species appears to have arisen as early as the early Miocene, during the aridification and formation of deserts in central Asia induced by the development of the Arctic ice-sheet and uplift of the Himalayan–Tibetan Plateau (Harrison et al., 1998; Guo et al., 1999). Desert vegetation that was better adapted to the lower temperature and more arid conditions gradually replaced the broadleaf forest that had developed in the warmer and moister global climate of the early Paleocene and mid-Eocene (∼65–45 Ma). Evidence from fossil pollen suggests that high percentages of grasses and herbs were present, and that grassy savannah or even desert conditions occurred as early as the mid-Miocene in the Gobi desert region of China (∼18–13 Ma) (Willis and McElwain, 2002). As an element of the ancient evergreen broadleaf forest, it has been speculated that the ancestral species of Ammopiptanthus was widely and continuously distributed from the eastern border of the Pamir Plateau to the Gobi desert during the Tertiary period (Liu, 1995). Desertification in the Ala-shan Plateau since the Miocene (Guo et al., 2002) and in the Tarim Basin since the early Pleistocene (Yan et al., 2000) may have been the main factors that caused the fragmentation of the continuous distribution of this ancestral Ammopiptanthus. Genetic differentiation of the two species probably occurred after the geographic barrier formed. The significant genetic difference between the two species is likely to be the result of long isolation.
Genetic diversity
Low levels of polymorphism within populations were revealed in both Ammopiptanthus species by ISSR markers. Their variation was similar with that of relict Cycas guizhouensis (14·21 %) (Xiao et al., 2004) and of the clonal plant Psammochloa villosa (15 %) (Li and Ge, 2001). The genetic diversity of a species or population is due to the combined effects of genealogical history and evolutionary processes (Comes and Kadereit, 1998). Although low levels of diversity have often been reported for rare and endemic plant species, such as Dendroseris spp. (Esselman et al., 2000), numerous allozyme studies and increasing numbers of cpDNA and mtDNA studies now provide substantial evidence that refugial plant populations can harbour higher levels of genetic diversity than their likely descendant populations (reviewed in Comes and Kadereit, 1998). Because deserts in the northern hemisphere are mostly located south of latitude 40°N, they have not been dramatically influenced by ice sheets or mountain glaciers (Brown and Gibson, 1983). In the Chinese desert region, no pollen evidence has been found to suggest that glaciation occurred during the Quaternary (Li, 1998). In addition, the western area of Inner Mongolia is characterized by high endemism, possibly because it was a Pleistocene refugium (Zhao, 1997). The extant distribution of Ammopiptanthus may indicate refugia for ancient broadleaf forest species and, if so, high ISSR diversity might be expected. Highly polymorphic ISSRs have been found in Tetraena mongolica (P = 48·1 %), which is also endemic to western Inner Mongolia and co-occurs with A. mongolicus (Ge et al., 2003). In contrast to the expectation of high genetic diversity, A. mongolicus and A. nanus revealed low levels of genetic variability at both the population and species levels.
The low levels of genetic diversity harboured in these two species may be due to the following four possible reasons. Firstly, they may be due to low inherent variability of the ancestral species (see, for instance, Godt et al., 1997). Although high levels of genetic diversity are expected for refugial plant populations (as reviewed in Comes and Kadereit, 1998), A. mongolicus and A. nanus may originate from genetically depauperate populations. Secondly, inbreeding could be one of the major factors responsible for the low genetic variation within the populations of these Ammopiptanthus species. Ammopiptanthus mongolicus and A. nanus have been found to be self-compatible, but pollinator-dependent (unpublished observations, Ge et al.). Ammopiptanthus mongolicus and A. nanus have numerous flowers on a single inflorescence (10–16 flowers per inflorescence), which could facilitate pollinator-mediated self-pollination and geitonogamous pollination. The gravity-disseminated seeds and the insect-dispersed pollen of Ammopiptanthus may promote mating between individuals in close proximity within populations. Thirdly, the repeated decrease and increase of temperature in climatic oscillations during the Pleistocene may have caused the repeated enlarging and decreasing of populations, hence causing founder effects. This might partly account for the lower levels of variation within populations of A. mongolicus despite its relatively wide distribution, and generally lower levels of variation within A. nanus due to the more limited distribution of this species. Finally, the explosive increase in the human population and destructive utilization for firewood have caused a dramatic decline of these two species. These small and isolated populations were probably subjected to genetic drift that may have contributed to the lack of genetic diversity observed today. In A. nanus, we observed only two bands (1·7 %) with a frequency lower than 50 %, indicative of such stochastic processes.
The differences in genetic variability between A. mongolicus and A. nanus could be related to their geographical ranges. Gitzendanner and Soltis (2000) summarized the results of studies in widespread and restricted congeners, and found that genetic variation was significantly lower in the rare species than in the widespread species. Both Ammopiptanthus species have similar biological and ecological characteristics, so the higher level of genetic diversity detected in A. mongolicus as compared to A. nanus could be due to the more restricted geographical distribution of the latter.
Genetic structure
The genetic structure of plant populations reflects the interactions of various factors, including the long-term evolutionary history of the species (shifts in distribution, habitat fragmentation and population isolation), genetic drift, mating system, gene flow and selection (Schaal et al., 1998). Ammopiptanthus mongolicus shows more differentiation between populations than A. nanus (Table 3). This is probably due to differences in the geographic fragmentation of populations of these two species, because the distribution of A. mongolicus is more fragmented than that of A. nanus. Ammopiptanthus nanus populations are generally in closer proximity to each other (5–85 km apart) than are A. mongolicus populations (3–362 km apart). It has been demonstrated that the level of genetic heterogeneity among populations is greater in species with geographically disjunct populations than in species with more continuous distributions (Hamrick and Godt, 1996; Premoli et al., 2001). Thus, this may account for the high between-region proportion of the total molecular variance found in A. mongolicus (24·93 % based on AMOVA). Within the Inner Mongolia region, the genetic differentiation between the populations of A. mongolicus was similar to that of A. nanus (28·4 % vs. 21·62 % according to AMOVA). Within Inner Mongolia alone, 28·4 % was attributable to populations (ΦST), indicating significant genetic differentiation.
Generally, the breeding system of flowering plant species greatly affects population genetic differentiation (Hamrick and Godt, 1989). Estimates of genetic differentiation between populations for outcrossing species based on AMOVA derived by analysing RAPD markers have usually been <28 %. For inbred species, estimates of interpopulation genetic variation have usually been >70 % (reviewed in Nybom and Bartish, 2000). The genetic variation between populations of A. nanus (AMOVA = 21·62 %) and between populations of A. mongolicus within the Inner Mongolia region (AMOVA = 28·4 %) are consistent with those observed in outcrossing species (Hamrick and Godt, 1989). Our preliminary field observations confirm that A. mongolicus and A. nanus are insect-pollinated species. Wasps (Vesta sp.) and honeybees (Apis sp.) were often found visiting their flowers (unpublished observations, Ge et al.).
The fact that estimates of Nm were <1 for both species suggests that gene flow between populations is insufficient to counter the effects of random drift (Real, 1994). In this study, the relatively high genetic differentiation and low levels of gene flow detected (Nm = 0·418 and 0·805 for A. mongolicus and A. nanus, respectively) strongly indicate that genetic drift has greatly affected the genetic composition of individual populations. Although there is a significant relationship between geographic and genetic distance for the 11 populations of A. mongolicus, some pairs of populations with low genetic distances are not geographic neighbours. This may be partly due to the effects of genetic drift on small populations. The lack of correlation between genetic distances and geographic distances in A. nanus suggests that there is low genetic flux between populations of this species, and that stochastic differentiation due to genetic drift has occurred. Between-population gene flow is limited by pollen and seed dispersal. Being an insect-pollinated plant, pollen dispersal is limited by the short flight ranges of the insects. Moreover, seed dispersal is not likely to be very efficient, given the weight of seeds typical for these two species (approx. 50 mg and 30 mg for A. mongolicus and A. nanus, respectively, effectively excluding wind transport) and their lack of dispersal structures. The limited seed dispersal contributes to the restricted gene flow and increases the probability that individuals in close physical proximity mated with one another. Both effects will promote inter-population differentiation.
The effects of desertification on genetic diversity
This study represents a unique opportunity to examine the effects of desertification on genetic diversity. Desertification has dramatic and comprehensive affects on the genetic diversity of plants living in this region, both at the specific and intraspecific levels. Lack of water and extreme temperature fluctuations make the desert a very harsh environment where few plant species can survive. Following the desertification in central Asia, most ancient Tertiary plant species in this region became extinct and only a few relict species survived in a limited number of refugia. The genus Ammopiptanthus represents a typical example. As mentioned above, desertification in central Asia and subsequent isolation led to the differentiation of A. mongolicus and A. nanus. At the intraspecific level, the desertification dramatically diminished the distribution and fragmented the once continuous populations. The harsh, and also homogeneous, environment imposes an extremely strong selection pressure on the plants. Any maladapted genotype could be eliminated rapidly. The genetic structure of extant populations therefore might reflect the cumulative results of this effect. As discussed above, the observed low genetic diversity in the two Ammopiptanthus species was suggested to be the result of low inherent variability, increased inbreeding, founder effects and genetic drift. Desertification might have played a fundamental role in shaping and linking these effects.
Conclusions and implications for conservation
The results reported here reveal distinct genetic differentiation between A. mongolicus and A. nanus. This differentiation might be due to a possible vicariant evolutionary event from a single common ancestor through the fragmentation of its natural distribution range. Both species displayed low levels of ISSR genetic variation and moderate genetic differentiation, but A. mongolicus showed higher genetic variation and differentiation than A. nanus.
The ultimate goal of conservation is to ensure the continuous survival of populations and to maintain their evolutionary potential. Information on current levels of genetic diversity of threatened and endangered species is essential for designing appropriate strategies for conservation (Falk and Holsinger, 1991). According to the results of this study, different strategies should be adopted for both the in situ and ex situ conservation of genetic diversity in the two Ammopiptanthus species. For A. mongolicus, the moderate genetic differentiation found among the regions, and the similarity between populations within each region, indicate that the most effective strategy for preserving its genetic variation would be to conserve a large number of individuals in a large population within each of as many regions as possible. For A. nanus, because of its highly uniform genetic make-up, any of the populations surveyed could represent a large proportion of the genetic variation within the species. Therefore, the most effective strategy for ex situ conservation of this species would be to sample a larger number of plants from one or two populations rather than to collect smaller samples from many different sites. The Biaoertuokuoyi and Tielieke populations harbour relatively high amounts of the genetic diversity within A. nanus and these two populations should therefore be a priority for in situ conservation action. Finally, because the level of genetic variation in selectively neutral marker loci is mainly determined by mutation and genetic drift (Kimura, 1983), the level of variation detected for marker loci, such as ISSR, will not necessarily be a direct reflection of the level of variation that determines adaptability or individual fitness (Booy et al., 2000). Therefore, samples from different habitats, such as from different mountain slopes and sandy deserts, should be considered in conservation.
Acknowledgments
This work was supported by the Key Project of the Chinese Academy of Sciences (CAS) (KSCX2-SW-104), the National Natural Foundation of China (Grant No. 30370282) and the Field Frontiers Project of the CAS Knowledge Innovation Program (Director Foundation of SCBG). We are grateful to Dr Gang Hao for his assistance and advice in the lab, and Dr Tzen-Yuh Chiang for helpful comments on previous drafts of this manuscript, Mr Yu-Guang Hao provided assistance in the field collecting samples; Ms Yun-Xiao Liu helped with map illustration. The authors also thank Dr Mitchell McGlaughlin and an anonymous reviewer for their valuable suggestions and comments on the manuscript.
LITERATURE CITED
- Barrett SC, Kohn JR. 1991. Genetic and evolutionary consequences of small population size in plants: implications for conservation. In: Falk DA, Holsinger KE, eds. Genetics and conservation of rare plants. New York: Oxford University Press, 3–30. [Google Scholar]
- Booy G, Hendriks RJJ, Smulders MJM, Van Groenendael JM, Vosman B. 2000. Genetic diversity and the survival of populations. Plant Biology 2: 379–395. [Google Scholar]
- Brown JH, Gibson AC. 1983.Quaternary events. Biogeography. St. Louis, MO: The C.V. Mosby Company, 412–435. [Google Scholar]
- Cheng SH. 1959.Ammopiptanthus Cheng f. A new genus of Leguminosae from central Asia. Journal of Botany USSR 44: 1381–1386. [Google Scholar]
- Comes HP, Kadereit JW. 1998. The effect of Quaternary climatic changes on plant distribution and evolution. Trends in Plant Science 3: 432–438. [Google Scholar]
- Culley TM, Wolfe AD. 2001. Population genetic structure of the cleistogamous plant species Viola pubescens Aiton (Violaceae), as indicated by allozyme and ISSR molecular markers. Heredity 86: 545–556. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. 1987. A rapid isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bulletin 19: 11–15. [Google Scholar]
- Esselman EJ, Crawford DJ, Brauner S, Stuessy TF, Anderson GJ, Silva OM. 2000. RAPD marker diversity within and divergence among species of Dendroseris (Asteraceae: Lactuceae). American Journal of Botany 87: 591–596. [PubMed] [Google Scholar]
- Esselman EJ, Jianqiang L, Crawford DJ, Windus JL, Wolfe AD. 1999. Clonal diversity in the rare Calamagrostis porteri ssp. insperata (Poaceae): comparative results for allozymes and random amplified polymorphic DNA (RAPD) and intersimple sequence repeat (ISSR) markers. Molecular Ecology 8: 443–451. [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM. 1992. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondria DNA restriction sites. Genetics 131: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DA, Holsinger KE. 1991.Genetics and conservation of rare plants. New York: Oxford University Press. [Google Scholar]
- FAO. 1997.FAO Environment and Energy Papers-15: Drylands development and combating desertification: Bibliographic study of experiences in China. Rome: Information Division, Food and Agriculture Organization of the United Nations. [Google Scholar]
- Fu LG. 1989.The rare and endangered plants in China. Shanghai: Shanghai Education Press, 183. [Google Scholar]
- Ge XJ, Yu Y, Zhao NX, Chen HS, Qi WQ. 2003. Genetic variation in the endangered Inner Mongolia endemic shrub Tetraena mongolica Maxim. (Zygophyllaceae). Biological Conservation 111: 427–434. [Google Scholar]
- Gemmill CEC, Ranker TA, Ragone D, Perlman SP, Wood KR. 1998. Conservation genetics of the endangered endemic Hawaiian genus Brighamia (Campanulaceae). American Journal of Botany 85: 528–539. [PubMed] [Google Scholar]
- Geng BY, Tao JR, Xie GP. 2001. Early Tertiary fossil plants and paleoclimate of Lanzhou Basin. Acta Phytaxonomica Sinica 39: 105–115. [Google Scholar]
- Gitzendanner MA, Soltis PS. 2000. Patterns of genetic variation in rare and widespread plant congeners. American Journal of Botany 87: 783–792. [PubMed] [Google Scholar]
- Godt MJW, Walker J, Hamrick JL. 1997. Genetic diversity in the endangered lily Harperocallis flava and a close relative, Tofieldia racemosa Conservation Biology 11: 361–366. [Google Scholar]
- Guo ZT, Peng SZ, Hao QZ, Chen XH, Liu TS. 1999. Late Tertiary development of aridification in Northwestern China: link with the arctic ice-sheet formation and Tibetan uplifts. Quaternary Science 19: 556–567. [Google Scholar]
- Guo ZT, Ruddiman WF, Hao QZ, Wu HB, Qiao YS, Zhu RX, Peng SZ, Wei JJ, Yuan BY, Liu TS. 2002. Onset of Asian desertification by 22 Myr ago inferred from loess deposits in China. Nature 416: 159–163. [DOI] [PubMed] [Google Scholar]
- Hamrick JL, Godt MJW. 1989. Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weir BS, eds. Plant population genetics, breeding and genetic resources. Sunderland, MA: Sinauer Associates, 43–63. [Google Scholar]
- Hamrick JL, Godt MJW. 1996. Conservation genetics of endemic plant species. In: Avise JC, Hamrick JL, eds. Conservation genetics, case histories from nature. New York: Chapman and Hall, 281–304. [Google Scholar]
- Harrison TM, Yin A, Ryerson FJ. 1998. Orographic evolution of the Himalaya and Tibetan plateau. In: Crowley TJ, Burke KC, eds. Tectonic boundary conditions for climate reconstruction. Oxford, UK: Oxford University Press, 39–73. [Google Scholar]
- Helldén U. 1991. Desertification—time for an assessment? Ambio 20: 372–383. [Google Scholar]
- Holsinger KE, Lewis PO, Dey DK. 2002. A Bayesian approach to inferring population structure from dominant markers. Molecular Ecology 11: 1157–1164. [DOI] [PubMed] [Google Scholar]
- Holsinger KE, Wallace LE. 2004. Bayesian approaches for the analysis of population genetic structure: an example from Plantanthera leucophaea (Orchiadaceae). Molecular Ecology 13: 887–894. [DOI] [PubMed] [Google Scholar]
- Kimura M. 1983.The neutral theory of molecular evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Li A, Ge S. 2001. Genetic variation and clonal diversity of Psammochloa villosa (Poaceae) detected by ISSR markers. Annals of Botany 87: 585–590. [Google Scholar]
- Li WC. 1998.The Chinese Quaternary vegetation and environment. Beijing: Science Press. [Google Scholar]
- Liu GH. 1998. Study on the endangered reasons of Ammopiptanthus mongolicus in the desert of Alashan. Bulletin of Botanical Research 18: 341–345. [Google Scholar]
- Liu JQ, Qiu MX. 1982. Ecological, physiological and anatomical traits of Ammopiptanthus mongolicus grown in desert of China. Acta Botanica Sinica 24: 568–573. [Google Scholar]
- Liu JQ, Qiu MX, Yang K, Shi QH. 1995. Studies on the plant community of Ammopiptanthus mongolicus Journal of Desert Research 15: 109–115. [Google Scholar]
- Liu YX. 1995. A study on origin and formation of the Chinese desert floras. Acta Phytaxonomica Sinica 3: 131–143. [Google Scholar]
- Miller MP. 1997.Tools for population genetic analysis. Version 1.3. Department of Biological Sciences, Northern Arizona University, Flagstaff. [Google Scholar]
- Nybom H, Bartish IV. 2000. Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspectives in Plant Ecology, Evolution and Systematics 3/2: 93–114. [Google Scholar]
- Pan BR, Huang SP. 1993. Cytological study of the genus Ammopipthanthus Act Botanica Sinica 35: 314–317. [Google Scholar]
- Pan BR, Yu QL, Yan C. 1992. Study for the ecological environment and vulnerable reasons of the Ammopiptanthus nanus Acta Phytecologia et Geobotanica Sinica 16: 276–282. [Google Scholar]
- Premoli AC, Souto CP, Allnutt TR, Newton AC. 2001. Effects of population disjunction on isozyme variation in the widespread Pilgerodendron uviferum Heredity 87: 337–343. [DOI] [PubMed] [Google Scholar]
- Real LA. 1994.Ecological genetics. Princeton, NJ: Princeton University Press. [Google Scholar]
- Schaal BA, Hayworth DA, Olsen KM, Rauscher JT, Smith WA. 1998. Phylogeographic studies in plants: problems and prospects. Molecular Ecology 7: 465–474. [Google Scholar]
- Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Research 18: 6531–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis KJ, McElwain JC. 2002. The evolution of plants. Oxford, UK: Oxford University Press. [Google Scholar]
- Wolfe AD, Liston A. 1998. Contributions of PCR-based methods to plant systematics and evolutionary biology. In: Soltis DE, Soltis PS, Doyle JJ, eds. Plant molecular systematics II. Boston, MA: Kluwer, 43–86. [Google Scholar]
- Xiao LQ, Ge XJ, Gong X, Hao G, Zheng SX. 2004. ISSR variation in the endemic and endangered plant Cycas guizhouensis (Cycaceae). Annals of Botany 94: 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, An L, Feng H, Wang X, Li X. 2002. The seasonal effects of water stress on Ammopiptanthus mongolicus in a desert environment. Journal of Arid Environment 51: 437–447. [Google Scholar]
- Yan S, Mu GJ, Xu YQ. 2000. Quaternary environmental evolution of the Lop Nur region, NW China. Acta Micropalaeontologica Sinica 17: 165–169. [Google Scholar]
- Yeh FC, Yang R, Boyle T. 1999. POPGENE. Microsoft Windows-based freeware for population genetic analysis. Release 1.31. University of Alberta, Edmonton, Canada. [Google Scholar]
- Yin LK, Wang Y. 1993. Preliminary study on the phonological characteristics of Ammopiptanthus during flowering period. Chinese Bulletin of Botany 10: 54–56. [Google Scholar]
- Zhao YZ. 1997. Endemic genera and their basic characteristics of the Mongolian Planteau plants. Acta Scientiarum Naturalium Universitatis NeiMongolicum 28: 547–552. [Google Scholar]


