Abstract
• Background and Aims Lepanthes is one of the largest angiosperm genera (>800 species). Their non-rewarding, tiny and colourful flowers are structurally complex. Their pollination mechanism has hitherto remained unknown, but has been subject of ample speculation; the function of the minuscule labellum appendix is especially puzzling. Here, the pollination of L. glicensteinii by sexually deceived male fungus gnats is described and illustrated.
• Methods Visitors to flowers of L. glicensteinii were photographed and their behaviour documented; some were captured for identification. Occasional visits to flowers of L. helleri, L. stenorhyncha and L. turialvae were also observed. Structural features of flowers and pollinators were studied with SEM.
• Key Results Sexually aroused males of the fungus gnat Bradysia floribunda (Diptera: Sciaridae) were the only visitors and pollinators of L. glicensteinii. The initial long-distance attractant seems to be olfactory. Upon finding a flower, the fly curls his abdomen under the labellum and grabs the appendix with his genitalic claspers, then dismounts the flower and turns around to face away from it. The pollinarium attaches to his abdomen during this pivoting manoeuvre. Pollinia are deposited on the stigma during a subsequent flower visit. The flies appear to ejaculate during pseudocopulation. The visitors of L. helleri, L. stenorhyncha and L. turialvae are different species of fungus gnats that display a similar behaviour.
• Conclusions Lepanthes glicensteinii has genitalic pseudocopulatory pollination, the first case reported outside of the Australian orchid genus Cryptostylis. Since most species of Lepanthes have the same unusual flower structure, it is predicted that pollination by sexual deception is prevalent in the genus. Several morphological and phenological traits in Lepanthes seem well suited for exploiting male fungus gnats as pollinators. Correspondingly, some demographic trends common in Lepanthes are consistent with patterns of male sciarid behaviour.
Keywords: Bradysia floribunda, Lepanthes glicensteinii, Lepanthes helleri, Lepanthes stenorhyncha, Lepanthes turialvae, mimicry, Orchidaceae, Pleurothallidinae, pollination, pseudocopulation, Sciaridae, sexual deception
INTRODUCTION
Orchids display a vast array of floral morphologies and pollination mechanisms, unparalleled in any other angiosperm family (Darwin, 1877; van der Pijl and Dodson, 1966; Dressler, 1981; Arditti, 1992; Endress, 1994; van der Cingel, 1995, 2001; Proctor et al., 1996). Among the most fascinating pollination syndromes is sexual deception, also known as pseudocopulation, in which the flower lures male insects by mimicking the sexual pheromones and appearance of their females. Although seemingly unique to the orchids, this syndrome has evolved independently in several unrelated groups within the family (Dressler, 1981; Singer et al., 2004; for a possible case outside of the Orchidaceae see Rudall et al., 2002).
In most cases of sexual deception, pollination occurs when the insect attempts (unsuccessfully) to copulate with the flower and brushes against the column (gynostemium) (e.g. Borg-Karlson, 1990; Singer et al., 2004). In a few Australian orchid genera, the labellum (the specialized median petal of orchids) imitates the wingless female insect and is attached to the rest of the flower by a flexible hinge; pollination occurs when the male insect tries to fly away with the female decoy and swings against the column (e.g. Peakall, 1990; Alcock, 2000). In other cases, the male insect inspects the flower looking for a female and falls into a pitfall trap (Trigonidium, Singer, 2002) or is imprisoned by an active-motion mechanism (Pterostylis, van der Cingel, 2001; but see Discussion) that forces the insect through a tight escape passage against the column. In all these cases, pollination takes place during the pre-copulatory behavioural phases of the insect's mating sequence (rapprochement and courtship, sensu Alexander et al., 1997).
In the most extreme case of pollination by sexual deception, here termed genitalic pseudocopulation, pollination takes place during the actual copulation phase of the mating sequence (sensu Alexander et al., 1997). That is, successful genitalic coupling of the male insect with the flower is a necessary step for pollen transfer. Heretofore, genitalic pseudocopulation was known only in the Australian orchid genus Cryptostylis (see Discussion). Here we describe a new case of pollination by genitalic pseudocopulation in orchids, this time in the genus Lepanthes.
More than 800 species of Lepanthes exist throughout the Neotropics (Luer, 1996, 2003; Salazar-Chávez and Soto-Arenas, 1996); they are small epiphytes, particularly diverse in cloud forests. The tiny and brightly coloured flowers have a complex but stereotyped structure: the labellum is transversally divided in two blades that curve over and surround the column (Fig. 1A). Most species have a diminutive structure, the appendix, at the junction of the blades of the labellum (Fig. 1D). As in most other orchids, the pollen is aggregated in a removable pair of masses (pollinia) with a sticky gland at its tip, the viscidium (together called a pollinarium). The flowers do not offer any legitimate rewards to potential pollinators. This unique suite of floral features has motivated ample speculation about the pollination mechanism in the genus (Dod, 1986; Christensen, 1994; Endress, 1994; Luer, 1996; Salazar-Chávez and Soto-Arenas, 1996; Tremblay, 1997a; Behar, 1999; Archila, 2001; C.H. Dodson, Missouri Botanical Garden, USA, pers. comm.), but until now there have been no corroborated observations (see Discussion).
Fig. 1.

Flower features of Lepanthes glicensteinii and pollination by male Bradysia floribunda flies. (A) Front view of flower. (B) Fly on top of flower; note closed gonostili at the tip of his abdomen. (C) The same fly with gonostili wide open bending his abdomen under the blades of the labellum. (D, E) Fly probing for the appendix, located under the labellum blades, lateral view. Scale bar = 2 mm. (F) Fly after securing the appendix with his gonostili; this flower is lying on its side against the leaf, with the column pointing toward the photographer. (G) The same fly pivoting around with his gonostili still securing the appendix. (H) Fly after completing the pivoting manoeuvre, assuming the ‘tail-to-tail’ position. (I) Fly ‘tail-to-tail’ with the flower; note column pressed against the dorsal part of his abdomen. (J) Fly ‘tail-to-tail’ with flower, with photographer's thumb for scale. (K) Fly probing for the appendix under the blades of the labellum, with pollinaria (bright yellow) attached to the ventral part of his abdomen. (L) Fly visiting flower with pollinaria attached to the dorsal part of his abdomen. Abbreviations: a, appendix; c, column; l, labellum blade; p, petal.
MATERIALS AND METHODS
Observations were made in the Monteverde Orchid Garden, located in the rural community of Cerro Plano, in Monteverde, Puntarenas province, Costa Rica (10°19′N, 84°49′W; 1400 m elevation). This site is a private display garden where approximately 200 orchid species native to the Monteverde area (including 20 species of Lepanthes) are cultivated in semi-natural conditions. Here, pollinator visits to flowers of Lepanthes were initially noticed by one of the authors (GB).
Detailed observations were performed on one plant of Lepanthes glicensteinii Luer in March–April 1999, and at another plant of the same species in May–July 2002 (only a single plant of this species was available on each occasion), between 0730–1800 h for 10 d, for a total of 95 h. Many more flower visits were observed, but not quantified, before and after this period. Both plants were collected in a nearby cloud forest at 1600 m elevation. Lepanthes glicensteinii is a rare species endemic to central Costa Rica (Luer, 1987, 2003).
Floral visitors were observed and notes were taken on their behaviour. Photographs were made with a 35 mm film camera equipped with a 100 mm macro lens, a 150 mm bellows extension, a ring macro flash, focusing sliders and a remote-release cable, all mounted on a tripod in front of the plant. Some insects were collected with an aspirator, or directly from the flowers with a killing jar with cyanide.
Some captured insects were glued to paper mounts and air-dried; other insects and flowers of L. glicensteinii were preserved in 70 % ethanol at the study site. Preserved samples were fixed in a 2·5 % glutaraldehyde and 2 % paraformaldehyde solution in a 0·1 m sodium phosphate buffer (pH 7·4) and were rinsed with the buffer solution. The samples were post-fixed with 1 % osmium tetraoxide in the sodium phosphate buffer, rinsed with distilled water, dehydrated in an ethanol gradient (30 %–100 %), washed four times with terbutylic alcohol, and finally dried by sublimation (Sublimate Eiko ID-2, Japan). The samples were placed on aluminium bases, covered with 20 nm platinum (Ionic Cover Eiko IB-5, Japan), and observed with an Hitachi S-570 scanning electron microscope (SEM) with an acceleration voltage of 15 kV. Two flowers and two insects were examined with SEM.
Vouchers of L. glicensteinii were deposited at the University of Costa Rica Herbarium (USJ, Barboza s.n.). Insect vouchers were deposited at the Entomological Museum of the University of Costa Rica and the personal collection of Werner Mohrig (Greifswald, Germany).
RESULTS
Plant features
Like in most species of Lepanthes (Luer, 1986, 1996; Salazar-Chávez and Soto-Arenas, 1996), plants of L. glicensteinii are small caespitose epiphytes with a sympodial growth habit. Each stem has a single apical leaf; the inflorescences are congested racemes borne at the base of the leaf, held against its lower surface, so that the flowers always face down. At any one time there usually is a single active raceme per leaf, and the flowers are produced sequentially; thus only one flower is open at a time per raceme. Each raceme produces flowers continuously. At any given day, the plants studied had 1–6 open flowers.
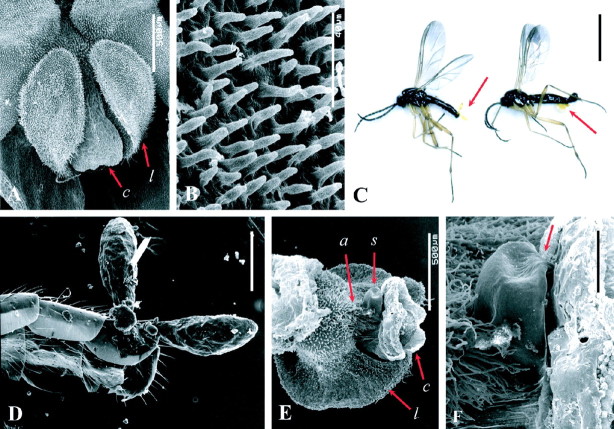
Individual flowers of L. glicensteinii measure 8–10 mm in length and 4–5 mm in width (Fig. 1A), with an appendix 0·4 mm long, and stay open for 2–4 d before withering and falling off. SEM examination of the flowers shows a dense cover of elongate papillae (20–40 µm long) on the labellum blades (Fig. 2A, B). The labellum appendix is densely covered with microscopic hairs.
Fig. 2.
Microstructural features of Lepanthes glicensteinii flowers, placement of pollinaria on Bradysia floribunda flies, and putative spermatophore. (A) SEM of central part of flower; scale bar = 500 μm. (B) Detail of labellum blade surface; scale bar = 40 μm. (C) Two flies with pollinaria of L. glicensteinii attached to their abdomens, indicated by the arrows. In the individual to the left the pollinarium is placed on the dorsal part of the abdomen, while in the individual to the right it is placed on the ventral part. Scale bar = 2 mm. (D) SEM of the terminal part of the abdomen of the first fly in (C), with pollinarium fastened to dorsal sclerite by viscidium. The gonostili in this fly were knocked off during specimen preparation, leaving a hole at the apex of the abdomen. Scale bar = 200 μm. (E) SEM of labellum and column of L. glicensteinii viewed from underside, with putative spermatophore of B. floribunda nested between the column and the appendix. Scale bar = 500 μm. (F) Close-up view of spermatophore, with narrow neck inserted in the stigma (arrow). Scale bar = 100 μm. Abbreviations: a, appendix; c, column; l, labellum blade; s, spermatophore.
For detailed information on the vegetative and floral morphology of L. glicensteinii see Luer (1987, 2003).
Pollinator behaviour and pollination mechanism
The only visitors to flowers of L. glicensteinii were males of a dark-winged fungus gnat (Diptera: Sciaridae) (Fig. 1B) that proved to be a new species; it was recently described as Bradysia floribunda (Mohrig, 2003). Throughout the paper, these insects will also be referred to simply as ‘flies’.
During our detailed observations (see Materials and Methods) 30 floral visits were witnessed. We also observed many additional visits to flowers of L. glicensteinii outside of this period. These additional visits were not quantified, but we estimate that we observed more than 100 of them. Visits occurred from 0900 h to 1600 h. The time of each visit was not noted, but there were noticeable peaks of visitation around 1000 and 1400 h.
The flies always approached the plant by flying from downwind, as if following a scent plume. Due to their diminutive size (3·5 mm body length), we noticed them only when they were within about 20 cm of the plant. They usually hovered around the plant for a few seconds before landing on the upper surface of a leaf. Immediately, they walked rapidly to the edge and to the underside of the leaf, constantly moving their antennae up and down in alternation. If there was not an open flower under that leaf, the flies rapidly walked down the stem and up another stem, instead of flying.
Once a flower was found, the fly walked frantically around it one to three times, fanning his wings. He positioned himself in front of the flower with his head toward the dorsal sepal, opening and closing his gonostili (genitalic claspers) several times, still fanning his wings. Then he mounted the flower labellum, curling his abdomen under the labellum blades and column with his gonostili wide open, and probed with them for the labellum appendix (Fig.1C–E). On many occasions the fly struggled for several minutes without securing the appendix, in which case he dismounted the labellum to rest for a few minutes before starting again. Other males grasped the labellum appendix quite promptly. After securing the appendix (Fig. 1F), the fly dismounted the labellum and turned around until facing the opposite direction (Fig. 1G, H), torsioning his abdomen 180° in the process. During this movement the abdomen usually made contact with the viscidium, and the pollinarium became attached to the sixth abdominal segment (Fig. 2C, D); on a few occasions the pollinarium attached itself to the abdomen when the fly was still probing for the appendix. The fly typically remained ‘tail-to-tail’ with the flower from 30 s to 3 min, but one individual spent over 20 min in this position (Fig. 1I, J). Slow peristaltic movements of the abdomen toward the labellum could be noticed at this stage. After this, the fly released the appendix by relaxing his gonostili, and stayed next to the flower grooming his antennae for about a minute before flying away without attempting to visit other flowers.
Some flies had pollinaria attached to their abdomens when first approaching a flower (Fig. 1K, L), indicating they had previously visited at least one other flower. Successful deposition of the pollinarium on the stigma was observed once, also during the pivoting manoeuvre. Further inspection of flowers revealed 83 % had their pollinaria removed, and 41 % had pollinaria deposited on their stigma (n = 24). However, neither of the two study plants of L. glicensteinii ever produced any fruits.
During the observations, plants of 13 additional species of Lepanthes were flowering at the study site. Bradysia floribunda was never seen to visit their flowers, indicating that they are attracted exclusively to flowers of L. glicensteinii. However, floral visits by males of different fungus gnat species were witnessed on a few occasions in L. turialvae, and once each in L. helleri and L. stenorhyncha. These species have the same flower structure as L. glicensteinii (see Introduction). In each case, the insects behaved in the same manner as described above, but they were easily disturbed when we came closer than 50 cm; their infrequency and shyness prevented our photographing them. Only one pollinator of L. stenorhyncha was captured, with pollinia adhered to the back of his abdomen; it is a different species of fungus gnat, still unidentified. In contrast, males of B. floribunda were almost oblivious of their surroundings when visiting flowers of L. glicensteinii, which permitted the manipulation of the plants after they assumed the tail-to-tail position (Fig. 1J).
In one of the two flowers examined with SEM, a semi-globular body was found nested between the labellum appendix and the column, with a narrow projection connected to the stigma (Fig. 2E, F). This object appears to be a fungus gnat spermatophore (compare with fig. 3 in Eberhard, 2001). This flower had been visited by at least one insect (observed in the field), which completed the sequence described above and removed the pollinarium.
DISCUSSION
Pollination mechanism and Sciarid mating behaviour
The exclusive attraction of male B. floribunda fungus gnats, their behaviour on the flowers and the absence of legitimate floral rewards indicate clearly that L. glicensteinii is pollinated by sexual deception, specifically by genitalic pseudocopulation. This is the first documented case of sexually deceptive pollination in the large Neotropical orchid subtribe Pleurothallidinae (>3800 species; Luer, 1986).
Sciarid flies (also known as dark-winged fungus gnats) comprise a diverse cosmopolitan group, sometimes treated as a subfamily of the true fungus gnats (Mycetophilidae) (Steffan, 1981; Menzel and Mohrig, 1997). Some species are economically important pests in plant and mushroom crops, and many show anomalous chromosome segregation patterns, which has prompted a significant amount of research on them (Harris et al., 1996). Their mating behaviour is rather stereotyped. Receptive females release pheromones to attract the slightly smaller males, usually from the underside of a leaf. Males fly following the pheromone signal and land close to the female, then approach her while fanning their wings and curling their abdomens underneath the thorax. The male positions himself directly behind the female and mounts her, and uses his gonostili to hold the female abdomen's terminalia. After coupling, the male dismounts and turns around to face the opposite direction, holding her abdomen with his gonostili and torsioning his own 180° along its axis. The couple remains attached in a tail-to-tail position for several minutes (see fig. 142b in McAlpine, 1981). The female usually remains passive, or occasionally flies away carrying the male behind her. An external spermatophore may be deposited by the male, with ducts connecting to the female's spermatheca. The male separates from the female and he might groom himself before he flies away (Binns, 1979; Alberts et al., 1981; Harris et al., 1996; Gotoh et al., 1999; Eberhard, 2001; Eberhard and Flores, 2002; Liu et al., 2002). This same sequence of events is mirrored in the visits of B. floribunda to flowers of L. glicensteinii (Figs 1A–L, 2E, F).
The male flies appear to confuse the flowers with their own females and are deceived into copulating with them, pollinating them in the process. The labellum appendix seems to mimic the abdominal terminalia of the pollinator's female, as it provides a tactile cue and an anchor point for the gonostili (see below). Genitalic coupling is necessary for male sciarids to start the pivoting manoeuvre that ends in the ‘tail-to-tail’ position with the female. It is precisely during the pivoting manoeuvre that the pollinarium is removed from the flower during pseudocopulation, and also any pollinaria previously attached to the pollinator is deposited on the stigma. Normally the pollinarium becomes attached to the dorsal part of the abdomen (while the fly is in the tail-to-tail position), but sometimes it attaches to the ventral part (Fig. 2C) or even to one of the sides.
We did not look for changes in size or orientation of the pollinia after removal from the anther, but such hygrometric changes are common in orchids (Darwin, 1877; Dressler, 1981; Arditti, 1992; Singer, 2002). Stenzel (2000) noted rapid dehydration and formation of furrows in pollinia of Lepanthes, which suggest that changes in orientation in relation to the viscidium are likely to occur.
It is notable that some flies spent long periods in the tail-to-tail position with the flower. Rhythmic squeezing by the male's gonostili during this stage as reported by Eberhard (2001) was not noted, but the camera magnification and the available illumination were not appropriate for such detailed observations. However, the peristaltic movements of the abdomen suggest that the flies were possibly attempting to find and penetrate the female's internal genitalia, and/or depositing a spermatophore. The globular object found in one of the flowers examined with SEM (Fig. 2E, F) is probably a spermatophore deposited by a male fly during pseudocopulation, based on its similarity with other sciarid spermatophores (e.g. Eberhard, 2001). Corroboration for this will require dissection of another object of the same kind found in a flower to look for sperm, or direct observation of spermatophore deposition by the insect. That the male flies ejaculate in the flowers is also supported by fact that they never attempt to copulate with another flower after completing a pseudocopulation, indicating they might experience a refractory period. Such post-copulatory refractory periods are common in insects (W. Eberhard, Universidad de Costa Rica, pers. comm.). Ejaculation in sexually deceptive flowers has previously been documented only in Cryptostylis (see below).
The sexual pheromones of Lycoriella mali, an economically important sciarid pest, were identified as heptadecane and other long-chain saturated hydrocarbons (Kostelc et al., 1980; but see Gotoh et al., 1999). The flowers of Lepanthes glicensteinii probably produce similar species-specific volatile compounds that attract males of B. floribunda. Corroboration for this will require the use of gas chromatography and electroantennographic detection (e.g. Schiestl et al., 2000, 2004). The volatile compounds produced by flowers in several species of Puerto Rican Lepanthes are currently under study (A. Cuevas, University of Puerto Rico-Rio Piedras, pers. comm.).
The surface of the labellum blades in L. glicensteinii possibly functions as an osmophore (Fig. 2A, B), as scent glands typically have papillate surfaces in order to increase their area for diffusion (Vogel, 1990). These papillae could also mimic the body texture of the female insect, as has been suggested for labellar trichomes in other sexually deceptive orchids (van der Pijl and Dodson, 1966; Borg-Karlson, 1990; van der Cingel, 1995, 2001; Singer et al., 2004).
Implications for pollination in the genus Lepanthes
Given that the vast majority of species in the genus Lepanthes share the same basic floral organization of L. glicensteinii and lack any detectable floral rewards, we predict that they also have pseudocopulatory pollination, possibly by species-specific fungus gnats. In this case Lepanthes would be by far the largest plant genus with sexually deceptive flowers; this more than triples the number of orchid species that are known or suspected to rely on pseudocopulation for pollination (M. Blanco, unpubl. data). The exclusive attraction of B. floribunda to L. glicensteinii, and our occasional observations of pollinators in other species of Lepanthes, suggest that each species attracts its own specific pollinator, in accordance with other sexually deceptive orchid genera (with the notable exception of Cryptostylis and, to a lesser degree, Ophrys).
Flowers of Lepanthes are popularly likened to insects, but those of L. glicensteinii do not bear an obvious resemblance to their pollinators. Females of B. floribunda are as yet unknown, but sciarid females are usually similar to their males, only slightly larger and with thicker abdomens. However, it is well known that male insects in general have low thresholds of sexual excitation when exposed to their specific female sexual pheromones, and they can occasionally attempt copulation with objects that bear only a crude resemblance to their females (Thornhill and Alcock, 1983). The flowers of some other orchids involved in sexual deception do not resemble the females of their pollinators either, at least to the human eye (e.g. Stoutamire, 1975; Steiner et al., 1994; Singer, 2002). In Lepanthes, the labellum blades or the petals could mimic the wings of the female insect, like the ‘speculum’ in the labellum of Ophrys (Dafni and Bernhardt, 1990), and provide a visual cue for proper positioning of the male with respect to the flower. The presence of a head decoy is not necessary, as sciarid males readily copulate with experimentally decapitated females (Eberhard, 2001). The contrast between the vividly coloured flower of L. glicensteinii and the dark body of B. floribunda is especially perplexing. It is possible that flowers of Lepanthes have an UV reflectivity pattern similar to that of the insect's body, which requires further investigation.
The role of the labellum appendix is critical, as it provides the anchor point for the pollinator's external genitalia. The appendix clearly substitutes the female abdominal terminalia, and the trichomes probably mimic the hairs in that part of the female body. Several authors have suggested that this organ might serve as a visual lure to the pollinators (Luer, 1996; Salazar-Chávez and Soto-Arenas, 1996; Behar, 1999; Archila, 2001), but at least in L. glicensteinii the pollinators do not have visual access to the appendix (Fig. 1B–F). Tactile stimuli must therefore be of critical importance. The shape and size of the labellum appendix is often species-specific, and can be quite complex in structure (Luer, 1996). In at least some species the appendix could mimic parts of the female genitalia; the highly intricate appendices of some Lepanthes certainly suggest this possibility. However, testing this hypothesis requires documenting pollinators in more species of Lepanthes and comparing the functional morphology of the pollinators' female genitalia and the labellum appendices.
Some species of Lepanthes only have a tuft of hairs in place of the appendix (e.g. L. aprica Catling & V. R. Catling) or lack it altogether (e.g. L. absens Luer & Hirtz). In those species, placement of the pollinarium on the insect's abdomen can conceivably occur when the gonostili probe under the blades of the labellum (circumventing the need for the pivoting manoeuvre), or alternatively the gonostili might clasp the base of the column. Observation of pollination events in these species is required to corroborate this idea. Yet the labellum of other species of Lepanthes is open and undivided (e.g. L. calodictyon Hooker and relatives) or is extremely reduced (e.g. L. rafaeliana Pupulin), and the flower organization of two small subgenera (Brachycladium and Marsipanthes, Luer 1994, 1996) is also somewhat different. The mechanism of pollination in these ‘unorthodox’ species of Lepanthes is likely to be different from that of L. glicensteinii and it might or might not rely on sexual deception.
Most species of Lepanthes produce their flowers in inflorescences that grow appressed against the lower (or less frequently the upper) surface of leaves (Luer, 1996; Salazar-Chávez and Soto-Arenas, 1996). This might be an adaptation to sciarid mating behaviour, as females generally attract males from the underside of a leaf, and the males do not alight directly on them (Binns, 1979; Harris et al., 1996; Eberhard and Flores, 2002). Interestingly, many species of Lepanthes with free-standing flowers (borne on inflorescences projected beyond the leaves) have expanded sepals (e.g. L. johnsonii Ames and relatives), conceivably to provide an alternative landing surface for the pollinators.
Fungus gnats have been traditionally regarded as inefficient pollinators (Proctor et al., 1996), but increasing evidence demonstrates otherwise (Vogel, 1978; Mesler et al., 1980; Christensen, 1994; Larson et al., 2001; Goldblatt et al., 2004, Okuyama et al., 2004). Their importance in pollination has been mostly related to brood-site deceptive systems (Vogel, 1978; Sugawara, 1988; Vogel and Martens, 2000) and some rewarding systems (Mesler et al., 1980; Goldblatt et al., 2004; Okuyama et al., 2004). However, in L. glicensteinii and possibly in most species of the genus, fungus gnats are efficient pollinators associated with a sexually deceptive system. Fungus gnats have also been suggested to carry out pseudocopulatory pollination in the Australasian orchid genus Pterostylis, but the evidence for this is not yet conclusive (Christensen, 1994; van der Cingel, 2001; and references therein).
Sciarids are short-lived as adults (3–8 d), but males usually live several days longer than females and can mate multiple times (Binns, 1979; Harris et al., 1996), which renders them appropriate agents for pollen transfer. Their entire life cycle is completed in few weeks, so populations are constantly renewed. This might explain why so many species of Lepanthes produce flowers throughout the year (cf. Trigonidium obtusum, Singer 2002). Sciarids are generally protandrous (adult males emerge 1 d prior to females; Harris et al., 1996), in common with most other groups of insects involved in pseudocopulatory pollination (van der Pijl and Dodson, 1966; Dafni, 1984; Dafni and Bernhardt, 1990); the orchids might benefit from this time window without competition for the males' attention from the females.
It is apparent that the frequency of pollinator visitation in many species of Lepanthes is very low. We observed numerous floral visits only in L. glicensteinii, despite the fact that many other species of Lepanthes were cultivated at the study site. Species in this genus commonly show low levels of fruit set and removal of pollinaria (Calvo, 1990; Salazar-Chávez and Soto-Arenas, 1996; Tremblay, 1997c; Tremblay et al., 1998; Esquilín and Tremblay, 1999; Tremblay and Ackerman, 2001; Tremblay and Salguero-Faría, 2001; Godden, 2002; L. Jost, pers. comm.; M. Blanco, pers. obs.), which also suggest infrequent pollinator visitation. (Old fruits fall off from their pedicels, but in those species with inflorescences appressed to the leaf surface the pedicels elongate substantially during fruit development [M. Blanco, pers. obs.], probably to maximize seed dispersal by wind. The pedicels of unpollinated flowers remain uniformly short. These elongated pedicels can be used to estimate the number of pollinated flowers even after they have abscised.) After knowing of our preliminary results, Godden (2002) placed sticky traps around plants of L. rupestris Stimson in Puerto Rico, in order to trap pollinating fungus gnats. No insects carrying pollinaria were found after 3 weeks. Though inconclusive, his results also suggest low rates of flower visitation; it is also possible that the pollinators of L. rupestris were not attracted to the type of sticky traps used.
Endress (1994) was the first to suggest that Lepanthes might be pollinated by pseudocopulation, but his was a speculation based on the odd floral morphology of the genus. There are only two previous accounts on the pollination of Lepanthes; both of them quite anecdotal. Dod (1986) suggested that aphids pollinate flowers of Lepanthes while walking on them, after observing one of these insects with a pollinarium attached to a leg. This could conceivably happen as a rare accident, but aphids remain stationary most of the time, feeding on the sap of their host plant. Their presence on the flowers more likely causes them to abort early. Archila (2001) reported observing ceratopogonid flies ‘entering’ and pollinating the flowers of various species of Lepanthes in Guatemala, but he did not provide photographs or voucher information, and only speculated about the details of the pollination mechanism. Both Archila (pers. comm.) and Christensen (1994) observed unidentified insect larvae in flowers of Lepanthes, that apparently did not cause damage. The significance of this phenomenon is unknown, but Christensen suggested that brood-site deception can occur in some species.
Cryptostylis is the only other plant genus in which genitalic pseudocopulatory pollination is known to occur. Despite obvious differences in floral morphology, all species of Cryptostylis are pollinated by a single species of ichneumonid wasp, Lissopimpla excelsa (synonym: L. semipunctata) (Coleman, 1928–1938; Dacy, 1974; Stoutamire, 1975; Schiestl et al., 2004). Males of L. excelsa alight on the labellum with their gonostili wide open, position themselves so that their abdomen points toward the column, and then back up. Coleman (1928b) demonstrated that the male wasps hold the base of the column with their gonostili, and that they ejaculate during at least some flower visits (they also fly away after a successful pseudocopulation without visiting other flowers, leaving sperm in the visited flower). The pollinaria are placed close to the apex of the insect's abdomen (see illustrations in Coleman, 1928a, 1929, 1930, 1938) These are remarkable similarities to the pollination process in Lepanthes glicensteinii. Sympatric species of Cryptostylis do not hybridize, possibly due to interspecific sterility (Stoutamire, 1975; Dafni and Bernhardt, 1990; Schiestl et al., 2004).
Observations on the breeding system
The absence of fruit production in our study plants suggests that L. glicensteinii requires cross-pollination for successful fertilization. The availability of a single plant per observation period prevented us from testing this hypothesis. The flies that arrived at flowers with pollinia already attached to their abdomens were most likely individuals that had visited flowers of the same plant some time (minutes or hours) earlier, because no other plants of L. glicensteinii occurred in the area. The closest natural habitat suitable for species of Lepanthes is at least 1 km away, and adult male sciarids tend not to disperse long distances (Binns, 1979; Eberhard and Flores, 2002).
In L. woodburyana Stimson from Puerto Rico, the probability of fruit set in the field is low (11–16 %) in both self- and cross-pollinations (Esquilín and Tremblay, 1999). It is possible that L. glicensteinii is likewise self-compatible but has a low proportion of successful fruit formation after pollination, but it is remarkable that both study plants survived in cultivation for several months with frequent visits by pollinators without ever producing fruits. Each plant probably produced many dozens of flowers during their time in cultivation. However, recent laboratory work by R. Tremblay (University of Puerto Rico-Humacao, pers. comm.) suggests that self-incompatibility is common in the genus.
Evolutionary considerations
Many species of Lepanthes have small populations and restricted distributions (Luer, 1996, 2003; Salazar-Chávez and Soto-Arenas, 1996; Tremblay, 1997a; Llamacho-Olmo, 2004) with low levels of gene flow among populations (Tremblay and Ackerman, 2001). This has a potential explanation in the sedentary habits of male sciarids, which in contrast to females, do not disperse far even as adults (Binns, 1979; Eberhard and Flores, 2002). Plants of Lepanthes are relatively short-lived in nature (1·7–7 years half-life in four species studied by Tremblay, 2000) and presumably have rapid life cycles; genetic drift appears to be important in geographical differentiation (Tremblay and Ackerman, 2001). This is in contrast to the suggestion that sexual-deceit pollination systems promote gene flow among populations (Dressler, 1981; Peakall, 1990; Peakall and Beattie, 1996; Soliva and Widmer, 2003). This is probably true in most cases; Lepanthes is atypical in that their male pollinators have a more stationary mate-seeking strategy. The high levels of genetic differentiation in Lepanthes can lead to morphological divergence of disjunct populations (Tremblay, 1997b). Thus, rapid allopatric speciation is likely to occur in the genus.
In addition, rapid speciation of Lepanthes can also occur in sympatry. With the exception of some species of Ophrys (Soliva and Widmer, 2003), sympatric species of sexually deceptive orchids avoid introgression by attracting different pollinators with species-specific pheromones (Bergström, 1978; Borg-Karlson, 1990; Paulus and Gack, 1990; Steiner et al., 1994; Bower, 1996; Schiestl and Ayasse, 2002; Schiestl et al., 2003). As suggested by Dressler (1981) and Schiestl and Ayasse (2002), mutational changes in the genes controlling floral fragrance compounds can attract a different species of insect. If the new visitor is capable of carrying out pollination, reproductive isolation is achieved instantly. In this respect, it is remarkable that putative natural hybrids are extremely rare in Lepanthes, despite the fact that several species often grow together in isolated phorophytes (Salazar-Chávez and Soto-Arenas, 1996; Tremblay, 1997a; Tremblay et al., 1998; Llamacho-Olmo, 2004; L. Jost, Ecuador, pers. comm.; M. Blanco and G. Barboza, pers. obs.) and that artificial hybrids are easily produced by hand pollination (Behar, 1999).
These patterns suggest that Lepanthes has experienced an explosive radiation that explains the large number of species in the genus. Evidence for rapid radiations has been found in other groups of sexually deceptive orchids (Soliva et al., 2001; Mant et al., 2002).
In a molecular phylogenetic analysis of subtribe Pleurothallidinae, Pridgeon et al. (2001) found that Lepanthes is closely related to the genera Frondaria, Lepanthopsis, Trichosalpinx, Zootrophion, and Pleurothallis subgenus Acuminatia and subgenus Specklinia section Muscosae (Anathallis sensu Pridgeon and Chase, 2001). However, the exact relationships among these genera could not be reconstructed unambiguously; additional sequencing to resolve intergeneric relationships in this clade is ongoing (A. Pridgeon, Royal Botanic Gardens, Kew, pers. comm.). Very little is known about pollinators and presence or absence of floral rewards in these genera, but it is interesting that Pleurothallis (Anathallis) sclerophylla Lindley is also pollinated by sciarids (Duque, 1993). Zootrophion might have brood-site deceptive flowers pollinated by flies that lay their eggs on mushrooms (Christensen, 1994), and the insectiform labellum of some species of Pleurothallis subgenus Specklinia section Muscosae suggest potential pseudocopulatory pollination. Nothing is known about pollinators in Frondaria, Lepanthopsis or Trichosalpinx. In any case, the vast majority of pleurothallid orchids are regarded as having non-rewarding flowers (Dressler, 1981), so it is likely that the sexually deceptive flowers of Lepanthes evolved from food deceptive or brood-site deceptive ancestors; a similar trend has been suggested for other groups of orchids (Dafni, 1987; Dafni and Bernhardt, 1990; Paulus and Gack, 1990; Steiner et al., 1994; Singer, 2002).
Supplementary Material
Acknowledgments
We are greatly indebted to Ethel Sánchez (Centro de Investigación en Estructuras Microscópicas, Universidad de Costa Rica) for help with preparation and examination of samples with SEM. Werner Mohrig (Greifswald, Germany) examined the insects and rushed the description of Bradysia floribunda at our request. Silvana Martén, Laura May and Richard Sander kindly hosted MAB during field study sessions at Monteverde. Several people provided valuable discussions, especially Calaway Dodson (Missouri Botanical Garden), William Eberhard (Universidad de Costa Rica), Lou Jost (Baños, Ecuador), Rodrigo Singer (Universidade Estadual de Campinas, Brazil), and Gerardo Salazar and Miguel Angel Soto (Universidad Nacional Autónoma de México). Comments by Robert Dressler, Mark Whitten and Norris Williams (University of Florida), and two anonymous reviewers helped improve earlier versions of the manuscript. Portions of this study were completed when MAB was supported by a Furniss Foundation graduate fellowship from the American Orchid Society, and by US National Science Foundation grant DB-0234064 to Norris H. Williams and W. Mark Whitten.
LITERATURE CITED
- Alberts SA, Kennedy MK, Cardé RT. 1981. Pheromone-mediated anemotactic flight and mating behavior of the sciarid fly Bradysia impatiens (Diptera, Sciaridae). Environmental Entomology 10: 10–15. [Google Scholar]
- Alcock J. 2000. Interactions between the sexually deceptive orchid Spiculaea ciliata and its wasp pollinator Thynnoturneria sp. (Hymenoptera: Thynninae). Journal of Natural History 34: 629–636. [Google Scholar]
- Alexander RD, Marshall DC, Cooley JR. 1997. Evolutionary perspectives on insect mating. In: Choe JC, Crespi BJ, eds. The evolution of mating systems in insects and arachnids. Cambridge: Cambridge University Press, 4–31. [Google Scholar]
- Archila F. 2001.Lepanthes de Guatemala. Guatemala: Editorial Kamar. [Google Scholar]
- Arditti J. 1992.Fundamentals of orchid biology. New York: John Wiley and Sons. [Google Scholar]
- Behar M. 1999. Die Gattung Lepanthes Sw. Die Orchidee (Hamburg) 50: 37–42. [Google Scholar]
- Bergström G. 1978. Role of volatile chemicals in Ophrys-pollinator interactions. In: Harborne JB, ed. Biochemical aspects of plant and animal coevolution. London: Academic Press, 207–232. [Google Scholar]
- Binns ES. 1979. Biology and behaviour of sciarid fungus gnats (Dipt. Sciaridae) in relation to swarming and migration. Entomologist's Monthly Magazine 115: 77–90. [Google Scholar]
- Borg-Karlson AK. 1990. Chemical and ethological studies of pollination in the genus Ophrys (Orchidaceae). Phytochemistry 29: 1359–1387. [Google Scholar]
- Bower CC. 1996. Demonstration of pollinator-mediated reproductive isolation in sexually deceptive species of Chiloglottis (Orchidaceae: Caladeniinae). Australian Journal of Botany 44: 15–33. [Google Scholar]
- Calvo RN. 1990. Inflorescence size and fruit distribution among individuals in three orchid species. American Journal of Botany 77: 1378–1381. [Google Scholar]
- Christensen DE. 1994. Fly pollination in the Orchidaceae. In: Arditti J, ed. Orchid biology: reviews and perspectives, VI. New York: John Wiley and Sons, 415–454. [Google Scholar]
- Coleman E. 1928. Pollination of Cryptostylis leptochila, F.v.M. Victorian Naturalist 44: 333–340. [Google Scholar]
- Coleman E. 1928. Pollination of an Australian orchid by the male ichneumonid Lissopimpla semipunctata, Kirby. Transactions of the Entomological Society of London 76: 533–539. [Google Scholar]
- Coleman E. 1929. Pollination of Cryptostylis subulata (Labill.) Reichb. Victorian Naturalist 46: 62–66. [Google Scholar]
- Coleman E. 1930. Pollination of Cryptostylis erecta, R.Br. Victorian Naturalist 46: 236–238. [Google Scholar]
- Coleman E. 1938. Further observations on the pseudocopulation of the male Lissopimpla semipunctata Kirby (Hymenoptera, Parasitica) with the Australian orchid Cryptostylis leptochila F.v.M. Proceedings of the Royal Entomological Society of London 13: 82–83. [Google Scholar]
- Dacy M. 1974. Pollination experiment, performed on Cryptostylis subulata Victorian Naturalist 91: 66–78. [Google Scholar]
- Dafni A. 1984. Mimicry and deception in pollination. Annual Review of Ecology and Systematics 15: 259–278. [Google Scholar]
- Dafni A. 1987. Pollination in Orchis and related genera: evolution from reward to deception. In: Arditti J, ed. Orchid biology, reviews and perspectives, IV. Ithaca, NY: Cornell University Press, 80–104. [Google Scholar]
- Dafni A, Bernhardt P. 1990. Pollination of terrestrial orchids of Southern Australia and the Mediterranean region: systematic, ecological, and evolutionary implications. Evolutionary Biology 24: 193–253. [Google Scholar]
- Darwin C. 1877. On the various contrivances by which orchids are fertilised by insects, 2nd edn. London: John Murray. [Google Scholar]
- Dod DD. 1986. Afidos y trípidos polinizan orquídeas en las Pleurothallidinae (Orchidaceae). Moscosoa 4: 200–202. [Google Scholar]
- Dressler RL. 1981.The orchids: natural history and classification. Cambridge, MA: Harvard University Press. [Google Scholar]
- Duque O. 1993. Polinización en Pleurothallis Orquideología 19: 55–69. [Google Scholar]
- Eberhard WG. 2001. Genitalic behavior in Hybosciara gigantea (Diptera: Sciaridae) and the evolution of species-specific genitalia. Journal of the Kansas Entomological Society 74: 1–9. [Google Scholar]
- Eberhard WG, Flores C. 2002. The behavior and natural history of Hybosciara gigantea (Diptera: Sciaridae). Journal of the Kansas Entomological Society 75: 8–15. [Google Scholar]
- Endress PK. 1994.Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press. [Google Scholar]
- Esquilín E, Tremblay RL. 1999. Reproductive biology of the orchid Lepanthes woodburyana Stimson. Plant Species Biology 14: 179. [Google Scholar]
- Godden GT. 2002. Pollination and speciation of Lepanthes: an approach to understanding orchid evolution. Pleurothallid News and Views 14: 52–54. [Google Scholar]
- Goldblatt P, Bernhardt P, Vogan P, Manning JC. 2004. Pollination by fungus gnats (Diptera: Mycetophilidae) and self-recognition sites in Tolmiea menziesii (Saxifragaceae). Plant Systematics and Evolution 244: 55–67. [Google Scholar]
- Gotoh T, Nakamuta K, Tokoro M, Nakashima T. 1999. Copulatory behavior and sex pheromones in sciarid fly, Lycoriella mali (Fitch) (Sciaridae: Diptera). Japanese Journal of Applied Entomology and Zoology 43: 181–184. [Google Scholar]
- Harris MA, Gardner WA, Oetting RD. 1996. A review of the scientific literature on fungus gnats (Diptera: Sciaridae) in the genus Bradysia Journal of Entomological Science 31: 252–276. [Google Scholar]
- Kostelc JG, Girard JE, Hendry LB. 1980. Isolation and identification of a sex attractant of a mushroom-infecting sciarid fly. Journal of Chemical Ecology 6: 1–11. [Google Scholar]
- Larson BMH, Kevan PG, Inouye DW. 2001. Flies and flowers: taxonomic diversity of anthophiles and pollinators. Canadian Entomologist 133: 439–465. [Google Scholar]
- Liu YN, Honda H, Kohno Y. 2002. Mating behavior and its regulatory factors in the black fungus gnat, Bradysia paupera (Diptera: Sciaridae). Japanese Journal of Applied Entomology and Zoology 46: 23–30. [Google Scholar]
- Llamacho-Olmo JA. 2004. Notas sobre ecología y distribución del género Lepanthes (Orchidaceae) en Cuba, con una lista actualizada y revisada. Lankesteriana 4: 61–66. [Google Scholar]
- Luer CA. 1986. Icones Pleurothallidinarum I. Systematics of the Pleurothallidinae (Orchidaceae). Monographs in Systematic Botany from the Missouri Botanical Garden, St. Louis 15: 1–81. [Google Scholar]
- Luer CA. 1987. New Lepanthes species from Costa Rica and Panama. Lindleyana 2: 185–217. [Google Scholar]
- Luer CA. 1994. Icones Pleurothallidinarum XI. Systematics of Lepanthes subgenus Brachycladium, and Pleurothallis subgenus Aenigma, subgenus Elongatia, and subgenus Kraenzlinella (Orchidaceae). Part One: Systematics of the subgenus Brachycladium genus Lepanthes Monographs in Systematic Botany from the Missouri Botanical Garden, St. Louis 52: 1–50. [Google Scholar]
- Luer CA. 1996. Icones Pleurothallidinarum XIV. Systematics of Draconanthes, Lepanthes subgenus Marsipanthes, and subgenus Lepanthes of Ecuador (Orchidaceae). Part Three: The genus Lepanthes subgenus Lepanthes in Ecuador. Monographs in Systematic Botany from the Missouri Botanical Garden, St. Louis 61: 1–255. [Google Scholar]
- Luer CA. 2003.Lepanthes In: Hammel BE, Grayum MH, Herrera C, Zamora N, eds. Manual de Plantas de Costa Rica. Volumen III: Monocotiledóneas (Orchidaceae-Zingiberaceae). Monographs in Systematic Botany from the Missouri Botanical Garden, St. Louis39: 216–255. [Google Scholar]
- Mant JG, Schiestl FP, Peakall R, Weston PH. 2002. A phylogenetic study of pollinator conservatism among sexually deceptive orchids. Evolution 56: 888–898. [DOI] [PubMed] [Google Scholar]
- McAlpine JF. 1981. Morphology and terminology—adults. In: McAlpine JF, Peterson BV, Shewell GE, Teskey JJ, Vockeroth JR, Wood DM, eds. Manual of Neartic Diptera. Vol. 1. Ottawa: Agriculture Canada, 9–63. [Google Scholar]
- Menzel F, Mohrig W. 1997. Family Sciaridae. In: Papp L, Darvas B, eds. Contributions to a manual of Paleartic Diptera. Vol. 2. Budapest: Science Herald, 51–69. [Google Scholar]
- Mesler MR, Ackerman JD, Lu KL. 1980. The effectiveness of fungus gnats as pollinators. American Journal of Botany 67: 564–567. [Google Scholar]
- Mohrig W. 2003. Black fungus gnats of Central America. Part I. (Diptera, Sciaridae). Beiträge zur Entomologie 53: 1–69. [Google Scholar]
- Okuyama Y, Kato M, Murakami N. 2004. Pollination by fungus gnats in four species of the genus Mitella (Saxifragaceae). Botanical Journal of the Linnean Society 144: 449–460. [Google Scholar]
- Paulus HF, Gack C. 1990. Pollinators as prepollinating isolation factors: evolution and speciation in Ophrys (Orchidaceae). Israel Journal of Botany 39: 43–79. [Google Scholar]
- Peakall R. 1990. Responses of male Zaspilothynnus trilobatus wasps to females and the sexually deceptive orchid it pollinates. Functional Ecology 4: 159–167. [Google Scholar]
- Peakall R, Beattie AJ. 1996. Ecological and genetic consequences of pollination by sexual deception in the orchid Caladenia tentaculata Evolution 50: 2207–2220. [DOI] [PubMed] [Google Scholar]
- Pridgeon AM, Chase MW. 2001. A phylogenetic reclassification of the Pleurothallidinae (Orchidaceae). Lindleyana 16: 235–271. [Google Scholar]
- Pridgeon AM, Solano R, Chase MW. 2001. Phylogenetic relationships in Pleurothallidinae (Orchidaceae): combined evidence from nuclear and plastid DNA sequences. American Journal of Botany 88: 2286–2308. [PubMed] [Google Scholar]
- Proctor M, Yeo P, Lack A. 1996.The natural history of pollination. Portland, OR: Timber Press. [Google Scholar]
- Rudall PJ, Bateman RM, Fay MF, Eastman A. 2002. Floral anatomy and systematics of Alliaceae with particular reference to Gilliesia, a presumed insect mimic with strongly zygomorphic flowers. American Journal of Botany 89: 1867–1883. [DOI] [PubMed] [Google Scholar]
- Salazar-Cháves GA, Soto-Arenas MA. 1996. El género Lepanthes Sw. en México. Orquídea (Mexico) 14: 1–231. [Google Scholar]
- Schiestl FP, Ayasse M. 2002. Do changes in floral odor cause speciation in sexually deceptive orchids? Plant Systematics and Evolution 234: 111–119. [Google Scholar]
- Schiestl FP, Ayasse M, Paulus HF, Lofstedt C, Hansson BS, Ibarra F, Francke W. 2000. Sex pheromone mimicry in the early spider orchid (Ophrys sphegodes): patterns of hydrocarbons as the key mechanism for pollination by sexual deception. Journal of Comparative Physiology A—Neuroethology, Sensory, Neural and Behavioral Physiology 186: 567–574. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Peakall R, Mant JG, Ibarra F, Schulz C, Francke S, Francke W. 2003. The chemistry of sexual deception in an orchid-wasp pollination system. Science 302: 437–438. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Peakall R, Mant JG. 2004. Chemical communication in the sexually deceptive orchid genus Cryptostylis Botanical Journal of the Linnean Society 144: 199–205. [Google Scholar]
- Singer RB. 2002. The pollination mechanism in Trigonidium obtusum Lindl. (Orchidaceae: Maxillariinae): sexual mimicry and trap-flowers. Annals of Botany 89: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RB, Flach A, Koehler S, Marsaioli AJ, Amaral MDCE. 2004. Sexual mimicry in Mormolyca ringens (Lindl.) Schltr. (Orchidaceae: Maxillariinae). Annals of Botany 93: 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliva M, Widmer A. 2003. Gene flow across species boundaries in sympatric, sexually deceptive Ophrys (Orchidaceae) species. Evolution 57: 2252–2261. [DOI] [PubMed] [Google Scholar]
- Soliva M, Kocyan A, Widmer A. 2001. Molecular phylogenetics of the sexually deceptive orchid genus Ophrys (Orchidaceae) based on nuclear and chloroplast DNA sequences. Molecular Phylogenetics and Evolution 20: 78–88. [DOI] [PubMed] [Google Scholar]
- Steffan WA. 1981. Sciaridae. In: McAlpine JF, Peterson BV, Shewell GE, Teskey JJ, Vockeroth JR, Wood DM, eds. Manual of Neartic Diptera. Vol. 1. Ottawa: Agriculture Canada, 247–255. [Google Scholar]
- Steiner KE, Whitehead VB, Johnson SD. 1994. Floral and pollinator divergence in two sexually deceptive South African orchids. American Journal of Botany 81: 185–194. [Google Scholar]
- Stenzel H. 2000. Pollen morphology of the subtribe Pleurothallidinae Lindl. (Orchidaceae). Grana 39: 108–125. [Google Scholar]
- Stoutamire WP. 1975. Pseudocopulation in Australian orchids. American Orchid Society Bulletin 44: 226–233. [Google Scholar]
- Sugawara T. 1988. Floral biology of Heterotropa tamaensis (Aristolochiaceae) in Japan. Plant Species Biology 3: 7–12. [Google Scholar]
- Thornhill R, Alcock J. 1983.The evolution of insect mating systems. Cambridge, MA: Harvard University Press. [Google Scholar]
- Tremblay RL. 1997. Distribution and dispersion patterns of individuals in nine species of Lepanthes (Orchidaceae). Biotropica 29: 38–45. [Google Scholar]
- Tremblay RL. 1997. Morphological variance among populations of three tropical orchids with restricted gene flow. Plant Species Biology 12: 85–96. [Google Scholar]
- Tremblay RL. 1997.Lepanthes caritensis, an endangered orchid: no sex, no future? Selbyana 18: 160–166. [Google Scholar]
- Tremblay RL. 2000. Plant longevity in four species of Lepanthes (Pleurothallidinae; Orchidaceae). Lindleyana 15: 257–266. [Google Scholar]
- Tremblay RL, Ackerman JD. 2001. Gene flow and effective population size in Lepanthes (Orchidaceae): a case for genetic drift. Biological Journal of the Linnean Society 72: 47–62. [Google Scholar]
- Tremblay RL, Salguero-Faría JA. 2001. The unkindest cut: the fate of Lepanthes woodburyana, a small neotropical orchid. Lindleyana 16: 38–42. [Google Scholar]
- Tremblay RL, Zimmerman JK, Lebrón L, Bayman P, Sastre I, Axelrod F, Alers-García J. 1998. Host specificity and low reproductive success in the rare endemic Puerto Rican orchid Lepanthes caritensis Biological Conservation 85: 297–304. [Google Scholar]
- van der Cingel NA. 1995.An atlas of orchid pollination: European orchids. Rotterdam: A. A. Balkema. [Google Scholar]
- van der Cingel NA. 2001.An atlas of orchid pollination: America, Africa, Asia and Australia. Rotterdam: A. A. Balkema. [Google Scholar]
- van der Pijl L, Dodson CH. 1966.Orchid flowers: their pollination and evolution. Coral Gables, FL: University of Miami Press. [Google Scholar]
- Vogel S. 1978. Pilzmückenblumen als Pilzmimeten. Flora (Germany) 167: 329–398. [Google Scholar]
- Vogel S. 1990.The role of scent glands in pollination: on the structure and function of osmophores. Washington, DC: Smithsonian Institution Libraries, and The National Science Foundation. [Google Scholar]
- Vogel S, Martens J. 2000. A survey of the function of the lethal kettle traps of Arisaema (Araceae), with records of pollinating fungus gnats from Nepal. Botanical Journal of the Linnean Society 133: 61–100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.