Abstract
• Background and Aims For the maintenance of gynodioecy (i.e. the coexistence of female and hermaphroditic plants), females need to compensate for the lack of pollen production through higher seed production or better progeny quality compared to hermaphrodites. In Geranium sylvaticum, females produce more seeds per flower than hermaphrodites. This difference in seed production might be modified by biological interactions with pollinators and herbivores that may favour one sex and thus affect the maintenance of gynodioecy.
• Methods Sexual dimorphism in flower size and flowering phenology, and in attractiveness to pollinators, pre-dispersal seed predators and floral herbivores were examined in natural populations of G. sylvaticum.
• Key Results Pollinators preferred hermaphrodites 25 % more often than females in two of the three study populations, and floral herbivores attacked hermaphrodites 15 % more often than females in two of the six study populations. These preferences might be explained by the larger flower size of hermaphrodites. In contrast, seed predators did not prefer either sex.
• Conclusions The data suggest that pollinator preference does not benefit females, whereas the higher floral herbivory of hermaphrodites might enhance the maintenance of females in G. sylvaticum. Thus, although the data support the view that ecological factors may contribute to the maintenance of gynodioecy, they also suggest that these contributions may vary across populations and that they may function in opposite directions.
Keywords: Floral herbivory, flowering phenology, Geraniaceae, Geranium sylvaticum, gynodioecy, petal size, pollinator attraction, seed predation
INTRODUCTION
Floral characters such as flower size, colour and number often influence plant attractiveness to pollinators and, in turn, these may affect plant reproductive success by selective visitation (Waser and Price, 1981, 1983; Zimmerman, 1983, 1988; Conner and Rush, 1996, and references therein). In sexually dimorphic populations, pollinators may also contribute to the maintenance of flower size dimorphism if higher allocation to attraction in one sex increases its reproductive success compared to the other sex (Ashman, 2000). On the other hand, different pollinators use different cues (e.g. petal size and number of open flowers) to discriminate between plants (Johnson et al., 1995; Conner and Rush, 1996; Strauss et al., 1996). Furthermore, the particular cue(s) that confer attractiveness to pollinators probably also affect attractiveness to other visitors. Pre-dispersal seed predators consume the seeds in the developing ovary, whereas floral herbivores forage on gametes, but may also have additional indirect effects on plant reproduction by reducing the size of floral display and consequent pollinator visitation rates (e.g. English-Loeb and Karban, 1992; Karban and Strauss, 1993; Cunningham, 1995; Krupnick et al., 1999). Thus, a large floral display may function in two ways: it may attract pollinators but it may also attract seed predators or other herbivores, and thus a balance between these two functions needs to be achieved.
To coexist with hermaphrodites, females of gynodioecious species need to compensate for the lack of male function, i.e. pollen production, either by higher seed production or better progeny quality compared to hermaphrodites (e.g. Lewis, 1941; Charlesworth and Charlesworth, 1978). The type of sex determination affects the fecundity advantage needed by females for sufficient compensation. If the gene conferring male sterility is in the nucleus, females must have at least a two-fold reproductive advantage to be maintained in a population (Lewis, 1941; Charlesworth and Charlesworth, 1978). Conditions for females to coexist with hermaphrodites are less strict if gender determination is cytoplasmic or nuclear-cytoplasmic (Lloyd, 1974; Charlesworth and Charlesworth, 1978; Charlesworth and Ganders, 1979). In our study species, Geranium sylvaticum, females produce 1·2 to 1·7 times more seeds per flower than hermaphrodites; the difference between the sexes varies between populations and years (Asikainen and Mutikainen, 2003). It has recently been suggested that natural enemies may contribute to the maintenance of gynodioecy by differentially preferring one of the two sexes (e.g. Marshall and Ganders, 2001; Ashman, 2002; Collin et al., 2002). For example, if seeds produced by females are naturally predated more often than those of hermaphrodites, calculations of seed production might overestimate the sexual difference. On the other hand, if the opposite occurs, sex-biased seed predation may contribute to the maintenance of gynodioecy. Indeed, sex-biased seed predation has been found to considerably reduce the reproductive output of hermaphrodites in the gynodioecious Sidalcea hendersonii and Dianthus sylvestris (Marshall and Ganders, 2001; Collin et al., 2002, respectively).
In addition to plant attractiveness, pollinators and natural enemies are affected by the flowering phenology of individual plants, which further affects plant reproductive success (Rathcke and Lacey, 1985). Competition for pollinator service with conspecifics or with other plants within the habitat area may modify flowering schedules (Waser, 1978; Rathcke and Lacey, 1985; Rathcke, 1988). Seed predation and herbivory may also affect flowering phenology (Brody, 1997). In Polemonium foliosissimum, for example, competition for pollination selects for early flowering, but since the risk of seed predation is also highest early in the season, seed predation selects for late flowering (Zimmerman, 1980a, b).
The aim of this study was to examine whether females and hermaphrodites differ in attractiveness to pollinators, pre-dispersal seed predators or floral herbivores in natural populations of Geranium sylvaticum and to evaluate the consequences of putative preferences for the maintenance of gynodioecy in this species. The following specific questions were addressed. (1) Is there sexual dimorphism in flower size in G. sylvaticum? (2) Does flowering phenology differ between females and hermaphrodites? (3) Do seed predators, floral herbivores and pollinators prefer one sex over the other?
MATERIALS AND METHODS
Study species and populations
Geranium sylvaticum (Geraniaceae) is a self-compatible, rhizomatous, perennial herb with a gynodioecious breeding system (Vaarama and Jääskeläinen, 1967; Asikainen and Mutikainen, 2003). It is common in Finland and occurs in meadows, at roadsides and in herb-rich forests. The flowers of G. sylvaticum are regular with five brightly coloured petals, varying from deep purple to white, and include five pistils and two whorls of five stamens. The anthers of female plants are either completely or partially reduced, and are always non-functional. Geranium sylvaticum starts flowering at the beginning of June in southern Finland and in July in northern Finland. Geranium sylvaticum is protandrous, presenting pollen before the stigmas become receptive. However, self-pollination by geitonogamy is possible. The flowers are visited by a variety of insects, including bumblebees, syrphid flies, and other dipterans. The fruit of G. sylvaticum is a schizocarp with five locules, each containing two ovules. Usually fewer than five seeds develop in each fruit. The seeds mature about 3 weeks after pollination. Just before the fruit matures, it changes from green to brown and the awns separate from the central axis to disperse the seeds.
Fieldwork was conducted during the summers of 2000–2002 in several populations located in south-western, central and northern Finland. For a more precise description of the study populations (P1–P13) see Asikainen and Mutikainen (2003). Female frequency within these populations varies between 4·4 % and 27·2 % (Asikainen and Mutikainen, 2003). Intermediate plants (i.e. plants with 1–9 functional anthers) were not included in this study since their frequency was less than 1 % in these populations. For each study presented here, only a part of the 13 populations was used (see below).
Flower size and flowering phenology
In each of 11 study populations (P1–P11), three fresh flowers were collected from each of 15 females and 15 hermaphrodites in 2000. Two petals from each of the flowers were measured and the average petal length was calculated for both sexes. The data on petal length was analysed with a mixed-model ANOVA with population (random) and sex (fixed) as factors.
In 2002, three of the populations (P3, P8 and P9) were followed in more detail. Large proportions of plants were marked in these populations (565, 320 and 766 plants, respectively; the whole population in case of P3) and flowering was monitored every third day at the beginning and end of the flowering season. Female frequencies of the monitored plants did not differ from the female frequency observed in 2000 (2000: 16·5 %, 4·6 % and 23·0 %; 2002: 17·0 %, 4·4 % and 26·2 %; χ2 = 0·415, d.f. = 2, P = 0·813; Asikainen and Mutikainen 2003). The sex and the dates of first and last flowering for each plant were recorded. Data on flowering phenology (start, end and duration of flowering) did not fulfill the assumptions of parametric ANOVA even after transformations, and so were analysed using ANOVA on ranked values with plant sex (fixed) and population (three populations, random) as factors.
Pollinator visits
Pollinator visits were observed in 2001 in populations P3, P4 and P8, which are located in a deciduous forest, a mixed forest and in a meadow, respectively. In each population, a total of 40 pollinators were followed for at least two observation periods when most of the plants were in full bloom (at the beginning of June) and when the pollinators were most active (between 1000 h and 1400 h). For each pollinator the following were recorded: (1) the type of visitor (bumblebee/syrphid fly/other type of pollinator); (2) the sex of each plant visited; and (3) the number of flowers visited per plant. Each visitor was observed until it left the population. Pollinators in the three populations were observed by one person for a total of 18 h. Only visits by bumblebees and syrphid flies are considered further, because together they accounted for 95 % of all observed visits. The data on the number of flowers visited per plant did not fulfill the assumptions of parametric ANOVA and thus we conducted a three-way ANOVA on ranked values using plant sex (fixed), pollinator type (fixed), and population (three populations, random) as factors. Further, we used a G-test to compare the sex ratio of plants visited by pollinators (observed) with the population sex ratio (expected) for each population separately, and in all three populations combined. We also tested for differences in pollinator visitation between populations with a G-test of heterogeneity (Sokal and Rohlf, 1981).
Seed predation and floral herbivory
Pre-dispersal seed predation was observed in ten populations (P2–P4, P6, P8–P13) in 2 years (2001 and 2002). Twenty female and 20 hermaphrodite plants were chosen randomly in each population. A few days before the fruits matured light mesh bags were installed around each study plant to collect all of the seeds produced during the season. The number of seeds produced were counted, distinguishing between undamaged and partly damaged seeds to determine the proportion predated. Seeds were consumed for example by larvae of the weevil Zacladus geranii in two ways: either a small hole had been bored in the seed or part of the seed was missing. The adults of this weevil forage and oviposit on the flowers of G. sylvaticum; they particularly damage flowers already open (e.g. petals; E. Asikainen, pers. obs.). Data on the proportion of seeds predated did not fulfill the assumptions of parametric ANOVA even after transformations, and were analysed using ANOVA on ranked values. Since we followed the same plants for 2 years, we used repeated-measures ANOVA with year as a factor. Population was used as a random factor and plant sex as a fixed factor. Population P11 was excluded from the analyses since the proportion of seeds predated was zero.
To estimate floral herbivory, all flowering plants were monitored in populations P2–P4, P6, P8 and P13 in 2001 at peak flowering. The sex of each plant was recorded and whether any flowers were damaged. The number of damaged female and hermaphroditic plants (observed) was compared to the population sex ratio (expected) using a G-test for each population separately, and in all six populations combined. Differences in floral herbivory between populations were also tested for with a G-test of heterogeneity (Sokal and Rohlf, 1981).
The error terms for the mixed-model ANOVAs were determined according to Zar (1996). All statistical analyses were performed with SPSS statistical software (Norusis, 1990).
RESULTS
Flower size and flowering phenology
Hermaphrodites had significantly larger petals (17·3 ± 0·1 mm, mean ± s.e.) than females (10·8 ± 0·12 mm; F1,10 = 549·8, P < 0·001; Fig. 1). Petal length also varied between populations (F10,305 = 10·541, P < 0·001). Furthermore, the difference in petal size between sexes varied between populations (sex × population: F10,305 = 3·304, P < 0·001).
Fig. 1.
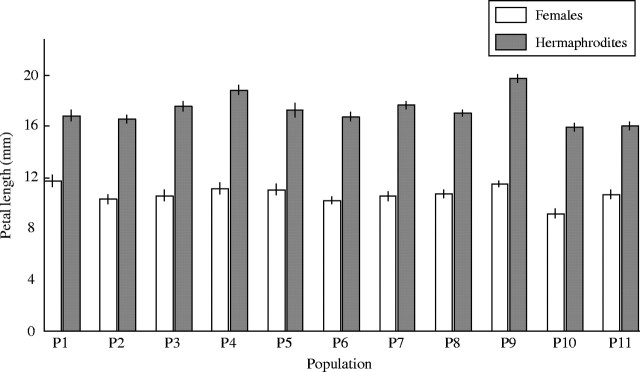
Petal length (mean ± s.e.) of female and hermaphroditic plants from 11 populations (P1–P11) of the gynodioecious Geranium sylvaticum.
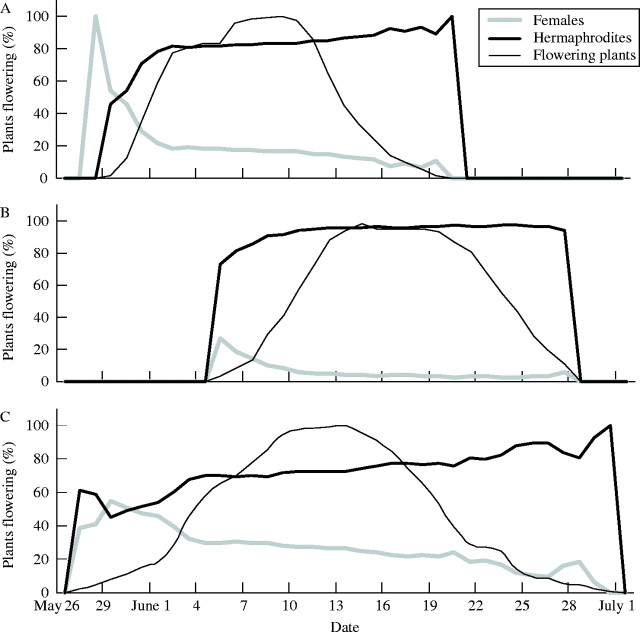
Females started flowering on average 3 d earlier than hermaphrodites (F1,2 = 112·83, P = 0·009; Fig. 2). Neither the end of flowering (F1,2 = 12·21, P = 0·073) nor duration of flowering differed significantly between females and hermaphrodites (F1,2 = 1·67, P = 0·326). The start of flowering, end of flowering and duration of flowering varied between the three populations (F2,1559 = 213·35, P < 0·001; F2,1559 = 552·37, P < 0·001; F2,1559 = 249·90, P < 0·001, respectively).
Fig. 2.
Proportion of females and hermaphrodites of all flowering plants, and proportion of plants flowering during the growing season in three populations of the gynodioecious Geranium sylvaticum: (A) P3, (B) P8 and (C) P9.
Pollinator visits
A total of 2197 plants (93 female and 2104 hermaphroditic plants) were visited by the 120 insects followed during the observation periods. The number of plants visited by individual insects varied between 2–43, 2–30 and 2–44 in populations P3, P4 and P8, respectively. The number of flowers visited per plant did not differ between females and hermaphrodites (mean ± s.e.: females, 1·98 ± 0·13; hermaphrodites, 2·47 ± 0·03), between pollinator species (bumblebees, 2·31 ± 0·10; syrphid flies, 2·47 ± 0·04) or between populations (P3, 2·27 ± 0·06, P4, 2·40 ± 0·06; P8, 2·61 ± 0·07; Table 1). The number of flowers per plant in the study populations varied in females from 25–284 (P3), from 17–78 (P4) and from 14–402 (P8), and in hermaphrodites from 25–110 (P3), from 7–61 (P4) and from 12–591 (P8) (E. Asikainen and P. Mutikainen, unpubl. data). Visitation by pollinators to female and hermaphroditic plants varied significantly between the three study populations (Table 2). In one population (P8) the observed visitation frequencies did not differ from the expected frequencies: pollinators visited females and hermaphrodites according to the population sex ratio (Table 2). In the other two populations (P3 and P4) hermaphrodites were visited more frequently than expected (Table 2). The results for pollinator visits were qualitatively similar when the frequencies for bumblebees and syrphid flies were analyzed separately (data not shown).
Table 1.
Results of analysis of variance for differences in the number of flowers visited per plant between sexes, between pollinator species and between populations of Geranium sylvaticum
| Source of variation |
df |
MS |
F |
P |
|---|---|---|---|---|
| Sexa | 1 | 23489·80 | 10·128 | 0·086 |
| Pollinator speciesb | 1 | 2040·94 | 1·629 | 0·330 |
| Population | 2 | 118·20 | 0·056 | 0·946 |
| Sex × pollinator speciesc | 1 | 7·19 | 0·003 | 0·965 |
| Sex × population | 2 | 2319·34 | 1·092 | 0·338 |
| Pollinator species × population | 2 | 1253·01 | 0·590 | 0·556 |
| Sex × pollinator species × population | 1 | 2787·67 | 1·312 | 0·254 |
| Error | 155 | 2124·31 |
Sex × population as error term;
pollinator species × population as error term;
sex × pollinator species × population as error term.
Table 2.
(a) Observed and expected visitation frequency of pollinators to female and hermaphroditic plants in three populations of Geranium sylvaticum. Expected frequencies are based on population sex ratio. Results of G-test for each population are shown at right. (b) Results of heterogeneity G test for between-population differences
| Observed visits (expected visits) |
||||||
|---|---|---|---|---|---|---|
| Population |
Females |
Hermaphrodites |
G |
|||
| (a) | ||||||
| P3 | 25 (98) | 567 (494) | 87·4*** | |||
| P4 | 23 (74) | 699 (648) | 51·6*** | |||
| P8 | 45 (41) | 838 (842) | 0·5 | |||
|
G-test |
df |
G |
P |
|||
|---|---|---|---|---|---|---|
| (b) | ||||||
| ΣG | 3 | 139·5 | <0·001 | |||
| Pooled | 1 | 91·7 | <0·001 | |||
| Heterogeneity among populations | 2 | 47·8 | <0·001 | |||
P < 0·001.
Seed predation and floral herbivory
Overall, the proportion of predated seeds varied from 0–44 % among plants (Fig. 3). Seed predators were present in all of the study populations except in the most northern population (P11). Significant differences were found in seed predation between years and between populations, whereas there were no significant differences between females and hermaphrodites (Table 3; Fig. 3). This result suggests that seed predators do not prefer one sex over the other.
Fig. 3.
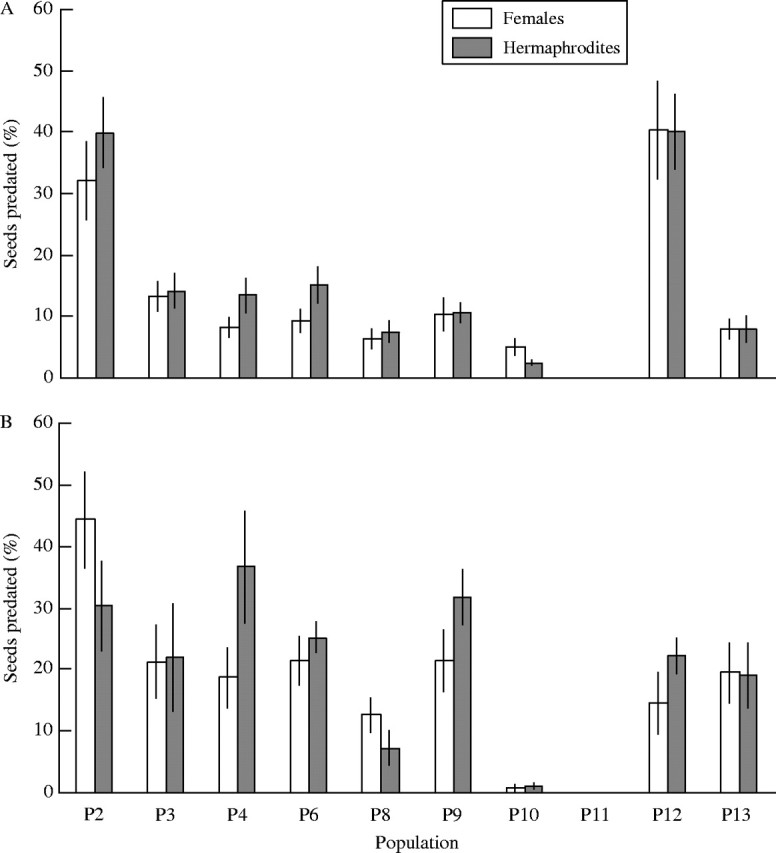
Proportion of seeds predated (%) in females and hermaphrodites in ten populations of the gynodioecious Geranium sylvaticum in (A) 2001 and (B) 2002.
Table 3.
Repeated-measures ANOVA for differences in proportion of predated seeds (a) between the sexes (between factor; fixed), and among populations (between factor; random), and (b) between the two study years (within factor)
| Source of variation |
df |
MS |
F |
P |
||||
|---|---|---|---|---|---|---|---|---|
| (a) Between subjects | ||||||||
| Sexa | 1 | 410·27 | 0·080 | 0·784 | ||||
| Population | 8 | 82039·65 | 16·097 | <0·001 | ||||
| Sex × population | 8 | 5117·11 | 1·004 | 0·435 | ||||
| Error | 158 | 5096·47 | ||||||
| Source of variation |
df |
Wilks' lambda |
F |
P |
||||
|---|---|---|---|---|---|---|---|---|
| (b) Within subjects | ||||||||
| Year | 1 | 0·907 | 16·161 | <0·001 | ||||
| Year × sexa | 1 | 0·998 | 0·668 | 0·437 | ||||
| Year × population | 8 | 0·771 | 5·872 | <0·001 | ||||
| Year × sex × population | 8 | 0·975 | 0·519 | 0·841 | ||||
Sex × population as error term.
Altogether, 4316 plants were checked, of which 1181 had one or more flowers damaged by herbivores; 105 were females and 1076 were hermaphrodites. Populations were marginally heterogeneous for the degree of floral herbivory (P = 0·064; Table 4). In the two populations (P2 and P4) where floral damage was disproportional to the population sex ratio, fewer female plants had damaged flowers than expected by their frequency.
Table 4.
(a) Observed and expected frequency of female and hermaphroditic plants with their flowers damaged by herbivores in six populations of Geranium sylvaticum. Expected frequencies are based on population sex ratio. Results of G-test for each population are shown at right. (b) Results of heterogeneity G-test
| Observed damaged (expected damaged) |
||||||
|---|---|---|---|---|---|---|
| Population |
Females |
Hermaphrodites |
G |
|||
| (a) | ||||||
| P2 | 26 (38) | 175 (163) | 5·012* | |||
| P3 | 17 (17) | 59 (59) | 0·0002 | |||
| P4 | 3 (14) | 203 (192) | 13·041** | |||
| P6 | 11 (14) | 281 (278) | 0·798 | |||
| P8 | 14 (20) | 192 (186) | 2·002 | |||
| P13 | 34 (38) | 166 (162) | 0·438 | |||
|
G-test |
df |
G |
P |
|||
|---|---|---|---|---|---|---|
| (b) | ||||||
| ΣG | 6 | 21·291 | 0·002 | |||
| Pooled | 1 | 10·845 | 0·001 | |||
| Heterogeneity among populations | 5 | 10·446 | 0·064 | |||
0·01 ≤ P < 0·05,
0·001 ≤ P < 0·01.
DISCUSSION
Flowers of Geranium sylvaticum show pronounced sexual dimorphism. In addition, hermaphrodites produce significantly more flowers per plant than females, although marginally so (Asikainen and Mutikainen, 2003). Thus, it is not surprising that insect pollinators visited hermaphrodites more frequently than expected based on the population sex ratio in two of the study populations. Pollinators also discriminate against female flowers in several other gynodioecious species (Ashman and Stanton, 1991; Eckhart, 1991; Delph and Lively, 1992; Delph, 1996; Ashman, 2000; Williams et al., 2000). However, even if the number of flowers visited per plant was not significantly different between females and hermaphrodites, the observed difference (i.e. 1·98 vs 2·47) suggests that pollinators' preference for hermaphrodites may be a direct response to both the considerably larger petals and more numerous flowers of hermaphrodites. Note that since the pollinators in general visited more than one flower per plant, some of the hermaphrodites' flowers might be pollinated geitonogamously. If self-pollination is related to inbreeding depression, such behavior might actually select against the hermaphrodites (Morgan et al., 1997; Eckert, 2000; Williams et al., 2000). In fact, the results of a recent experiment suggest there is inbreeding depression in this species in terms of germination and juvenile survival (E. Asikainen and P. Mutikainen, unpubl. data). If inbreeding depression is also expressed in the early stages of seed development, geitonogamy might partly explain the lower seed set of hermaphrodites.
In one of the study populations, the sex ratio of visited plants did not differ from the population sex ratio. Since female frequency in this population is very low (4·6 %), and considerably lower than that of the two other populations studied here, it is possible that pollinators visited female plants in this population just by chance. Furthermore, this population differs from the other two in terms of habitat; the two populations where pollinators preferred hermaphrodites are located in forest habitats whereas the population where we found no preference is located in a meadow. Since both the habitat and sex ratio of this particular population differ from the other two and since we only used three populations, it is not possible to conclude whether pollinator preference is affected more by habitat type or by population sex ratio.
Hermaphrodite-biased visitation may contribute to pollen limitation of female plants and may further reduce the reproductive output of females. In several populations of G. sylvaticum, however, seed production has been found to be pollen-limited in both females and hermaphrodites (Asikainen and Mutikainen, 2005), despite the hermaphrodite-biased visitation of pollinators. Therefore, selection for pollinator attraction through female function should be expected in both sex morphs (Bawa, 1980; Campbell, 1989; Johnston, 1991). Thus, the dimorphism in petal size may not necessarily indicate stronger selection for pollinator attraction in hermaphrodites but, for example, differences in hormonal regulation or in constraints and correlations between petal size and other reproductive traits between the sex morphs (Delph et al., 1996; Ashman, 2000).
In addition to pollinators, large floral displays may attract other plant visitors, including pre-dispersal seed predators (Zimmerman, 1980a, b; Augspurger, 1981). For example, in the gynodioecious Dianthus sylvestris, the larger flowers of hermaphrodites have been found to suffer from higher risk of predation by seed predators than the smaller flowers of females (Collin et al., 2002). However, our results suggest that although pollinators prefer hermaphroditic flowers, seed predators of G. sylvaticum do not discriminate between the two sexes. Seed predators may not use the same cues as pollinators in their flower choice. It would be advantageous for an ovipositing insect to be able to choose flowers with a high probability of initiating a fruit with a high number of seeds in order to ensure good larval performance. Thus, one possible cause for not discriminating against female flowers would be the higher seed production of females. In G. sylvaticum, females produce from 1·2 to 1·7 times more seeds per flower than hermaphrodites (Asikainen and Mutikainen, 2003). However, the seed predators did not seem to prefer females. In addition, it is not known whether ovipositing insects can choose between fruits with different numbers of initiating seeds. A significant difference was also found in the average level of seed predation between the two study years, indicating that insect abundance at the flowering period is subject to yearly variation. However, it should be noted that since there were no significant interactions between year and plant sex or between year, plant sex and population, these results suggest that the non-selective behaviour of the seed-eating weevil was constant between the years. Thus, yearly variation in seed predation is highly unlikely to affect the relative seed fitness of females and hermaphrodites.
In four populations the floral herbivores did not discriminate between females and hermaphrodites. However, in two populations hermaphrodites were chosen more often than would be expected on the basis of population sex ratio. Floral herbivores have not always been observed to respond to floral display (e.g. Willson and Price, 1977; Augspurger, 1981; English-Loeb and Karban, 1992). In addition, Alonso (2003) found no preferences of leaf herbivores for either female or hermaphrodite plants in the gynodioecious Daphne laureola. On the other hand, floral herbivores that forage, for example, on the stamens are obviously expected to prefer the pollen-filled hermaphroditic flowers (Uno, 1982; Ashman, 2002). However, even if the hermaphrodites of G. sylvaticum have considerably larger petals and pollen-filled flowers, our results suggest that floral herbivores do not strictly prefer hermaphrodites over females.
Since the reproductive success of females depends on pollen supply, the flowering schedule of females should be strongly affected by the flowering schedule of hermaphrodites. If females flower too early the risk of pollen limitation increases. In fact, in all of our study populations female plants started flowering earlier than hermaphrodites. Thus, it seems that the flowering schedule of females is also affected by other biotic or abiotic factor(s). For example, pollinator discrimination may affect flowering schedule of females if pollinators discriminate against females less when only a small fraction of plants is flowering. On the other hand, early flowering may be a way to avoid floral herbivory. However, with the data available so far, it is not possible to conclude whether pollination success, predation or abiotic factors present the strongest selection pressures on flowering phenology in G. sylvaticum.
Maintenance of gynodioecy in Geranium sylvaticum
We studied whether pollinators, seed predators and floral herbivores choose between females and hermaphrodites of the gynodioecious Geranium sylvaticum. First, it was found that pollinators preferred hermaphrodites over female plants. This preference might increase the seed fitness of hermaphrodites relative to females since both females and hermaphrodites have been found to suffer from pollen limitation (Asikainen and Mutikainen, 2005). In spite of the pollinators' preference for hermaphrodites, females of G. sylvaticum produce significantly more seeds per flower than hermaphrodites (Asikainen and Mutikainen, 2003), which might partly be explained by geitonogamy (as suggested by the present data) and inbreeding depression or simply by a difference between females and hermaphrodites in the propensity to set fruit. Second, it was found that seed predators did not choose between females and hermaphrodites. This suggests that sex-biased seed predation does not contribute to the maintenance of females. Third, it was found that floral herbivores sometimes preferred hermaphrodites over female plants. Floral herbivores that prefer hermaphrodites may reduce the seed production of hermaphrodites more than that of females by consuming or damaging developing ovules, thus contributing to the difference in relative seed fitness between the two. Overall, pollinators preferred hermaphrodites on the whole 13 % more often than female plants whereas hermaphrodites were predated on the whole 8 % more often than female plants by floral herbivores. However, since the effects of pollinator visits on seed production were not measured, it is not possible to directly compare the effects of herbivore and pollinator preference on the maintenance of gynodioecy in G. sylvaticum. Taken together, our data supports the view that ecological factors may contribute to the maintenance of gynodioecy, but they also suggest that these contributions may vary across populations and that they may function in opposite directions.
Acknowledgments
We thank Riitta Ahonen, Mia Vuomajoki, Anne Muola, Niina Nurminen and Satu Ramula for their help in the field, and Lynda Delph, Veikko Salonen, Don Levin and two anonymous reviewers for their valuable comments on the manuscript. This study was funded by the Academy of Finland and the Graduate School of Evolutionary Ecology.
LITERATURE CITED
- Alonso C. 2003. Herbivores do not discriminate between leaves of female and hermaphrodite individuals of gynodioecious Daphne laureola (Thymelaeaceae). Oikos 101: 505–510. [Google Scholar]
- Ashman T.-L. 2000. Pollinator selectivity and its implications for the evolution of dioecy and sexual dimorphism. Ecology 81: 2577–2591. [Google Scholar]
- Ashman T.-L. 2002. The role of herbivores in the evolution of separate sexes from hermaphroditism. Ecology 83: 1175–1184. [Google Scholar]
- Ashman T.-L, Stanton ML. 1991. Seasonal variation in pollination dynamics of sexually dimorphic Sidalcea oregana ssp. spicata (Malvaceae). Ecology 72: 993–1003. [Google Scholar]
- Asikainen E, Mutikainen P. 2003. Female frequency and relative fitness of females and hermaphrodites in gynodioecious Geranium sylvaticum (Geraniaceae). American Journal of Botany 90: 226–234. [DOI] [PubMed] [Google Scholar]
- Asikainen E, Mutikainen P. 2005. Pollen and resource limitation in a gynodioecious species. American Journal of Botany (in press). [DOI] [PubMed] [Google Scholar]
- Augspurger CK. 1981. Reproductive synchrony of a tropical shrub: experimental studies on effects of pollinators and seed predators on Hybanthus pronifolius (Violaceae). Ecology 62: 775–788. [Google Scholar]
- Bawa KS. 1980. Mimicry of male by female flowers and intra-sexual competition for pollinators in Jacaratia dolichaula (D. Smith) Woodson (Caricaceae). Evolution 34: 467–474. [DOI] [PubMed] [Google Scholar]
- Brody AK. 1997. Effects of pollinators, herbivores, and seed predators on flowering phenology. Ecology 78: 1624–1631. [Google Scholar]
- Campbell DR. 1989. Inflorescence size: test of the male function hypothesis. American Journal of Botany 76: 730–738. [Google Scholar]
- Charlesworth B, Charlesworth D. 1978. A model for the evolution of dioecy and gynodioecy. American Naturalist 112: 975–997. [Google Scholar]
- Charlesworth D, Ganders FR. 1979. The population genetics of gynodioecy with cytoplasmic-genic male-sterility. Heredity 43: 213–218. [Google Scholar]
- Collin CL, Pennings PS, Rueffler C, Widmer A, Shykoff JA. 2002. Natural enemies and sex: how seed predators and pathogens contribute to sex-differential reproductive success in a gynodioecious plant. Oecologia 131: 94–102. [DOI] [PubMed] [Google Scholar]
- Conner JK, Rush S. 1996. Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum Oecologia 105: 509–516. [DOI] [PubMed] [Google Scholar]
- Cunningham SA. 1995. Ecological constraints on fruit initiation by Calyptrogyne ghiesbreghtiana (Arecaceae): floral herbivory, pollen availability, and visitation by pollinating bats. American Journal of Botany 82: 1527–1536. [Google Scholar]
- Delph LF. 1996. Flower size dimorphism in plants with unisexual flowers. In: Lloyd DG, Barrett SCH, eds. Floral biology: studies on floral evolution in animal pollinated plants. New York: Chapman and Hall, 217–237. [Google Scholar]
- Delph LF, Galloway LF, Stanton ML. 1996. Sexual dimorphism in flower size. American Naturalist 148: 299–320. [Google Scholar]
- Delph LF, Lively CM. 1992. Pollinator visitation, floral display, and nectar production of the sexual morphs of a gynoecious shrub. Oikos 63: 161–170. [Google Scholar]
- Eckhart VM. 1991. The effects of floral display on pollinator visitation vary among populations of Phacelia linearis (Hydrophyllaceae). Evolutionary Ecology 5: 370–384. [Google Scholar]
- Eckert CG. 2000. Contributions of autogamy and geitonogamy to self-fertilization in a mass-flowering, clonal plant. Ecology 81: 532–542. [Google Scholar]
- English-Loeb GM, Karban R. 1992. Consequences of variation in flowering phenology for seed head herbivory and reproductive success in Erigeron glaucus (Compositae). Oecologia 89: 588–595. [DOI] [PubMed] [Google Scholar]
- Johnson SE, Delph LF, Elderkin CL. 1995. The effect of petal-size manipulation on pollen removal, seed set, and insect behaviour in Campanula americana Oecologia 102: 174–179. [DOI] [PubMed] [Google Scholar]
- Johnston MO. 1991. Natural selection on floral traits in two species of Lobelia with different pollinators. Evolution 45: 1468–1480. [DOI] [PubMed] [Google Scholar]
- Karban R, Strauss SY. 1993. Effects of herbivores on growth and reproduction of their perennial host, Erigeron glaucus Ecology 74: 39–46. [Google Scholar]
- Krupnick GA, Weis AE, Campbell DR. 1999. The consequences of floral herbivory for pollinator service to Isomeris arborea Ecology 80: 125–134. [Google Scholar]
- Lewis D. 1941. Male sterility in natural populations of hermaphroditic plants. New Phytologist 40: 56–63. [Google Scholar]
- Lloyd DG. 1974. Theoretical sex ratios of dioecious and gynodioecious angiosperms. Heredity 32: 11–32. [Google Scholar]
- Marshall M, Ganders FR. 2001. Sex-biased seed predation and the maintenance of females in a gynodioecious plant. American Journal of Botany 88: 1437–1443. [PubMed] [Google Scholar]
- Morgan MT, Schoen DJ, Bataillon TM. 1997. The evolution of self-fertilization in perennials. American Naturalist 150: 618–638. [DOI] [PubMed] [Google Scholar]
- Norusis MJ. 1990.SPSS Advanced statistics user's guide. Chicago, IL: SPSS. [Google Scholar]
- Rathcke B. 1988. Flowering phenologies in a shrub community: competition and constraints. Journal of Ecology 76: 975–994. [Google Scholar]
- Rathcke B, Lacey EP. 1985. Phenological patterns of terrestrial plants. Annual Review of Ecology and Systematics 16: 179–214. [Google Scholar]
- Sokal RR, Rohlf FJ. 1981.Biometry, 2nd edn. New York: Freeman. [Google Scholar]
- Strauss SY, Conner JK, Rush S. 1996. Foliar herbivory affects floral characters and plant attractiveness to pollinators: implications for male and female plant fitness. American Naturalist 147: 1098–1107. [Google Scholar]
- Uno GE. 1982. Comparative reproductive biology of hermaphrodite and male-sterile Iris douglasiana Herb. (Iridaceae). American Journal of Botany 69: 818–823. [Google Scholar]
- Vaarama A, Jääskeläinen O. 1967. Studies in gynodioecism in the Finnish populations of Geranium sylvaticum L. Annales Academiae Scientiarum Fennicae. Series A. IV. Biologica 108: 1–39. [Google Scholar]
- Waser NM. 1978. Competition for hummingbird pollination and sequential flowering in two Colorado wildflowers. Ecology 59: 934–944. [Google Scholar]
- Waser NM, Price MV. 1981. Pollinator choice and stabilizing selection for flower color in Delphinium nelsonii Evolution 35: 376–390. [DOI] [PubMed] [Google Scholar]
- Waser NM, Price MV. 1983. Optimal and actual outcrossing in plants and the nature of plant-pollinator interaction. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold, 341–359. [Google Scholar]
- Williams CF, Kuchenreuther MA, Drew A. 2000. Floral dimorphism, pollination, and self-fertilization in gynodioecious Geranium richardsonii (Geraniaceae). American Journal of Botany 87: 661–669. [PubMed] [Google Scholar]
- Willson MF, Price PW. 1977. The evolution of inflorescence size in Asclepias (Asclepiadaceae). Evolution 31: 495–511. [DOI] [PubMed] [Google Scholar]
- Zar JH. 1996.Biostatistical analysis, 3rd edn. Upper Saddle River, New York: Prentice Hall. [Google Scholar]
- Zimmerman M. 1980. Reproduction in Polemonium: competition for pollinators. Ecology 61: 497–501. [Google Scholar]
- Zimmerman M. 1980. Reproduction in Polemonium: pre-dispersal seed predation. Ecology 61: 502–506. [Google Scholar]
- Zimmerman M. 1983. Plant reproduction and optimal foraging: experimental nectar manipulations in Delphinium nelsonii Oikos 41: 57–63. [Google Scholar]
- Zimmerman M. 1988. Nectar production, flowering phenology, and strategies for pollination. In: Lovett-Doust J, Lovett-Doust L, eds. Plant reproductive ecology: patterns and strategies. New York: Oxford University Press, 157–178. [Google Scholar]