Abstract
• Background and Aims The subfamily Salicornioideae (Chenopodiaceae) are a taxonomically difficult group largely due to the lack of diagnostic characters available to delineate tribal- and generic-level boundaries; a consequence of their reduced floral and vegetative features. This study examined the variation in fruits and seeds across both tribes of the Salicornioideae to assess if characters support traditional taxonomic sections.
• Methods Light microscopy, environmental scanning electron microscopy and anatomical ultra-thin sectioning were employed to examine variation in fruits and seeds. Sixty-eight representatives across 14 of the 15 genera currently recognized within the tribes Halopeplideae and Salicornieae were examined to determine whether characters support current taxonomic groups.
• Key Results Characters such as seed coat structure, embryo shape, seed orientation, the forms of seed storage proteins and carbohydrates show variation within the Salicornioideae and may be phylogenetically useful. The campylotropous ovule typical of the Chenopodiaceae generally results in a curved embryo; however, many Halosarcia and Sclerostegia species have straight embryos and in Salicornia and Sarcocornia the large peripheral embryo appears bent rather than curved. Seed coat ornamentation of Microcnemum and Arthrocnemum is distinct from other Salicornioideae as the elongated epidermal cells of the exotesta have convex walls. Histochemical stains of anatomical sections of cotyledon cells showed protein bodies were variable in shape, and starch grains were present in some species, namely Salicornia bigelovii, S. europaea and Allenrolfea occidentalis.
• Conclusions While fruits and seeds were found to be variable within the subfamily, no synapomorphic characters support the tribe Halopeplideae as these genera have crustaceous seed coats, curved embryos and abundant perisperm; features characteristic of many of the tribe Salicornieae. The endemic Australian genera are closely related and few seed and fruit characters are diagnostic at the generic level. Nineteen characters identified as being potentially informative will be included in future phylogenetic analyses of the subfamily.
Keywords: Morphology, anatomy, histochemistry, fruits, seeds, Salicornioideae, Chenopodiaceae, Sarcocornia, Salicornia, Arthrocnemum, Halosarcia
INTRODUCTION
The subfamily Salicornioideae Kostel. (Chenopodiaceae Juss.; APG, 2003), more commonly known as samphires or glassworts, are characterized by their distinctive reduced succulent leaves, which may be modified to form an articulated, photosynthetic stem (de Fraine, 1912). These specialized plants generally have spike-like compound inflorescences, comprised of paired cymules of tiny flowers that are sessile within succulent free or fused bracts (Scott, 1977; Kühn et al., 1993). Each cymule has (1–) 3 (–12) flowers. These flowers have 2–4 perianth lobes that may be free or fused almost to the apex, 1–2 stamens and a single ovary. In the Amaranthaceae the solitary ovule is basal, bitegmic, crassinucellar and campylotropous: where the embryo is curved and the micropyle is in relatively close proximity to the hilum (Bocquet, 1959; Connor, 1984; Johri et al., 1992). The ovule is comprised of an outer integument and an inner integument, each being 2–3 cells thick (Corner, 1976) and the micropyle is formed from the endostome (inner integument) (Johri et al., 1992; Werker, 1997).
The Salicornioideae are among the most salt-tolerant land plants (Short and Colmer, 1999; English, 2004) and frequently occur in saline areas associated with coastlines, tidal floodways and salt lakes (Waisel, 1972; Wilson, 1980; Davy et al., 2001). These halophytes are globally distributed, being found on every continent with the exclusion of Antarctica. Currently there are two tribes (Halopeplideae and Salicornieae), 15 genera (recognizing Sarcocornia as distinct from Salicornia) and approximately 80 species within the subfamily (Kühn et al., 1993) (Table 1).
Table 1.
The circumscription of the subfamily Salicornioideae Ulbr. sensu Kühn (1993), except Sarcocornia is recognized from Salicornia (Scott, 1977), including total number of species currently recognized and number of taxa sampled in the present study
| Taxon |
Distribution |
Total no. of species |
Total no. sampled |
|||
|---|---|---|---|---|---|---|
| Tribe Halopeplideae Ulbr. | ||||||
| Halopeplis Bunge ex Ung.-Sternb. | N and S Africa, W Asia, Mediterranean Europe | 3 | 1 | |||
| Kalidium Moq. in DC. | W and C Asia, Mediterranean Europe | 5 | 1 | |||
| Tribe Salicornieae Dumort | ||||||
| Allenrolfea O. Kuntze | N, C and S America | 2–3 | 1 | |||
| Arthrocnemum Moq. | S Africa, Asia, Mediterranean Europe, N America | 2 | 2 | |||
| Halocnemum M. Bieb. | W and C Asia, Mediterranean Europe | 1 | 1 | |||
| Halosarcia Paul G. Wilson | Australia, rarely S Asia and Africa | 23 | 40 | |||
| Halostachys C. Meyer ex Schrenk | SW and C Asia | 1 | ||||
| Heterostachys Ung.-Sternb. | S and C America | 2 | 1 | |||
| Microcnemum Ung.-Sternb. | Caucasus, Iran, Spain, Turkey | 1 | 1 | |||
| Pachycornia Hook.f. | Australia | 1 | 1 | |||
| Salicornia L. | Worldwide, except Australia | approx. 13 | 3 | |||
| Sarcocornia A.J. Scott | Worldwide | approx. 15 | 6 | |||
| Sclerostegia Paul G. Wilson | Australia | 5 | 6 | |||
| Tecticornia Hook.f. | Australia, New Guinea | 3 | 3 | |||
| Tegicornia Paul G. Wilson | Australia | 1 | 1 | |||
While long recognized as a natural group (Dumortier, 1827), the floral and vegetative features characteristic of the Salicornioideae are so modified that it is difficult to determine their phylogenetic relationships relative to other Chenopodiaceae. Moreover, recent molecular phylogenetic studies also show that the sister group relationship of the Salicornioideae is equivocal. Bienertia cycloptera Bunge (subfamily Suaedoideae Ulbr.) was placed sister to the monophyletic Salicornioideae based on both nrDNA Internal Transcribed Spacer (ITS) sequence data (Schütze et al., 2003) and cpDNA rbcL sequences (Kadereit et al., 2003). However, Bienertia cycloptera placed sister to Suaeda Forsk. ex Scop. in the same study by Schütze et al. (2003) based on analysis of cpDNA atpB-rbcL cpDNA sequence data. The lack of a clear sister group precludes the analysis of character evolution within the Salicornioideae.
The reduced morphology of the Salicornioideae also limits the availability of easily recognized diagnostic characters at the tribal and generic levels. Furthermore, the Salicornioideae exhibit considerable phenotypic variation at the population level (Wilson, 1980; Davy et al., 2001; Freitag et al., 2001) and taxonomic confusion is exacerbated by the occurrence of species complexes (Wilson, 1980) and polyploids (Contandriopoulos, 1968; Castroviejo and Coello, 1980; Castroviejo and Lago, 1992; Martínez and Herrera, 1996; Shepherd and Yan, 2003). The succulent vegetative morphology may also become modified when dried, limiting the use of herbarium material. As a result, the subfamilial and infrageneric relationships of the Salicornioideae are not fully clarified.
Although few vegetative and floral features are diagnostic in the Salicornioideae, seed characters have been recognized as potentially useful at both the generic and species levels (Moss, 1954; Tölken, 1967; Wilson, 1980). For example, key characters separating Sarcocornia A. J. Scott and Salicornia L. from other Salicornioideae include the presence of a horseshoe-shaped embryo and the absence of perisperm (Cooke, 1912; Standley, 1914; Scott, 1977; Kühn et al., 1993; Judd and Ferguson, 1999). In some respects mature fruits and seeds of the Salicornioideae are more convenient to use as taxonomic characters than vegetative and floral features as they are less inclined to exhibit variability in the field, are frequently retained on perennial plants for months or even years, and are likely to remain relatively unmodified upon drying.
The perianth of the Salicornioideae is persistent, and is retained in the mature fruit with the pericarp and seed. In the Chenopodiaceae the mature embryo is peripheral and annular (Martin, 1946; Prego et al., 1998), due to the development of a curved embryo sac through the elongation of the chalazal caecum (Pal et al., 1990). During embryo maturation the endosperm is largely consumed and may only persist as 1–2 layers near the micropyle surrounding the radicle. As the embryo fully develops it fills the horseshoe-shaped sac. This sac curves around the remaining nucellus material, which eventually becomes perisperm (Pal et al., 1990). The outer layer (exotesta) of the bitegmic seed coat develops from the outer epidermis of the outer integument while the endotegmen is derived from the inner epidermis of the inner integument (Wunderlich, 1968; Corner, 1976). Within the family there is considerable variation among genera in the shape of the outer epidermal cells of the exotesta (Corner, 1976), the size and shape of the embryo, and position and amount of perisperm.
There have been few global taxonomic treatments of the Salicornioideae and to our knowledge there has been no detailed survey examining morphological and anatomical characters of fruits and seeds of the subfamily. To gain a better understanding of the phylogenetic relationships in any focus group it is essential to determine diagnostic characters that are reliable and consistent across a wide range of taxa. This study examined the variation in fruits and seeds of both tribes of the Salicornioideae to identify characters that support current taxonomic classification of the subfamily sensu Kühn et al. (Kühn et al., 1993) (recognizing Sarcocornia). Furthermore, the fruit and seed features determined to be phylogenetically useful are included, along with other vegetative and floral characters, in a morphological phylogenetic analysis of the Salicornioideae (Shepherd et al., 2005).
MATERIALS AND METHODS
Fruit and seed characters were recorded from 68 taxa across 14 of the 15 genera currently recognized within the subfamily Salicornioideae (Table 1). Characters were scored from fresh material collected from 45 representatives of the six genera present within Australia: Halosarcia Paul G. Wilson, Pachycornia Hook.f., Sarcocornia, Sclerostegia Paul G. Wilson, Tecticornia Hook.f. and Tegicornia Paul G. Wilson. These included eight undescribed Halosarcia species and one undescribed Sclerostegia, identified with phrase names and collection numbers. Voucher specimens of the maternal plants were lodged with the Western Australian State Herbarium (PERTH). Characters of a further six described species and two undescribed species were scored using herbarium specimens already lodged at PERTH. Fresh specimens from the following countries were obtained: Arthrocnemum subterminalis Standl. and Sarcocornia pacifica (Standl.) A. J. Scott from Mexico; Salicornia procumbens Sm. from the Netherlands; Arthrocnemum macrostachyum (Morich.) Koch and Sarcocornia fruticosa (L.) A. J. Scott from Spain. Herbarium specimens of the following species were examined including: Halopeplis amplexicaulis Ung.-Sternb. ex Cesati, Passer. & Gibelli and Halocnemum strobilaceum M. Bieb. from the Institut Botánic de Barcelona (BC); Allenrolfea occidentalis Kuntze, Heterostachys ritteriana Ung.-Sternb., Salicornia bigelovii Torr., S. europaea L., and Sarcocornia perennis (Miller) A. J. Scott from the United States National Herbarium at the Smithsonian Institute (USNH) and Halosarcia cupuliformis Paul G. Wilson from the Royal Botanic Gardens Melbourne (MEL). Kalidium foliatum Moq. seed characters were scored from the literature (Gorshkova et al., 1970; Scott, 1977; Kühn et al., 1993).
Mature perianth, pericarp and seed coat morphology were examined using a dissection microscope and environmental scanning electron microscopy (ESEM) (Danilastos, 1993). Seeds of 23 species from 12 genera of the Salicornioideae were examined in detail after being embedded in glycol methacrylate (GMA) resin and sectioned using a Sorvall semi-automatic microtome following the methods of O'Brien and McCully (1981). Seeds were nicked using a scalpel and immediately fixed in 2·5–5·0 % glutaraldehyde in 0·05 m phosphate buffer (pH 7·0) for a minimum of 72 h at room temperature. Ultra-thin (2–4 µm) sections of the resin blocks were made using a glass knife and a Sorvall microtome in both the longitudinal (LS) and transverse (TS) planes. The thin sections were floated in water on a glass slide and melted onto the slide surface using a hotplate. Sections were stained with Toluidine Blue (pH 4·4), Periodic Acid-Schiff's reagent (PAS), Amido Black 10B (1 % in 7 % acetic acid) and Sudan Black B (in 70 % ethanol). Images were taken using a digital camera mounted on a Zeiss Akioskop Microscope.
RESULTS
Seed morphology
Perianth, pericarp and seed characters such as exotesta ornamentation, embryo shape and perisperm presence may be phylogenetically informative (Table 2), as they are variable amongst the Salicornioideae (Table 3).
Table 2.
Morphological and anatomical fruit and seed characters and character states that may be phylogenetically informative for the Salicornioideae (Chenopodiaceae)
| 1. Fruiting perianth texture: membranous; pithy; chartaceous; crustaceous; corky; woody. |
|---|
| 2. Fruiting perianth enclosure of the pericarp and seed: not enclosed; enclosed. |
| 3. Fruiting perianth dehiscence: does not split down the centre; splits down the centre. |
| 4. Pericarp texture: membranous; pithy; chartaceous; crustaceous; woody. |
| 5. Pericarp fusion to mature perianth: fused; free. |
| 6. Pericarp enclosure of the seed: not enclosed; enclosed. |
| 7. Pericarp fusion to the seed: completely fused, 1 = partially fused; free. |
| 8. Seed orientation 〈relative to the stem axis〉: Type 1; Type 2; Type 3; Type 4; Type 5 (see Fig. 1). |
| 9. Seed length/width ratio: 0·1–0·9; 1·0–1·9; 1·9–2·0; 2–2·9. |
| 10. Seed colour: fawn-white; light gold brown; reddish brown; brown; very dark red-brown to black; green. |
| 11. Seed transparency: opaque; translucent; transparent. |
| 12. Seed hairs: absent; present (see Fig. 2). |
| 13. Seed ornamentation: absent; present. |
| 14. Seed ornamentation type: Type 1; Type 2; Type 3; Type 4; Type 5 (see Fig. 3). |
| 15. Seed ornamentation distribution: all over; on the outer edge and part of central area; on the extreme outer margin; on the apex. |
| 16. Seed coat tannins: absent; present. |
| 17. Embryo shape: straight-slightly curved; curved; bent. |
| 18. Perisperm: present; absent. |
| 19. Perisperm volume: trace; medium to abundant. |
Table 3.
Fruit and seed characters of the Australian Salicornioideae and related genera
| Taxon |
Collection details* |
Collection country† |
Perianth texture |
Perianth enclosure |
Perianth dehiscence |
Pericarp texture |
Pericarp fusion to perianth |
Pericarp enclosure |
Pericarp adherence to seed |
Seed orientation |
Seed length (mm) |
Seed length/width ratio |
Seed colour |
Seed transparency |
Seed hair presence |
Seed ornamentation |
Seed ornamentation type |
Seed ornamentation distribution |
Seed coat tannins |
Embryo shape |
Perisperm presence |
Perisperm volume |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tribe Halopeplideae | ||||||||||||||||||||||||||||||||||||||||||||
| Halopeplis amplexicaulis Ung.-Sternb.ex Cesati, Passer. & Gibelli | BC# 653314 | EU | me | ne | ns | me | fu | ne | fr | 4 | 0·8 | 1·0–1·9 | gb | tp | ab | pr | 1 | oe | ab | cu | pr | an | ||||||||||||||||||||||
| Kalidium foliatum Moq. | pi | ne | ns | me | ne | fr | 4 | 1·0 | ab | pr/ab | cu | pr | an | |||||||||||||||||||||||||||||||
| Tribe Salicornioideae | ||||||||||||||||||||||||||||||||||||||||||||
| Allenrolfea occidentalis O. Kuntze | US# 1634018 | EU | me | ne | – | me | fr | ne | fr | 1 | 1·0 | 1·0–1·9 | br | op | ab | ab | – | – | ab | cu | pr | an | ||||||||||||||||||||||
| Arthrocnemum macrostachyum (Moric.) Koch | BD1 | EU | pi | ne | ns | me | fu | ne | fr | 4 | 1·3 | 1·0–1·9 | bl | op | ab | pr | 2 | al | pr | cu | pr | an | ||||||||||||||||||||||
| A. subterminalis Standl. | EST1 | AM | pi | ne | ns | ch | fr | ec | fr | 4 | 1·2 | 1·0–1·9 | br | op | ab | pr | 2 | oc | ab | cu | pr | an | ||||||||||||||||||||||
| Halocnemum strobilaceum M. Bieb. | BC# 640555 | EU | me | ne | – | me | fr | ne | fr | 3 | 0·9 | 1·0–1·9 | br | tl | ab | pr | 1 | oe | pr | cu | pr | an | ||||||||||||||||||||||
| Halosarcia auriculata Paul G. Wilson | KS814 | AU | cr | ec | sp | me | fr | ne | fr | 4 | 1·0 | 1·0–1·9 | gb | tl | ab | pr | 1 | oc | st | pr | an | |||||||||||||||||||||||
| H. bulbosa Paul G. Wilson | KS907 | AU | cr | ec | sp | ch | fr | ne | fr | 4 | 1·5 | 1·0–1·9 | gb | op | ab | ab | – | – | st | pr | an | |||||||||||||||||||||||
| H. calyptrata Paul G. Wilson | PGW8942 | AU | cr | ec | ns | wd | fr | ne | fr | 4 | 1·0–1·1 | 1·0–1·9 | br | tl | ab | pr | 1 | oc | st | pr | an | |||||||||||||||||||||||
| H. calyptrata Paul G. Wilson | KS822 | AU | wd | ec | ns | wd | fr | ne | fr | 4 | 0·9 | 1·0–1·9 | gb | tp | ab | ab/pr | 1 | ap | st | pr | an | |||||||||||||||||||||||
| H. chartacea Paul G. Wilson | KS539 | AU | ch | ec | ns | me | fr | ne | fr | 1/2 | 1·5 | 1·0–1·9 | gb | tp | ab | ab | – | – | cu | pr | an | |||||||||||||||||||||||
| H. cymbiformis MS | KS869 | AU | ch | ec | ns | ch | fr | ne/ec | pa | 4 | 1·0 | 0·1–0·9 | br | op | ab | ab | – | – | st | pr | an | |||||||||||||||||||||||
| H. cupuliformis Paul G. Wilson | MEL# 651604 | AU | me | ne | – | me | fu | ne | fr | 4 | 1·3 | br | op/tl | ab | pr | al/oc | st | pr | an | |||||||||||||||||||||||||
| H. doleiformis Paul G. Wilson | KS762 | AU | pi | ne | ns | me | fu | ne | fr | 4 | 0·9 | 1·0–1·9 | bl | op | ab | pr | 3 | al | st | pr | an | |||||||||||||||||||||||
| H. entrichoma Paul G. Wilson | KS891 | AU | cr | ec | ns | me | fr | ne | fr | 4 | 2·0–2·2 | 1·0–1·9 | gb | tp | ab | ab | – | cu | pr | an | ||||||||||||||||||||||||
| H. fimbriata Paul G. Wilson | KS873 | AU | me | ne | ns | me | fu | ne | fr | 4 | 0·7 | 1·0–1·9 | gb | tl | ab | pr | 1 | oe | cu | pr | an | |||||||||||||||||||||||
| H. flabelliformis Paul G. Wilson | KS883 | AU | me | ne | – | me | fr | ne | fr | 1/2 | 1·2 | 1·0–1·9 | br | tl | ab | pr | 1 | oe | cu | pr | an | |||||||||||||||||||||||
| H. halocnemoides (Nees) Paul G. Wilson subsp. catenulata Paul G. Wilson | PGW8585 | AU | me | ne | ns | me | fu | ne | fr | 4 | 0·9 | 1·0–1·9 | rb | op | ab | pr | 1 | oe | st | pr | an | |||||||||||||||||||||||
| H. halocnemoides (Nees) Paul G. Wilson subsp. caudata Paul G. Wilson | ASG9357 | AU | me | ne | ns | me | fu | ne | fr | 4 | 0·9–1·0 | 1·0–1·9 | rb | op | ab | pr | 1 | oc | st | pr | an | |||||||||||||||||||||||
| H. halocnemoides (Nees) Paul G. Wilson subsp. halocnemoides | KS740 | AU | me | ne | ns | me | fu | ne | fr | 4 | 1·0 | 1·0–1·9 | rb | op | ab | pr | 1 | oe | pr | st | pr | an | ||||||||||||||||||||||
| H. halocnemoides (Nees) Paul G. Wilson subsp. longispicata Paul G Wilson | IC3163 | AU | me | ne | ns | me | fu | ne | fr | 4 | 0·9 | 1·0–1·9 | br | op | ab | pr | 1 | oe | st | pr | an | |||||||||||||||||||||||
| H. halocnemoides (Nees) Paul G. Wilson subsp. tenuis Paul G. Wilson | KS791 | AU | me | ne | ns | me | fu | ne | fr | 4 | 0·7–1·0 | 1·0–1·9 | rb | op | ab | pr | 1 | al | st | pr | an | |||||||||||||||||||||||
| H. indica (Willd.) Paul G. Wilson subsp. bidens (Nees) Paul G. Wilson | KS761 | AU | pi | ec | ns | wd | fu | ec | fr | 4 | 0·9 | 1·0–1·9 | gb | tl | ab | ab | – | – | st | pr | an | |||||||||||||||||||||||
| H. indica (Willd.) Paul G. Wilson subsp. indica | KS783 | AU | pi | ec | ns | wd | fu | ec | fr | 4 | 1·1 | 1·0–1·9 | gb | tl | ab | ab | – | – | cu | pr | an | |||||||||||||||||||||||
| H. indica (Willd.) Paul G. Wilson subsp. julacea Paul G. Wilson | KS776 | AU | pi | ec | ns | ch | fr | ne | fr | 4 | 0·9–1·4 | 0·1–0·9 | gb | op | ab | ab | – | – | cu | pr | an | |||||||||||||||||||||||
| H. indica (Willd.) Paul G. Wilson subsp. leiostachya (Benth.) Paul G. Wilson | IC3157 | AU | pi | ne | ns | wd | fu | ec | fr | 4 | 1·1 | 1·0–1·9 | gb | tl | ab | ab | – | – | st | pr | an | |||||||||||||||||||||||
| H. lepidosperma Paul G. Wilson | KS704 | AU | pi | ne | ns | me | fu | ne | fr | 1 | 1·4 | 1·0–1·9 | fw | op | ab | pr | 4 | al | ab | cu | pr | an | ||||||||||||||||||||||
| H. leptoclada Paul G. Wilson subsp. inclusa Paul G. Wilson | KS888 | AU | ch | ec | ns | me | fr | ne | fr | 4 | 1·2 | 1·0–1·9 | gb | tl | ab | ab | – | – | ab | cu | pr | an | ||||||||||||||||||||||
| H. lylei (Ewart et White) Paul G. Wilson | KS711 | AU | pi | ne | ns | me | fu | ne | fr | 1 | 0·8 | 1·0–1·9 | rb | op | ab | pr | 3 | oe | cu | pr | an | |||||||||||||||||||||||
| H. peltata Paul G. Wilson | KS571 | AU | pi | ec | ns | ch | fr | ec | fr | 1 | 0·9 | 1·0–1·9 | br | op | ab | ab | – | – | st | pr | an | |||||||||||||||||||||||
| H. pergranulata (Black) Paul G. Wilson subsp. divaricata Paul G. Wilson | IC3189 | AU | me | ne | ns | me | fu | ne | fr | 4 | 1·0 | 1·0–1·9 | bl | op | ab | pr | 3 | al | st | pr | an | |||||||||||||||||||||||
| H. pergranulata (Black) Paul G. Wilson subsp. elongata Paul G. Wilson | IC3154 | AU | me | ne | ns | me | fu | ne | fr | 4 | 0·8 | 1·0–1·9 | rb/bl | op | ab | pr | 3 | al | st | pr | an | |||||||||||||||||||||||
| H. pergranulata (Black) Paul G. Wilson subsp. pergranulata | KS738 | AU | me | ne | ns | me | fu | ne | fr | 4 | 0·8–1·1 | 1·0–1·9 | rb/bl | op | ab | pr | 3 | al | pr | cu | pr | an | ||||||||||||||||||||||
| H. pergranulata (Black) Paul G. Wilson subsp. queenslandica Paul G. Wilson | KS788 | AU | me | ne | ns | me | fu | ne | fr | 4 | 0·8 | 1·0–1·9 | rb/bl | op | ab | pr | 3 | al | cu | pr | an | |||||||||||||||||||||||
| H. pruinosa (Paulsen) Paul G. Wilson | KS769 | AU | cr | ec | sp | me | fr | ne | fr | 4 | 0·7–0·8 | 1·0–1·9 | gb | tl | ab | ab | – | – | ab | st | pr | an | ||||||||||||||||||||||
| H. pterygosperma (Black) Paul G. Wilson subsp. pterygosperma | KS666 | AU | me | ne/ec | ns | me | fu | ne | fr | 1 | 1·0 | 1·0–1·9 | fw | op | ab | pr | 4 | al | ab | st | pr | an | ||||||||||||||||||||||
| H. syncarpa Paul G. Wilson | KS721 | AU | ch | ec | sp | ch | fr | ne | fu | 4 | 1·0 | 1·0–1·9 | gb | op | ab | pr | 1 | oc | st | pr | an | |||||||||||||||||||||||
| H. undulata Paul G. Wilson | KS766 | AU | me | ec | sp | ch | fr | ne | fr | 4 | 0·8 | 1·0–1·9 | gb | tp | ab | ab | – | – | st | pr | an | |||||||||||||||||||||||
| H. sp. Angelfish Island (B. Davey 4) | KSQ7 | AU | me | ec | sp | me | fr | ec | pa | 2 | 1·0 | 1·0–1·9 | br | op | ab | pr | 1 | oc | st | pr | an | |||||||||||||||||||||||
| H. sp. Angelfish Island (B. Davey 4) affn. | KS864 | AU | me | ec | sp | me | fr | ec | fr | 2 | 0·9–1·0 | 1·0–1·9 | br | op | ab | pr | 1 | oc | st | pr | an | |||||||||||||||||||||||
| H. sp. Gunyidi (M.N. Lyons 2607) | KS897 | AU | me | ec | sp | cr | fu | ec | fr | 4 | 2·2–2·5 | 2·0–2·9 | gr | tp | ab | ab | – | – | st | pr | an | |||||||||||||||||||||||
| H. sp. Little Sandy Desert (K. Shepherd & C. Wilkins KS830) | KS829 | AU | me | ec | ns | me | fr | ne | fr | 4 | 0·9 | 1·0–1·9 | bl | op | ab | pr | 3 | oe | cu | pr | an | |||||||||||||||||||||||
| H. sp. Lake Moore (M.N. Lyons 2603) | KS901 | AU | me | ne | ns | me | fu | ne | fr | 4 | 0·9 | 1·0–1·9 | br | op | ab | pr | 1 | oc | st | pr | an | |||||||||||||||||||||||
| H. sp. Roy Hill (H. Pringle) | HP62 | AU | me | ne | ns | me | fu | ne | fr | 4 | 0·9 | 1·0–1·9 | rb | op | ab | pr | 3 | oe | cu | pr | an | |||||||||||||||||||||||
| H. sp Sunshine Lake (K. Shepherd KS867) | KS867 | AU | me | ne | ns | me | fu | ne | fr | 4 | 0·7 | 1·0–1·9 | gb | op | ab | ab | – | – | st | pr | an | |||||||||||||||||||||||
| H. sp. Yanneri Lake (S. van Leeuwen 3002) | KS836 | AU | pi | ec | ns | wd | fu | ec | fr | 4 | 1·0 | 1·0–1·9 | gb | tl | ab | ab | – | – | st | pr | an | |||||||||||||||||||||||
| Heterostachys ritteriana Ung.-Sternb. | US# 1930104 | SM | me | ne | ns | me | fr | ne | fr | 3 | 0·9 | 1·0–1·9 | br | op | ab | pr | 1 | al | pr | cu | pr | an | ||||||||||||||||||||||
| Microcnemum coralloides (Loscos & Pardo) Font Quer | BC# 84814 | EU | me | ne | ns | me | fr | ne | fr | 4 | 0·9 | 1·0–1·9 | bl | op | ab | pr | 2 | al | cu | pr | an | |||||||||||||||||||||||
| Pachycornia triandra (F. Muell.) Black | KS675 | AU | me | ne | sp | wd | fr | ec | fr | 4 | 2·8–3·1 | 1·0–1·9 | gb | op | ab | ab | – | – | ab | cu | pr | an | ||||||||||||||||||||||
| Salicornia bigelovii Torr. | US# 788805 | AM | pi | ne | ns | me | fu | ne | fr | 4 | 1·6 | 1·0–1·9 | gb | op | ab | pr | 5 | al | be | ab | ab | |||||||||||||||||||||||
| S. europaea L. | US# 1203027 | EU | pi | ne | ns | me | fu | ne | fr | 4 | 1·3–1·7 | 1·0–1·9 | gb | op | pr | ab | – | – | be | ab | ab | |||||||||||||||||||||||
| S. procumbens Sm. | BPK | EU | pi | ne | ns | me | fu | ne | fr | 4 | 1·7 | 1·0–1·9 | gr | tp | pr | ab | – | – | be | ab | ab | |||||||||||||||||||||||
| Sarcocornia blackiana (Ulbr.) A. J. Scott | KS734 | AU | pi | ne | ns | me | fu | ne | fr | 4 | 1·0–1·4 | 1·0–1·9 | fw | op | ab | pr | 5 | al | ab | be | pr | tr | ||||||||||||||||||||||
| S. fruticosa (L.) A. J. Scott | BD9 | EU | pi | ne | ns | me | fu | ne | fr | 4 | ab | ab | ab | |||||||||||||||||||||||||||||||
| S. globosa Paul G. Wilson | KS644 | AU | pi | ne | ns | me | fu | ec | pa | 4 | 1·8–2·3 | 1·0–1·9 | gb | op | ab | ab | – | – | ab | be | pr | tr | ||||||||||||||||||||||
| S. pacifica Standl. (A. J. Scott) | EST3 | AM | pi | ne | ns | me | fu | ne | fr | 4 | 1·1 | 1·0–1·9 | br | op | ab | pr | 5 | al | be | ab | ab | |||||||||||||||||||||||
| S. perennis (Miller) A. J. Scott | US# 1222247 | AM | pi | ne | ns | me | fu | ne | fr | 4 | 1·0 | 1·0–1·9 | gb | op | ab | pr | 5 | al | be | ab | ab | |||||||||||||||||||||||
| S. quinqueflora (Ung.-Sternb.) A. J. Scott subsp. quinqueflora | KS774 | AU | pi | ne | ns | me | fr | ne | fr | 4 | 1·1 | 1·0–1·9 | fw | op | ab | pr | 5 | al | ab | be | pr | tr | ||||||||||||||||||||||
| Sclerostegia arbuscula (R. Br.) Paul G. Wilson | JGC39 | AU | me | ec | sp | cr | fr | ec | fr | 4 | 1·8 | 2·0–2·9 | gb | tp | ab | ab | – | – | st | pr | an | |||||||||||||||||||||||
| S. disarticulata Paul G. Wilson | KS903 | AU | me | ne | ns | cr | fu | ec | fr | 4 | 1·0 | 1·0–1·9 | gb | tp | ab | ab | – | – | st | pr | an | |||||||||||||||||||||||
| S. moniliformis Paul G. Wilson | KS732 | AU | me | ne | sp | cr | fr | ec | fr | 4 | 2·0 | 2·0–2·9 | gb | op | ab | ab | – | – | ab | st | pr | an | ||||||||||||||||||||||
| S. tenuis (Benth.) Paul G. Wilson | KS482 | AU | me | ne | sp | cr | fr | ec | fr | 4 | 2·7 | 2·0–2·9 | gb | tp | ab | ab | – | – | st | pr | an | |||||||||||||||||||||||
| S. sp. Blue Hill (D. J. Edinger Nats 61) | DJE 61 | AU | me | ne | ns | cr | fu | ec | fr | 4 | 1·0 | 1·0–1·9 | gb | tp | ab | ab | – | – | st | pr | an | |||||||||||||||||||||||
| S. sp. Hamersley Lake (K. Shepherd KS887) | KS887 | AU | me | ne | sp | me | fr | ec | fr | 4 | 1·4 | 1·0–1·9 | gb | op | ab | pr | 1 | oe/ap | st | pr | an | |||||||||||||||||||||||
| Tecticornia arborea Paul G. Wilson | RJC7548 | AU | me | ec | – | me | fr | ne | fr | 1 | 1·4 | 1·0–1·9 | rb | op | ab | pr | 1 | al | pr | cu | pr | an | ||||||||||||||||||||||
| T. australasica (Moq.) Paul G. Wilson | PKL 10830 | AU | me | ec | – | me | fr | ne | pa | 1 | 1·4 | 1·0–1·9 | gr | tp | ab | pr | 1 | al | cu | pr | an | |||||||||||||||||||||||
| T. verrucosa Paul G. Wilson | KS556 | AU | me | ec | – | me | fr | ne | fr | 1 | 1·9 | 1·0–1·9 | rb | op | ab | pr | 1 | al | pr | cu | pr | an | ||||||||||||||||||||||
| Tegicornia uniflora Paul G. Wilson | KS749 | AU | me | ec | ns | me | fu | ne | fr | 5 | 1·6 | 1·0–1·9 | bl | op | ab | pr | 4 | al | pr | cu | pr | an | ||||||||||||||||||||||
Character states: – = not applicable; me = membranous; pi = pithy; ch = chartaceous; cr = crustaceous; wd = woody; ne = not enclosed; ec = enclosed; ns = does not split centrally; sp = splits down the centre;fu = fully adhere; fr = free; pa = partially adhere; fw = fawn-white; gb = light gold brown; rb = reddish brown; br = brown; bl = black; gr = green; op = opaque; tl = translucent; tp = transparent; pr: present; ab = absent;al = all over; oc = on outer edge and central area; oe = on outer edge; ap = apex; st = straight – slightly curved; cu = curved; be = bent; tr = trace; an = abundant.
Collectors: KS = Kelly Shepherd; PGW = Paul G. Wilson; ASG = Alex S. George; IC = Ian Clarke; JGC = J. Gibbs-Clema; HP = Hugh Pringle; DJE = DJ Edinger; RJC = Ray J. Cranfield; PKL = PK Latz;BPK = BP Koustaal; BD = Bernard Dudley; EST = Michelle Waycott; Herbaria: US = United States National Herbarium at the Smithsonian Institute, BC = Institut Botánic de Barcelona, MEL = Royal Botanic Gardens Melbourne.
Collection distribution: EU = Europe; AM = North America and Mexico; SM = South America, AU = Australia.
Mature perianth and pericarp
The texture of the mature perianth of the Salicornioideae may be soft and characterized as membranous, pithy or chartaceous or hardened, appearing crustaceous, corky or woody. At maturity, the perianth may or may not enclose the pericarp and seed. If the perianth encloses the pericarp and seed, it can dehisce centrally (Table 2). Perianth dehiscence was not scored for Allenrolfea occidentalis and Halocnemum strobilaceum as the perianth lobes are completely free at anthesis. Similarly, the perianth lobes in the Australian species Halosarcia cupuliformis, H. flabelliformis Paul G. Wilson and the Tecticornia species separate into free lobes prior to development of the fruit (Table 3).
The pericarp of the Salicornioideae is also variable in texture and in some instances may be fused to the perianth. In such cases, the pericarp may be difficult to discern. The pericarp may also partially or fully adhere to the outer surface of the seed (Tables 2 and 3).
Seed orientation
Salicornioideae seeds have a basal placentation, congruent with other representatives of the Chenopodiaceae. However, seed orientation is variable at the species level. The position of each seed can be described relative to the axis of the inflorescence stem and a perpendicular line pointing outwards (represented by the x-axis) (Fig. 1). There are five types of seed orientation evident in the Salicornioideae. Type 1 seeds are horizontal, with the longitudinal plane of the seed aligned along the x-axis, perpendicular to the inflorescence axis (Fig. 1A). Ten of the Salicornioideae taxa examined have horizontal seeds (Table 3). In these species, the longitudinal plane of the flower may also be aligned horizontally or tend towards a more vertical angle relative to the seed. Type 2 seeds are also aligned horizontally but each seed is rotated 90° to the left or right around the pole of the x-axis to lie flat on one side (Fig. 1B), while the longitudinal plane of the flower remains horizontal. Type 2 seeds were observed in only four species including Halosarcia chartacea Paul G. Wilson. Type 3 seeds are similarly aligned on the horizontal plane and rotated flat to one side, but the seed is twisted a further 45° to the left or right away from the x-axis (Fig. 1C). Type 3 seeds were observed only in Halocnemum strobilaceum and Heterostachys ritteriana. Their flowers appear to remain in a horizontal position. Type 4 seeds are orientated vertically, with the longitudinal plane of the seed aligned along a 45–90° angle from the x-axis (Fig. 1D). Of the 68 Salicornioideae taxa examined in this study, 53 have vertical seeds (Table 3). The orientation of the flower in these species is variable and it may be aligned horizontally or more vertically relative to the seed. Type 5 seeds are also orientated in the vertical position; however, the seed is rotated 90° to the left or right around the pole of the y-axis (Fig. 1E). Type 5 seeds have only been observed in Tegicornia uniflora Paul G. Wilson.
Fig. 1.
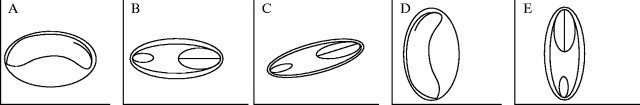
Diagram of the orientation of Salicornioideae seeds showing the position of the embryo as viewed from the side. Seed orientation is described relative to the orientation of the main inflorescence stem (y-axis) and a perpendicular line pointing outwards (x-axis). The number of taxa that exhibit each orientation type are given in brackets (see Table 3): (A) Type 1 seeds are horizontal (10); (B) Type 2 seeds are horizontal but have rotated to lie flat on one side (4); (C) Type 3 seeds are similar to Type 2 seeds but have rotated a further 45° away from the x-axis (2); (D) Type 4 seeds are vertical in orientation (53), and (E) Type 5 seeds are also vertical but have rotated to lie flat on one side (1).
Seed orientation is usually consistent within a species; however, there are notable exceptions. Whilst the mature seeds of Pachycornia triandra (F. Muell.) Black are generally orientated vertically (Type 4, Fig. 1D), some seeds may rotate upside down relative to the stem axis within the lignified fruit. In Halosarcia chartacea and H. flabelliformis, the seeds within each triad of fruits orientate differently. The central seed has a Type 1 configuration, whilst the two lateral seeds have a Type 2 configuration.
Seed size and shape
The seeds of the Salicornioideae range from 0·7–3·1 mm in length (Table 3). Halosarcia halocnemoides (Nees) Paul G. Wilson subsp. tenuis Paul G. Wilson, H. fimbriata Paul G. Wilson, H. pruinosa (Paulsen) Paul G. Wilson and H. sp. Sunshine Lake (K. Shepherd 867) have the smallest seeds at 0·7 mm long, while Pachycornia triandra seeds are the largest at 2·8–3·1 mm long. The majority of Salicornioideae seeds are less than or equal to 1·5 mm in length; however, nine species had longer seeds.
Salicornioideae seeds are round to ellipsoid in shape and may be categorized based on length–width ratios (Table 2). In most species the length-to-width ratio (L : W) is 1·0–1·9 : 1 (Table 3). Exceptions are Halosarcia cymbiformis ms and H. indica (Willd.) Paul G. Wilson subsp. julacea Paul G. Wilson where the seed L : W is 0·1–0·9 : 1. In contrast, long narrow seeds are present in H. sp. Gunyidi (M.N. Lyons 2607), Sclerostegia arbuscula (R. Br.) Paul G. Wilson, S. moniliformis Paul G. Wilson and S. tenuis (Benth.) Paul G. Wilson, which have a L : W of 2·0–2·9 : 1.
Seed colour and transparency
Salicornioideae seeds are monochrome ranging from pale fawn to light gold-brown, reddish brown, brown, or black (Tables 2 and 3). Halosarcia sp. Gunyidi (M.N. Lyons 2607), Salicornia procumbens and Tecticornia australasica (Moq.) Paul G. Wilson have green seeds. The colour of Halosarcia pergranulata (Black) Paul G. Wilson seeds may vary within an inflorescence, appearing either reddish brown or black.
The transparency of the exotesta also varies (Table 2). Most Salicornioideae seeds appear opaque but some are translucent with a faint outline of the embryo and perisperm visible. Other species have an almost transparent exotesta and the embryo is clearly evident. In the almost transparent seeds, the seed colour is determined by the colour of the embryo; for example species with a green embryo have green seeds (Table 3).
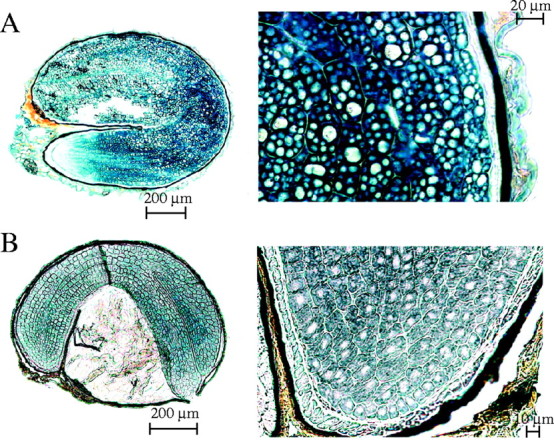
Seed hairs and exotesta ornamentation
Salicornia europaea and S. procumbens are the only species examined where the outer epidermal cells of the outer integument have elongated to form simple hairs (Fig. 2; Table 2). The remaining Salicornioideae seeds are glabrous, as exemplified by Sclerostegia moniliformis (Fig. 3A), or ornamented with papilloid projections formed by radial elongation of outer epidermal cells of the exotesta (Fig. 3B–F). The form and distribution of exotesta ornamentation varies significantly (Table 3). Forty-one taxa with exotesta ornamentation were classified into five types (Table 2). In seeds with Type 1 ornamentation, single outer epidermal cells of the exotesta elongate radially to form small, rounded bumps. The elongation of these cells occurs in longitudinal rows that are separated by epidermal cells that have not elongated. Twenty-one taxa have this form of ornamentation as exemplified by Halosarcia halocnemoides (Nees) Paul G. Wilson subsp. caudata Paul G. Wilson (Fig. 3B) (Table 3). In seeds with Type 2 ornamentation the walls of the outer epidermal cells of the exotesta are convex with undulate walls, which form uneven bumps. This ornamentation type was observed in Arthrocnemum macrostachyum (Fig. 3C), A. subterminalis and Microcnemum coralloides. In Type 3 ornamented seeds all of the outer testa epidermal cells have elongated radially to form uniserate longitudinal ridges that form densely packed mammilate projections. Eight taxa have Type 3 ornamentation including all of the subspecies of H. pergranulata (Fig. 3D). Similarly, in seeds with Type 4 ornamentation there is radial elongation of the outer epidermal cells of the exotesta but these cells have continued to elongate, narrowing near the apex to form fan-like ridges as evident in Tegicornia uniflora (Fig. 3E), H. pterygosperma (Black) Paul G. Wilson and H. lepidosperma Paul G. Wilson (Fig. 3F). In Type 5 ornamented seeds the outer exotesta epidermal cells exhibit uneven elongation to form rounded or pointed projections, as illustrated in Sarcocornia quinqueflora (Ung.-Sternb.) A. J. Scott subsp. quinqueflora (Fig. 3G). Sarcocornia blackiana (Ulbr.) A. J. Scott, Salicornia bigelovii, S. pacifica and S. perennis also have Type 5 seed ornamentation.
Fig. 2.
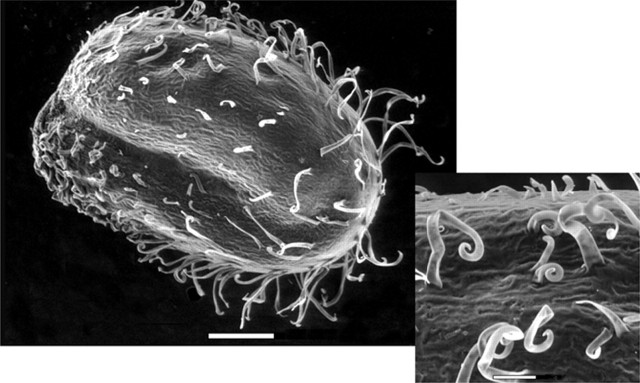
Environmental scanning electron micrograph of Salicornia europaea L. seed showing the elongation of the outer epidermal cells of the testa to form simple hairs. Scale bars = 200 μm, and 50 μm in the inset.
Fig. 3.
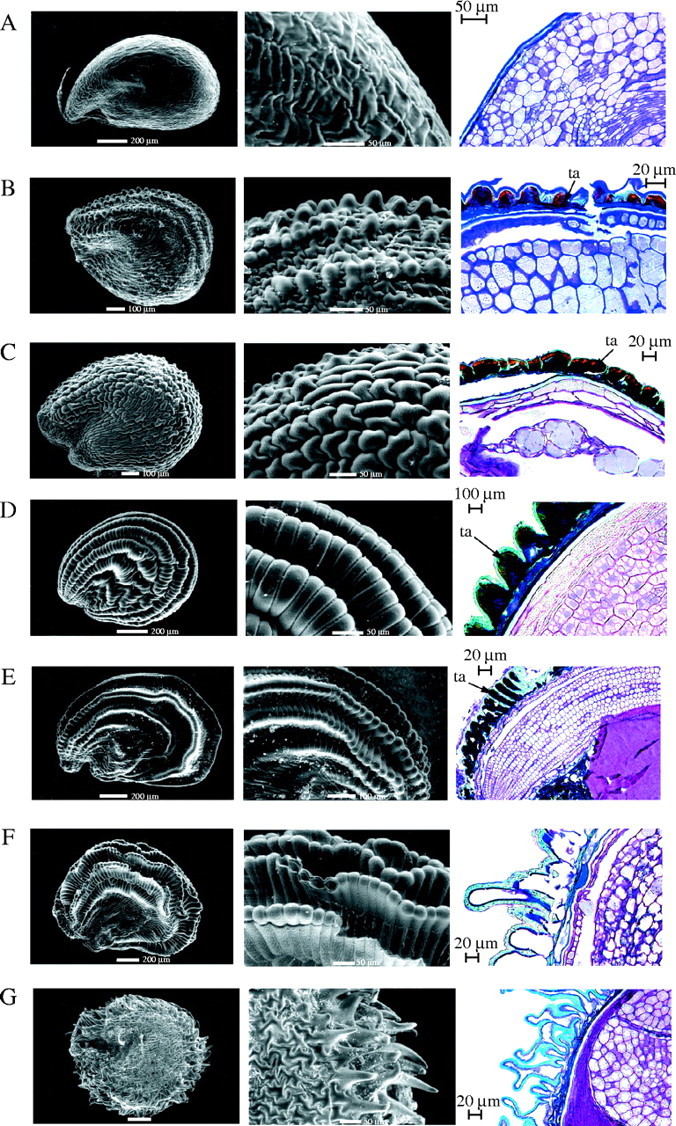
Environmental scanning electron micrographs and light micrographs of longitudinal anatomical sections of Salicornioideae seeds showing seed coat ornamentation, as recorded in Table 3. The number of taxa examined which exhibit each type is given in brackets: (A) absent in Sclerostegia moniliformis Pau G. Wilson (25); (B) raised into small rounded bumps (Type 1) in Halosarcia halocnemoides (Nees) Paul G. Wilson subsp. caudata Paul G. Wilson (21); (C) uneven and cone-shaped (Type 2) in Arthrocnemum macrostachyum (Moric.) Koch (3); (D) mammilate projections closely packed together (Type 3) in Halosarcia pergranulata (Black) Paul G. Wilson subsp. pergranulata (9); (E) fan-like ridges (Type 4) in Tegicornia uniflora Paul G. Wilson and (F) Halosarcia lepidosperma Paul G. Wilson (3); and (G) uneven finger-like projections (Type 5) in Sarcocornia quinqueflora (Ung.-Sternb.) A. J. Scott subsp. quinqueflora (5). ta = tannin filled epidermal cells of the outer exotesta. Scale bar in left and middle columns, respectively; 200 μm and 50 μm in (A), (D), (F) and (G); 100 μm and 50 μm in (B) and (C); and 200 μm and 100 μm in (E).
The position of the ornamentation of the exotesta also varies. Some taxa exhibit patterns over the entire seed surface, whereas other taxa are ornamented on the central region and outer edge (margin opposite the hilum), the outer edge only, or at the apex of the seed (Tables 2 and 3). Areas of the seed surface without ornamentation are glabrous.
Seed coat ornamentation is generally related to the hardness of the perianth or pericarp. Seeds with either a firm perianth or pericarp usually have a glabrous seed coat and, conversely, seeds with a soft-textured perianth and pericarp are ornamented. Exceptions include taxa that have a soft perianth or pericarp and yet have glabrous seeds, as observed in Allenrolfea occidentalis, Halosarcia chartacea, H. cupuliformis, H. leptoclada Paul G. Wilson subsp. inclusa Paul G. Wilson, H. peltata Paul G. Wilson, H. sp. Sunshine Lake (K. Shepherd 867) and Sarcocornia globosa. In addition, ornamented seeds are present in H. auriculata Paul G. Wilson, which has a crustaceous perianth, and in H. calyptrata Paul G. Wilson that has a woody pericarp.
Seed anatomy
Seed coat structure
Longitudinal sections stained with Sudan Black enabled observations of the cell layers that comprise the outer and inner integuments of the seed coat. In seeds with a glabrous and almost transparent exotesta, such as Sclerostegia moniliformis, the outer and inner integument cells are compressed and difficult to discern (Fig. 4A). In contrast, in the micropylar region of Tecticornia verrucosa Paul G. Wilson (Fig. 4B) the outer epidermal cells of the outer integument are subtended by a row of cells filled with tannin-like material and 2–4 rows of thin-walled, tangentially oblong unstained cells. The cuticle of the inner epidermis of the outer integument and the cuticle of the outer epidermis of the inner integument occur together and were stained blue. Furthermore, in T. verrucosa an unstained row of cells with slightly thickened walls comprising the inner integument was evident above the embryo cuticle, which stained black.
Fig. 4.
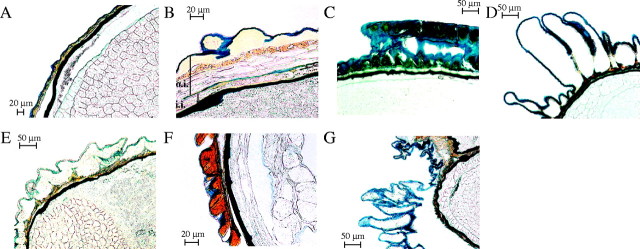
Longitudinal sections showing seed coat cell layers stained with Sudan Black in (A) Sclerostegia moniliformis Paul G. Wilson; (B) Tecticornia verrucosa; (C) Tegicornia uniflora Paul G. Wilson; (D) Halosarcia pterygosperma (Black) Paul G. Wilson; (E) Arthrocnemum subterminalis Standl.; (F) Arthrocnemum macrostachyum (Moric.) Koch; and (G) Sarcocornia blackiana (Ulbr.) A. J. Scott. Near the micropylar region in the seed of (B), Tecticornia verrucosa, multiple cell layers of the outer integument (o.i.) and inner integument (i.i.) are visible either side of the cuticle of the inner epidermis of the outer integument and the cuticle of the outer epidermis of the inner integument, which occur together and are stained blue.
Seed colour may be influenced by the presence of tannins in the outer epidermal cells of the exotesta. Tegicornia uniflora (Fig. 4C) and Halosarcia pterygosperma (Fig. 4F) have Type 4 seed ornamentation. However, T. uniflora seeds are brown and tannins are present in the outer epidermal cells of the exotesta (Table 3). In contrast, H. pterygosperma has fawn-coloured seeds and the outer epidermal cells of the exotesta are clear, as also observed in Sarcocornia blackiana (Fig. 4G) and H. lepidosperma (Fig. 3F). By contrast, even though the seeds of Arthrocnemum subterminalis are brown, tannins are absent from exotesta outer epidermal cells (Fig. 4E), whereas these cells are tannin-filled in Arthrocnemum macrostachyum (Fig. 4F). It is of interest that a space occurs between the outer epidermal cells and the underlying seed coat layers of S. blackiana (Fig. 4G), and also in other taxa with fawn seeds, such as Sarcocornia quinqueflora and Halosarcia lepidosperma. When examined closely using a dissecting microscope, this outermost layer falls away to reveal a smooth, brown layer beneath, which appears superficially similar to the outer surface of a glabrous seed.
Endosperm and perisperm
The storage material of Salicornioideae seeds is predominantly nucellus-derived perisperm. Traces of endosperm may be present comprising one or two living cell layers, most evident near the radicle above the micropylar region, as seen in Halosarcia halocnemoides (Fig. 5). Cell walls stain positive with the PAS reaction and proteins stain with Amido Black. In contrast, the perisperm consists of thin-walled, non-living cells densely packed with starch grains as indicated by positive reaction to PAS. The perisperm is present in abundance in seeds of 59 of the Salicornioideae taxa examined (Table 3), as observed in Halosarcia pruinosa (Fig. 6F), H. pergranulata (Fig. 6E) and Pachycornia triandra (Fig. 6D). Perisperm is absent from Sarcocornia fruticosa, S. perennis (Fig. 6A) and the three Salicornia species. Small amounts of PAS positive perisperm are evident in the Australian Sarcocornia species; Sarcocornia blackiana (Fig. 6B), S. quinqueflora subsp. quinqueflora (Fig. 6C) and in S. globosa Paul G. Wilson.
Fig. 5.
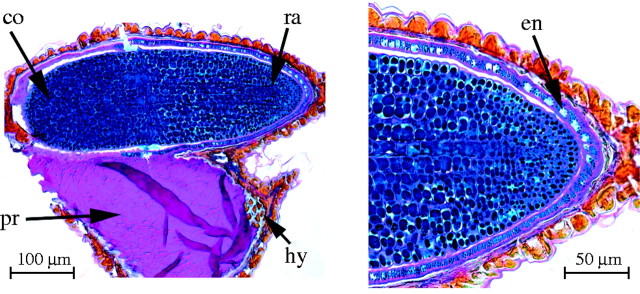
Longitudinal section of a seed of Halosarcia halocnemoides (Nees) Paul G. Wilson highlighting the single endosperm cell layer near the embryo radicle, the presence of cell wall polysaccharides stained pink from PAS, and small protein bodies stained blue with Amido Black. ra = radicle, co = cotyledons, pr = perisperm, hy = hypostase and en = endosperm.
Fig. 6.
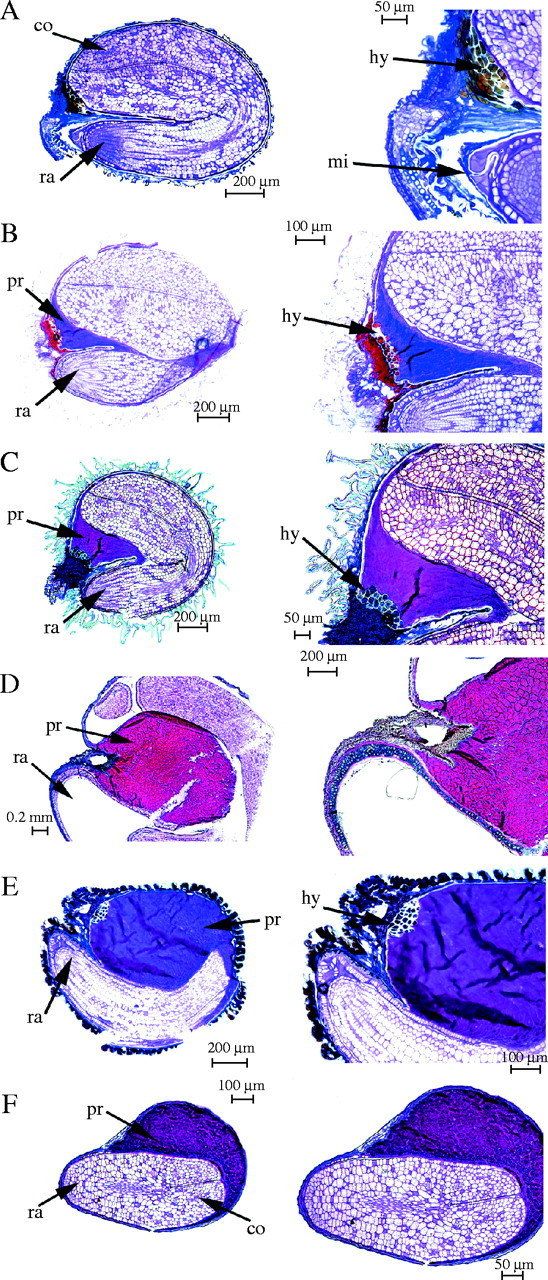
Longitudinal sections of Salicornioideae seeds showing embryo curvature and perisperm presence, as recorded in Table 3. (A) Perisperm absent, embryo bent in Sarcocornia perennis (Miller) A. J. Scott; (B) trace amount of perisperm present, embryo bent in Sarcocornia blackiana (Ulbr.) A. J. Scott and (C) S. quinqueflora (Ung.-Sternb.) A. J. Scott subsp. quinqueflora; (D) perisperm abundant, embryo curved in Pachycornia triandra (F. Muell.) Black and (E) Halosarcia pergranulata (Black) Paul G. Wilson; and (F) embryo straight in Halosarcia pruinosa (Paulsen) Paul G. Wilson. ra = radicle, co = cotyledons, hy = hypostase, mi = micropyle and pr = perisperm.
Embryo shape
Like other Chenopodiaceae, the embryo is peripheral in the Salicornioideae seeds, with the outer edge of the embryo aligned against the inner surface of the bitegmic seed coat (Fig. 6). However, the embryo of Tecticornia australasica is slightly displaced towards the centre of the seed with a layer of perisperm between the outer edge of the embryo and the seed coat inner surface.
Salicornioideae embryos are campylotropous and therefore develop in a curve from the micropyle region toward the chalazal end of the seed, characterized by the tannin-filled cells of the hypostase in mature seeds. However, Salicornioideae embryos exhibit varying degrees of curvature. In Salicornia and Sarcocornia the peripheral embryo almost completely occupies the internal area of the seed. The cotyledons are similar in length to the hypocotyl-root axis and the horse-shoe shaped embryo appears bent in half (with the base of the cotyledons touching the top of the hypocotyl-root axis) exemplified by Sarcocornia perennis (Fig. 6A). In this species the perisperm is entirely consumed and the cotyledons occur adjacent to the hypostase. In the Australian Sarcocornia species the embryo is also strongly bent; however, the cotyledons do not elongate completely to fill the entire length of the seed. Instead there is a small amount of perisperm present between the hypostase area and the apex of the embryo, as seen in Sarcocornia blackiana (Fig. 6B) and Sarcocornia quinqueflora (Fig. 6C). While the Pachycornia triandra embryo also occupies the entire length of the seed, with the radicle adjacent to the micropylar end and the cotyledons extending to the chalazal region (Fig. 6D), the embryo is curved rather than bent as observed in Salicornia and Sarcocornia. The curved embryo surrounds a large amount of centrally positioned perisperm. The embryo of Halosarcia pergranulata is not as strongly curved (Fig. 6E) as the cotyledons do not extend as far towards the hypostase of the chalazal region and an abundant amount of perisperm is also evident. In contrast, the embryo of Halosarcia pruinosa is straight (Fig. 6F) and the cotyledons of the embryo are much shorter than the hypocotyl-root axis with the remaining internal region of the seed occupied by perisperm.
Embryo lipids, proteins and carbohydrates
Only the embryos of Sarcocornia perennis (Fig. 7A) and Allenrolfea occidentalis (Fig. 7B) showed a strong positive reaction to Sudan Black, indicating the presence of lipids (Table 4). These lipids occupied the cell cytoplasm throughout most of the embryo. The only region that stained positively with Sudan Black in the remaining Salicornioideae seeds was the cuticle present below the bitegmic seed coat.
Fig. 7.
Salicornioideae seeds in longitudinal section stained with Sudan Black to highlight lipids present in the embryo cell cytoplasm of (A) Sarcocornia perennis (Miller) A. J. Scott and (B) Allenrolfea occidentalis Kuntze.
Table 4.
Presence of lipids, proteins, polysaccharides, starch and unstained inclusions in cotyledon cells of Salicornioideae seeds
| Collector, collection no., herbarium number, location |
Lipids |
Protein form |
Cell wall polysaccharides |
Starch grains in protein bodies |
Starch grains in cell cytoplasm |
Inclusions |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tribe Halopeplideae | ||||||||||||||
| Halopeplis amplexicaulis Ung.-Sternb.ex Cesati, Passer.&Gibelli | J. Molero, BC# 653314, Zaragoza Spain | − | 1 | + | + | − | + | |||||||
| Tribe Salicornieae | ||||||||||||||
| Allenrolfea occidentalis O. Kuntze | R. H. Peebles 10605, US# 1634018, Arizona USA | + | 1 | + | − | + | − | |||||||
| Arthrocnemum macrostachyum (Moric.) Koch | B. Dudley BD1, Aragón, Spain | − | 1 | + | + | − | + | |||||||
| Arthrocnemum subterminalis Standl. | F. R. Fosberg S4888, US# 1766320, California, USA | − | 1 | + | − | − | − | |||||||
| Halocnemum strobilaceum M. Bieb. | BC# 640555, Almería, Spain | − | 1 | + | + | − | + | |||||||
| Halosarcia halocnemoides (Nees) Paul G. Wilson | K. Shepherd KS698, W of Wubin, Western Australia | − | 6 | + | − | − | − | |||||||
| Halosarcia lepidosperma Paul G. Wilson | K. Shepherd KS726, N of Scadden, Western Australia | − | 1 | + | few | − | + | |||||||
| Halosarcia leptoclada Paul G. Wilson subsp. inclusa Paul G. Wilson | K. Shepherd KS646, Lake Koobabie Western Australia | − | 5 | + | few | − | + | |||||||
| Halosarcia. pergranulata (Black) Paul G. Wilson subsp. pergranulata Paul G. Wilson | K. Shepherd KS737, Pallarup Reserve, Western Australia | − | 5 | + | + | − | + | |||||||
| Halosarcia pruinosa Paul G. Wilson | K. Shepherd KS707, S of Coolgardie, Western Australia | − | 5 | + | + | − | + | |||||||
| Halosarcia pterygosperma (Black) Paul G. Wilson subsp. pterygosperma | K. Shepherd KS666, West of Menzies, Western Australia | − | 1 | + | few | − | + | |||||||
| Heterostachys ritteriana Ung.-Sternb. | H. H. Bartlett 20664, US# 1930104, Argentina | − | 5 | + | − | − | + | |||||||
| Pachycornia triandra (F. Muell.) Black | K. Shepherd KS675, S of Sandstone, Western Australia | − | 4 | + | few | − | + | |||||||
| Salicornia bigelovii Torr. | W. L. McAttie, US# 788805, Massachusetts, USA | − | 1 | + | − | + | − | |||||||
| Salicornia europaea L. | B. P. Koustaal, E of Vlissingen, Netherlands | − | 1 | + | − | + | − | |||||||
| Sarcocornia globosa Paul G. Wilson | K. Shepherd KS644, Lake Koobabie, Western Australia | − | 1 | + | − | − | rare | |||||||
| Sarcocornia blackiana (Ulbr.) A. J. Scott | T. Aplin, P# 02618400, Dumbleyung, Western Australia | − | 2 | + | − | − | − | |||||||
| Sarcocornia perennis (Mill.) A. J. Scott | M. C. Johnston, US# 1222247, Texas, USA | + | 1 | + | − | − | few | |||||||
| Sarcocornia quinqueflora (Ung.-Sternb.) A. J. Scott subsp. quinqueflora | K. Shepherd KS755, Camel Lake, Western Australia | − | 2 | + | + | − | + | |||||||
| Sclerostegia moniliformis Paul G. Wilson | K. Shepherd KS732, N of Scadden, Western Australia | − | 4 | + | + | − | − | |||||||
| Tecticornia arborea Paul G. Wilson | R. J. Cranfield 7548, Australia | − | 3 | + | − | − | − | |||||||
| Tecticornia verrucosa Paul G. Wilson | K. Shepherd KS625, N of Wyalkatchem, Western Australia | − | 3 | + | few | − | few | |||||||
| Tegicornia uniflora Paul G. Wilson | K. Shepherd KS746, Camel Lake, Western Australia | − | 2 | + | − | − | + | |||||||
Character states: − = absent, + = present. Protein body Types (1–5) are described in Figs 8 and 9. Storage compounds identified by the following histochemical stains: Sudan Black for lipids, Amido Black for protein, Periodic Acid-Schiff's Reagent for cell wall polysaccharides and starch grains. Herbaria: US = United States National Herbarium at the Smithsonian Institute, BC = Institut Botánic de Barcelona, P = Perth.
All embryo cells of the taxa examined contained abundant proteins that stained positive with Amido Black, although the shape and form of the bodies varied. The protein bodies have been categorized into six types on the basis of their size, shape and arrangement (Table 4). Type 1 protein bodies are small and densely packed together as seen in Halopeplis amplexicaulis (Fig. 8C) and in ten other taxa including Allenrolfea occidentalis (Fig. 9A), A. subterminalis (Fig. 8E), Salicornia bigelovii (Fig. 8A) and Sarcocornia perennis (Fig. 8B). Type 2 protein bodies are small and round, scattered through the cell cytoplasm and are found in three Australian species: Sarcocornia blackiana (Fig. 8D), S. quinqueflora and the monotypic Tegicornia uniflora. Type 3 protein bodies appear crescent-shaped with large unstained bodies also apparent, as observed only in Tecticornia australasica and T. verrucosa (Fig. 9B). Type 4 protein bodies are clearly outlined polygons evident only in Sclerostegia moniliformis (Fig. 9C) and the monotypic Pachycornia triandra. Type 5 protein bodies are darkly stained and variable in size, as apparent in Halosarcia leptoclada, H. pergranulata subsp. pergranulata, H. pruinosa (Fig. 9D) and Heterostachys ritteriana. Type 6 protein bodies are very large and occupy most of the cytoplasm and were observed only in Halosarcia halocnemoides (Fig. 9E).
Fig. 8.
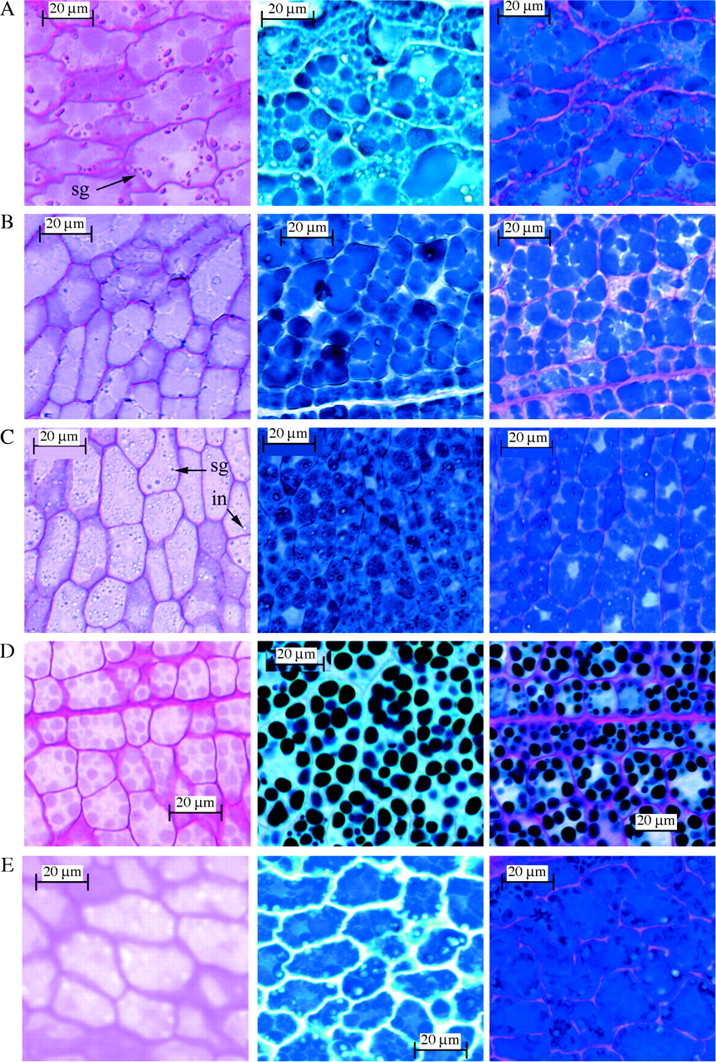
Salicornioideae seeds in longitudinal section through the cotyledon stained with PAS (left column), Amido Black (middle column) and PAS + Amido Black (right column). (A) Salicornia bigelovii Torr.; (B) Sarcocornia perennis (Miller) A. J. Scott; (C) Halopeplis amplexicaulis Ung.-Sternb. ex Cesati, Passer. & Gibeli; (D) Sarcocornia blackiana (Ulbr.) A. J. Scott; and (E) Arthrocnemum subterminalis Standl. sg = starch grains, in = inclusions. Protein bodies recorded in Table 4 are classified from the examples shown in the middle column; small and densely packed together (Type 1) in (A), (B), (C) and (E); small, round protein bodies scattered through the cell cytoplasm (Type 2) in (D).
Fig. 9.
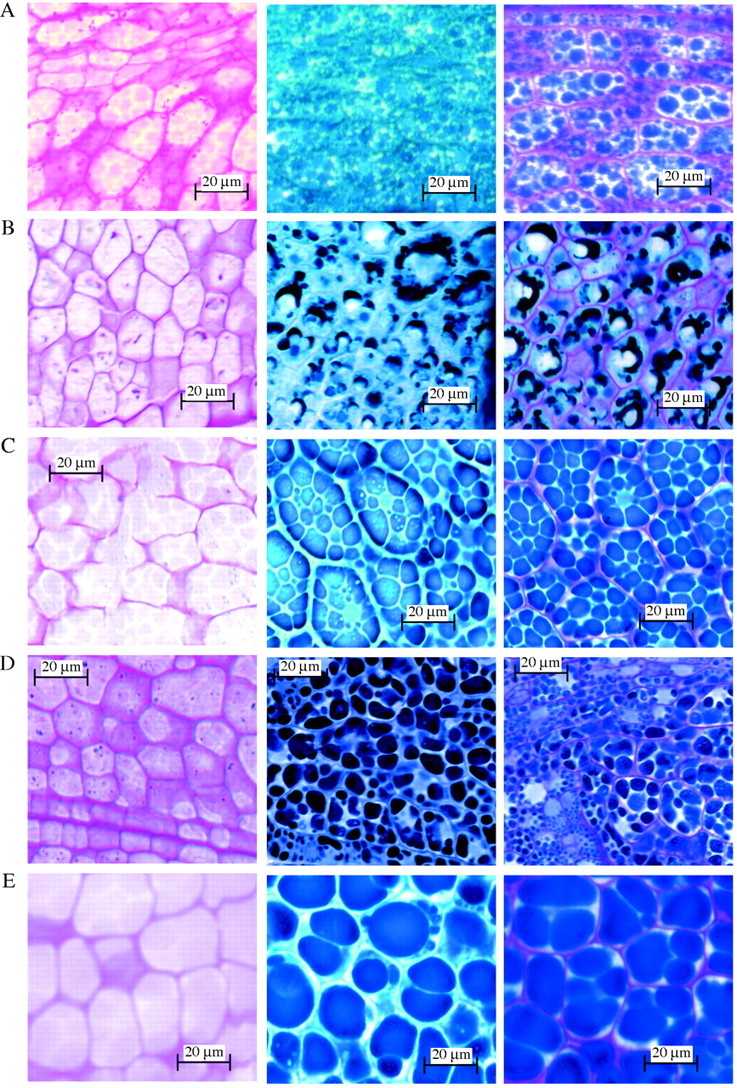
Salicornioideae seeds in longitudinal section through the cotyledon stained with PAS (left column), Amido Black (middle column) and PAS + Amido Black (right column). (A) Allenrolfea occidentalis Kuntze; (B) Tecticornia verrucosa Paul G. Wilson; (C) Sclerostegia moniliformis Paul G. Wilson; (D) Halosarcia pruinosa (Paulsen) Paul G. Wilson; and (E) H. halocnemoides (Nees) Paul G. Wilson. Protein bodies recorded in Table 4 are classified from the examples shown in the middle column: small and densely packed (Type 1) in (A), crescent-shaped with unstained bodies (Type 3) in (B), clearly outlined polygon (Type 4) in (C), darkly-stained bodies variable in size (Type 5) in (D) and large bodies that occupy most of the cell cytoplasm (Type 6) in (E).
All embryo cells stained positively to PAS, indicating the presence of cell wall polysaccharides. Protein body polysaccharides are also evident as the internal contents of the cells of several taxa that stained light pink with PAS. In some taxa, for example Sarcocornia blackiana (Fig. 8D) and Sclerostegia moniliformis (Fig. 9C) the protein body polysaccharides are clearly outlined. Starch grains, when present, may occur as tiny granules inside the protein bodies, as seen in Halopeplis amplexicaulis (Fig. 8C) and Halosarcia pruinosa (Fig. 9D) or as large grains in the cell cytoplasm, as observed in Salicornia bigelovii (Fig. 8A), Salicornia europaea and Allenrolfea occidentalis (Fig. 9A). Small inclusions of varying frequency and size that did not react to histochemical stains were clearly evident in Halopeplis amplexicaulis (Fig. 8C) and a number of other taxa (Table 4).
DISCUSSION
This study examined representatives from 14 of the 15 genera from both tribes of the subfamily Salicornioideae and documented for the first time many perianth, pericarp and seed characters that were found to be variable at the genus and species levels. Although some fruit and seed features may be potentially phylogenetically informative, none appear to be definitive at the tribal level as Halopeplis amplexicaulis and Kalidium foliatum of the tribe Halopeplideae have abundant perisperm as well as similar seed coat structure and embryo curvature to many species in the tribe Salicornieae.
Generic level variation
The absence of perisperm was one of the diagnostic characters previously used to delimit Salicornia and Sarcocornia from all other Salicornioideae (Scott, 1977; Wilson, 1980). This study has shown that small amounts of perisperm are evident in three Australian Sarcocornia species. These three species are closely related, as they form a monophyletic group based on ITS molecular sequence data, sister to Salicornia within a paraphyletic Sarcocornia clade (Shepherd et al., 2004). Thus, the diagnostic character that distinguishes Salicornia and Sarcocornia from other Salicornioideae should be redefined to: perisperm being absent or present only in trace amounts versus perisperm being abundant. Accordingly, the abundant perisperm detected in seeds of the North American Arthrocnemum subterminalis in this study indicates that this species should not be placed into synonymy under Salicornia, as suggested by some authors (Wiggins, 1980; Wilken, 1993).
The campylotropous ovule typical of the Chenopodiaceae results in a curved embryo. However, this study has shown that Salicornioideae embryos vary in size, shape and curvature. The embryos of Salicornia and Sarcocornia are relatively large and occupy almost the entire seed. Moreover, these horse-shoe shaped embryos appear ‘bent’, as the base of the inner cotyledon touches the apex of the hypocotyl region as the embryo bends. This even occurs in the Australian Sarcocornia species, which have trace amounts of perisperm present at the apex of the cotyledons near the chalazal region. While the embryos of Salicornia and Sarcocornia may be considered distinct, the large curved embryo of Pachycornia triandra is simply an extreme on a continuum of curvature, rather than a unique autapomorphy. It is apparent that many species of Halosarcia and Sclerostegia have straight embryos. While these embryos still develop from the micropylar end, they do not continue to elongate and curve towards the chalazal end of the seed. Therefore, a straight embryo represents a secondary loss of curvature in these seeds. Accordingly, the peripheral embryos of the Salicornioideae may be further characterized as bent, curved or straight.
Seed ornamentation, a result of radial extension of individual epidermal cells, occurs in many of the Salicornioideae. This study identified five types of exotesta ornamentation. None of these ornamentation types were found to be diagnostic at the genus level; however, the epidermal cells of Microcnemum had convex outer periclinal and undulate anticlinal walls (Type 2) similar to that observed in Arthrocnemum seeds. The absence of tannins in the exotesta of brown Arthrocnemum subterminalis seeds was of interest. However, as tannin may appear at the late stages of development this character should be confirmed, since the seeds examined in this study might not have been fully matured.
Wilson (1980) suggested that the ‘hardness of testa in [seeds of] Halosarcia is inversely proportional to the hardness of the pericarp or fruiting perianth’. Dark-coated seeds that are ornamented appear much ‘harder’ than glabrous seeds, but it is difficult to measure this accurately. Moreover, the difference in transparency of glabrous seeds indicates that seed coat thickness varies even among taxa with ‘soft’ seeds. Anatomical sections highlight the difference in seed coat structure; for example, the multiple cell layers of both the outer and inner integuments were apparent near the micropyle of the Tecticornia verrucosa seed; in contrast, these layers were highly compressed in the Sclerostegia moniliformis seed. Similarly, the position of seed ornamentation is also highly variable in the Salicornioideae but this variation does not appear to be related to specific perianth or pericarp characters.
The orientation of seeds within Salicornioideae fruits has rarely been reported (Wilson, 1980) and may be a useful diagnostic character. While the placentation of Salicornioideae seeds is basal, the longitudinal axis of the seed may orientate toward the horizontal or vertical plane. Seeds may also subsequently rotate to the left or right in either plane. The flowers of Halocnemum strobilaceum and Heterostachys ritteriana are sessile within free bracts. The inflorescence of Halocnemum strobilaceum is compact and globular, while the bracts of Heterostachys ritteriana are arranged alternately; factors which may cause the seeds to be positioned in the horizontal plane but lying flat and rotated back towards the inflorescence axis. The polymorphic orientation of both Halosarcia chartacea and H. flabelliformis seeds, where the seed from the central fruit of the triad is horizontal while the seeds from the two lateral fruits are horizontal and lie flat on either the left or right side, may also be a consequence of inflorescence structure. The inflorescence of Halosarcia chartacea is compressed and the fused bracts form plate-like rings as the fruits mature, which might cause the lateral seeds to flatten during development.
The relatively large seeds of Pachycornia were sometimes found to be orientated upside down relative to the inflorescence axis, and the seeds of Tegicornia were orientated in the vertical position relative to the stem axis rotated to the left or right side. No other fruit or seed features were found to be diagnostic at the genus level for the remaining Australian genera; Halosarcia, Sclerostegia and Tecticornia. In a recent molecular phylogenetic analysis based on ITS sequence data, none of the Australian genera were supported as monophyletic as the smaller genera were all nested within Halosarcia (Shepherd et al., 2004). On the basis of low molecular sequence variation amongst the Australian genera, it was hypothesised that they radiated rapidly from a single common ancestor (Shepherd et al., 2004; Kadereit et al., 2005). A rapid radiation could account for a lack of diagnostic characters between genera, but does not explain the considerable variation, particularly in seed coat morphology, expressed at lower levels. The heterogeneity of Australian salt lakes, which exhibit differences in soil type, salinity levels, area and depth, provide infinite habitat variability. Species complexes and hybridization are reported (Wilson, 1980) and polyploidy is also evident with diploids, triploids and tetraploids reported within a single subspecies (Shepherd and Yan, 2003). All these factors may influence diversity at the population level among the Australian Salicornioideae.
Storage compounds in Salicornioideae seeds
Halophytic plants with seeds rich in proteins, oils and carbohydrates may provide invaluable alternative crops for agricultural regions affected by salinity. Positive histochemical stains for lipids were observed in Sarcocornia perennis and Allenrolfea occidentalis embryos. However, lipid bodies were not detected in Salicornia bigelovii, a North American annual targeted as a saltwater irrigation crop with seeds containing up to 33 % protein and 33 % oil (Glenn et al., 1991, 1999; Attia et al., 1997). Similarly, lipid bodies were not detected in Salicornia europaea seed even though it is reported to have a similar oil content to that of S. bigelovii (Austenfeld, 1986, cited in Glenn et al., 1991), or in Halocnemum strobilaceum seeds which have been reported to contain 12·6 % oil (Barclay and Earle, 1974). Various factors may account for the lack of positive reaction to Sudan Black in species known to have oil-rich seeds, such as the age of the seed or form of the oils present. Extraction and chemical analytical methods, such as those described by Glenn et al. (1991), should be employed to more accurately quantify the presence of oils and fatty acid compounds in other Salicornioideae species to assess their potential for oilseed crops.
Salicornia and Sarcocornia species lack starchy perisperm in the seed, so they must rely on energy stores within the embryo for the initial stages of germination. The large bent embryo characteristic of these genera is effectively twice the length of the seed, increasing the length of the cotyledons and hypocotyl-radicle axis in the young seedling. A larger embryo in annual species may confer an advantage, facilitating rapid germination and establishment to optimize growth before flowering and seed set. Salicornia procumbens and two other Salicornioideae species have green embryos. The presence of green pigments in mature seeds is not common, although this has been documented for representatives from five other genera within the Chenopodiaceae and the cotyledons of a Kochia childsii (Chenopodiaceae) seed embryo were shown to contain chloroplasts and chlorophyll (Marin and Dengler, 1972). It is possible that species with green embryonic cotyledons are capable of becoming actively photosynthetic at a very early stage of establishment. Since the majority of Salicornioideae species have opaque seeds, it is not apparent how many have green embryos. It would be of interest to assess the relationship between cotyledon size, embryo colour, seed storage materials and germination strategies in the wide-ranging genera of the Salicornioideae.
The compartmentalization of protein into distinct bodies is evident in both the endosperm and embryo cells of the Salicornioideae taxa investigated in this study. The variability in form, size and distribution of these protein bodies has not previously been reported for this subfamily. Protein bodies may be potentially useful taxonomic characters (Lott, 1981) and closely related Salicornioideae species exhibit similar forms. For example, Sarcocornia blackiana and S. quinqueflora have protein body Type 2, while Tecticornia arborea and T. verrucosa have protein body Type 3. However, these characters are difficult to quantify, as protein bodies may vary in size and structure within different tissues of an embryo (Lott, 1981; Coimbra and Salema, 1994).
The size and shape of starch grains within seeds are also potentially taxonomically useful, but a wide-ranging survey by Gill et al. (1991) showed little phylogenetic correlation of starch grain characteristics among 49 angiosperm families. Perisperm starch grains are relatively homogeneous among the 14 genera of Salicornioideae examined. However, two different forms of starch were found within cotyledon cells. Tiny PAS-positive grains in the protein bodies were present in 12 of the 23 taxa examined anatomically. Three other species had larger grains evident in the cotyledon cell cytoplasm and the grains observed in Salicornia bigelovii and S. europaea were larger than those observed in Allenrolfea occidentalis. Unstained inclusions were also apparent in 15 taxa. Anatomical examination of protein bodies, starch grains and inclusions at varying stages of embryo development in a wider range of Salicornioideae species will test if these characters are systematically informative for the subfamily.
The endosperm is usually absent in Chenopodiaceae seeds as it is completely consumed by the developing embryo (Pal et al., 1990). However, a thin endosperm layer was observed in Halosarcia halocnemoides near the radicle region of the embryo. Traces of endosperm have also been documented in other Chenopodiaceae and Amaranthaceae species, such as Chenopodium quinoa (Prego et al., 1998) and Amaranthus hypochondriacus (Coimbra and Salema, 1994).
In conclusion, there are no unique fruit or seed characters that support the traditional tribes recognized in the subfamily Salicornioideae. This study has highlighted fruit and seed characters that may be informative at the genus and species level. Furthermore, anatomical sectioning of seeds has demonstrated differences in seed coat structure and embryo size and shape. Histochemical staining of seeds revealed previously unreported variability in protein bodies, starch grains and the presence of unstained inclusions in the embryo cells of various Salicornioideae taxa. Further anatomical studies including more representatives of the Salicornioideae are required to assess if these new characters are also phylogenetically useful.
Acknowledgments
We thank the Centre for Microscopy and Microanalysis (UWA) for use of facilities; John Kuo for his guidance on anatomical methods and enthusiastic discussion of the observed results; Sharon Platten for instruction on the use of the Environmental Scanning Electron Microscope and John Murphy for instruction on the use of the Sorvall Microtome. K.S. thanks the field assistance provided by various friends and colleagues and is especially grateful to Ian Clarke from Royal Botanical Gardens Melbourne for collecting specimens from the Northern Territory and South Australia. Special thanks go to Paul Wilson for sharing his knowledge of the Australian Salicornioideae, to Carol Wilkins and Grant Whiteman for comments and to Wolfgang Stuppy for his immensely helpful and insightful review of the manuscript. This work was supported by an ARC Grant with linkage support from MERIWA, Normandy Mining Limited, Placer (Granny Smith), Acacia Resources, KCGM and the Western Australian Herbarium.
LITERATURE CITED
- APG. 2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society 141: 399–436. [Google Scholar]
- Attia FM, Alsobayel AA, Kriadees MS, Al-Saiady MY, Bayoumi MS. 1997. Nutrient composition and feeding value of Salicornia bigelovii Torr meal in broiler diets. Animal Feed Science Technology 65: 257–263. [Google Scholar]
- Barclay A, Earle F. 1974. Chemical analyses of seeds III: oil and protein content of 1253 species. Economic Botany 28: 178–236. [Google Scholar]
- Bocquet G. 1959. The campylotropous ovule. Phytomorphology 9: 222–227. [Google Scholar]
- Castroviejo S, Coello P. 1980. Datos cariológicos y taxonómicos sobre las Salicorniinae A.J.Scott Ibéricas. Anales Jardín Botánico de Madrid 37: 41–73. [Google Scholar]
- Castroviejo S, Lago E. 1992. Datos acerca de la hibridación en el género Sarcocornia (Chenopodiaceae). Anales Jardín Botánico de Madrid 50: 163–170. [Google Scholar]
- Coimbra S, Salema R. 1994.Amaranthus hypochondriacus: seed structure and localization of seed reserves. Annals of Botany 74: 373–379. [Google Scholar]
- Connor HE. 1984. Gynodioecism in Sarcocornia quinqueflora (Salicornieae) in New Zealand. New Zealand Journal of Botany 22: 433–439. [Google Scholar]
- Contandriopoulos J. 1968. A propos des nombres Chromosomiques des Salicornia de la région méditerranéenne. Bulletin du Museum D'Histoire Naturelle de Marseille 28: 45–52. [Google Scholar]
- Cooke FW. 1912. Observations on Salicornia australis. Transactions and Proceedings of the New Zealand Institute 44: 349–362. [Google Scholar]
- Corner E. 1976.The seeds of dicotyledons. London: Cambridge University Press. [Google Scholar]
- Danilastos GD. 1993. Microscopy research and technique. In: Johnson Jnr JE, ed. Introduction to the environmental scanning electron microscope instrument. New York: Wiley-Liss Inc., 354–361. [Google Scholar]
- Davy AJ, Bishop GF, Costa CSB. 2001.Salicornia L. (Salicornia pusilla J. Woods, S. ramosissima J. Woods, S. europaea L., S. obscura P.W. Ball & Tutin, S. nitens P.W. Ball & Tutin, S. fragilis P.W. Ball & Tutin and S. dolichostachya Moss). Journal of Ecology 89: 681–707. [Google Scholar]
- de Fraine E. 1912. Anatomy of the genus Salicornia Journal of the Linnean Society of London Botany 41: 317–348. [Google Scholar]
- Dumortier DC. 1827.Flora Belgica. Tournai. [Google Scholar]
- English J. 2004.Ecophysiology of salinity and waterlogging tolerance in selected species of Halosarcia. PhD Thesis. The University of Western Australia. Perth. [Google Scholar]
- Freitag H, Hedge IC, Jafri SMH, Kothe-Heinrich G, Omer S, Uotila P. 2001. Chenopodiaceae. In: Ali SI, Qaiser M, eds. Flora of Pakistan. Karachi and St Louis, Missouri, USA: Department of Botany, University of Karachi and Missouri Botanical Press, Missouri Botanical Garden. 1–213. [Google Scholar]
- Gill LS, Nyawuame HGK, Aibangbee MI, Agho DA. 1991. Nature of ergastic substances in some Mediterranean angiospermous seeds—vi. Feddes Repertorium 102: 613–628. [Google Scholar]
- Glenn E, Brown J, Blumwald E. 1999. Salt tolerance and crop potential of halophytes. Critical Reviews in Plant Sciences 18: 227–255. [Google Scholar]
- Glenn EP, O'Leary JW, Watson MC, Thompson TL, Kuehl RO. 1991.Salicornia bigelovii Torr: an oilseed halophyte for seawater irrigation. Science 251: 1065–1067. [DOI] [PubMed] [Google Scholar]
- Gorshkova SG, Il'in MM, Knorring OE, Kuzeneva OI, Murav'eva OA, Tolmachev AI, Shishkin BK, Shteinberg EI, Vasil'chenko IT. 1970. Centrospermae. In: Komarov VL, Shishkin BK, eds. Flora of the USSR. Jerusalem: Israel Program for Scientific Translations, 127–134. [Google Scholar]
- Johri BM, Ambegaokar KB, Srivastava PS. 1992.Comparative embryology of angiosperms. Berlin, Heidelberg, New York: Springer-Verlag. [Google Scholar]
- Judd WS, Ferguson IK. 1999. The genera of Chenopodiaceae in the southeastern United States. Harvard Papers in Botany 4: 365–416. [Google Scholar]
- Kadereit G, Borsch T, Weising K, Freitag H. 2003. Phylogeny of Amaranthaceae and Chenopodiaceae and the evolution of C4 photosynthesis. International Journal of Plant Sciences 164: 959–986. [Google Scholar]
- Kadereit G, Gotzek D, Jacobs S, Freitag H. 2005. Origin and age of Australian Chenopodiaceae. Organisms, Diversity and Evolution In Press. [Google Scholar]
- Kühn U, Bittrich V, Carolin R, Freitag H, Hedge IC, Uotila P, Wilson P. 1993. Chenopodiaceae. In: Kubitzki K, Rohwer JG, Bittrich V, eds. The families and genera of vascular plants. Flowering plants—Dicotyledons. 2nd edn. Berlin: Springer-Verlag, 253–280. [Google Scholar]
- Lott JNA. 1981. Protein bodies in seeds. Nordic Journal of Botany 1: 421–432. [Google Scholar]
- Marin L, Dengler RE. 1972. Granal plastids in the cotyledons of the dry embryo of Kochia childsii. Canadian Journal of Botany 50: 2049–2052. [Google Scholar]
- Martin AC. 1946. The comparative internal morphology of seeds. American Midland Naturalist 36: 513–660. [Google Scholar]
- Martínez SR, Herrera M. 1996. Datos sobre Salicornia L. (Chenopodiaceae) en España. Anales Jardín Botánico de Madrid 54: 149–154. [Google Scholar]
- Moss CE. 1954. The species of Arthrocnemum and Salicornia in Southern Africa. The Journal of South African Botany 20: 1–22. [Google Scholar]
- O'Brien TP, McCully ME. 1981.The study of plant structure: principles and selected methods. Melbourne: Termarcarphi. [Google Scholar]
- Pal A, Singh RP, Pal M. 1990. Development and structure of seeds in Amaranthus hypochaondriacus L. and its wild progenitor A. hybridus L. Phytomorphology 40: 145–150. [Google Scholar]
- Prego I, Maldonado S, Otegui M. 1998. Seed structure and localization of reserves in Chenopodium quinoa. Annals of Botany 82: 481–488. [Google Scholar]
- Schütze P, Freitag H, Weising K. 2003. An integrated molecular and morphological study of the subfamily Suaedioideae Ulbr. (Chenopodiaceae). Plant Systematics and Evolution 239: 257–286. [Google Scholar]
- Scott AJ. 1977. Reinstatement and revision of Salicorniaceae J. Agardh (Caryophyllales). Botanical Journal of the Linnean Society 75: 357–374. [Google Scholar]
- Shepherd KA, Macfarlane TD, Waycott M. 2005. Phylogenetic analysis of the Australian Salicornioideae (Chenopodiaceae) based on morphology and nuclear DNA. Australian Systematic Botany 18: 1–32. [Google Scholar]
- Shepherd KA, Waycott M, Calladine A. 2004. Radiation of the Australian Salicornioideae (Chenopodiaceae)—based on evidence from nuclear and chloroplast DNA sequences. American Journal of Botany 91: 1387–1397. [DOI] [PubMed] [Google Scholar]
- Shepherd KA, Yan G. 2003. Chromosome number and size variations in the Australian Salicornioideae (Chenopodiaceae)—evidence of polyploidisation. Australian Journal of Botany 51: 1–12. [Google Scholar]
- Short DC, Colmer TD. 1999. Salt tolerance in the halophyte Halosarcia pergranulata subsp. pergranulata. Annals of Botany 83: 207–213. [Google Scholar]
- Standley PC. 1914. The genus Arthrocnemum in North America. Journal of the Washington Academy of Sciences 4: 398–399. [Google Scholar]
- Tölken HR. 1967. The species of Arthrocnemum and Salicornia (Chenopodiaceae) in Southern Africa. Bothalia 9: 255–307. [Google Scholar]
- Waisel Y. 1972.Biology of halophytes. New York and London: Academic Press. [Google Scholar]
- Werker E. 1997.Seed anatomy. Berlin: Gebrüder Borntraeger. [Google Scholar]
- Wiggins IL. 1980. Chenopodiaceae. In: Flora of Baja California. Stanford, California: Stanford University Press, 100–110. [Google Scholar]
- Wilken DH. 1993. Chenopodiaceae. In: Hickman JC, ed. The Jepson Manual: higher plants of California. Berkeley: University of California Press. [Google Scholar]
- Wilson PG. 1980. A revision of the Australian species of Salicornieae (Chenopodiaceae). Nuytsia 3: 1–154. [Google Scholar]
- Wunderlich R. 1968. Some remarks on the taxonomic significance of the seed coat. Phytomorphology 17: 301–311. [Google Scholar]


