Abstract
• Background and Aims Summer dormancy in perennial grasses has been studied inadequately, despite its potential to enhance plant survival and persistence in Mediterranean areas. The aim of the present work was to characterize summer dormancy and dehydration tolerance in two cultivars of Dactylis glomerata (dormant ‘Kasbah’, non-dormant ‘Oasis’) and their hybrid using physiological indicators associated with these traits.
• Methods Dehydration tolerance was assessed in a glasshouse experiment, while seasonal metabolic changes which produce putative protectants for drought, such as carbohydrates and dehydrins that might be associated with summer dormancy, were analysed in the field.
• Key Results The genotypes differed in their ability to survive increasing soil water deficit: lethal soil water potential (Ψs) was −3·4 MPa for ‘Kasbah’ (although non-dormant), −1·3 MPa for ‘Oasis’, and −1·6 MPa for their hybrid. In contrast, lethal water content of apices was similar for all genotypes (approx. 0·45 g H2O g d. wt−1), and hence the greater survival of ‘Kasbah’ can be ascribed to better drought avoidance rather than dehydration tolerance. In autumn-sown plants, ‘Kasbah’ had greatest dormancy, the hybrid was intermediate and ‘Oasis’ had none. The more dormant the genotype, the lower the metabolic activity during summer, and the earlier the activity declined in spring. Decreased monosaccharide content was an early indicator of dormancy induction. Accumulation of dehydrins did not correlate with stress tolerance, but dehydrin content was a function of the water status of the tissues, irrespective of the soil moisture. A protein of approx. 55 kDa occurred in leaf bases of the most dormant cultivar even in winter.
• Conclusions Drought avoidance and summer dormancy are correlated but can be independently expressed. These traits are heritable, allowing selection in breeding programmes.
Keywords: Orchard grass; drought tolerance; avoidance; dehydration; dehydrins; carbohydrates; Dactylis glomerata, summer dormancy
INTRODUCTION
Dormancy of plants is a temporary suspension of visible growth of any structure containing a meristem (Lang et al., 1987). In vegetative tissues, dormancy is often associated with winter. This response assists in survival of cold and associated stresses and has been extensively studied in tree (Bigras, 1996; Wisniewski et al., 1996; Erez, 2000) and herbaceous species (Brummer et al., 2000; Cunningham et al., 2001). Dormancy also occurs in summer in some perennial grasses originating from Mediterranean climates (Laude, 1953; Biddiscombe et al., 1977). The ecophysiology of this response was studied extensively in the geophyte Poa bulbosa (Ofir, 1986; Ofir and Kigel, 1988). Little is known about this response in forage grasses, although in Dactylis glomerata the cultivar ‘Kasbah’ both ceased to grow and allowed most of its aerial tissues to die under both drought and summer irrigation, and survived drought through dormant buds (Volaire, 2002). This response can be defined as ‘endodormancy’ since it results from physiological changes within the bud, ensuring that meristems of vegetative buds will not resume growth when environmental conditions are unfavourable. This trait is associated with reduced water consumption (Lolicato, 2000) and superior survival (Oram, 1990) and could be of great value in developing grasses for Mediterranean areas. Other research developed hybrids between parents of northern European and Mediterranean origin to combine the desirable features of both for use in Mediterranean regions (Knight, 1966), e.g. better persistence under summer drought from a Mediterranean parent, ability to respond to summer rain from a northern European parent. However, the physiological responses associated with dormancy were not described. Therefore, in order to understand better how germplasm responds to dormancy, and to assess the potential for plant improvement, the response of a hybrid developed between two populations of Mediterranean origin—one a strongly summer-dormant population and the other a highly summer-active population—was compared with that of the original populations.
As dormancy was associated with reproductive stages in the grasses Poa bulbosa and Hordeum bulbosum (Ofir and Koller, 1972; Ofir, 1976, Ofir and Kerem, 1982), sowing dates were compared in autumn and spring, with the latter treatment included to avoid vernalization and consequent floral induction. The experiment was done for an entire year, to detect potential indicators of early dormancy induction. While summer dormancy is common in herbaceous plants growing in semi-arid conditions, it has been shown that some of these plants are very dehydration tolerant even when not dormant (Volaire et al., 2001; Volaire, 2002). Although the molecular aspects of endodormancy are poorly understood (Horvath et al., 2003), it is hypothesized that traits associated with dehydration tolerance may be involved in summer dormancy. For example, it is known that certain late embryogenesis abundant (LEA) proteins (dehydrins) are associated with dehydration tolerance in seeds. As dormancy often occurs in seeds, it could be surmised that dehydrin expression might be associated with the induction of dormancy in the adult plant. This study investigated whether seasonal metabolic changes that produce putative protectants for drought and desiccation such as complex carbohydrates and dehydrins (Ingram and Bartels, 1996; Bray, 1997; Phillips et al., 2002) may also be involved in summer dormancy.
MATERIALS AND METHODS
Plant material
In the field experiment, two cultivars of cocksfoot and their hybrid were compared. The cultivar ‘Kasbah’ was selected in Australia from Moroccan germplasm collected in an area with 270 mm average annual rainfall. It is very summer dormant and drought resistant (Oram, 1990). The productive cultivar ‘Oasis’ was bred in New Zealand from Portuguese material, originating in an environment similar to where the parents of cultivar ‘Porto’ were collected (Oram, 1990). The hybrid PG325 was obtained by crossing ‘Kasbah’ and ‘Oasis’ with the aim of combining good persistence with high productivity. Preliminary results show this F3 generation hybrid has some potential (A. Stewart, Pyne Gould Guinness Seeds, pers. comm.) but its dormancy has not been tested. In the glasshouse experiment, the drought-resistant and summer-active cultivar ‘Medly’, of Mediterranean origin (INRA, Institut National de Recherche Agronomique, Montpellier, France) was also tested.
Experimental design and conditions
The first experiment was done in the field at INRA (Mauguio, France), in a deep loamy clay soil. Due to limited quantities of PG325 seed, the full irrigation and drought treatment plots for both autumn- (24 October 2002) and spring-sown treatments (17 March 2003) consisted of three 1·5-m-long replicate rows in each of the three populations. Seed was sown at 1 g m−2. All plots were fertilized using accepted practice for semi-intensive Mediterranean grasslands (40 kg N ha−1 at sowing and in February). Plants were defoliated on 28 May in the autumn-sown treatment, on 21 July, 18 August and 11 September in irrigated plots of both sowing dates, and on 22 October after rehydration following a drought of 143 d (22 May to 22 September) maintained by a ventilated rain shelter. The control treatment was irrigated weekly throughout the summer. Mean vapour pressure deficit (VPD) was −1·7 kPa for the period June to August, with a mean air temperature of 26·4 °C, while at meristem level the temperature was 29·4 °C.
The second experiment was done in a glasshouse at INRA (Montpellier, France) in spring and summer 2004, using 18-cm PVC pots containing the same quantity of substrate (80 % sand, 10 % loam, 10 % clay ). Tillers of cultivars ‘Medly’, ‘Oasis’, ‘Kasbah’ and the hybrid were transplanted from the field, into separate pots (25 per pot) on 25 March 2004. These plants had all experienced winter temperatures sufficiently low to induce flowering and dormancy. Pots of each genotype were fully irrigated and were defoliated when necessary until 15 June, when the soil moisture was adjusted to 12 % and irrigation stopped. One or two pots of each genotype were successively re-watered after 13, 17, 20, 22, 24 and 27 d of drought, and after 35 d of drought for ‘Kasbah’. Two pots of each genotype were irrigated throughout the period as controls. All droughted pots were fully randomized. For 3 weeks following each rehydration, the number of plants that regrew from among the 20 remaining plants was counted. Over the duration of the experiment the mean VPD was −1 kPa and the mean air temperature 24·4 °C.
Soil and plant measurements
In the glasshouse experiment, all pots subjected to drought were weighed to determine soil water content (SWC) two or three times a week. The relationship between SWC (%) and soil water potential (MPa) was defined previously (Volaire and Lelièvre, 2001) as: Ψs = −104·66 × exp(−1·44 × SWC) – 0·003.
Five (glasshouse) to ten (field) plants per genotype were sampled on days 1, 17, 20, 22, 24, 27 and 35 in the glasshouse and on a monthly basis, from January 2003, in the field experiment. As the only aerial tissues that remained alive during most of the drought periods were bases of immature leaves, they were divided into two fractions: (1) the first 20 mm above root insertion (mainly sheaths and leaf bases); (2) the remaining upper tissues (mainly mature lamina). Fraction 2 was divided into green and senescent tissues only during the summer period of the field experiment. Fresh and dry weights (after 48 h at 60 °C) were measured to assess green and senescent biomass. In fraction 1, bases of surviving immature leaves were dissected out, immediately weighed fresh and dried (48 h at 60 °C) to determine tissue water content. In the field experiment, three other subsamples of leaf bases were collected. One was for psychrometric determination of osmotic potential (Wescor HR33T dewpoint microvoltmeter) after freezing, thawing and equilibration. Another was frozen in liquid nitrogen before measurements of dehydrins, and the third frozen for measurement of water-soluble carbohydrate contents.
Protein extraction, electrophoresis and Western blotting
Plants were dissected, dead tissues discarded, and bases of immature leaves frozen in liquid nitrogen and stored at −25 °C. Total protein was extracted from seeds of ‘Medly’ and used as a control in all blots. Plant tissues were homogenized in a mortar with liquid nitrogen and extracted using a buffer containing 5 mm Tris–HCl, pH 8, 500 mm NaCl, 2 mm ascorbic acid, 0·5 mm phenylmethylsulfonyl fluoride and 1 mm dithiothreitol. The homogenate was heated at 80 °C for 10 min and centrifuged at 17000 g. Supernatants were thawed and heat-stable proteins quantified by a dye-binding assay (Bradford, 1976). Proteins were separated by 10 % SDS–PAGE using a Mini protean 3 electrophoresis cell (Biorad, CA, USA), with the same weight of heat-stable proteins (10 µg) loaded into each well of each gel. Separated polypeptides were transferred onto a nitrocellulose filter (Sartorius 0·2 μm) using a semi-dry transblot cell (Biorad) for 2 h. Blotted proteins were checked by Ponceau S staining. After blocking with 0·2 % casein (Aurora Western Blotting kit; ICN Biomedicals, Aurora, OH, USA) overnight at 4 °C, the membrane was incubated for 2 h with antidehydrin polyclonal antibody (StressGen Biotechnologies Corp., Victoria, BC, Canada) at a dilution of 1:1000 in phosphate-buffered saline (Close et al., 1993). After three consecutive washes of 15 min each in PBST, the membrane was incubated for 1 h with the secondary antibody, goat anti-rabbit IgG alkaline phosphatase conjugate at a dilution of 1:1000 in phosphate-buffered saline, and the Aurora Western Blotting kit (ICN) was used for detection. Gels and Western blots were repeated at least three times. Densitometric analyses (image analyser, Scion image and Photoshop packages) were performed on the best representative blots. Seed of ‘Medly’ was used as the control for dehydrin expression and was common to all blots.
Water-soluble carbohydrate content (WSC)
For the monthly samplings, bases of the last immature leaves were sampled, frozen, freeze-dried and the WSC extracted in 40 % ethanol at 85 °C and purified with activated charcoal. WSC was quantified by HPLC using an Aminex HPX 42-C column and a differential refractometer calibrated against glucose, fructose, sucrose and inulin.
Statistical analysis
The data were analysed using the appropriate analysis of variance and regression models in the Genstat package. The figures were drawn and curves fitted with the Fig.P package (Biosoft, Cambridge, UK) and with the FITCURVE procedure in the Genstat package.
RESULTS
Dehydration tolerance
The glasshouse experiment aimed to rank the cultivars according to their dehydration tolerance. As SWC decreased in pots, aerial tissues of the cultivar ‘Kasbah’ senesced significantly more slowly (Fig. 1A) than the other cultivars (P = 0·05). In addition, ‘Kasbah’ reached full aerial senescence and had more hydrated tissue at lower SWC than the other cultivars (Fig. 1B) since, in particular, the dehydration of leaf bases progressed more quickly in the sensitive ‘Oasis’ (P < 0·001). Responses of the hybrid and ‘Medly’ were intermediate (Fig. 1A and B). However, mortality was substantial in all genotypes, especially when the last surviving leaf bases had a water content below 0·45 g H20 g−1 d. wt (30 % f. wt). As a result, the plant survival curves after rehydration following progressive soil water deficit differed markedly between genotypes (Fig. 1C). A four parameter logistic model using the FITCURVE procedure from Genstat Version 5 accounted for 96 % of the variation in the data, relating soil moisture to plant survival and indicated the necessity of fitting a separate curve for each of the four cultivars in the glasshouse experiment. It can be confidently asserted (P = 0·05) that the soil moisture content at which 50 % mortality occurs is different for each of the cultivars except ‘Medly’ and the hybrid. Plants of ‘Oasis’ died at a higher SWC than those of‘Kasbah’, the other genotypes being intermediate. The SWC associated with 50 % mortality (SWC50) was3·14 % (±0·005), 2·98 % (±0·04), 2·95 % (±0·01), and 2·62 % (±0·02) for ‘Oasis’, the hybrid, ‘Medly’ and ‘Kasbah’, respectively.
Fig. 1.
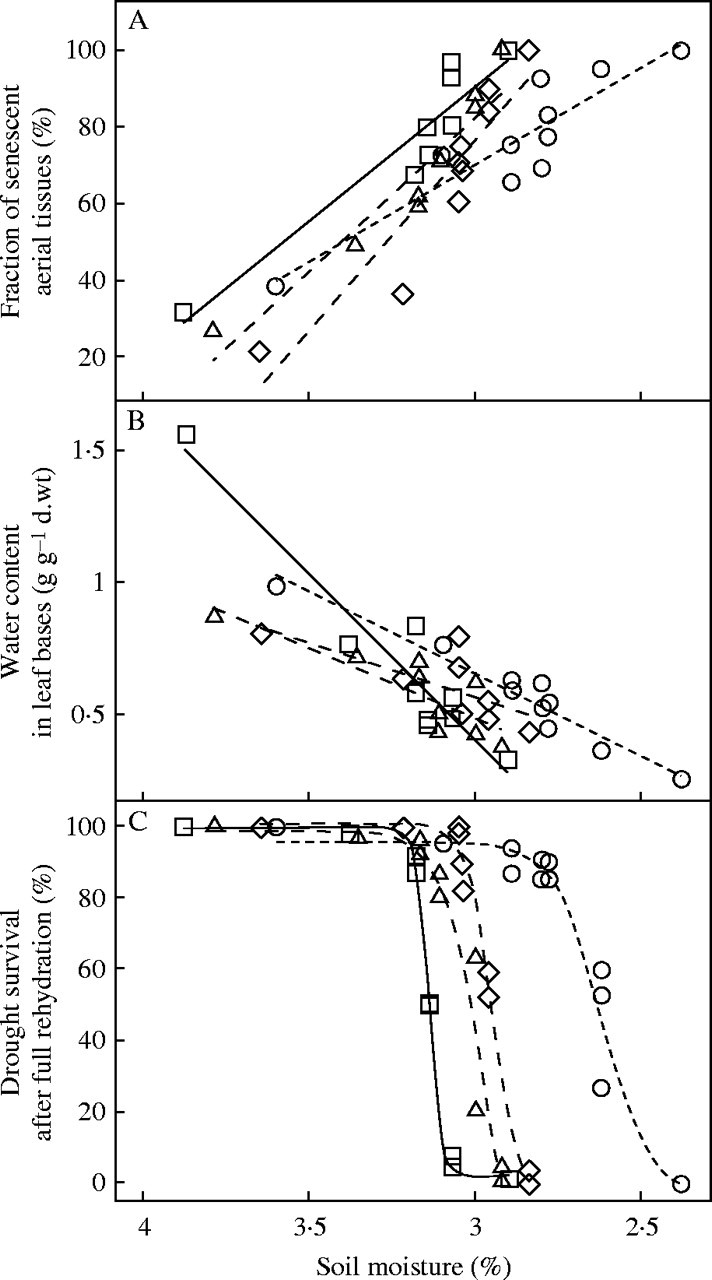
Relationships between soil water content (%) and (A) fraction of senescent shoot tissues, (B) water content in leaf bases (g H2O g−1 d. wt) and (C) drought survival rate after successive full rehydration in four genotypes of cocksfoot: a drought-resistant cultivar ‘Medly’ (open diamonds); a drought-resistant cultivar ‘Kasbah’ (open circles); a drought-sensitive cultivar ‘Oasis’ (open squares) and the ‘Oasis’ × ‘Kasbah’ hybrid PG325 (open triangles), subjected to progressive drought in a glasshouse experiment.
No sign of dormancy was detected in control plants that were maintained fully hydrated, and senescence of shoots was limited to 20–30 % in these plants at both sampling dates at the beginning and at the end of the experiment.
Shoot biomass and senescence
Biomass was harvested mainly from irrigated field plots since almost no growth occurred on the droughted plots between 30 May and 22 September. Sown in autumn, the hybrid was significantly more productive than the other cultivars during the following spring (Table 1). Conversely, in both autumn and spring sown treatments, ‘Kasbah’did not grow during the first 2 months of drought (21 July cut) and produced very little biomass in August and September. After rewatering the droughted plots, autumn-sown plants of ‘Kasbah’ produced significantly more shoot biomass than when sown in the previous spring.
Table 1.
Mean biomass production (g d. wt m−2) in autumn- and spring-sown cultivars of cocksfoot (cultivars ‘Oasis’, hybrid PG325 and ‘Kasbah’) subjected to full irrigation or rehydrated after a drought period between 30 May and 22 September 2003
| Autumn sowing |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Production period |
||||||||||||||
| 24 October to 28 May | 28 May to 21 July | 21 July to 18 August | 18 August to 11 September | 28 May to 11 September | 22 September to 22 October | 22 October autumn/spring | ||||||||
| Irrigated |
Irrigated |
Irrigated |
Irrigated |
Drought |
After rehydration |
|||||||||
| ‘Oasis’ | 32·7 (2·0) | 37·0 (2·3) | 10·1 (0·6) | 7·0 (0·4) | 0 | 16·4 (4·3) | 0·91 (0·3) | |||||||
| Hybrid | 48·3 (7·9) | 24·7 (4·0) | 7·2 (1·2) | 8·2 (1·3) | 0 | 35·4 (21·7) | 1·33 (0·4) | |||||||
| ‘Kasbah’ | 30·7 (3·5) | 0 (0) | 0·7 (0·1) | 2·4 (0·3) | 0 | 19·6 (6·1) | 2·44 (0·5) | |||||||
| l.s.d. | 10·23 | 5·4 | 1·5 | 1·7 | 0·76 | |||||||||
| Spring sowing |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Production period |
||||||||||
| 17 March to 21 July | 21 July to 18 August | 18 August to 11 September | 28 May to 11 September | 22 September to 22 October | ||||||
| Irrigated |
Irrigated |
Irrigated |
Drought |
After rehydration |
||||||
| ‘Oasis’ | 55·2 (14·9) | 12·0 (3·2) | 4·4 (1·2) | 0 | 19·5 (9) | |||||
| Hybrid | 24·5 (0·5) | 10·6 (0·2) | 6·5 (0·1) | 0 | 25·7 (10·1) | |||||
| ‘Kasbah’ | 0 (0) | 0·5 (0·01) | 1·6 (0·2) | 0 | 7·9 (0·9) | |||||
| l.s.d. | 17·2 | 3·7 | ||||||||
The last column presents the ratio of autumn biomass (on 22 October following rehydration) between plants previously sown in autumn and those previously sown in spring.
Standard deviations are given in parenthesis. Least significant difference values (l.s.d.) are given when significant differences occur between cultivars at P < 0·05.
Irrespective of the sowing date, plants of ‘Oasis’ exhibited similar patterns of shoot senescence which increased from 20 to 60 % as drought progressed, but remained <40 % in control plants (Fig. 2). In contrast, the fraction of senescent tissues exceeded 80 % in both irrigated and droughted plants of autumn-sown ‘Kasbah’ but was <50 % in spring-sown plants. A similar pattern occurred in spring-sown plants of the hybrid PG325, although in the autumn-sown treatment, senescent tissues were 80 % of the above-ground biomass in August; which then decreased in September in the droughted plants. No induced senescence occurred in control plants of this genotype.
Fig. 2.
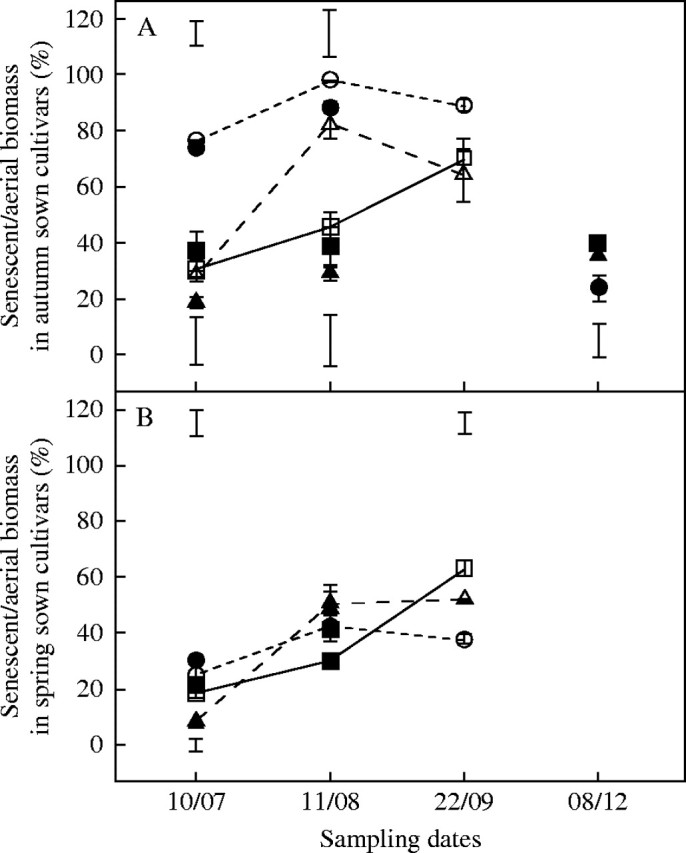
The time course of the development of senescence in tissues of shoot biomass during and after the summer in three genotypes of cocksfoot: (A) drought-resistant cultivar ‘Kasbah’ (circles); a drought sensitive cultivar ‘Oasis’ (squares) and the ‘Oasis’ × ‘Kasbah’ hybrid, PG325 (triangles) subjected to progressive drought from 31 May to 22 September 2003 (open symbols) or fully irrigated during the same period (closed symbols) in a field experiment when plants were sown in autumn (A) or in spring (B). Bars indicate l.s.d. when differences between genotypes are significant at P < 0·05. Bars at the bottom of the figure refer to irrigated treatments, bars at the top of the figure refer to the drought treatment.
Water relations
In winter, and throughout most of spring and autumn, plants were fully hydrated and the water content (WC) of leaf bases was 5 g H2O g−1 d. wt (Fig. 3A). However, before drought, the WC in leaf bases of autumn-sown plants decreased to 3 g H2O g−1 d. wt on 23 May, although the osmotic potential (OP) was not affected significantly in either sowing treatment (Fig. 4). Tissue hydration decreased even earlier in April in ‘Kasbah’ and was significantly less than in ‘Oasis’ for most of the summer (1 g H2O g−1 d. wt in May) whether under irrigation or drought, and in plants from both sowing dates. Prior to autumn rehydration, the tissue hydration of ‘Oasis’ continued to decrease and reached approx. 1 g H2O g−1 d. wt, without causing any tiller mortality. Under drought the OP of all plants decreased from −2 MPa to −6 MPa without significant differences between genotypes. However, the OP of the control plants of ‘Kasbah’ was significantly lower in July (autumn sowing) and August (spring sowing) than in the other two cultivars
Fig. 3.
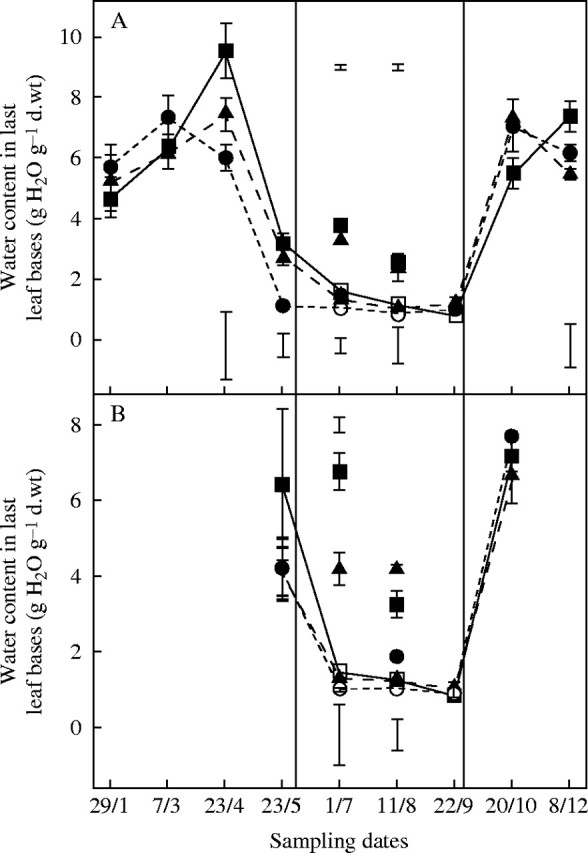
Time course of water content in last leaf bases (g H2O g−1 d. wt) over the year 2003 in three genotypes of cocksfoot: the drought-resistant cultivar ‘Kasbah’ (circles); the drought-sensitive ‘Oasis’ (squares) and the ‘Oasis’ × ‘Kasbah’ hybrid PG325 (triangles) subjected to progressive drought from 31 May to 22 September (open symbols) or fully irrigated over the same period and the rest of the year (closed symbols) in a field experiment when plants were sown in autumn (A) or in spring (B). Bars indicate l.s.d. when differences between genotypes are significant at P < 0·05. Bars at the bottom of the figure refer to irrigated treatments, while those at the top of the figure refer to the drought treatment.
Fig. 4.
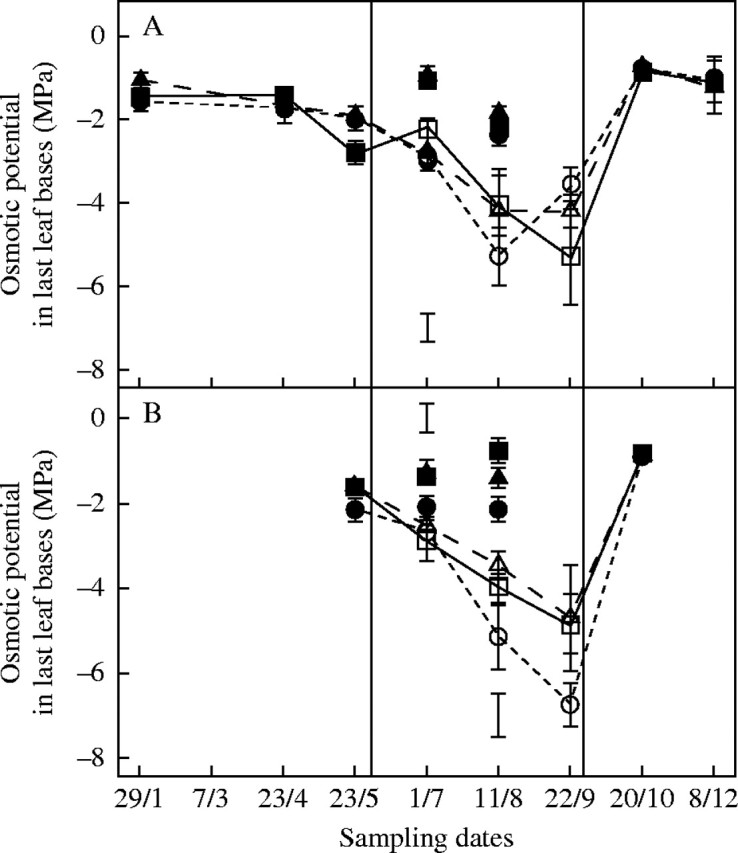
Time course of osmotic potential (MPa) in last leaf bases over the year 2003 in three genotypes of cocksfoot: the drought-resistant cultivar ‘Kasbah’ (circles); the drought-sensitive cultivar ‘Oasis’ (squares) and the ‘Oasis’ × ‘Kasbah’ hybrid PG325 (triangle) subjected to progressive drought from 31 May to 22 September (open symbols) or fully irrigated over the same period and the rest of the year (closed symbols) in a field experiment when plants were sown in autumn (A) or in spring (B). Bars indicate l.s.d. when differences between genotypes are significant at P < 0·05. Bars at the bottom of the figure refer to irrigated treatments, while those at the top of the figure refer to the drought treatment.
Water-soluble carbohydrates
Total WSC in leaf bases of all cultivars was between 200 and 300 mg g−1 d. wt over the year, increasing to over 350 mg g−1 d. wt in both droughted and control plants in summer. Fructans were the major component of total WSC and differences between cultivars (Fig. 5A and B) paralleled those found for total WSC. In May, the fructan content of ‘Oasis’ (autumn and spring sown) was significantly lower than ‘Kasbah’; similarly before rehydration the fructan content of spring-sown ‘Oasis’ was less than in ‘Kasbah’ (143 cf. 307 mg g d. wt−1, respectively). Leaf bases of ‘Oasis’ also accumulated twice as much sucrose as the other cultivars at the end of the drought (Fig. 5C and D). In ‘Kasbah’ a significant decline in the monosaccharide concentrations occurred from early April, and remained close to zero over the entire summer in this dormant variety (Fig. 5E and F), while in the hybrid the monosaccharide content was intermediate between the two parents over the period.
Fig. 5.
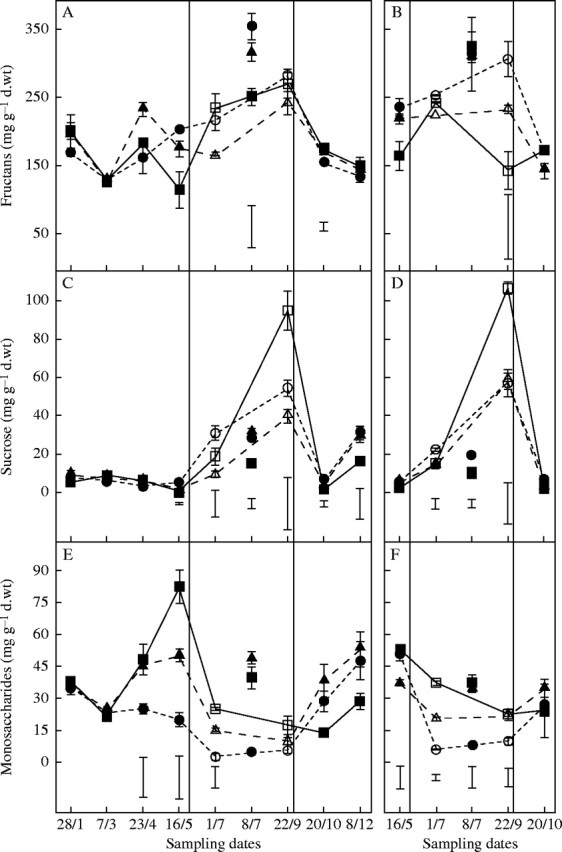
Time course of water-soluble carbohydrate contents (mg g−1 d. wt) (A and B, fructans; C and D, sucrose; E and F, monosaccharides) in last leaf bases over the year 2003 in three genotypes of cocksfoot: the drought-resistant cultivar ‘Kasbah’ (circles); the drought-sensitive cultivar ‘Oasis’ (squares) and the ‘Oasis’ × ‘Kasbah’ hybrid PG325 (triangles) subjected to progressive drought from 31 May to 22 September (open symbols) or fully irrigated during the same period and the rest of the year (closed symbols) in a field experiment when plants were sown in autumn (A, C and E) or in spring (B, D and F). Bars indicate l.s.d. when differences between genotypes are significant at P < 0·05.
Accumulation of dehydrins
The pattern of dehydrin accumulation in leaf bases of ‘Oasis’ was similar for both sowing dates (Fig. 6A) with a late appearance of two major proteins (approx. 24 and 43 kDa) after 2 months of drought (lane 4, 11 August), which were still present on 22 September, (lane 5) although dehydrins were not detected under irrigation (lanes 2, 6 and 7).
Fig. 6.
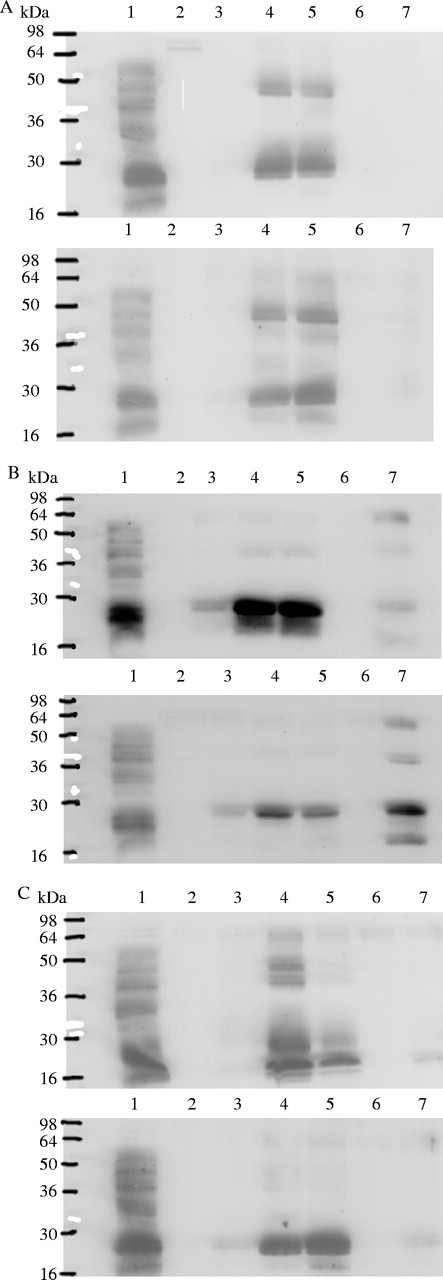
Immunoblots of three genotypes of Dactylis glomerata ‘Oasis’ (A), ‘Kasbah’ (B) and an ‘Oasis’ × ‘Kasbah’ hybrid, PG325 (C) either sown in spring (top) or in autumn (bottom) and subjected to a progressive and severe drought from 31 May to 22 September in a field experiment. Dehydrins expressed in 10 μg of heat-stable proteins from seeds of ‘Medly’ (lane 1), and last surviving leaf bases of plants on 26 May, irrigated plants (lane 2), 1 July, droughted plants (lane 3), 11 August, droughted plants (lane 4), 22 September, droughted plants (lane 5), 20 October after rehydration (lane 6), 8 July, irrigated control plants (lane 7).
In ‘Kasbah’, a dehydrin (approx. 55 kDa) was detected as traces in early spring in autumn-sown plants (Fig. 6B, bottom, lane 2). This dehydrin occurred at low and constant amounts per unit of tissue on all sampling dates, except for summer irrigation when the accumulation increased 10-fold (Fig. 6B, bottom, lane 7). After 1 month of drought, a protein of 24 kDa was strongly expressed, with a 51-kDaprotein less so, especially in spring-sown plants; their expression increased after 2 months (11 August, lane 4) to a maximum which was maintained until the end of drought (22 September, lane 5). Dehydrins were also detected in leaf bases of plants irrigated in summer where they were twice as abundant as those in droughted tissues of autumn-sown (Fig. 6B, bottom, lane 7) and spring-sown plants (Fig. 6B, top, lane 7).
In the hybrid, at both sowing dates (Fig. 6C), the approx. 24-kDa dehydrin was detected from 11 July (lane 3), accumulating until 11 August (lane 4). It was also detected in plants irrigated in summer (lane 7), although less than in ‘Kasbah’ (concentration 14 times lower than in autumn-sown ‘Kasbah’ at the same date and treatment). Traces of the approx. 55-kDa proteins were detected in all plants sampled during and after August in both irrigated and droughted conditions.
Under full irrigation, dehydrins were not detectable in autumn-sown plants sampled on 28 January, 7 March, 23 April and 8 December in ‘Oasis’, although traces of the approx. 55 kDa-protein were detected on 8 December in the hybrid (data not shown). This 55-kDa dehydrin was detected in ‘Kasbah’ on 8 December when its accumulation was half the amount (Fig. 7, lane 8) of that exhibited under irrigation in the middle of summer (Fig. 7, lane 6).
Fig. 7.
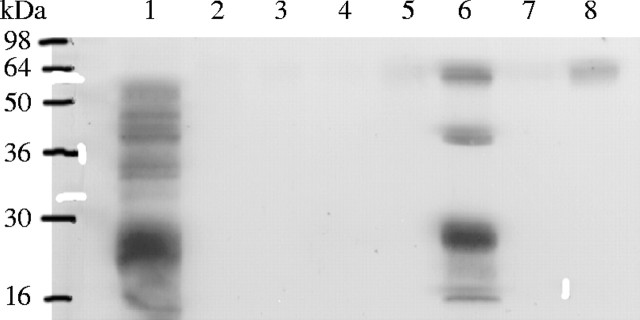
Immunoblot of Dactylis glomerata ‘Kasbah’ sown in autumn and subjected to full irrigation over 1 year in a field experiment. Dehydrins expressed in 10 μg of heat-stable proteins from seeds of ‘Medly’ (lane 1), and last surviving leaf bases of plants on 28 January (lane 2), 7 March (lane 3), 23 April (lane 4), 26 May (lane 5), 8 July (lane 6), 20 October (lane 7) and 8 December (lane 8).
DISCUSSION
The experiments aimed to analyse summer dormancy, dehydration tolerance and physiological indicators associated with these traits in two cultivars of cocksfoot and their hybrid, PG325. The ‘Medly’ was used as a control to be able to compare the levels of dehydration tolerance with those found in previous experiments (Volaire, 1995; Volaire and Lelièvre, 2001).
Dehydration tolerance and dormancy
When a long drought was imposed under controlled conditions, there was a relationship between survival rate and soil water content which differed for each genotype. As expected ‘Kasbah’ survived until very low SWC since its lethal Ψs was −3·4 MPa, compared with −1·3 MPa for ‘Oasis’, −1·6 MPa for the hybrid and −1·7 MPa for ‘Medly’. Previous data showed that ‘Medly’ exhibited a lethal Ψs of −3·8 MPa when subjected to 150 d drought concurrent with low temperatures (Volaire and Lelièvre, 2001). Therefore, these values seem dependant on the environmental conditions of the experiment but nevertheless allow a ranking of the cultivars. Our results show that none of the tested genotypes could be considered desiccation tolerant since each required continued access to soil water at a physiologically tolerable water potential for survival (Proctor and Pence, 2002). It confirms that summer-dormant cocksfoot exhibits responses to drought that are very different from those of resurrection plants (Ingram and Bartels, 1996). In addition, there were no differences between genotypes in the lethal water content of apices (around 30 %, 0·45 g H2O g−1 d. wt). Consequently, under the experimental conditions tested, ‘Kasbah’ is not more tolerant of tissue dehydration and while able to avoid the effects of drought for longer, cannot be regarded as more dehydration tolerant (Levitt, 1972; Sugiyama and Nikara, 2004). Previous experiments which compared the drought-resistant Mediterranean cultivar ‘Medly’ with the drought-sensitive cultivar ‘Lutetia’ which is of maritime origin (Volaire, 2002) did not find differences in lethal tissue water content. Therefore, it appears that as one moves across the zone of origin of D. glomerata from the wetter to the drier margins the primary response to drought among the resident populations is an ever-intensifying dehydration avoidance and not dehydration tolerance.
Although the glasshouse experiment was designed to test fully vernalized adult plants, at a post-flowering stage, the transplanting of plants into pots appeared to have removed dormancy. It can be hypothesized that ‘Kasbah’ would have exhibited even greater drought avoidance if the senescence and dehydration of aerial biomass induced by dormancy from the end of spring had been expressed. While it has been shown that leaf senescence contributes to plant survival under drought, since it allows a remobilization of nutrients from senescing to young leaves and reductions in water loss at the whole plant level (Munné-Bosch and Alegre, 2004), the ranking of summer dormancy in the field nevertheless, paralleled that found for drought tolerance in pots. This confirms the finding that tolerance to intense soil dehydration is independent of dormancy, although they often occur in the same genotypes (Volaire et al., 2001).
Dormancy appears to be partly heritable since in autumn-sown plants, dormancy was high in ‘Kasbah’, intermediate in the hybrid and absent in ‘Oasis’. The responses associated with dormancy have been analysed previously on plants fully irrigated over summer (Volaire, 2002). Under these conditions, cessation of leaf elongation, total absence of biomass production during the first 2 months of summer, senescence of aerial tissues and dehydration of surviving organs were the main morpho/physiological characteristics of dormant ‘Kasbah’ plants. In the hybrid, notwithstanding the high yield potential in spring, its biomass production was reduced during the first two summer months and lower than that of ‘Oasis’. This can be related to the pattern of carbohydrate in leaf bases that was intermediate in the hybrid compared with both parents (discussed later). In addition, droughted plants of the hybrid senesced actively in August compared with the non-dormant parent. Knight (1966) also showed that some Mediterranean × northern European hybrids of cocksfoot could exhibit an ability to survive drought that may equal that of Mediterranean germplasm.
The comparison of sowing dates in the field showed that all autumn-sown plants of ‘Kasbah’ formed bulbs at the tiller bases in spring. Bulbs are associated with potential dormancy since they can develop a state of rest in which they do not exhibit any visible external growth under adverse conditions (Le Nard and de Hertogh, 1993; Okubo, 2000). Although spring-sown plants did not flower very actively, some bulb formation also occurred in ‘Kasbah’ (particularly in those plants that flowered) as in Hordeum bulbosum, another perennial grass, for which flowering was a condition for bulb initiation (Ofir and Koller, 1972).
A similar pattern of biomass production was found over summer (zero in ‘Kasbah’), although there was a significantly greater regrowth after the drought in autumn-sown ‘Kasbah’ plants compared with those sown in the spring. Spring-sown ‘Kasbah’ exhibited responses that may be ascribed to partial dormancy induction with no early senescence, but nevertheless greater tissue dehydration under summer irrigation than the other cultivars. In addition, the osmotic potential of ‘Kasbah’ leaf bases fell below −6 MPa at the end of the drought, indicating a higher water stress than in the case of autumn-sown plants (−4 MPa). It could be hypothesized that only active flowering and sufficient plant growth and development in spring resulted in full dormancy induction that proved essential in facilitating active growth resumption after drought.
Biochemical indicators associated with dormancy
The pattern of accumulation of soluble carbohydrates in leaf bases paralleled those associated with growth rhythms over the year. As drought intensified over summer, fructans and sucrose tended to accumulate, while monosaccharides declined related to reduction of leaf growth and increase of osmotic potential in the tissues (Volaire, 1995). The comparison of genotypes of contrasting dormancy showed the following distinctive patterns: (1) in early April under full irrigation, the monosaccharide contents declined in the dormant genotype but peaked for the non-dormant genotype before declining later in summer. The differences were maintained in all treatments, irrespective of water supply and sowing date, for most of the summer; (2) conversely, the fructan contents were significantly lower in non-dormant genotypes of irrigated plants both in May and August, at a time when dormancy was fully expressed in the autumn-sown treatment; (3) the fructan content was also higher in spring-sown droughted ‘Kasbah’ than ‘Oasis’, confirming previous findings that fructan conservation is correlated with drought resistance (Volaire and Lelievre, 1997); (4) the sucrose content at the end of the summer in the drought-sensitive ‘Oasis’ was double that of the other genotypes, even though a high concentration of sucrose has been shown to be associated with improved drought and desiccation tolerance in higher plants (Oliver and Bewley, 1997) and seeds (Leprince et al., 1993). Dormancy and stress acclimation are expressed at the same time which makes it difficult to associate physiological and molecular changes with one or the other states (Wisniewski and Arora, 2000). The present results indicate that the dormancy of genotypes is correlated with the early cessation of metabolic activity in spring. In particular, the monosaccharide content appears to be an early indicator of dormancy induction, its decrease signalling onset of dormancy. The role of sugar in the control of growth and development is just beginning to be understood but the interplay between bud dormancy status and cell division suggests that these two fundamental processes are probably regulated by common signalling pathways (Horvath et al., 2002, 2003). The combination of protective substances such as fructan, sucrose and dehydration proteins allows vitrification of the cell contents on drying and therefore maintenance of membrane integrity (Crowe et al., 1998; Buitink et al., 2000).
The time course of dehydrin appearance showed a similar pattern of accumulation of two major proteins (approx. 24 and 51 kDa) in all genotypes whatever their sowing date over the last 2 months of drought. The amount of dehydrins produced was not correlated with stress tolerance of these genotypes, in contrast to other cocksfoot germplasm (Volaire, 2002). However, three main responses (partially exhibited by the hybrid but completely absent in the non-dormant parent) characterized the dormant genotype. (1) Significant early accumulation at the beginning of the drought was associated with low water content in tissues. (2) There was an abundance of the same dehydrins synthesized during summer, even in dehydrated tissues subjected to summer irrigation and especially when fully dormant (i.e. autumn-sown plants). In general, the dehydrin content was a function of the water status of plant tissues and was independent of soil moisture status. It raises the question whether such a metabolic response is a consequence of drying or is a protective strategy (Walters et al., 2002), although there is mounting evidence for the involvement of LEA proteins in desiccation tolerance (Alpert and Oliver, 2002). (3) The presence of an approx. 55-kDa protein in winter is an intriguing result since this protein appears to be expressed constitutively in the most dormant cultivar, whatever the severity of drought. It may be associated with the potential to respond rapidly to photoperiod and temperature as both play a significant role in the induction and breaking of endodormancy (Horvath et al., 2003).
In conclusion, it was confirmed that drought avoidance and dormancy are correlated but can be independently exhibited. These plant strategies are both characterized by the expression of various morphological and biochemical traits greatly dependent on seasonal growth patterns. These traits appear to be heritable insofar as partial expression occurs in the hybrid studied. This opens the way to the development of a breeding programme with the involvement of Mediterranean research groups, currently supported by the European Union (project INCO-MED, PERMED).
Supplementary Material
Acknowledgments
We thank Meat and Livestock Australia Pty, Ltd for provision of a Fellowship to M.R.N. and financial support for the research, Alan Stewart (Pyne Gould Guinness Seeds, New Zealand) for providing seeds and Pascal Chapon for excellent technical assistance.
LITERATURE CITED
- Alpert P, Oliver MJ. 2002. Drying without dying. In: Black M, Pritchard HW, eds. Desiccation and survival in plants—drying without dying. Wallingford: CAB International, 3–44. [Google Scholar]
- Biddiscombe EF, Rogers AL, Mallers RA. 1977. Summer dormancy, regeneration and persistence of perennial grasses in south-western Australia. Australian Journal of Experimental Agriculture and Animal Husbandry 17: 795–801. [Google Scholar]
- Bigras FJ. 1996. Conifer bud dormancy and stress resistance: a forestry perspective. In: Lang GA, ed. ‘Plant dormancy: physiology, biochemistry and molecular biology’. Wallingford: CAB International, 171–192. [Google Scholar]
- Bradford M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Annals of Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Bray E. 1997. Plant responses to water deficit. Trends in Plant Science 2: 48–54. [Google Scholar]
- Brummer EC, Shah MM, Luth D. 2000. Re-examining the relationship between fall dormancy and winter hardiness in alfalfa. Crop Science 40: 971–977. [Google Scholar]
- Buitink J, Hemmings MA, Hoekstra FA. 2000. Is there a role for oligosaccharides in seed longevity? An assessment of intracellular glass stability. Plant Physiology 122: 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close TJ, Fenton RD, Moonan F. 1993. A view of plant dehydrins using antibodies specific to the carboxy terminal peptide. Plant Molecular Biology 23: 279–286. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. 1998. The role of vitrification in anhydrobiosis. Annual Reviews of Physiology 60: 73–103. [DOI] [PubMed] [Google Scholar]
- Cunningham SM, Gana JA, Volenec JJ, Teuber R. 2001. Winter hardiness, root physiology, and gene expression in successive fall dormancy selections from ‘Mesilla’ and ‘CUF 101’ alfalfa. Crop Science 41: 1091–1099. [Google Scholar]
- Erez A. 2000. Bud dormancy: a suggestion for the control mechanism and its evolution. In: Viémont JD, Crabbé J, eds. Dormancy in plants: from whole plant behaviour to cellular control. Wallingford: CAB International, 23–34. [Google Scholar]
- Horvath DP, Anderson JV, Chao WS, Foley ME. 2003. Knowing when to grow: signals regulating bud dormancy. Trends in Plant Science 8: 534–540. [DOI] [PubMed] [Google Scholar]
- Horvath DP, Chao WS, Anderson JV. 2002. Molecular analysis of signals controlling dormancy and growth in underground adventitious buds of leafy spurge. Plant Physiology 128: 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram J, Bartels D. 1996. The molecular basis of dehydration tolerance in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 377–403. [DOI] [PubMed] [Google Scholar]
- Knight R. 1966. The performance of hybrids between Mediterranean and Northern European parents of cocksfoot (Dactylis glomerata L.) in a Mediterranean type environment. Australian Journal of Agricultural Research 17: 105–117. [Google Scholar]
- Lang GA, Early JD, Martin GC, Darnell RL. 1987. Endo-, para-, and eco-dormancy: physiological terminology and classification for dormancy research. Horticultural Science 22: 371–377. [Google Scholar]
- Laude HM. 1953. The nature of summer dormancy in perennial grasses. Botanical Gazette 114: 284–292. [Google Scholar]
- Le Nard M, de Hertogh AA. 1993. Bulb growth and development and flowering. In: De Hertogh AA, Le Nard, M, eds. The physiology of flower bulbs. Amsterdam: Elsevier, 29–43. [Google Scholar]
- Leprince O, Hendry GAF, McKersie BD. 1993. The mechanisms of desiccation tolerance in developing seeds. Seed Science Research 3: 231–246. [Google Scholar]
- Levitt J. 1972.Responses of plants to environmental stresses. New York, Academic Press. [Google Scholar]
- Lolicato SJ. 2000. Soil water dynamics and growth of perennial pasture species for dryland salinity control. Australian Journal of Experimental Agriculture 40: 37–45. [Google Scholar]
- Munné-Bosch S, Alegre L. 2004. Die and let live: leaf senescence contributes to plant survival under drought stress. Functional Plant Biology 31: 203–216. [DOI] [PubMed] [Google Scholar]
- Ofir M. 1976. Interaction of gibberellin with photoinduction in the initiation of the dormant phase in vernalized Hordeum bulbosum L. Australian Journal of Plant Physiology 3: 827–832. [Google Scholar]
- Ofir M. 1986. Seasonal changes in the response to temperature of summer-dormant Poa bulbosa L. bulbs. Annals of Botany 58: 81–89. [Google Scholar]
- Ofir M, Kerem D. 1982. The effects of temperature and photoperiod on the onset of summer-dormancy in Poa bulbosa L. Annals of Botany 50: 259–264. [Google Scholar]
- Ofir M, Kigel J. 1988. Abscisic acid involvement in the induction of summer-dormancy in Poa bulbosa, a grass geophyte. Physiologia Plantarum 102: 163–170. [Google Scholar]
- Ofir M, Koller D. 1972. A kinetic analysis of the relationships between flowering and initiation of the dormant state in Hordeum bulbosum L.—a perennial grass. Israel Journal of Botany 21: 21–34. [Google Scholar]
- Okubo H. 2000. Growth cycle and dormancy in plants. In: Viémont JD, Crabbé J, eds. Dormancy in plants: from whole plant behaviour to cellular control. Wallingford: CAB International, 1–22. [Google Scholar]
- Oliver MJ, Bewley JD. 1997. Desiccation tolerance of plant tissues: amechanistic overview. Horticultural Reviews 18: 171–214. [Google Scholar]
- Oram RN. (ed.) 1990.Register of Australian herbage plant cultivars, 3rd edn. CSIRO, East Melbourne, Australia. ISBN 0643 05054X. [Google Scholar]
- Phillips JR, Oliver MJ, Bartels D. 2002. Molecular genetics of desiccation and tolerant systems. In: Black M, Pritchard HW, eds. Desiccation and survival in plants—drying without dying. Wallingford: CAB International, 319–342. [Google Scholar]
- Proctor MCF, Pence VC. 2002. Vegetative tissues: bryophytes, vascular resurrection plants and vegetative propagules. In: Black M, Pritchard HW, eds. Desiccation and survival in plants—drying without dying. Wallingford: CAB International, 207–238. [Google Scholar]
- Sugiyama S, Nikara C. 2004. Differential contribution of avoidance and tolerance to dehydration resistance in populations of perennial ryegrass, Lolium perenne L. Australian Journal of Agricultural Research 55: 33–37. [Google Scholar]
- Volaire F. 1995. Growth, carbohydrate reserves and drought survival strategies of contrasting Dactylis glomerata populations in a Mediterranean environment. Journal of Applied Ecology 32: 56–66. [Google Scholar]
- Volaire F. 2002. Drought survival, summer dormancy and dehydrin accumulation in contrasting cultivars of Dactylis glomerata Physiologia Plantarum 116: 42–51. [DOI] [PubMed] [Google Scholar]
- Volaire F, Lelièvre F. 1997. Production, persistence and water-soluble carbohydrate accumulation in 21 contrasting populations of Dactylis glomerata L. subjected to severe drought in the south of France. Australian Journal of Agricultural Research 48: 933–944. [Google Scholar]
- Volaire F, Lelièvre F. 2001. Drought survival in Dactylis glomerata and Festuca arundinacea under similar rooting conditions in tubes. Plant and Soil 229: 225–234. [Google Scholar]
- Volaire F, Conéjero G, Lelièvre F. 2001. Drought survival and dehydration tolerance in Dactylis glomerata and Poa bulbosa Australian Journal of Plant Physiology 28: 743–754. [Google Scholar]
- Wisniewski M, Arora R. 2000. Seasonally regulated proteins in peach (Prunus persica L. Batsch): what are they and what do they do? In: Viémont JD, Crabbé J, eds. Dormancy in plants: from whole plant behaviour to cellular control. Wallingford: CAB International, 161–172. [Google Scholar]
- Wisniewski M, Fuchigamie LH, Sauter JJ, Shirazi A, Zhen L. 1996. Near-lethal stress and bud dormancy in woody plants. In: Lang GA, ed, Plant dormancy: physiology, biochemistry and molecular biology. Wallingford: CAB International, 201–212. [Google Scholar]
- Walters C, Farrant JM, Pammenter NW, Berjak P. 2002. Desiccation stress and damage. In: Black M, Pritchard HW, eds. Desiccation and survival in plants—drying without dying. Wallingford: CAB International, 263–292. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


