Abstract
• Background and Aims Ammonium can result in toxicity symptoms in many plants when it is supplied as the sole source of N. In this work, influences of different nitrogen forms at two levels (2 and 15 mm N) on growth, water relations and uptake and flow of potassium were studied in plants of Nicotiana tabacum ‘K 326’.
• Methods Xylem sap from different leaves was collected from 106-d-old tobacco plants cultured in quartz sand by application of pressure to the root system. Whole-shoot transpiration for each of the treatments was measured on a daily basis by weight determination.
• Key Results Total replacement of 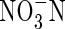 by
by 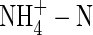 caused a substantial decrease in dry weight gain, even when plants grew under nutrient deficiency. Increasing nutrient concentration resulted in a greater net dry weight gain when nitrogen was supplied as
caused a substantial decrease in dry weight gain, even when plants grew under nutrient deficiency. Increasing nutrient concentration resulted in a greater net dry weight gain when nitrogen was supplied as 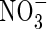 or NH4NO3, but resulted in little change when nitrogen was supplied as
or NH4NO3, but resulted in little change when nitrogen was supplied as 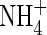 .
. 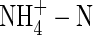 as the sole N-source also caused reduction in transpiration rate, changes in plant WUE (which depended on the nutrient levels) and a decrease in potassium uptake. However, the amount of xylem-transported potassium in the plants fed with
as the sole N-source also caused reduction in transpiration rate, changes in plant WUE (which depended on the nutrient levels) and a decrease in potassium uptake. However, the amount of xylem-transported potassium in the plants fed with 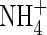 was not reduced: it was 457 % or 596 % of the potassium currently taken up at low or high nutrient level, respectively, indicating a massive export from leaves and cycling of potassium in the phloem.
was not reduced: it was 457 % or 596 % of the potassium currently taken up at low or high nutrient level, respectively, indicating a massive export from leaves and cycling of potassium in the phloem.
• Conclusions Ammonium reduces leaf stomatal conductance of tobacco plants. The flow and partitioning of potassium in tobacco plants can be changed, depending on the nitrogen forms and nutrient levels.
Keywords: Carbon-isotope discrimination (δ13C), Nicotiana tabacum, nitrogen forms, nutrient levels, potassium uptake, potassium flow, transpiration, water use efficiency
INTRODUCTION
Ammonium (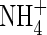 ) and nitrate (
) and nitrate (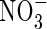 ) are two main forms of nitrogen (N) that can be absorbed by higher plants from the natural environment. Because the two forms are fundamentally different in charge, they have quite different effects on the physiological and metabolic processes of plants (Haynes and Goh, 1978; Marschner, 1995). Although
) are two main forms of nitrogen (N) that can be absorbed by higher plants from the natural environment. Because the two forms are fundamentally different in charge, they have quite different effects on the physiological and metabolic processes of plants (Haynes and Goh, 1978; Marschner, 1995). Although 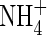 is an intermediate in many metabolic reactions, it can result in toxicity symptoms in many plants when it is supplied as the sole nitrogen source (Britto and Kronzucker, 2002).
is an intermediate in many metabolic reactions, it can result in toxicity symptoms in many plants when it is supplied as the sole nitrogen source (Britto and Kronzucker, 2002).
One of the inhibitory effects of 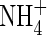 on plant growth is its influence on water relations. It has been reported that
on plant growth is its influence on water relations. It has been reported that 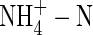 caused an increase in stomatal conductance in white clover (Høgh-Jensen and Schjoerring, 1997), and enhanced the transpiration rate in alfalfa (Khan et al., 1994) and tomatoes (Lugert et al., 2001). In both cases, leaf water and osmotic potential was lower than that of plants grown under
caused an increase in stomatal conductance in white clover (Høgh-Jensen and Schjoerring, 1997), and enhanced the transpiration rate in alfalfa (Khan et al., 1994) and tomatoes (Lugert et al., 2001). In both cases, leaf water and osmotic potential was lower than that of plants grown under 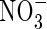 conditions (Ashraf, 1999). However, in Phaseolus vulgaris,
conditions (Ashraf, 1999). However, in Phaseolus vulgaris, 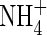 was found to decrease stomatal conductance and reduce root water uptake (Guo et al., 2002). Other research using Beta vulgaris indicated that the N-source had no effect on stomatal conductance, although use of
was found to decrease stomatal conductance and reduce root water uptake (Guo et al., 2002). Other research using Beta vulgaris indicated that the N-source had no effect on stomatal conductance, although use of 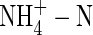 did reduce water uptake in muskmelon and sugar beet (Raab and Terry, 1994; Adler et al., 1996), with a slight reduction in leaf conductance in castor beans (Peuke et al., 1994a). The dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is expressed not only in roots, but also in guard cells of Arabidopsis thaliana plants and functions as a
did reduce water uptake in muskmelon and sugar beet (Raab and Terry, 1994; Adler et al., 1996), with a slight reduction in leaf conductance in castor beans (Peuke et al., 1994a). The dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is expressed not only in roots, but also in guard cells of Arabidopsis thaliana plants and functions as a 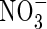 transporter, and it has been demonstrated that CHL1 supports stomatal function in the presence of
transporter, and it has been demonstrated that CHL1 supports stomatal function in the presence of 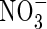 (Guo et al., 2003). The results imply that
(Guo et al., 2003). The results imply that 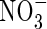 plays a role in stomatal opening, since in the absence of
plays a role in stomatal opening, since in the absence of 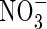 stomatal aperture and transpiration are reduced.
stomatal aperture and transpiration are reduced.
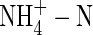 nutrition also causes a decrease in water use efficiency (WUE) in tomatoes (Lugert et al., 2001; Claussen, 2002), white clover (Høgh-Jensen and Schjoerring, 1997), Phaseolus vulgaris and Ricinus communis (Raven et al., 1992), but increases WUE in wheat (Morgan, 1986; Yin and Raven, 1998). It seems that the influence of
nutrition also causes a decrease in water use efficiency (WUE) in tomatoes (Lugert et al., 2001; Claussen, 2002), white clover (Høgh-Jensen and Schjoerring, 1997), Phaseolus vulgaris and Ricinus communis (Raven et al., 1992), but increases WUE in wheat (Morgan, 1986; Yin and Raven, 1998). It seems that the influence of 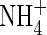 on water relations varies depending on the plant species and the experimental conditions. Given these variable responses, it would be useful to determine within species how water relations behave when plants grow under various nutritional regimes and supplied with different nitrogen forms. Stable carbon isotope discrimination (δ13C) is a useful method to study plant photosynthesis (Farquhar et al., 1989). It is known that variations in the 13C/12C ratio of the organic carbon of plants are a result of genetic and environmental factors (Farquhar et al., 1989; Raven et al., 1992). N-sources, for example, can affect plant δ13C by influencing plant WUE and carbon assimilation (Yin and Raven, 1998). A common finding is that a lower WUE is correlated with a more negative δ13C value (Farquhar et al., 1989; Raven and Farquhar, 1990; Raven et al., 1992; Yin and Raven, 1997, 1998).
on water relations varies depending on the plant species and the experimental conditions. Given these variable responses, it would be useful to determine within species how water relations behave when plants grow under various nutritional regimes and supplied with different nitrogen forms. Stable carbon isotope discrimination (δ13C) is a useful method to study plant photosynthesis (Farquhar et al., 1989). It is known that variations in the 13C/12C ratio of the organic carbon of plants are a result of genetic and environmental factors (Farquhar et al., 1989; Raven et al., 1992). N-sources, for example, can affect plant δ13C by influencing plant WUE and carbon assimilation (Yin and Raven, 1998). A common finding is that a lower WUE is correlated with a more negative δ13C value (Farquhar et al., 1989; Raven and Farquhar, 1990; Raven et al., 1992; Yin and Raven, 1997, 1998).
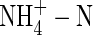 has been found to cause a strong inhibition in potassium uptake by plants (Allen and Raven, 1987). Potassium, as a macro-element in the cells of higher plants, exhibits high mobility. Its flow, however, is quite different depending on plant type (Armstrong and Kirkby, 1979; Jeschke et al., 1987; Jiang et al., 2001) and can be changed by stress. Under saline conditions, for example, plants have been found to have higher potassium cycling rates compared with plants grown under normal conditions. This phenomenon may contribute to the plant's ability to adjust to saline stress (Jeschke et al., 1987; Jeschke and Pate, 1991). Plants infected by a parasitic angiosperm (Hibberd et al., 1999) or that have been decapitated (Jiang et al., 2001) have also shown changes in potassium flow and cycling patterns within them. However, the extent to which translocation in the xylem and circulation of potassium is altered by the supply of N in different forms under varying nutrient conditions is not clear.
has been found to cause a strong inhibition in potassium uptake by plants (Allen and Raven, 1987). Potassium, as a macro-element in the cells of higher plants, exhibits high mobility. Its flow, however, is quite different depending on plant type (Armstrong and Kirkby, 1979; Jeschke et al., 1987; Jiang et al., 2001) and can be changed by stress. Under saline conditions, for example, plants have been found to have higher potassium cycling rates compared with plants grown under normal conditions. This phenomenon may contribute to the plant's ability to adjust to saline stress (Jeschke et al., 1987; Jeschke and Pate, 1991). Plants infected by a parasitic angiosperm (Hibberd et al., 1999) or that have been decapitated (Jiang et al., 2001) have also shown changes in potassium flow and cycling patterns within them. However, the extent to which translocation in the xylem and circulation of potassium is altered by the supply of N in different forms under varying nutrient conditions is not clear.
The present experiments were carried out to address these questions. The influence of different N-forms at two nutrient levels on the growth, water relations, and potassium uptake and flow in tobacco plants was investigated to better understand the underlying mechanisms of the inhibitory effects of 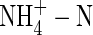 on plant growth.
on plant growth.
MATERIALS AND METHODS
Plant cultivation
Tobacco seeds (Nicotiana tabacum L. ‘K 326’) were germinated in a mixture consisting of 60 % (w/w) peat culture substrate, 20 % (w/w) ground maize stalk and 20 % (w/w) perlite, and grown in a seedbed in a naturally illuminated greenhouse for 65 d. The plants were then washed with tap water to remove all substrate from the roots, and transferred to 2·3 L pots (one plant per pot) containing quartz sand (0·25–0·5 mm in diameter). They were supplied initially with a half-strength nutrient solution as used by Walch-Liu et al. (2000). The solution consisted of (in mm for full strength): KH2PO4 0·5, MgSO4 1·2, CaCl2 2·0, KNO3 2, H3BO3 1·0 × 10−2, MnSO4 5 × 10−4, ZnSO4 5 × 10−4, CuSO4 1 × 10−4, (NH4)6Mo7O24 1 × 10−5, Fe-EDTA 1·5 × 10−2. After 2 d a full strength solution was provided. The pots were watered every morning with an excess of the nutrient solution, a hole in the bottom of the pot allowing drainage. Plants were grown in a growth room with a 14-h photoperiod. The photosynthetically active radiation at the surface of the pots was 220–270 mmol m−2 s−1 provided by reflector sunlight dysprosium lamps (DDF 400, Nanjing, China). The first harvest was made 106 d after sowing and 41 d after transferring to constant environmental conditions; the second harvest was performed 10 d later.
Treatments and harvest procedures
For harvest, the plants were divided into seven groups of five plants, each of similar size and development. One group was used for the first harvest, the other six groups for the second harvest. On the day of the first harvest, the remaining six groups of plants were treated with either a low (as used by Walch-Liu et al., 2000) or high (Hoagland solution) concentration of nutrient solution combined with different forms of nitrogen (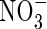 ,
, 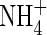 and NH4NO3) as shown in Table 1.
and NH4NO3) as shown in Table 1.
Table 1.
Macronutrient composition of the solutions with low or high concentration of nutrients combined with different N-forms (in mm)
| Treatment |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition |
Low 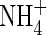 |
Low 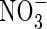 |
Low NH4NO3 |
High 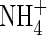 |
High 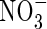 |
High NH4NO3 |
|||||
| KH2PO4 | 0·5 | 0·5 | 0·5 | 1 | 1 | 1 | |||||
| KNO3 | 2 | 5 | |||||||||
| NH4NO3 | 1 | 7·5 | |||||||||
| (NH4)2SO4 | 1 | 7·5 | |||||||||
| Ca(NO3)2 | 5 | ||||||||||
| CaCl2 | 2·0 | 2·0 | 2·0 | 5 | 5 | ||||||
| MgSO4 | 1·2 | 1·2 | 1·2 | 2 | 2 | 2 | |||||
| K2SO4 | 1 | 1 | 2·5 | 2·5 | |||||||
In addition, the media with low concentration of nutrients contained the same micronutrient as mentioned in the section ‘Plant cultivation’. The high concentration of nutrients contained the following micronutrients (mm): H3BO3 4·6 × 10−2, ZnSO4.7H2O 7·6 × 10−4, CuSO4.5H2O 3·2 × 10−4, (NH4)6MoO24 1·6 × 10−5, MnCl2.4H2O 9·0 × 10−3, FeEDTA 3·7 × 10−2.
Plant leaves were numbered in ascending order, starting with the lowest mature leaf, which was designed as leaf 1. Smaller leaves, which had already senesced, were removed. At the first harvest, the youngest unfolded leaf was number 9, and the top part of the plant above this was incorporated into the upper leaves because of its small size. At harvest, the plants were separated into the lower, middle and upper stratum of three leaves each, and also into roots, stem and top. For the second harvest the top included new leaves that had grown out above leaf 9. Roots were washed free of sand with tapwater. The root and the three strata of leaves were divided into two lateral symmetrical parts: one was kept at −20 °C until analysis of tissue 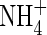 and
and 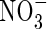 contents, and the other was dried (70 °C) for determination of ions present in different organs with Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP) (Perkin Elmer 3300 DV, USA).
contents, and the other was dried (70 °C) for determination of ions present in different organs with Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP) (Perkin Elmer 3300 DV, USA).
For the measurement of tissue 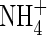 and
and 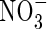 contents, samples were homogenized with distilled water and centrifuged. The residue was washed twice with distilled water by repeating the homogenization/centrifugation procedure. The combined supernatants were used for analysis of tissue
contents, samples were homogenized with distilled water and centrifuged. The residue was washed twice with distilled water by repeating the homogenization/centrifugation procedure. The combined supernatants were used for analysis of tissue 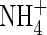 and
and 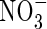 contents with a TRAACS-2000 autoanalyser (Bran+Luebbe, Germany).
contents with a TRAACS-2000 autoanalyser (Bran+Luebbe, Germany).
Measurement of transpiration
Whole-shoot transpiration was measured on a daily basis by weighing five pots of each of the treatments at the beginning of the light period, after the daily addition of nutrient solution and draining, and at the end of the light period (08:00 h, 10:00 h and 22:00 h, respectively). The transpiration between 08:00 h and 10:00 h was calculated by averaging the amount of transpiration between 10:00 h and 22:00 h. Corrections were applied for water loss from pots without plants. In all cases, evaporation from the pots was minimized by covering the exposed surface with a layer of filter paper.
Collection of xylem sap
For collection of xylem sap, plants were grown in special pots in order to apply pressure to the root system (Seel and Jeschke, 1999), but were otherwise treated the same as the plants for harvest. The xylem sap collection procedure was essentially the same as described by Jeschke and Pate (1991). Samples were taken from leaf numbers 2, 5 and 8 (counting up from the base). In brief, approximately midway along the length of the leaf an incision was made into the midrib. The cut surface was washed carefully with distilled water and a Teflon tube attached. Pressure was applied to both the sand substrate and the roots of the treated plants. After slowly applying pressure, xylem sap started to exude from the midrib after reaching a balancing pressure (Seel and Jeschke, 1999), and sap was collected 50 kPa above this pressure. The first exudate was discarded to avoid contamination from damaged cells. Xylem sap was also collected from the stem base of the pressurized plants, either from an incision into the stem (Hibberd et al., 1999) or by inserting a syringe needle into the stem until it reached the xylem vessels. Xylem sap was kept on ice during collection and stored at −20 °C before analysis. After appropriate dilution, ions were analysed directly using ICP (PE 3300 DV, USA).
Measurement of carbon isotope discrimination (δ13C)
Leaves (including leaf veins and petioles) were dried at 70 °C and ground into a fine powder. They were then treated and analysed for carbon isotope composition using a mass spectrometer (Delta S, Finnigan MAT, Germany) according to the method described by Farquhar et al. (1989), with three internal replications. The 13C/12C ratios of the samples were expressed as δ13C relative to the isotope composition of the fossil belemnite standard from the Pee Dee Formation (PDB).
Estimation of the net flows of potassium through xylem and phloem in the whole plant
Net flows of potassium within plants were estimated using the method described by Armstrong and Kirkby (1979). Based on the assumption that nutrients were transported solely through xylem and phloem, Ca2+ could only be transported apically through the xylem and had no mobility in the phloem.
The net xylem flow of Ca2+, JCa,x, transported to various plant parts during a given period was the same of that of the net increment of Ca2+, ΔCa, in the same position during the same phase, where
 |
The content of K+ and calcium had a stable ratio in the xylem during relatively stable growth conditions. The [K/Ca]x ratio was obtained by determining the content of these two elements in the xylem sap collected.
For a given period, the net xylem flow of potassium, JK,x, towards a site was calculated from the net increment of Ca2+, ΔCa, and the ratio of potassium and calcium, [K/Ca]x, in the xylem by:
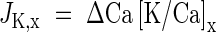 |
The amount of potassium exported through the phloem, JK,p, was equal to the difference between the measured potassium increment, ΔK, in each organ and the net xylem import according to:
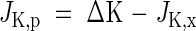 |
A positive difference indicated net phloem import, while a negative difference implied net phloem export from an organ. Working progressively along the plant from the root to the leaves and top, the net flows of potassium within the whole plant were obtained as shown in Figs 3 and 4. The differences between the quantities of potassium translocated in the phloem and in the xylem also allowed the estimation of transfer processes between these translocation streams.
Fig. 3.
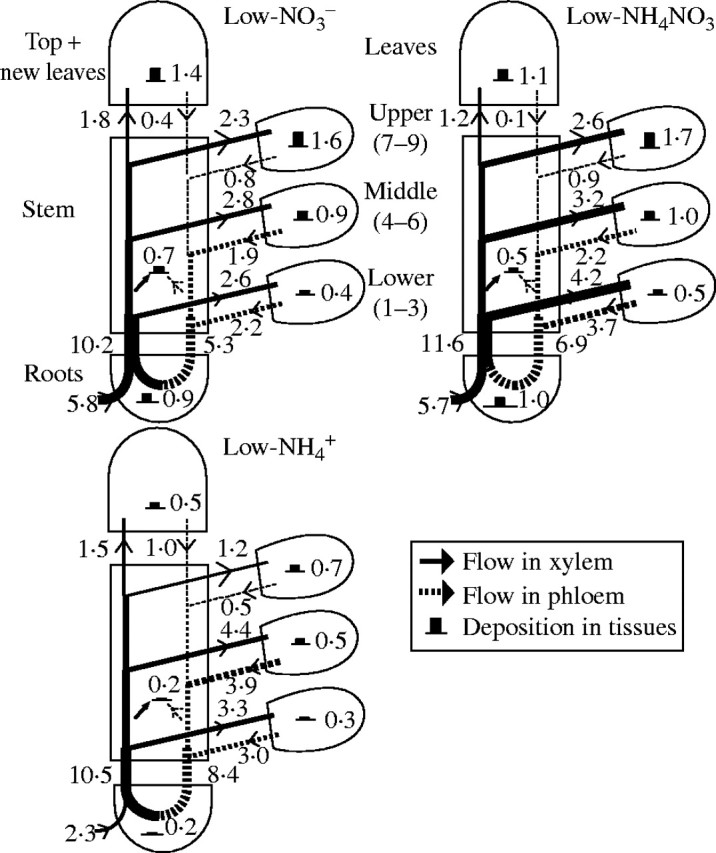
Flow profiles for uptake, transport and utilization of K+ in tobacco plants supplied with a low nutrient concentration (2·5 mm K and 2 mm N) over a 10 d experimental period, starting 106 d after sowing. The values of K+ deposition and the statistical significance are given in Table 3. The width of arrows and the height of histograms are drawn in proportion to the net flows and deposition of K+. The numbers indicate the values of uptake, transport and utilization (mmol K+ per plant over the 10 d study period).
Fig. 4.
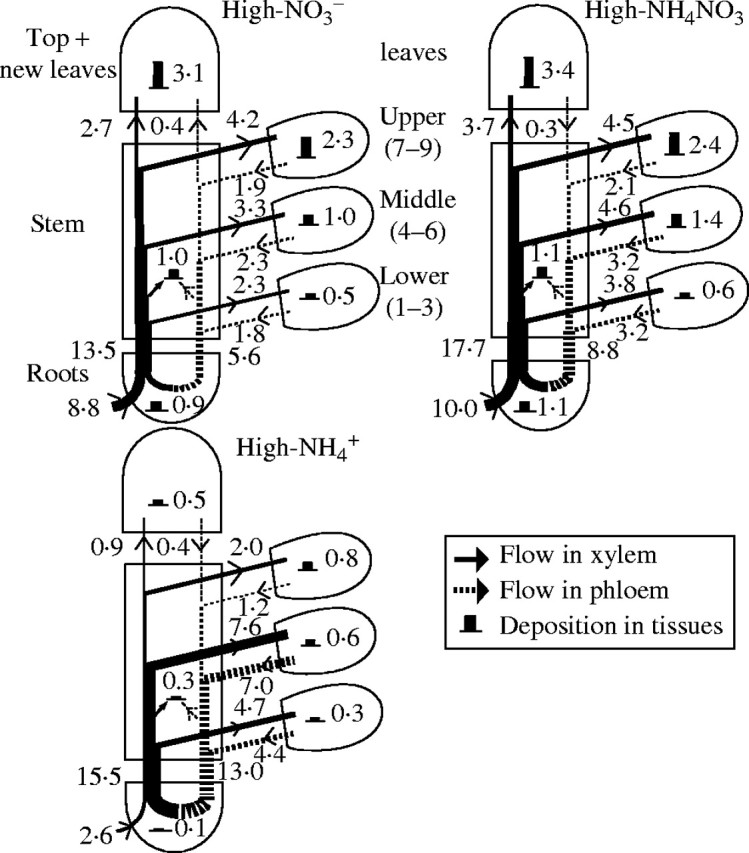
Flow profiles for uptake, transport and utilization of K+ in tobacco plants supplied with a high nutrient concentration (6 mm K and 15 mm N) over a 10 d experimental period, starting 106 d after sowing. The values of K+ deposition and the statistical significance are given in Table 3. The width of arrows and the height of histograms are drawn in proportion to the net flows and deposition of K+. The numbers indicate the values of uptake, transport and utilization (mmol K+ per plant over the 10 d study period).
Statistical treatment
Dry weight increments were obtained from the five replicates of each treatment at the first and second harvest. All further analyses were made with five individual samples for each organ. For statistical analysis of the data, SAS for Windows (version 6.12) was used (SAS, 1987). Differences between data in all tables were tested with ANOVA.
RESULTS
Plant growth and development
At low nutrient levels, all plants treated with the three forms of nitrogen showed symptoms of N deficiency, such as thin leaves and light-green lower leaf colour, and small plant size at the second harvest compared with those grown at higher N-levels. However, the growth of the 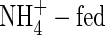 plants was more inhibited than those of either NO3- or NH4NO3-fed plants. The total net increment in dry weight of the
plants was more inhibited than those of either NO3- or NH4NO3-fed plants. The total net increment in dry weight of the 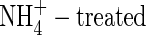 plants was 23·5 % and 22·7 % lower in comparison with that of the NO3- and NH4NO3-fed plants, respectively. Increasing nutrient concentration resulted in a greater net dry weight gain when nitrogen was supplied as
plants was 23·5 % and 22·7 % lower in comparison with that of the NO3- and NH4NO3-fed plants, respectively. Increasing nutrient concentration resulted in a greater net dry weight gain when nitrogen was supplied as 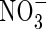 or NH4NO3, but resulted in little change when nitrogen was supplied as
or NH4NO3, but resulted in little change when nitrogen was supplied as 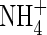 . The total net dry weight gain of the
. The total net dry weight gain of the 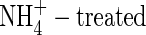 plants was 38·8 % and 47·9 % lower than that of the NO3- and NH4NO3-fed plants, respectively (Table 2). Under the
plants was 38·8 % and 47·9 % lower than that of the NO3- and NH4NO3-fed plants, respectively (Table 2). Under the 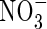 and NH4NO3 treatments at both nutrient-levels, the plant top (including new leaves and upper leaves) contributed the most to the dry weight increment, while under the
and NH4NO3 treatments at both nutrient-levels, the plant top (including new leaves and upper leaves) contributed the most to the dry weight increment, while under the 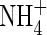 treatment, the upper and middle leaves were the main sites for assimilate deposition. In spite of the inhibited growth of the plants treated with different N-forms at low nutrient-level during the study period, their root net increments were not reduced in comparison with the respective treatments at high nutrient-level (Table 2), resulting in a decreased shoot/total dry weight ratio.
treatment, the upper and middle leaves were the main sites for assimilate deposition. In spite of the inhibited growth of the plants treated with different N-forms at low nutrient-level during the study period, their root net increments were not reduced in comparison with the respective treatments at high nutrient-level (Table 2), resulting in a decreased shoot/total dry weight ratio.
Table 2.
Initial values and net increments in dry weights of component organs and of whole tobacco plants under different treatments over a 10 d study period
| Net increase in DW (g organ−1) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low concentration |
High concentration |
|||||||||||
| Position |
Initial DW (g organ−1) |
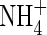 |
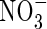 |
NH4NO3 |
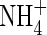 |
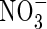 |
NH4NO3 |
|||||
| Top + new leaves | 0·53bc | 0·95b | 0·79bc | 0·42c | 2·05a | 2·21a | ||||||
| Upper leaves | 0·43 ± 0·05 | 0·77c | 1·15b | 1·16b | 0·80c | 1·49a | 1·61a | |||||
| Middle leaves | 0·48 ± 0·08 | 0·81ab | 0·79ab | 0·88a | 0·84ab | 0·71b | 0·90a | |||||
| Lower leaves | 0·43 ± 0·15 | 0·40a | 0·44a | 0·53a | 0·53a | 0·39a | 0·51a | |||||
| Stem | 0·58 ± 0·15 | 0·64b | 0·69ab | 0·54b | 0·62b | 0·71ab | 0·84a | |||||
| Roots | 0·38 ± 0·18 | 0·62bc | 0·90abc | 0·98ab | 0·57c | 0·82abc | 1·18a | |||||
| Whole plant | 2·30 ± 0·52 | 3·77d | 4·93c | 4·88c | 3·78d | 6·18b | 7·26a | |||||
Values in rows followed by the same letter are not significantly different (P > 0·05).
Total transpiration and leaf δ13C
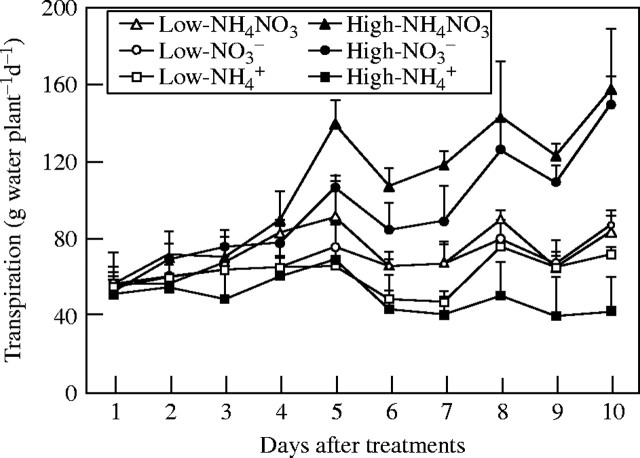
When a high level of nutrients was supplied, the shoot transpiration of the NO3- and NH4NO3-fed plants increased soon after the beginning of the treatment, remaining stable until the end of the experiment. Shoot transpiration declined during the same time for plants fed with 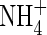 (Fig. 1). At low nutrient level, the shoot transpiration of the NO3- and NH4NO3-treated plants did not increase as much as that of the plants supplied with the same N-forms at higher nutrient level, but was still higher than that of the
(Fig. 1). At low nutrient level, the shoot transpiration of the NO3- and NH4NO3-treated plants did not increase as much as that of the plants supplied with the same N-forms at higher nutrient level, but was still higher than that of the 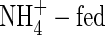 plants.
plants.
Fig. 1.
Time-course of whole-shoot transpiration of tobacco plants as dependent on N-forms and nutrient. Bars denote s.e. of the mean, n = 5.
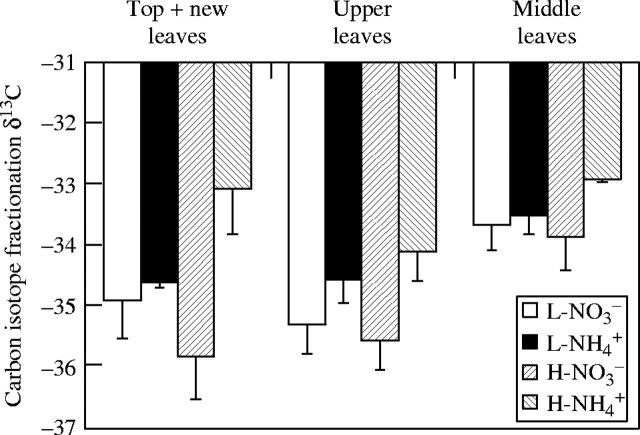
At the end of the experiment, samples collected from the middle and upper leaf strata and top and new leaves of the 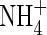 - and NO3-fed plants were used to compare their stable carbon isotopes δ13C. Tissue δ13C in the
- and NO3-fed plants were used to compare their stable carbon isotopes δ13C. Tissue δ13C in the 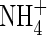 treatment was less negative (smaller absolute value) than that in the
treatment was less negative (smaller absolute value) than that in the 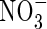 treatment at either nutrient level; especially in the top and new leaves under high nutrient level (Fig. 2).
treatment at either nutrient level; especially in the top and new leaves under high nutrient level (Fig. 2).
Fig. 2.
Carbon isotope fractionation (δ13C, ‰) of leaf tissues of 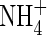 - and NO3-fed plants at both low and high nutrient levels during a 10 d study period. L and H represent the low and high nutrient level, respectively. Bars denote s.e. of the mean, n = 5.
- and NO3-fed plants at both low and high nutrient levels during a 10 d study period. L and H represent the low and high nutrient level, respectively. Bars denote s.e. of the mean, n = 5.
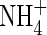 and
and 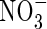 contents in leaf tissues and roots
contents in leaf tissues and roots
As shown in Table 3, no significant differences in 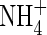 and
and 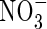 content was observed in the leaves and roots of the plants treated with three N-forms at low nutrient level. At high nutrient level, however, the
content was observed in the leaves and roots of the plants treated with three N-forms at low nutrient level. At high nutrient level, however, the 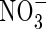 content in the upper and middle leaves of the
content in the upper and middle leaves of the 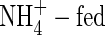 plants was substantially lower than that of the NO3- and NH4NO3-treated plants, and
plants was substantially lower than that of the NO3- and NH4NO3-treated plants, and 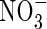 could not be detected in lower leaves and roots. The
could not be detected in lower leaves and roots. The 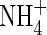 content, on the other hand, was obviously higher in the upper leaves and roots of both
content, on the other hand, was obviously higher in the upper leaves and roots of both 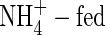 (
(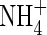 and NH4NO3) plants than that of the NO3-fed plants.
and NH4NO3) plants than that of the NO3-fed plants.
Table 3.
Contents of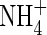 and
and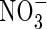 in different leaves and roots of tobacco plants grown under different nutrient concentrations and nitrogen forms over a 10 d study period (mmol organ−1)
in different leaves and roots of tobacco plants grown under different nutrient concentrations and nitrogen forms over a 10 d study period (mmol organ−1)
| Treatment |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position |
Low 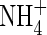 |
Low 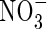 |
Low NH4NO3 |
High 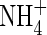 |
High 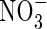 |
High NH4NO3 |
||||||
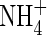 content content |
||||||||||||
| Upper leaves | 2·65b | 2·71b | 2·37b | 35·17a | 4·34b | 23·25a | ||||||
| Middle leaves | 1·81b | 1·29b | 1·68b | 16·41a | 6·81ab | 12·75ab | ||||||
| Lower leaves | 1·81a | 0·92a | 2·01a | 3·64a | 1·30a | 2·22a | ||||||
| Roots | 12·55bc | 4·52c | 3·78c | 21·90ab | 6·18c | 28·32a | ||||||
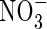 content content |
||||||||||||
| Upper leaves | – | – | – | 0·70c | 231·87a | 96·96b | ||||||
| Middle leaves | – | – | – | 1·77b | 58·88a | 40·84a | ||||||
| Lower leaves | – | – | – | – | 20·49a | 21·97a | ||||||
| Roots | – | – | – | – | 52·92a | 24·06b | ||||||
Values in rows followed by the same letter are not significantly different (P > 0·05). – indicates not detected.
Effect of N supply on contents and changes in potassium
The changes in net potassium increment as affected by different N-forms and nutrient levels were similar to, but clearly more dramatic than those in net dry weight gain. The total net uptake of potassium in 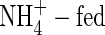 plants was markedly smaller than that in the NO3- or NH4NO3-fed plants at both nutrient levels (Table 4). The greatest increments of potassium were found in the rapidly growing organs, i.e. top and new leaves, and upper leaves of the NO3- and NH4NO3-fed plants, and top and new leaves, and upper and middle leaves of the
plants was markedly smaller than that in the NO3- or NH4NO3-fed plants at both nutrient levels (Table 4). The greatest increments of potassium were found in the rapidly growing organs, i.e. top and new leaves, and upper leaves of the NO3- and NH4NO3-fed plants, and top and new leaves, and upper and middle leaves of the 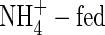 plants. Substantial increments were also found in the middle leaves, stem and roots of the NO3- and NH4NO3-treated plants, while that in stem and roots of the
plants. Substantial increments were also found in the middle leaves, stem and roots of the NO3- and NH4NO3-treated plants, while that in stem and roots of the 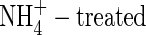 plants was much less at both nutrient levels during the study period. Again, as with dry weight gain, the total potassium K+ contents in the NO3- and NH4NO3-treated plants increased when potassium supply was elevated. The potassium content of the
plants was much less at both nutrient levels during the study period. Again, as with dry weight gain, the total potassium K+ contents in the NO3- and NH4NO3-treated plants increased when potassium supply was elevated. The potassium content of the 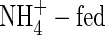 plants was relatively unchanged under the same conditions.
plants was relatively unchanged under the same conditions.
Table 4.
Initial values and net increments in K+ contents of component organs and of whole tobacco plants under different treatments over a 10 d study period
| Net K+ increment (mmol organ−1) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low concentration |
High concentration |
|||||||||||
| Position |
Initial values (mmol organ−1) |
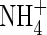 |
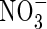 |
NH4NO3 |
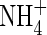 |
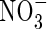 |
NH4NO3 |
|||||
| Top+new leaves | 0·52c | 1·37b | 1·08bc | 0·46c | 3·05a | 3·37a | ||||||
| Upper leaves | 0·49 ± 0·07 | 0·68c | 1·56b | 1·65b | 0·81c | 2·29a | 2·45a | |||||
| Middle leaves | 0·53 ± 0·06 | 0·52c | 0·88b | 0·96b | 0·60c | 1·01b | 1·36a | |||||
| Lower leaves | 0·39 ± 0·12 | 0·25d | 0·41bc | 0·49ab | 0·33cd | 0·50ab | 0·58a | |||||
| Stem | 0·37 ± 0·06 | 0·18d | 0·68b | 0·49bc | 0·25cd | 1·02a | 1·14a | |||||
| Roots | 0·17 ± 0·06 | 0·15b | 0·88a | 1·00a | 0·11b | 0·92a | 1·06a | |||||
| Whole plant | 1·96 ± 0·21 | 2·30d | 5·78c | 5·67c | 2·56d | 8·81b | 9·97a | |||||
Values in rows followed by the same letter are not significantly different (P > 0·05).
Water use efficiency of plants
The WUE is presented as net increase in plant dry weight per unit water used (g DW increase kg−1 H2O) during the experimental period. The WUE of the NO3- and NH4NO3-fed plants was almost same under both nutrient levels, but that of the 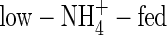 plants was the lowest and that of the
plants was the lowest and that of the 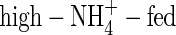 plants was the highest among the treatments (Table 5).
plants was the highest among the treatments (Table 5).
Table 5.
Water use efficiency (g DW increase kg−1 H2O) in different treatments over a 10 d study period
| Low concentration |
High concentration |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
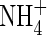 |
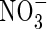 |
NH4NO3 |
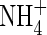 |
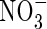 |
NH4NO3 |
|||||
| WUE | 5·69 | 6·49 | 6·12 | 6·89 | 5·89 | 6·07 | ||||
Estimation of net flows of potassium within tobacco plants
As shown in Figs 3 and 4, in spite of the inhibited K+ uptake in the 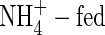 plants, K+ transport in the xylem was still more than that in the NO3-fed plants. The highest amount of the xylem-transported potassium was found in the NH4NO3-treated plants at both nutrient levels. The amount of the xylem-transported potassium was much more than accounted for by uptake, and was higher at high nutrient level than that at low nutrient level. Phloem retranslocation of potassium from shoot to root contributed to the xylem-transported potassium. The amount of the phloem-retranslocated potassium in the
plants, K+ transport in the xylem was still more than that in the NO3-fed plants. The highest amount of the xylem-transported potassium was found in the NH4NO3-treated plants at both nutrient levels. The amount of the xylem-transported potassium was much more than accounted for by uptake, and was higher at high nutrient level than that at low nutrient level. Phloem retranslocation of potassium from shoot to root contributed to the xylem-transported potassium. The amount of the phloem-retranslocated potassium in the 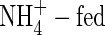 plants was four times more than that of the total uptake under high nutrient levels and 2·6 times more under low levels.
plants was four times more than that of the total uptake under high nutrient levels and 2·6 times more under low levels.
The phloem-recycled potassium from shoot to roots came mainly from leaves (Figs 3, 4). The proportion of potassium exported via the phloem to that imported via the xylem of a leaf depended on leaf age, nutrient levels and N-forms. In plants fed with a high level of NO3-N, for example, it was 45 %, 70 % and 78 % in the upper, middle and lower leaves, respectively; while under high 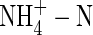 conditions, it was increased to 60 %, 92 % and 94 %, respectively. Also at low nutrient level, the value was 35 %, 68 % and 85 % respectively in the NO3-fed plants, and 42 %, 89 % and 91 % in the
conditions, it was increased to 60 %, 92 % and 94 %, respectively. Also at low nutrient level, the value was 35 %, 68 % and 85 % respectively in the NO3-fed plants, and 42 %, 89 % and 91 % in the 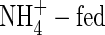 plants.
plants.
DISCUSSION
Effect on plant growth
The reported symptoms of 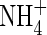 toxicity generally appeared with external
toxicity generally appeared with external 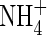 concentrations above 0·1–0·5 mm (reviewed in Britto and Kronzucker, 2002). In spite of the inhibited growth under low nutrient levels (2 mm N), as represented by lower net dry weight gain (Table 2), lower shoot/total dry weight ratio and obvious N-deficient symptoms (photographs not shown), plant growth was further suppressed when
concentrations above 0·1–0·5 mm (reviewed in Britto and Kronzucker, 2002). In spite of the inhibited growth under low nutrient levels (2 mm N), as represented by lower net dry weight gain (Table 2), lower shoot/total dry weight ratio and obvious N-deficient symptoms (photographs not shown), plant growth was further suppressed when 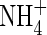 was supplied as the sole N-source. Increasing nutrient concentration (Hoagland, 15 mm N) resulted in a greater net dry weight increment when nitrogen was supplied as
was supplied as the sole N-source. Increasing nutrient concentration (Hoagland, 15 mm N) resulted in a greater net dry weight increment when nitrogen was supplied as 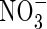 or NH4NO3, but produced no change when nitrogen was supplied as
or NH4NO3, but produced no change when nitrogen was supplied as 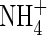 (Table 2). These results could not simply be explained by
(Table 2). These results could not simply be explained by 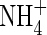 toxicity. In the present study, the same amount of
toxicity. In the present study, the same amount of 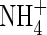 was found in the leaf tissues and roots of the plants grown under the low nutrient level with different N-forms. Furthermore, at the high nutrient level, a similar
was found in the leaf tissues and roots of the plants grown under the low nutrient level with different N-forms. Furthermore, at the high nutrient level, a similar 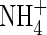 content was detected in the tissues of both
content was detected in the tissues of both 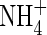 - and NH4NO3-fed plants (Table 3): the latter were able to grow well and even obtained the highest net increment in dry weight (Table 2). As shown in Table 3, a large amount of NO3-N was also present in the tissues of the NH4NO3-fed plants grown under the high nutrient level but not in the
- and NH4NO3-fed plants (Table 3): the latter were able to grow well and even obtained the highest net increment in dry weight (Table 2). As shown in Table 3, a large amount of NO3-N was also present in the tissues of the NH4NO3-fed plants grown under the high nutrient level but not in the 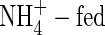 plants. These results support the conclusion that co-provision of
plants. These results support the conclusion that co-provision of 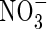 can not only alleviate
can not only alleviate 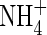 toxicity, but may also induce a synergistic growth response that can surpass the maximal growth rates produced by either N-source alone (Britto and Kronzucker, 2002). As long as there was
toxicity, but may also induce a synergistic growth response that can surpass the maximal growth rates produced by either N-source alone (Britto and Kronzucker, 2002). As long as there was 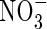 ,
, 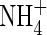 did not inhibit plant growth (Walch-Liu et al., 2000). One of the important roles of
did not inhibit plant growth (Walch-Liu et al., 2000). One of the important roles of 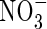 is as a signal to stimulate (or optimize) a multitude of biochemical responses (Tischner, 2000; Britto and Kronzucker, 2002).
is as a signal to stimulate (or optimize) a multitude of biochemical responses (Tischner, 2000; Britto and Kronzucker, 2002).
Transpiration and water use efficiency
NO3-N and NH4NO3 had similar effects on plant transpiration, while 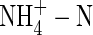 caused a marked reduction in transpiration (Fig. 1), although all of the treated plants were watered daily and showed no signs of water stress. The present results do not agree with the observation that use of
caused a marked reduction in transpiration (Fig. 1), although all of the treated plants were watered daily and showed no signs of water stress. The present results do not agree with the observation that use of 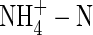 increased leaf transpiration in alfalfa (Khan et al., 1994), tomato (Lugert et al., 2001), maize and wheat (Lewis et al., 1989), and increased stomatal conductance of white clover (Høgh-Jensen and Schjoerring, 1997). Although
increased leaf transpiration in alfalfa (Khan et al., 1994), tomato (Lugert et al., 2001), maize and wheat (Lewis et al., 1989), and increased stomatal conductance of white clover (Høgh-Jensen and Schjoerring, 1997). Although 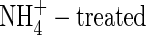 plants have smaller leaf area (Khan et al., 1994; Raab and Terry, 1994) and lower leaf area ratio (Magalhaes and Wilcox, 1984), these could not be the reasons to explain the reduced transpiration in the present study. At the second harvest, the
plants have smaller leaf area (Khan et al., 1994; Raab and Terry, 1994) and lower leaf area ratio (Magalhaes and Wilcox, 1984), these could not be the reasons to explain the reduced transpiration in the present study. At the second harvest, the 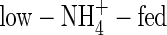 shoots increased in dry weight by 3·15 g, 1·64 times more than that at the first harvest, and daily transpiration increased by only 33 %. The
shoots increased in dry weight by 3·15 g, 1·64 times more than that at the first harvest, and daily transpiration increased by only 33 %. The 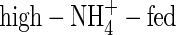 shoot increased in dry weight by 3·21 g, 1·67 times more than that at the first harvest, while daily transpiration was reduced by 13 % compared with the first harvest (Fig. 1). Furthermore, results of carbon isotope discrimination for different leaves of the NO3- and
shoot increased in dry weight by 3·21 g, 1·67 times more than that at the first harvest, while daily transpiration was reduced by 13 % compared with the first harvest (Fig. 1). Furthermore, results of carbon isotope discrimination for different leaves of the NO3- and 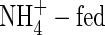 plants indicated a reduced stomatal conductance in the
plants indicated a reduced stomatal conductance in the 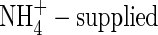 plants, especially in the top and new leaves, and upper leaves, meaning that the stomata in the leaves were at least partly closed. Also in the NO3-fed plants, a smaller stomatal conductance at the low nutrient level than that at the high nutrient level was observed (Fig. 2). The results suggest that the reduced leaf transpiration in the
plants, especially in the top and new leaves, and upper leaves, meaning that the stomata in the leaves were at least partly closed. Also in the NO3-fed plants, a smaller stomatal conductance at the low nutrient level than that at the high nutrient level was observed (Fig. 2). The results suggest that the reduced leaf transpiration in the 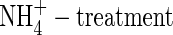 was not due to the reduced leaf area, but due to reduced stomatal conductance (Guo et al., 2002). There is some evidence that ABA may be responsible for the reduced stomatal conductance in leaves of the
was not due to the reduced leaf area, but due to reduced stomatal conductance (Guo et al., 2002). There is some evidence that ABA may be responsible for the reduced stomatal conductance in leaves of the 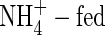 plants, since elevated biosynthesis of ABA and an obvious increase in the xylem and phloem flow of ABA in
plants, since elevated biosynthesis of ABA and an obvious increase in the xylem and phloem flow of ABA in 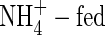 plants have been observed in comparison with NO3-grown plants in castor beans (Peuke et al., 1994b; Jeschke and Hartung, 2000). On the other hand, Guo et al. (2003) provided evidence for a role of
plants have been observed in comparison with NO3-grown plants in castor beans (Peuke et al., 1994b; Jeschke and Hartung, 2000). On the other hand, Guo et al. (2003) provided evidence for a role of 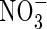 in stomatal opening, and demonstrated by using chl1 mutants of A. thaliana that the reduced stomatal aperture is not the result of effects on ABA responses. There is also an early report that supplies evidence that KCl supports stomatal opening as well as KNO3 in Vicia epidermal strips (Humble and Hsiao, 1969).
in stomatal opening, and demonstrated by using chl1 mutants of A. thaliana that the reduced stomatal aperture is not the result of effects on ABA responses. There is also an early report that supplies evidence that KCl supports stomatal opening as well as KNO3 in Vicia epidermal strips (Humble and Hsiao, 1969).
N-sources can influence plant WUE and δ13C value. A common finding is that a lower WUE is correlated with a more negative δ13C value (Farquhar et al., 1989; Raven and Farquhar, 1990; Raven et al., 1992; Yin and Raven, 1997, 1998). Different nutrient levels may also influence WUE of plants. In winter oilseed rape (Brück et al., 2001) and sweet potato plants (Kelm et al., 2001), a low N level led to lower WUE, since N stress led to increased transpiration per unit leaf area and decreased WUE (Kelm et al., 2001). In the present study, however, opposite results were obtained. The WUE of the low-NO3- and low-NH4NO3-fed plants was higher than that of the high-NO3- and high-NH4NO3-fed plants (Table 5). By comparison of the transpiration rate on a leaf dry weight basis at the second harvest, it was found that the transpiration rate for 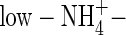 , low-NO3- and low-NH4NO3-fed plants was 18·6, and 17·8 g d−1 g−1 leaf dry weight, respectively, and for
, low-NO3- and low-NH4NO3-fed plants was 18·6, and 17·8 g d−1 g−1 leaf dry weight, respectively, and for 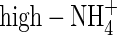 -, high-NO3- and high-NH4NO3-fed plants it was 10·6, 25·0 and 23·9 g d−1 g−1 leaf dry weight, respectively (calculated based on the results in Table 2 and Fig. 1), showing a decreased transpiration rate for the low-NO3- and low-NH4NO3-fed plants compared with that for the high-NO3- and high-NH4NO3-fed plants. The WUE of the NO3- or NH4NO3-fed plants was almost same under both nutrient levels (Table 5), which was in agreement with previous results for wheat (Yin and Raven, 1998) and peanut (Hubick, 1990). The WUE of the
-, high-NO3- and high-NH4NO3-fed plants it was 10·6, 25·0 and 23·9 g d−1 g−1 leaf dry weight, respectively (calculated based on the results in Table 2 and Fig. 1), showing a decreased transpiration rate for the low-NO3- and low-NH4NO3-fed plants compared with that for the high-NO3- and high-NH4NO3-fed plants. The WUE of the NO3- or NH4NO3-fed plants was almost same under both nutrient levels (Table 5), which was in agreement with previous results for wheat (Yin and Raven, 1998) and peanut (Hubick, 1990). The WUE of the 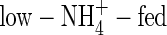 plants was the lowest and that of the
plants was the lowest and that of the 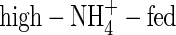 plants the highest among the treatments. Apart from the negative influence on leaf stomatal conductance discussed above, the effect of
plants the highest among the treatments. Apart from the negative influence on leaf stomatal conductance discussed above, the effect of 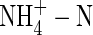 on root water uptake rate relative to assimilation of carbon dioxide might also contribute to the difference in WUE under both nutrient levels. At the second harvest, the water uptake rate on a root dry weight basis for
on root water uptake rate relative to assimilation of carbon dioxide might also contribute to the difference in WUE under both nutrient levels. At the second harvest, the water uptake rate on a root dry weight basis for 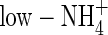 -, low-NO3- and low-NH4NO3-fed plants was 76, 70 and 62 mL d−1 g−1 dry weight, respectively; i.e. the
-, low-NO3- and low-NH4NO3-fed plants was 76, 70 and 62 mL d−1 g−1 dry weight, respectively; i.e. the 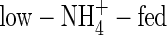 plants had the highest water uptake rate. For
plants had the highest water uptake rate. For 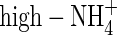 -, high-NO3- and high-NH4NO3-fed plants the water uptake rate on a root dry weight basis was 45, 127 and 106 mL d−1 g−1 dry weight, respectively; i.e. the
-, high-NO3- and high-NH4NO3-fed plants the water uptake rate on a root dry weight basis was 45, 127 and 106 mL d−1 g−1 dry weight, respectively; i.e. the 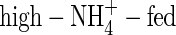 plants had the lowest water uptake rate, and the lowest leaf transpiration (Fig. 1).
plants had the lowest water uptake rate, and the lowest leaf transpiration (Fig. 1).
Uptake, flow and partitioning of potassium
There was no difference in the amount of K+ taken up by NO3- and NH4NO3-fed plants at the low nutrient level (2·5 mm potassium). By increasing the nutrient concentration (6 mm K; where the concentration of potassium supplied was 1·4 times more), potassium uptake increased by only 52 % and 75 % in the NO3- and NH4NO3-fed plants, respectively (Table 4). This is in agreement with the proposed theory that cycling of potassium in plants can act as an important signal for feedback control of nutrient uptake (Drew et al., 1990; Engels and Marschner, 1992). However, when NO3-N was replaced by 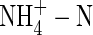 , potassium uptake was substantially reduced, and it was and unchanged when the potassium supply was increased (high nutrient level). This is consistent with earlier results from castor bean (Van Beusichem et al., 1988) and wheat (Wang and Below, 1998), because of the strong inhibition of potassium uptake by
, potassium uptake was substantially reduced, and it was and unchanged when the potassium supply was increased (high nutrient level). This is consistent with earlier results from castor bean (Van Beusichem et al., 1988) and wheat (Wang and Below, 1998), because of the strong inhibition of potassium uptake by 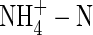 (Marschner, 1995; Britto and Kronzucker, 2002).
(Marschner, 1995; Britto and Kronzucker, 2002).
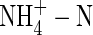 not only inhibited potassium uptake, but also markedly influenced its flow and partitioning within plants. In the
not only inhibited potassium uptake, but also markedly influenced its flow and partitioning within plants. In the 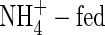 plants grown under both nutrient levels, the amount of potassium transported in the xylem was almost the same compared with the NO3- and NH4NO3-fed plants. It was 457 % or 596 % of the potassium currently taken up at low or high nutrient level, respectively (Figs 3, 4), a situation that can be explained only by massive export from leaves and retranslocation of potassium in the phloem. Retranslocation of potassium from the shoot via the phloem and cycling through the roots also took place in the NO3- and NH4NO3-fed plants (Figs 3, 4). It contributed to the potassium transported in the xylem by 52 %, 59 % and 80 % in the low-NO3-, low-NH4NO3- and
plants grown under both nutrient levels, the amount of potassium transported in the xylem was almost the same compared with the NO3- and NH4NO3-fed plants. It was 457 % or 596 % of the potassium currently taken up at low or high nutrient level, respectively (Figs 3, 4), a situation that can be explained only by massive export from leaves and retranslocation of potassium in the phloem. Retranslocation of potassium from the shoot via the phloem and cycling through the roots also took place in the NO3- and NH4NO3-fed plants (Figs 3, 4). It contributed to the potassium transported in the xylem by 52 %, 59 % and 80 % in the low-NO3-, low-NH4NO3- and 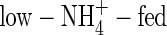 plants, respectively, and 41 %, 50 % and 84 % in the high-NO3-, high-NH4NO3- and
plants, respectively, and 41 %, 50 % and 84 % in the high-NO3-, high-NH4NO3- and 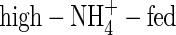 plants, respectively. Thus, retranslocation of potassium in the phloem of
plants, respectively. Thus, retranslocation of potassium in the phloem of 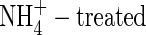 plants exceeded that of the NH4NO3- and NO3-fed plants.
plants exceeded that of the NH4NO3- and NO3-fed plants.
The results from the present study indicate that total replacement of NO3-N by 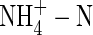 caused a substantial decrease in dry weight gain, even when plants were grown under nutrient deficiency. This suggests that the inhibitory effect of
caused a substantial decrease in dry weight gain, even when plants were grown under nutrient deficiency. This suggests that the inhibitory effect of 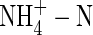 on plant growth might not be directly caused by ammonium toxicity. In addition,
on plant growth might not be directly caused by ammonium toxicity. In addition, 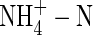 as the sole N-source caused a reduction in transpiration rate, changes in plant WUE (depending on the concentration of nutrient solution) and a decrease in potassium uptake. This leads to a massive export of K+ from leaves and recycling in the phloem. The results highlight the high degree of reutilization by retranslocation via the phloem, and provide further evidence that the partitioning and retranslocation of potassium in plants can be changed, depending on environmental conditions (Jeschke et al., 1987; Jeschke and Pate, 1991; Hibberd et al., 1999; Jiang et al., 2001).
as the sole N-source caused a reduction in transpiration rate, changes in plant WUE (depending on the concentration of nutrient solution) and a decrease in potassium uptake. This leads to a massive export of K+ from leaves and recycling in the phloem. The results highlight the high degree of reutilization by retranslocation via the phloem, and provide further evidence that the partitioning and retranslocation of potassium in plants can be changed, depending on environmental conditions (Jeschke et al., 1987; Jeschke and Pate, 1991; Hibberd et al., 1999; Jiang et al., 2001).
Acknowledgments
The authors thank the NNSFC (No. 30070452) and NKBRSF (No G1999011707) for financial support.
LITERATURE CITED
- Adler PR, Wilcox GE, Markhart AH. 1996. Ammonium decreases muskmelon root system hydraulic conductivity. Journal of Plant Nutrition 19: 1395–1403. [Google Scholar]
- Allen S, Raven JA. 1987. Intracellular pH regulation in Ricinus communis grown with ammonium or nitrate as N source: the role of long distance transport. Journal of Experimental Botany 38: 580–596. [Google Scholar]
- Armstrong MJ, Kirkby EA. 1979. Estimation of potassium recirculation in tomato plants by comparison of potassium and calcium accumulation in the tops with their flux in the xylem stream. Plant Physiology 63: 1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M. 1999. Interactive effect of salt (NaCl) and nitrogen form on growth, water relations and photosynthetic capacity of sunflower (Helianthus annuus L.). Annals of Applied Biology 135: 509–513. [Google Scholar]
-
Britto DT, Kronzucker HJ.
2002./article/back/ref-list/ref/citation/inline-formula
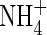 toxicity in higher plants: a critical review. Journal of Plant Physiology
159: 567–584. [Google Scholar]
toxicity in higher plants: a critical review. Journal of Plant Physiology
159: 567–584. [Google Scholar] - Brück H, Lugert I, Zhou W, Sattelmacher B. 2001. Why is physiological water-use efficiency lower under low nitrogen supply? In: Horst WJ, Schenk MK, Buerkert A, et al, eds. Food security and sustainability of agro ecosystems through basic and applied research. Dordrecht: Kluwer, 400–401. [Google Scholar]
- Claussen W. 2002. Growth, water use efficiency, and proline content of hydroponically grown tomato plants as affected by nitrogen source and nutrient concentration. Plant and Soil 247: 199–209. [Google Scholar]
- Drew MC, Webb J, Saker LR. 1990. Regulation of K+ uptake and transport to the xylem in barley roots: K+ distribution determined by electron probe X-ray microanalysis of frozen-hydrated cells. Journal of Experimental Botany 41: 815–825. [Google Scholar]
- Engels C, Marschner H. 1992. Adaptation of potassium translocation into the shoot of maize (Zea mays) to shoot demand: evidence for xylem loading as a regulating step. Physiologia Plantarium 86: 263–268. [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick TK. 1989. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40: 503–537. [Google Scholar]
- Guo FQ, Young J, Crawford NM. 2003. The nitrate transporter AtNRT1·1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in arabidopsis. The Plant Cell 15: 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Brük H, Sattelmacher B. 2002. Effects of supplied nitrogen from on growth and water uptake of French bean (Phaseolus vulgaris L.) plants. Plant and Soil 239: 267–275. [Google Scholar]
- Haynes RJ, Goh KM. 1978. Ammonium and nitrate nutrition of plants. Biological Review 53: 465–510. [Google Scholar]
- Hibberd JM, Quick WP, Press MC, Scholes JD, Jeschke WD. 1999. Solution fluxes from tobacco to the parasitic angiosperm Orobanche cernua and the influence of infection on host carbon and nitrogen relations. Plant, Cell and Environment 22: 937–947. [Google Scholar]
- Høgh-Jensen H, Schjoerring JK. 1997. Effects of drought and inorganic N form on nitrogen fixation and carbon isotope discrimination in Trifolium repens Plant Physiology and Biochemistry 35: 55–62. [Google Scholar]
- Hubick KT. 1990. Effects of nitrogen source and water limitation on growth, transpiration efficiency and carbon-isotope discrimination in peanut cultivars. Australian Journal of Plant Physiology 17: 413–430. [Google Scholar]
- Humble GD, Hsiao TC. 1969. Specific requirement of potassium for light-activated opening of stomata in epidermal strips. Plant Physiology 44: 230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke WD, Hartung W. 2000. Root–shoot interactions in mineral nutrition. Plant and Soil 226: 57–69. [Google Scholar]
- Jeschke WD, Pate JS. 1991. Modelling the uptake, flow and utilization of C, N and H2O within whole plants of Ricinus communis L. based on empirical data. Journal of Plant Physiology 137: 488–498. [Google Scholar]
- Jeschke WD, Pate JS, Atkins CA. 1987. Partitioning of K+, Na+, Mg2+, and Ca2+ through xylem and phloem to component organs of nodulated white lupin under mild salinity. Journal of Plant Physiology 128: 77–93. [Google Scholar]
- Jiang F, Li CJ, Jeschke WD, Zhang FS. 2001. Effect of top excision and replacement by 1-naphthylacetic acid on partition and flow of potassium in tobacco plants. Journal of Experimental Botany 52: 2143–2150. [DOI] [PubMed] [Google Scholar]
- Khan MG, Silberbush M, Lips SH. 1994. Physiological studies on salinity and nitrogen interaction in alfalfa. II. Photosynthesis and transpiration. Journal of Plant Nutrition 17: 669–682. [Google Scholar]
- Kelm M, Brük H, Hermann M, Sattelmacher B. 2001. The effect of low nitrogen supply on yield and water-use efficiency of sweet potato (Ipomoea batatas L.). In: Horst WJ, Schenk MK, Buerkert A, et al, eds. Food security and sustainability of agro ecosystems through basic and applied research. Dordrecht: Kluwer, 402–403. [Google Scholar]
- Lewis OAM, Leidi EO, Lips SH. 1989. Effects of nitrogen source on growth response to salinity stress in maize and wheat. New Phytologist 111: 155–160. [DOI] [PubMed] [Google Scholar]
- Lugert I, Gerendas J, Bruech H, Sattelmacher B. 2001. Influence of N form on growth and water status of tomato plants. In: Horst WJ, Schenk MK, Buerkert A, et al, eds. Food security and sustainability of agro ecosystems through basic and applied research. Dordrecht: Kluwer, 306–307. [Google Scholar]
- Magalhaes JR, Wilcox GE. 1984. Growth, free amino acids, and mineral composition of tomato plants in relation to nitrogen form and growth media. Journal of America Society Horticultural Science 109: 406–411. [Google Scholar]
- Marschner H. 1995.Mineral nutrition of higher plants, 2nd edn. London: Academic Press. [Google Scholar]
- Morgan JA. 1986. The effects of N nutrition on the water relations and gas exchange characteristics of wheat (Triticum aestivum L.). Plant Physiology 80: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuke AD, Hartung W, Jeschke WD. 1994. The uptake and flow of C, N and ions between roots and shoots in Ricinus Communis L. II. Grown with low or high nitrate supply. Journal of Experimental Botany 45: 733–740. [Google Scholar]
- Peuke AD, Jeschke WD, Hartung W. 1994. The uptake and flow of C, N and ions between roots and shoots in Ricinus communis L. III. Long distance transport of abscisic acid depending on nitrogen nutrition and salt stress. Journal of Experimental Botany 45: 741–747. [Google Scholar]
- Raab TK, Terry N. 1994. Nitrogen source regulation of growth and photosynthesis in Beta vulgaris L. Plant Physiology 105: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA, Farquhar GD. 1990. The influence of N metabolism and organic acid synthesis on the natural abundance of isotopes of carbon in plants. New Phytologist 116: 505–529. [DOI] [PubMed] [Google Scholar]
- Raven JA, Wollenweber B, Handley L. 1992. A comparison of ammonium and nitrate as nitrogen sources for photolithotrophs. New Phytologist 121: 19–32. [Google Scholar]
- SAS. 1987.SAS/STAT guide for personal computers, 6th edn. Cary, NC: SAS Institute Inc. [Google Scholar]
- Seel WE, Jeschke WD. 1999. Simultaneous collection of xylem sap from Rhinanthus minor and the hosts Hordeum and Trifolium: hydraulic properties, xylem sap composition and effects of attachment. New Phytologist 143: 281–298. [Google Scholar]
- Tischner R. 2000. Nitrate uptake and reduction in higher and lower plants. Plant, Cell and Environment 23: 1005–1024. [Google Scholar]
- Van Beusichem ML, Kirkby EA, Baas R. 1988. Influence of nitrate and ammonium on the uptake, assimilation and distribution of nutrients in Ricinus communis Plant Physiology 86: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Liu P, Neumann G, Bangerth F, Engels C. 2000. Rapid effects of nitrogen form on leaf morphogenesis in tobacco. Journal of Experimental Botany 51: 227–237. [DOI] [PubMed] [Google Scholar]
- Wang XT, Below FE. 1998. Accumulation and partitioning of mineral nutrients in wheat as influenced by nitrogen form. Journal of Plant Nutrition 21: 49–61. [Google Scholar]
- Yin ZH, Raven JA. 1997. A comparison of the impacts of various nitrogen sources on acid-dase balance in C3 Triticum aestivum L. and C4 Zea mays L. plants. Journal of Experimental Botany 48: 315–324. [Google Scholar]
- Yin ZH, Raven JA. 1998. Influences of different nitrogen sources on nitrogen- and water-use efficiency, and carbon isotope discrimination in C3 Triticum aestivum L. and C4 Zea mays L. plants. Planta 205: 574–580. [Google Scholar]