Abstract
• Background and Aims Orobanche species represent major constraints to crop production in many parts of the world as they reduce yield and alter root/shoot allometry. Although much is known about the histology and effect of Orobanche spp. on susceptible hosts, less is known about the basis of host resistance to these parasites. In this work, histological aspects related to the resistance of some legumes to Orobanche crenata have been investigated in order to determine which types of resistance responses are involved in the unsuccessful penetration of O. crenata.
• Methods Samples of resistance reactions against O. crenata on different genotypes of resistant legumes were collected. The samples were fixed, sectioned and stained using different procedures. Sections were observed using a transmission light microscope and by epi-fluorescence.
• Key Results Lignification of endodermal and pericycle host cells seems to prevent parasite intrusion into the root vascular cylinder at early infection stages. But in other cases, established tubercles became necrotic and died. Contrary to some previous studies, it was found that darkening at the infection site in these latter cases does not correspond to death of host tissues, but to the secretion of substances that fill the apoplast in the host–parasite interface and in much of the infected host tissues. The secretions block neighbouring host vessels. This may interfere with the nutrient flux between host and parasite, and may lead to necrosis and death of the developing parasite.
• Conclusions The unsuccessful penetration of O. crenata seedlings into legume roots cannot be attributed to cell death in the host. It seems to be associated with lignification of host endodermis and pericycle cells at the penetration site. The accumulation of secretions at the infection site, may lead to the activation of xylem occlusion, another defence mechanism, which may cause further necrosis of established tubercles.
Keywords: Orobanche crenata, Pisum sativum, Vicia faba, Vicia sativa, Cicer arietinum, Lens spp., legumes, hypersensitive response, necrosis, resistance to parasitic plants
INTRODUCTION
Broomrapes (Orobanche spp.) are obligate root holoparasites of the genus Orobanche. These parasites lack chlorophyll and depend entirely on their hosts for their supply of carbon, nitrogen and inorganic solutes. Orobanche species parasitize a wide range of economically important hosts, e.g. legumes, sunflower, tomato and tobacco (Parker and Riches, 1993). These parasites represent major constraints to crop production in many parts of the world as they reduce yield and alter root/shoot allometry (Barker et al., 1996; Hibberd et al., 1999). Although much is known about the effect of Orobanche species on susceptible hosts, less is known about the basis of host resistance to these parasites (Joel et al., 1996).
Following invasion of plant tissues by some pathogenic fungi and bacteria, a range of defence mechanisms may be triggered to restrict their growth. These include the production of reactive oxygen species, the death of host cells in a hypersensitive response (HR) or the induction of pathogenesis-related proteins (Hammond-Kosack and Jones, 1996). It has been suggested that a hypersensitive reaction may also take place to some extent after invasion of host plants by some species/races of parasitic plants, e.g. in cowpea (Vigna unguiculata) that exhibits resistance to Striga gesnerioides (Lane and Bailey, 1992), cotton (Gossypium hirsutum) resistant to Cuscuta lupuliformis (Capdepon et al., 1985), poplar (Populus sp.) resistant to Viscum album (Hariri et al., 1991), sunflower (Helianthus annuus) resistant to Orobanche cumana (Dörr et al., 1994), vetch (Vicia athropurpurea) resistant to O. aegyptiaca (Goldwasser et al., 1997) and many others. In these interactions it is hypothesized that the HR prevented penetration of the haustorium into the cortex and the further development of the parasite.
Although resistance to parasitic plants has been reported, few detailed histological studies of the resistant interactions have been undertaken and there is no conclusive evidence that an HR really occurs in all these interactions in a manner similar to that described for fungal attack (Heath, 1999; Richael and Gilchrist, 1999). In some resistant interactions host cell death is not seen immediately after penetration of the host root. For example, following penetration of Helianthus roots by Orobanche cumana the parasite succeeded in establishing connections with host tissues. An initial vascular connection was established and tubercles developed but they then became dark and the parasite died at an early developmental stage (Dörr et al., 1994; Labrousse et al., 2001).
In the present work, the histology of defensive reactions found in resistant genotypes to O. crenata are studied and described.
MATERIALS AND METHODS
Plant material and growth conditions
Orobanche crenata was grown on resistant and susceptible genotypes of vetch (Vicia sativa L., ‘A01’ and ‘V27’, respectively), faba bean (V. faba L., ‘Baraca’ and ‘Prothabon’), pea (Pisum sativum, ‘Messire’ and ‘Ps139’), chickpea (Cicer arietinum, ‘P2245’ and ‘CA2065’) and lentil (Lens orientalis, ‘L317’ and L. culinaris, ‘Eston’). Plants were grown in a controlled environment chamber with a day/night temperature of 20 ± 0·5 °C, a 14-h photoperiod and an irradiance of 200 µmol m−2 s−1. Legume seeds were germinated in water (after fungicide treatment) and then transferred to Petri dishes (15 cm diameter) containing perlite and glass fibre paper (GF/A Whatman). Orobanche crenata seeds were collected from infected faba beans during 1999. Surface-sterilized seeds of O. crenata (70 % ethanol for 30 s, 1 % sodium hypochlorite for 20 min) were conditioned for 10 d in a sealed Petri dish with glass fibre paper moistened with distilled water. After conditioning the seeds (8 mg) were then spread homogeneously on the paper in the vicinity of the roots (Rubiales et al., 2003). Ten replicates of each genotype were placed on trays containing Hoagland nutrient solution (Hoagland and Arnon, 1950) and covered with aluminium foil in order to prevent exposure of the roots to direct light (100 trays in total).
Collection and fixation of samples
Observations were taken every week on host–parasite development using a binocular microscope (Olympus SZ60; Olympus Optical Ltd, London, UK). Germinated Orobanche seeds near host roots, attached seedlings and developed tubercles were photographed using a reflex camera (Olympus OM-4 Ti). At 20, 30 and 40 d after inoculation, tubercles and seedlings of O. crenata were sampled with the corresponding attached parts of host roots. A minimum of 20 samples of each genotype was taken at random. The sampled material was fixed in FAA (50 % ethanol + 5 % formaldehyde + 10 % glacial acetic acid in water) for 48 h. Fixed samples were then dehydrated in an ethanol series (50 %, 80 %, 95 %, 100 %, 100 %; 12 h each) and transferred to an embedding solvent (xylene; Panreac Quimica SA, Montcada i Reixac, Spain) through a xylene–ethanol series (30 %, 50 %, 80 %, 100 %, 100 %; 12 h each) and finally saturated with paraffin (Paraplast Xtra; Sigma, St Louis, USA). Sections (7 µm) were cut with a rotary microtome (Reichert, Austria) and attached to adhesive-treated microscope slides (polysine slides; Menzel GmbH & Co KG, Braunschweig, Germany).
Staining methods
After removal of paraffin with xylene (20 min twice) and rehydration with an ethanol-water series (100 %, 100 %, 95 %, 70 %, 50 %, 30 %, 0 %: 20 min each), sections were stained with alcian green–safranin (AGS) (Joel, 1983). Slides were dried on a hot plate at 45 °C for 1 h and mounted with DePeX (BDH). With this staining method, carbohydrates (including cell walls and mucilage) appeared green, yellow or blue, while lignified, cutinized and suberized walls, as well as tannin and lipid material inside cells, appeared red (Joel, 1983). Non-stained sections were kept as controls, and for examination under the fluorescence microscope. Acid staining with phloroglucinol (2 % in ethanol)–HCl (35 %) was used for identification of lignified cell walls (Sherwood and Vance, 1976).
Sections were observed using a transmission microscope (Leica DM-LB, magnification ×100 to ×400; Leica Microsystems Wetzlar GmbH, Wetzlar, Germany) and photographed using a digital camera (Nikon DXM1200F; Nikon Europe BV, Badhoevedorp, The Netherlands). Samples were also observed by epi-fluorescence under excitation at 450–490 nm (blue-violet).
RESULTS
The two main incompatible interactions found between O. crenata and the host legumes were characterized by darkening of host and/or parasite tissues around the point of attachment and darkening of developing tubercles. These symptoms were more frequent on resistant legume cultivars (about 40 % darkening of the attached seedlings and 60 % darkening of developing tubercles, depending on the species; data not shown) but could also be observed at lower frequencies on susceptible cultivars (about 15 % darkening of the attached seedlings and 20 % darkening of developing tubercles, depending on the species; data not shown). Similarly, as resistance is only incomplete, some parasites also develop on resistant genotypes (see Fig. 2A).
Fig. 2.
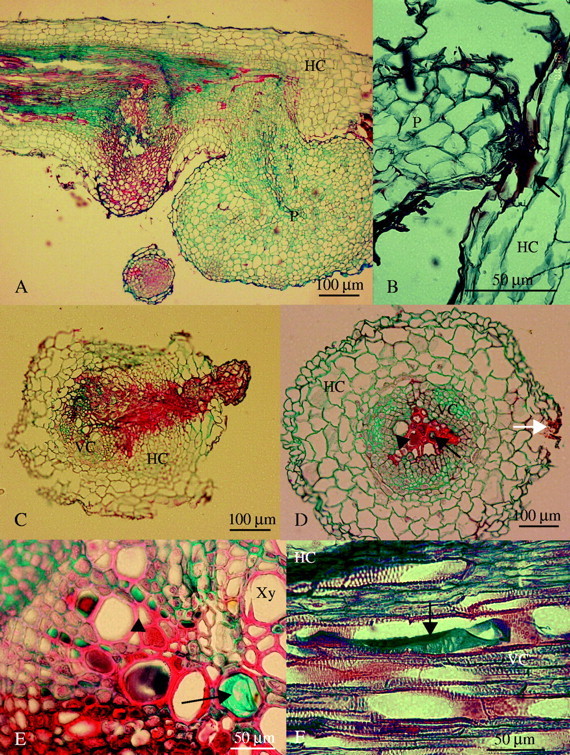
(A) Longitudinal section of a resistant vetch root showing both a necrotic (stained red) and a healthy (stained blue) tubercle (30 d after inoculation). (B) Light micrograph of a broomrape seedling attached and trying to penetrate into a resistant faba bean root (10 d after inoculation). Arrow indicates accumulation of a red staining substance that did not show any fluorescence. (C) Cross-section of an unsuccessful seedling penetration on resistant lentil (20 d after inoculation). (D) Cross-section of a resistant pea root away from the haustorium of a necrotic tubercle (30 d after inoculation). The white arrow indicates the side where the tubercle and the haustorium were located in other consecutive sections, the black arrow indicates accumulation of safranin-staining substance with fluorescence and the arrowhead indicates accumulation of alcian-staining carbohydrates inside vessel elements. (E) Detailed cross-section of a resistant vetch root near the haustorium of a necrotic tubercle (30 d after inoculation). The arrow indicates accumulation of alcian-staining carbohydrates and arrowhead indicates accumulation of red staining substance with fluorescence inside vessel elements. (F) Longitudinal section of a resistant vetch root (30 d after inoculation) showing a xylem vessel filled with the alcian-staining carbohydrates (arrow). P, Parasite; HC, host cortex; VC, host vascular cylinder; Xy, xylem vessel.
Figure 1A and B shows the compatible interaction between O. crenata and a susceptible legume (pea). A healthy tubercle developed on the host roots with 10 d of attachment. In contrast, when O. crenata attacked the roots of resistant faba bean and pea the parasite usually died at an early stage of tubercle development (Fig. 1C and D). Figure 1C shows the penetration point on the root of a resistant faba bean. The incompatible interaction was characterized by darkening of host and parasite tissues around the point of attachment. The seedling lost its hyaline appearance (as shown in Fig. 1A) and the parasite died before a tubercle had formed. Figure 1D shows the resistance reaction on a resistant pea root. Initially, the parasite developed a healthy tubercle with an orange to light brown coloration (as illustrated in Fig. 1B). However, 30 d after inoculation the tubercle stopped growing and became dark in colour. Darkening started from the basis of the radicle in the seedling remains and proceeded acropetally towards the haustorium inside the host tissue.
Fig. 1.
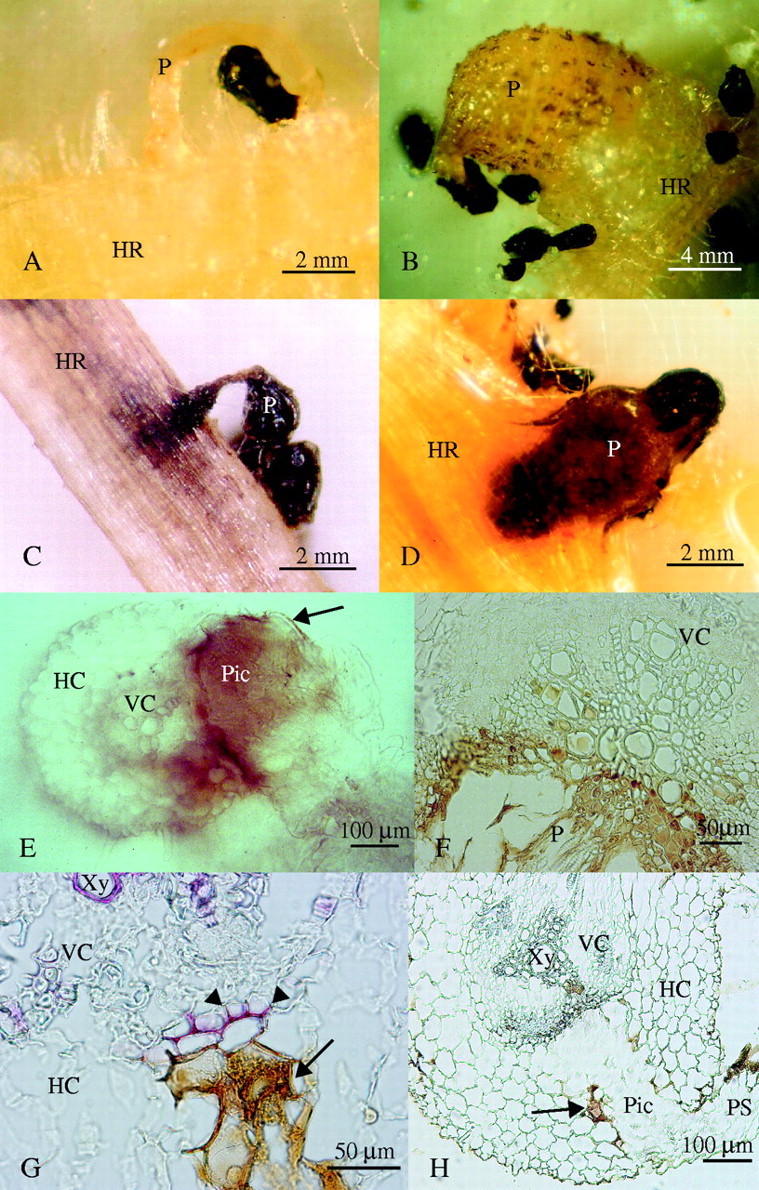
Resistant reaction found in response to O. crenata attack. (A) Compatible Orobanche crenata development on susceptible pea: early attachment showing hyaline radicle (10 d after inoculation), and (B) healthy tubercle (20 d after inoculation). (C) Surface view of incompatible seedling penetration on resistant faba bean (20 d after inoculation). (D) Necrotic O. crenata tubercle on resistant pea (30 d after inoculation). (E) Non-stained hand-cut section of an unsuccessful seedling penetration on resistant vetch (20 d after inoculation) showing accumulation of a dark brown material. Arrow indicates brown material in contact only with parasite tissues. (F) Detail of a non-stained microtome cross-section of a necrotic tubercle on resistant vetch (30 d after inoculation) showing accumulation of a brown material inside host vessels. (G) Detail of an unsuccessful penetration on resistant vetch (20 d after inoculation) showing the lignified host cells (arrowheads) in contact with the parasite intrusive cells, after phloroglucinol–HCl staining. The arrow indicates accumulation of a brown secreted material. (H) Non-stained section of an unsuccessful penetration attempt on resistant vetch (20 d after inoculation). The arrow indicates accumulation of brown material. HR, Host root; P, parasite; PS, parasite seedling; Pic, parasite intrusive cells; HC, host cortex; VC, host vascular cylinder; Xy, xylem vessel.
When sections of the above Orobanche material were examined under the light microscope, accumulation of a brown substance was observed around the penetration pathway of abortive haustoria in both the cortex and the central cylinder of the host root. This phenomenon can clearly be observed in non-stained sections (Fig. 1E, F and H). The substance covered the whole tissue and looked dark brown, almost black in thick sections, resembling the appearance of necrotic tissue (Fig. 1E). However, in thin sections the substance was restricted to the apoplast and its colour looked reddish-brown (Fig. 1F, G and H).
A substance that stained red with AGS accumulated at the attachment area of O. crenata seedlings on the host root (Fig. 2B). In cases where the parasite had already established vascular connections with the host, a similar substance was also visible surrounding both the haustorium and the nearby host tissues (Fig. 2C). In addition, a significant part of the apoplast of host root tissues at the infection site, including the xylem vessel cavities, was filled with two or more kinds of substances. These substances were found in infected roots of resistant vetch, pea, lentil and chickpea (Fig. 2D and E). One substance, possibly a carbohydrate, as it stained with alcian green, accumulated inside host vessels away from the core of the haustorium (Fig. 2E and F). The other substance that stained red with safranin, was usually present close to the haustorium, within xylem vessels and also in intercellular spaces and cell walls in the central cylinder of the root (Fig. 2D and E). Following consecutive cross-sections of infected roots both substances always appeared inside vessels that were in contact with the haustorium or adjacent to affected vessels (Fig. 2D). While the lignified cell walls of vessel elements in all these legumes usually fluoresced green under blue excitation (Fig. 3E), they gave yellow or red fluorescence when associated with the red-staining substance (Fig. 3F).
Fig. 3.
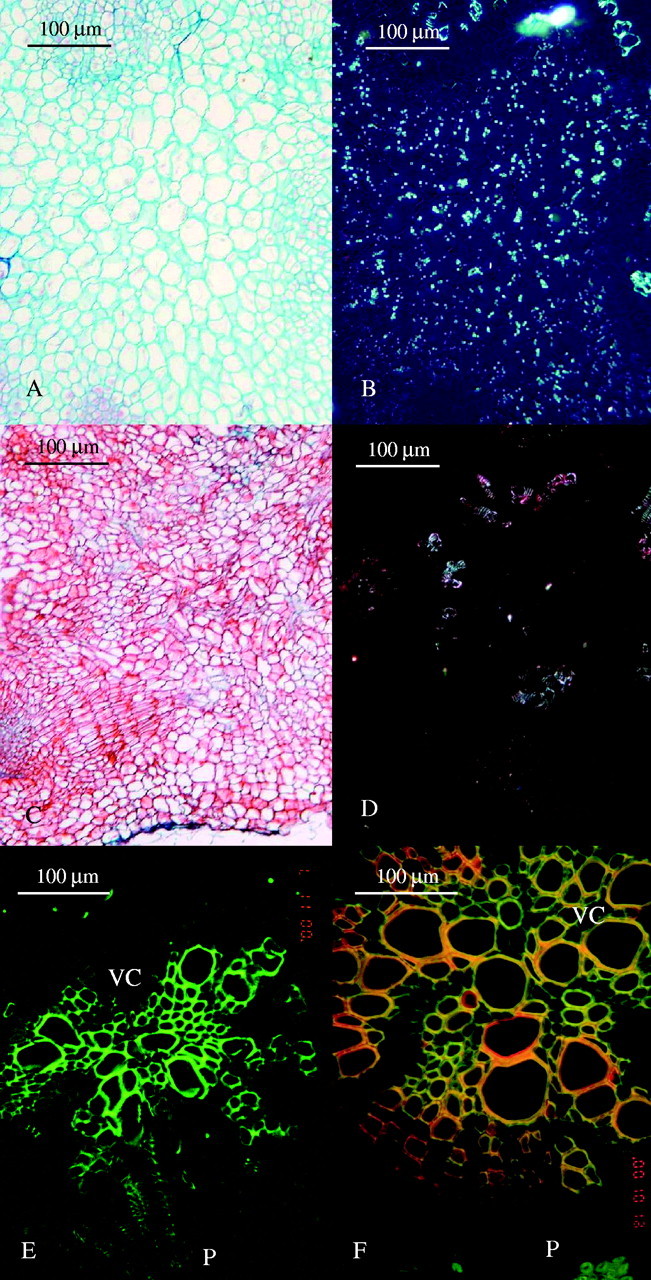
(A) Light micrograph of a cross-section in a healthy broomrape tubercle on susceptible pea (30 d after inoculation). (B) The same as (A) under polarized light showing accumulation of starch grains. (C) Light micrograph of a cross-section in a necrotic broomrape tubercle on resistant pea (30 d after inoculation). (D) The same as (C) under polarized light showing the lack of starch grains. (E) Cross-section of a susceptible pea root with the haustorium of a healthy tubercle under fluorescence microscopy (30 d after inoculation). (F) Cross-section of a resistant pea root and the haustorium of a necrotic tubercle under fluorescence microscopy (30 d after inoculation). Red colour corresponds to the red staining substance which is seen in Fig. 2C, the cell wall itself fluoresces in yellow and green fluorescence is seen only in a few unaffected xylem cells. P, Parasite; HC, host cortex; VC, host vascular cylinder.
An important feature of the Orobanche tubercle is the accumulation of starch inside its parenchymatous tissues (Joel and Losner-Goshen, 1994) (Fig. 3B). In contrast to healthy tubercles, most of the tubercles in an incompatible interaction lacked any starch (Fig. 3D). In addition, the cell walls in abortive Orobanche tubercles stained red with AGS, whereas in healthy tubercles they usually stained green or blue (Figs 2A and 3A and C).
Using phloroglucinol–HCl, which specifically stains lignins in plant cell walls, it was found that, in some cases, lignification developed in cell walls of host root pericycle and in inner walls of the endodermal cells that were in contact with an abortive haustorium [the identification of the endodermal cells was based on the detection of the Casparian strips in their anticlinal walls (not shown)]. Extra lignified thickenings did not occur in cell walls of host xylem or in other cells in the central cylinder that were in contact with the haustorium (Fig. 1G). In addition, the host pericycle and endodermis in contact with abortive haustoria were deformed in many cases (Fig. 1H).
DISCUSSION
In the present work, darkening of the infected tissues and of Orobanche tubercles has been observed in all tested resistant legume genotypes, i.e. faba bean, pea, chickpea, lentil and vetch. In all these legumes belonging to different Fabaceae genera it was found that resistance does not necessarily include the death of host cells or tissues. Thus, evidence supporting the idea that cell death is responsible for the resistance of legumes to O. crenata was not found in any of the examined samples. The data point to a different mechanism and it is suggested that the darkening of the tissue is a secondary symptom; it developed as a result of the operation of a different type of resistance mechanism that stopped the development of the parasite. Contrary to previous studies, the darkening in the infection zone was clearly found to be the result of a secretion that seems to originate from haustorial cells, and occupies the apoplast in the host–parasite interface and much of the infected host tissues, including xylem. This secretion was also observed only on the external parasite interface near the attachment and penetration point (Fig. 1E), which indicates that it must have originated from the parasite.
During the early stages of penetration, the parasitic plant releases enzymes that allow penetration of intrusive cells between host cells (Losner-Goshen et al., 1998) and an adhesive substance that facilitates internal anchoring of the parasite to host cell walls (Joel et al., 1996). Once the parasite has penetrated host tissues the host can detect the presence of this foreign organism and react to it. This was found to apply also in compatible interactions (Joel and Portnoy, 1998; Vieira Dos Santos et al., 2003). In the present study, lignification of host pericycle and endodermis has been observed in incompatible interactions between legumes and O. crenata, which seems to prevent penetration of the parasite to the root vascular cylinder. Previous reports described lignification of host cortex and xylem elements (Dörr et al., 1994; Antonova and Ter Borg, 1996) and unspecific tissues (De Ruck et al., 1995; Goldwasser et al., 1999). This may explain why, in some cases, the haustorium developed in the cortex around the vascular cylinder but could not penetrate the cylinder itself as was previously shown in the O. aegyptiaca–Vicia spp. interaction (Goldwasser et al., 1996). The deformation of the host pericycle in contact with the haustorium can also be explained by the pressure exerted by the parasite.
Whether penetrating the vascular cylinder or not, in all incompatible interactions the intrusive cells release a secretion that not only fills the gaps between host and parasite tissues, it also flows through the apoplast to neighbouring host tissues, and fills nearby vessel elements of the host. Under low magnification this dark secretion can be easily confused with a hypersensitive cell death of host tissues. The dark secretion was also found in mature vessel elements that are already dead.
During the penetration attempt the parasite should succeed in breaking the barrier or it will eventually die. If it succeeds and the intrusive cells reach the host vascular system, then the pathogen has the chance to establish successfully inside the host. It is assumed that the parasite may overcome some host barriers by releasing its secretion at the point of resistance, as earlier suggested by Joel et al. (1996). The secretion that is released when resistance is met, either at early penetration stages or later when some vascular connections have already been established, functions as an internal anchoring device and as a fulcrum for further physical efforts to break into ‘stubborn’ host tissues. The secretion that contains enzymes like peroxidases (Antonova and Ter Borg, 1996) and pectin methyl esterases (Losner-Goshen et al., 1998) may also serve in changing the composition of host cell walls, which may make them weaker and more vulnerable to the attack. This is consistent with the changes that were found in the autofluorescence of host xylem vessels that are in contact with the secreted substances (Fig. 3E and F). An analogous phenomenon was previously described for the interaction between parasites of the Santalaceae and incompatible hosts. Unlike the Orobanche strategy of secreting anchoring substance when meeting a barrier, in the Santalaceae additional attachment organs develop when the previously developed attachment ‘mantle’ failed to provide sufficient support for haustorial penetration (Kuijt, 1969). In both cases anchoring devices (‘mantles’ or secretion) accumulate at the infection zone in incompatible interactions (Joel et al., 1996). The occurrence of the secretion only at some limited internal host–parasite interface areas in the few compatible infection cases on resistant legumes is not surprising; in these cases the parasite was first obstructed, but managed to overcome the barriers.
The excess secretion that is released by Orobanche when it connects to resistant legumes leaks through the apoplast and reaches the neighbouring host xylem elements. Once it accumulates in these vessels it may block some of them and interfere with the nutrient flux between host and parasite (Fig. 2D and E), thereby obstructing its own supply channel. It is believed that this secondary effect may in some cases cause the death of the parasite. The lack of starch grains in the dying tissues of the parasite indicates that the parasite has exhausted its reserves and has not been able to replenish it with supplies from the host.
The secretion found in vessel elements was of two kinds, one stained red with safranin, the other stained green-blue with alcian dyes. The safranin staining substance was also observed only on the external parasite interface near the attachment and penetration point (Fig. 1E), and as stated above it indicates an origin from the parasite. Since the alcian-positive substance was only found in vessel elements, and only away from the haustorium, not in the host–parasite interface itself, one may assume that this carbohydrate is derived from the host. It may be possible that the presence of foreign substances inside the host vessels activates the release of the carbohydrate into the infected vessels, an additional defense mechanism, similar to that known to be associated with wilt diseases. Vascular sealing of similar type prevented other pathogens from further spread along the plant (Vander Molen et al., 1983; Beckman, 2000). In this latter case the occlusion gel contains carbohydrates that are found in cell walls, and it was therefore suggested that it congeals in host vessels after being solubilized by pathogen enzymes (Vander Molen et al., 1983). Obviously the vessel elements do not possess any cytoplasm and can thus not produce any substance by themselves. Therefore it is assumed that the origin of these carbohydrates needs to be in neighbouring living cells in the xylem tissue.
Accumulation of substances inside host tissues have been reported by authors in the case of various parasitic plants: Dörr et al. (1994), Goldwasser et al. (1996, 2000), Joel and Losner-Goshen (1994), Joel et al. (1996) and Labrousse et al. (2001) for Orobanche; Heide-Jorgensen and Kuijt (1993) for Triphysaria; Neumann et al. (1998) for Rhamphicarpa; Arnaud et al. (1999) for Striga. Usually these substances are regarded as defensive materials from the host, but it would be possible to differentiate several substances in the Orobanche–legume interaction that may play different roles and seem to originate from both the host and the parasite.
In conclusion, the death of O. crenata seedlings during unsuccessful penetration into the studied resistant legume roots cannot be attributed to cell death in the host. It seems to be associated with lignification of host endodermis and pericycle cells at the penetration site. The reaction of the young parasite to these barriers, i.e. the secretion of further amounts of anchoring glue and possibly also enzymes at the infection site, may lead to the activation of xylem occlusion, another defence mechanism, which may cause necrosis of established tubercles.
Supplementary Material
Acknowledgments
We thank all the people working in the groups at Newe-Ya'ar Research Center, University of Sheffield and IAS-CSIC, especially A. Moral, for their help in the realization of this work. This research was supported by the Spanish project AGL2002-03248, and by the BARD Project IS-1421. D.M.J. was a Visiting Professor at Utsunomiya University (Japan) in 2002.
LITERATURE CITED
- Antonova TS, Ter Borg SJ. 1996. The role of peroxidase in the resistance of sunflower against Orobanche cumana in Russia. Weed Research 36: 113–121. [Google Scholar]
- Arnaud MC, Veronesi C, Thalouarn P. 1999. Physiology and histology of resistance to Striga hermonthica in Sorghum bicolor var. Framida. Australian Journal of Plant Physiology 26: 63–70. [Google Scholar]
- Barker ER, Press MC, Scholes JD, Quick WP. 1996. Interactions between parasitic angiosperm Orobanche aegyptiaca and its tomato host: growth and biomass allocation. New Phytologist 133: 637–642. [Google Scholar]
- Beckman CH. 2000. Phenolic-storing cells: keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiological and Molecular Plant Pathology 57: 101–110. [Google Scholar]
- Capdepon M, Fer A, Ozenda P. 1985. Sur un systèm inédit de seject d'un parasite: exemple de la Cuscute sur Cotonnier (C. lupuliformis Krock. sur Gossypium hirsutum L.). Comptes Rendus de l'Académie des Sciences 6: 227–232. [Google Scholar]
- De Ruck E, Tena M, Jorrín J. 1995. La lignificación como respuesta defensiva de girasol (Helianthus spp.) frente a la infección por plantas parásitas (Orobanche cernua). In: XIX Congreso de la Sociedad Española de Bioquímica. Córdoba, 225. [Google Scholar]
- Dörr I, Staack A, Kollmann R. 1994. Resistance of Helianthus to Orobanche—histological and cytological studies. In: Pieterse AH, Verkleij JAC, ter Borg SJ, eds. Proceedings of the 3rd International Workshop on Orobanche and Related Striga Research. Amsterdam: Royal Tropical Institute, 276–289. [Google Scholar]
- Goldwasser Y, Hershenhorn J, Plakhine D, Kleifeld Y, Rubin B. 1999. Biochemical factors involved in vetch resistance to Orobanche aegyptiaca Physiological and Molecular Plant Pathology 54: 87–96. [Google Scholar]
- Goldwasser Y, Kleifeld Y, Joel DM, Plakhine D, Rubin B. 1996. Variation in vetch (Vicia spp.) response to Orobanche aegyptiaca Pers. In: Moreno MT, Cubero JI, Berner D, Joel D, Musselman LJ, Parker C, eds. Advances in parasitic plant research. Proceedings of the 6th International Symposium on Parasitic Weeds. Sevilla: Junta de Andalucía, Consejería de Agricultura y Pesca, 616–623. [Google Scholar]
- Goldwasser Y, Kleifeld Y, Plakhine D, Rubin B. 1997. Variation in vetch (Vicia spp.) response to Orobanche aegyptiaca Weed Science 45: 756–762. [Google Scholar]
- Goldwasser Y, Plakhine D, Kleifeld Y, Zamski E, Rubin B. 2000. The differential susceptibility of vetch (Vicia spp.) to Orobanche aegyptiaca: anatomical studies. Annals of Botany 85: 257–262. [Google Scholar]
- Hammond-Kosack KE, Jones JDG. 1996. Resistance gene-dependent plant defense responses. Plant Cell 8: 1773–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri EB, Sallé G, Andrary C. 1991. Involvement of flavonoids in the resistance of two poplar cultivars to mistletoe (Viscum album L.). Protoplasma 162: 20–26. [Google Scholar]
- Heath MC. 1999. The enigmatic hypersensitive response: induction, execution, and role. Physiological and Molecular Plant Pathology 55: 1–3. [Google Scholar]
- Heide-Jorgensen HS, Kuijt J. 1993. Epidermal derivatives as xylem elements and transfer cells: a study of the host–parasite interface in two species of Triphysaria (Scrophulariaceae). Protoplasma 174: 173–183. [Google Scholar]
- Hibberd JM, Quick WP, Press MC, Scholes JD, Jeschke WD. 1999. Solute fluxes from tobacco to the parasitic angiosperm Orobanche cernua and the influence of infection on host carbon and nitrogen relations. Plant, Cell and Environment 22: 937–947. [Google Scholar]
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. University of California Agricultural Experimental Station Circular No. 347. [Google Scholar]
- Joel DM. 1983. AGS (Alcian Green Safranin)—a simple differential staining of plant material for the light microscope. Proceedings the Royal Microscopical Society 18: 149–151. [Google Scholar]
- Joel DM, Losner-Goshen D. 1994. The attachment organ of the parasitic angiosperms Orobanche cumana and O. aegyptiaca and its development. Canadian Journal of Botany 72: 564–574. [Google Scholar]
- Joel DM, Losner-Goshen D, Hershenhorn J, Goldwasser Y, Assayag M. 1996. The haustorium and its development in compatible and resistant host. In: Moreno MT, Cubero JI, Berner D, Joel D, Musselman LJ, Parker C, eds. Advances in parasitic plant research. Proceedings of the 6th International Symposium on Parasitic Weeds. Sevilla: Junta de Andalucía, Consejería de Agricultura y Pesca, 531–541. [Google Scholar]
- Joel DM, Portnoy VH. 1998. The angiospermous root parasite Orobanche L. (Orobanchaceae) induces expression of pathogenesis related (PR) gene in susceptible tobacco roots. Annals of Botany 81: 779–781. [Google Scholar]
- Kuijt J. 1969.The biology of parasitic flowering plants. Berkeley: University of California Press. [Google Scholar]
- Labrousse P, Arnaud MC, Serieys H, Bervillé A, Thalouarn P. 2001. Several mechanisms are involved in resistance of Helianthus to Orobanche cumana Wallr. Annals of Botany 88: 859–868. [Google Scholar]
- Lane JA, Bailey JA. 1992. Resistance of cowpea and cereals to the parasitic angiosperm Striga Euphytica 63: 85–93. [Google Scholar]
- Losner-Goshen D, Portnoy VH, Mayer AM, Joel DM. 1998. Pectolytic activity by the haustorium of the parasitic plant Orobanche L. (Orobanchaceae) in host roots. Annals of Botany 81: 319–326. [Google Scholar]
- Neumann U, Sallé G, Weber HC. 1998. Development and structure of the haustorium of the parasite Rhamphicarpa fistulosa (Scrophulariaceae). Botanica Acta 111: 354–365. [Google Scholar]
- Parker C, Riches CR. 1993.Parasitic weeds of the world. Wallingford: CAB International. [Google Scholar]
- Richael C, Gilchrist D. 1999. The hypersensitive response: a case of hold or fold? Physiological and Molecular Plant Pathology 55: 5–12. [Google Scholar]
- Rubiales D, Alcántara C, Joel DM, Pérez-de-Luque A, Sillero JC. 2003. Characterization of the resistance to Orobanche crenata in chickpea. Weed Science 51: 702–707. [Google Scholar]
- Sherwood RT, Vance CP. 1976. Histochemistry of papillae formed in reed canarygrass leaves in response to noninfecting pathogenic fungi. Phytopathology 66: 503–510. [Google Scholar]
- Vander Molen GE, Labavitch JM, Strand LL, DeVay JE. 1983. Pathogen-induced vascular gels: ethylene as a host intermediate. Physiologia Plantarum 59: 573–580. [Google Scholar]
- Vieira Dos Santos C, Delavault P, Letousey P, Thalouarn, P. 2003. Identification by suppression subtractive hybridization and expression analysis of Arabidopsis thaliana putative defence genes during Orobanche ramosa infection. Physiological and Molecular Plant Pathology 62: 297–303. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


