Abstract
• Background and Aims A reliable protocol for flowering and fruiting in cuttings was developed with the aim of (a) studying inflorescence and flower development in grapevine cuttings and field plants, and (b) assisting haploid plant production.
• Methods Inflorescence and flower development was studied in ‘Gewurztraminer’ (GW) and ‘Pinot Noir’ (PN) grape vines and cuttings grown in a glasshouse, along with variations in starch in the flowers. As there is a strong relationship between flower development and starch, the starch content of reproductive structures was estimated.
• Key Results Inflorescence and flower development were similar in the vines and cuttings with consistent differences between the two cultivars. Indeed, the ontogenesis of male and female organs is not synchronous in GW and PN, with both female and male meiosis occurring earlier in PN than in GW. Moreover, changes of starch reserves were similar in the two plant types.
• Conclusions Cuttings have a similar reproductive physiology to vines, and can be used to study grape physiology and to develop haploid plants.
Keywords: Cuttings, flowering, reproductive structures, starch, vines, Vitis vinifera L
INTRODUCTION
The life cycle of higher plants varies greatly, depending on the species. In small herbaceous plants such as Arabidopsis thaliana, the cycle from seed to seed is 6 weeks, whereas woody plants with a long juvenile phase may not start flowering for several decades. Hence, most of the information on reproduction in plants is obtained from herbaceous models using Arabidopsis thaliana (Boss et al., 2004; Jack, 2004), Nicotiana tabacum or similar species (Rieu et al., 2003).
There is rich interest in the reproductive physiology of woody plants. For example, it has been shown that there is a strong relationship between flower development and carbohydrates (Rodrigo et al., 2000; Jean and Lapointe, 2001; Ruiz et al., 2001; Iglesias et al., 2003). In particular, the concentration of starch in the ovule at specific steps of development is closely correlated to fertility. In Prunus armeniaca, secondary ovules that degenerate often display starch degradation in their tissues (Rodrigo and Herrero, 1998). Moreover, carbohydrate physiology in flowers is affected by environmental stresses such as drought (Lalonde et al., 1997), chilling (Ebadi et al., 1995) or heat (Young et al., 2004), which lead to partial or total sterility.
In Vitis vinifera L., the pathways of inflorescence and flower development contrast with those for many other species in several aspects (Boss et al., 2003), with stresses delaying or stopping reproductive development. For example, low temperatures near flowering affect ovule development and pollen tube growth (Ebadi et al., 1995). These responses are mediated by perturbations in carbohydrate physiology. Indeed, shading the leaves reduces photosynthesis and carbohydrate supply to the developing inflorescences, causing flower abscission and lower yields (Jackson, 1991). The reaction to stresses differs according to the genotypes (Gu et al., 1996; Zapata et al., 2004). ‘Gewurztraminer’ and ‘Pinot Noir’ have different rates of flower abscission, related to the sugar content in the inflorescence during flower development (Lebon et al., 2004). Efforts to understand this behaviour would be improved by developing a reliable model of flowering and carbohydrate physiology.
Grapevine cuttings can flower when grown under controlled conditions (Mullins, 1966; Mullins and Rajasekaran, 1981), although the available protocols are complicated. Moreover, the correspondence between the development of inflorescences in vitro and in the vineyard is not well explained. The objective of this work was to provide a simple protocol for flowering in in vitro cuttings that mimic sexual reproduction in the field. The development of male and female tissues and the presence of starch within the flowers were followed during ontogenesis in cuttings and the response compared in vineyards. The study was performed in ‘Gewurztraminer’ and ‘Pinot Noir’, which differ in fertility and sensitivity to flower abscission (Lebon et al., 2004).
MATERIALS AND METHODS
Plant material
Investigations were carried out on Vitis vinifera L. using the flower abscission-sensitive ‘Gewurztraminer’ (GW) and the non-sensitive ‘Pinot Noir’ (PN) varieties. Dormant cuttings, which had been cane-pruned were collected from the INRA vineyard in Bergheim, France (Mullins and Rajasekaran, 1981).
Cuttings were collected at the proximal part of the cane and limited to three nodes, successively named N0 (the proximal), N1 and N2. A key point in the protocol is the sampling of the cane fragments generating the cuttings. The physiology of this fragment, in particular the amount of reserves in the wood, is important for obtaining predictable results. Harvesting fragments from any part of the annual cane provides cuttings with different sugar contents that vary in reproduction. Similarly, fragments from the distal part of the cane have low carbohydrate reserves and flower less (data not shown).
Cuttings were treated with cryptonol (2 % v/v) to prevent contamination, stored in the dark and forced for at least 2 weeks at 4 °C. After 16 h of hydration at 25–30 °C, N0 and N1 were removed and the proximal extremity of the cutting immerged in indole-3-butyric acid (IBA) at 1 g L−1 for 30 s to promote rhizogenesis. This protocol was developed from Mullins (1966) and Mullins and Rajasekaran (1981) with IBA favouring rhizogenesis and thus eliminating the need for a 4-week pre-treatment in which the cuttings are warmed at the base. The IBA-treated cuttings developed within 1 week instead of 4 weeks.
Cuttings were planted in 0·5-L pots containing perlite : sand (1 : 1) and transferred to a greenhouse at 20/30 °C (night/day), with the pots placed on a 30 °C warming blanket. The photoperiod was 16 h using natural daylight or artificial 400 W ‘Saudiclaude’ lamps supplying 1000 μE m−2 s−1, and a relative humidity of 50 %. Each pot was watered daily with 100 mL of Coïc and Lesaint medium (Coïc and Lesaint, 1971). Leaves were removed until the first inflorescence appeared (each cuttings was limited to four leaves).
Inflorescences were collected from vineyard and glasshouse cuttings (Eichhorn and Lorenz, 1977), from the visible cluster (stage 12) up to fruit set (stage 27). Since meiosis occurs in these cultivars between stages 15 and 17 in vineyards (Lebon et al., 2004), this period was subdivided (15 + 2 d and 15 + 8 d). Development was quicker in the cuttings and the intermediate stages were defined as 15 + 1 d and 15 + 3 d.
Each developmental stage occurred when at least half of the cuttings were at this stage. The numbers of flowers, berries and seeds per fruit were counted on each cutting (n = 10).
Microscopy
Samples were fixed in 2 % glutaraldehyde (v/v in a 0·1 m phosphate buffer) at pH 7·2 in 2 % sucrose (w/v) and 1 % Tween 20 (v/v) for 24 h and agitated at room temperature. After three rinses (5 min) in buffer, the flowers were post-fixed with 1 % osmium tetroxide (w/v) in the buffer without Tween 20 for 4 h. Flowers were then rinsed three times (5 min) in the buffer, dehydrated in an alcohol series, transferred to acetone, and embedded in Araldite.
For starch, 1 µm sections were collected on glass slides, bleached for 30 min with hydrogen peroxide (H2O2 10 volumes), and the periodic acid Schiff (PAS) polysaccharide-specific reaction carried out according to Clément et al. (1994). Sections were plunged in 1 % periodic acid (w/v) for 4 h, in Schiff's reagent without rinsing for 16 h, and in 5 % sodium metabisulfite (w/v) for 20 min. Sections were rinsed in distilled water, air-dried, and mounted in Eukitt®. For each stage, ten flowers from five inflorescences were used, each inflorescence sampling from a different vine or cutting.
Statistical analysis
For the number of flowers, berries and seeds per fruit, ten inflorescences were used, each inflorescence sampling from a different vine or cutting. Statistical analyses were carried out using Student's t-test. A 5 % probability was considered significant. For morphology and starch content, ten flowers from five different inflorescences per stage were sampled, each inflorescence sampling from a different vine or cutting; for these parameters, no statistical analysis was performed because observations from the ten flowers considered were similar.
RESULTS AND DISCUSSION
It is possible to mimic flowering and fruit set in the woody grapevine (Vitis vinifera) planted under controlled conditions. Both the male and female flowers were similar in the cuttings and field vines.
Phenology
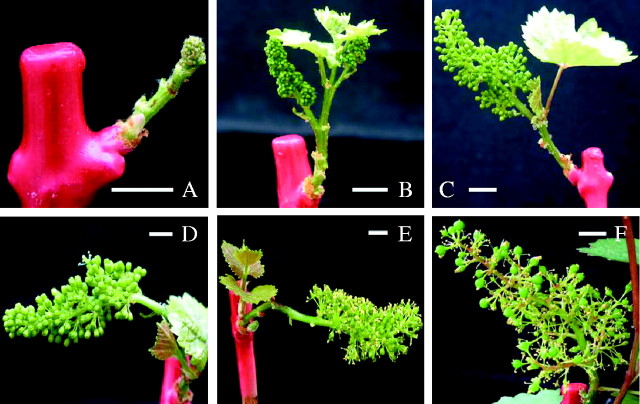
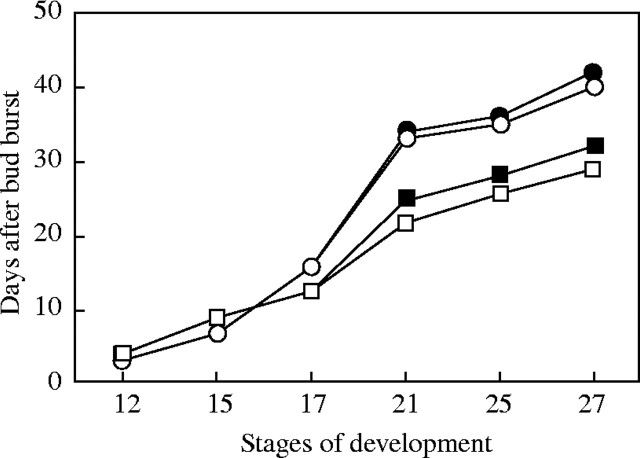
Previous studies on cuttings of grapevine (Mullins, 1966; Mullins and Rajasekaran, 1981) and apple (Höfer and Lespinasse, 1996) were not focused on the ontogenesis of reproductive organs, although it is a key issue in models of flowering. The grapes could be distinguished at stage 15 (Fig. 1B), whereas each flower bud was separated at stage 17 (Fig. 1C). At least 10 % and 90 % of the flowers were opened, respectively, at stages 21 and 25 (Fig. 1D and E), and thereafter fruit set occurred (Fig. 1F). Development was similar in the two cultivars, and both in vineyards and cuttings (Fig. 2). However, development after stage 17 was more rapid in the field than in the glasshouse.
Fig. 1.
Phenology of the ‘Gewurztraminer’ grape cuttings: (A) visible clusters (stage 12); (B) separated clusters (stage 15); (C) separated floral buds (stage 17); (D) flowering onset (stage 21); (E) end of flowering (stage 25); and (F) fruit set (stage 27). Scale bars = 1 cm.
Fig. 2.
Development of grape inflorescences in ‘Gewurztraminer’ (open symbols) and ‘Pinot Noir’ (closed symbols), in vines (circles) and cuttings (squares). Stage 12, visible clusters; stage 15, separated clusters; stage 17, separated floral buds; stage 21, early bloom (10 % opened flowers); stage 25, late bloom (90 % opened flowers); and stage 27, fruit set. Ten inflorescences were used, each inflorescence sampling from a different vine or cutting. Data are the mean of ten plants per treatment. The standard errors were smaller than the symbols, and therefore are not shown.
The PN cuttings had more flowers than the vines, whereas there were no differences in the two types of GW plants (Table 1). There were also no differences between the two cultivars within a plant type. In contrast, the GW plants had more berries than the PN plants, and a higher rate of fruit set. The number of seeds per berry was not affected by cultivar or plant type, with general means of 1·35 ± 0·49 and 1·79 ± 0·97, respectively (Table 1). Final yield was similar in the cuttings and vineyard, with respect to number of flowers, number of fruits and seed set.
Table 1.
Number of flowers, berries per bunch, seeds per berries, and fruit set in ‘Gewurztraminer’ (GW) and ‘Pinot Noir’ (PN) grapes
| PN |
GW |
|||
|---|---|---|---|---|
| No. of flowers | ||||
| Vine | 206 ± 24a | 231 ± 32a | ||
| Cutting | 295 ± 28b | 255 ± 40ab | ||
| No. of berries | ||||
| Vine | 135 ± 20c | 188 ± 18d | ||
| Cutting | 162 ± 3c | 226 ± 38d | ||
| Fruit set (%) | ||||
| Vine | 65 ± 3e | 82 ± 6f | ||
| Cutting | 57 ± 6e | 94 ± 6f | ||
| Number seeds per berry | ||||
| Vine | 1·8 ± 0·8g | 1·6 ± 0·7g | ||
| Cutting | 1·4 ± 0·5g | 1·8 ± 1·0g | ||
For each stage, ten flowers from five inflorescences were used, each inflorescence sampling from a different vine or cutting.
Values are means of ten inflorescences ± s.e.
Statistical analyses were carried out using Student's t-test. Means for a considered parameter were not significantly different when followed by the same letter (P ≥ 0·05).
Development of reproductive organs
Inflorescences in the cuttings developed more rapidly than those from the vineyard plants, with a difference of 12 and 13 d in GW and PN, respectively (Fig. 2). The rate of flower development is dependent on temperature, with extreme temperatures damaging these tissues (Ebadi et al., 1995; Young et al., 2004). The vineyards are exposed to daily fluctuations of temperature, with relatively low temperatures in the night and early morning, whereas the cuttings were exposed to constant temperature ranging from 20–30 °C.
The chronology of pollen development was identical in the vines and cuttings (Table 2). In GW, stage 12 sporogenous cells (Fig. 3A) multiplied and gave rise to microspore mother cells at stage 15. Male meiosis occurred between stages 15 and 15 + 2 d (Fig. 3B) with microspores released from the tetrads at stage 15 + 8 d (Fig. 3C). Microspores vacuolated thereafter and pollen mitosis took place between stages 17 and 21 (Fig. 3D). At stage 21 (anthesis) mature pollen grains were released from the anthers. Pollen development occurred earlier in PN than in GW (Table 2). Meiosis was registered between stages 12 and 15, whereas pollen mitosis took place between stages 15 + 8 d and 17. These results indicate that cuttings grown under controlled conditions can be used to study pollen development in grape.
Table 2.
Reproductive stages according to the classification of Eichhorn and Lorenz (1977)
| Developmental stage |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 15 | 15 + 2 d | 15 + 8 d | 17 | 21 | |||||||
| 15 + 1 d |
15 + 3 d |
|||||||||||
| Male | ||||||||||||
| GW | ||||||||||||
| Vine | SC | mMC | T | m | Vm | MPG | ||||||
| Cutting | SC | mMC | T | T | Vm | MPG | ||||||
| PN | ||||||||||||
| Vine | SC | T | m | Vm | YPG | MPG | ||||||
| Cutting | SC | T | m | m | YPG | MPG | ||||||
| Female | ||||||||||||
| GW | ||||||||||||
| Vine | ST | ST | MMC | MMC | ESMC | ES | ||||||
| Cutting | ST | ST | A | A | MMC | ES | ||||||
| PN | ||||||||||||
| Vine | ST | A | MMC | ESMC | ES | ES | ||||||
| Cutting | ST | A | MMC | MMC | ES | ES | ||||||
A, Archeospore; SC, sporogenous cells; m, microspores; mMC, microspore mother cell; MMC, macrospore mother cell; Vm, vacuolated microspores; MPG, mature pollen grain; ESMC, embryo sac mother cell; ES, embryo sac; ST, sporogenous tissue; T, tetrads; YES, young embryo sac; YPG, young pollen grain.
For vine and cutting, data are the means of ten flowers from five different inflorescences, and each inflorescence was sampled from a different vine or cutting. For each stage of development, the ten flowers considered show similar results.
Fig. 3.
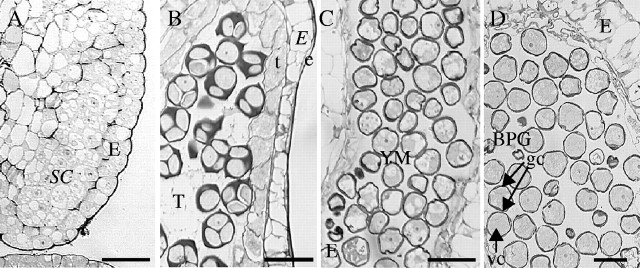
Development of anthers in ‘Gewurztraminer’ (GW) cuttings: (A) stage 12, sporogenous cells; (B) stage 15 + 2 d, tetrads; (C) stage 15 + 8 d, young microspores; and (D) stage 17, bicellular pollen grains. BPG, Bicellular pollen grains; e, epidermis; E, endothecium; gc, generative cell; SC, sporogenous cells; t, tapetum; T, tetrads; vc, vegetative cell; YM, young microspores. Scale bars = 500 μm.
There was a slight delay in female meiosis in the cuttings compared with the vines (Table 2). In GW cuttings, within the sporogenous tissue (Fig. 4A) an archespore developed at stage 15 + 1 d (Fig. 4B), which remained until stage 15+3 d. Stage 17 was characterized by the presence of the macrospore mother cell (Fig. 4C) and meiosis occurred thereafter. The embryo sac was formed at stage 21 (Fig. 4D). In the GW vines, the sporogenous tissue multiplied at early stages of development. The macrospore mother cell was seen from stage 15 + 2 d to stage 15 + 8 d. Between stages 15 + 8 d and 17 meiosis occurred, and the three micropylar macrospores degenerated, leading to a single macrospore generating the whole embryo sac at stage 17. At anthesis, the embryo sac contained eight cells and was ready for fertilization.
Fig. 4.
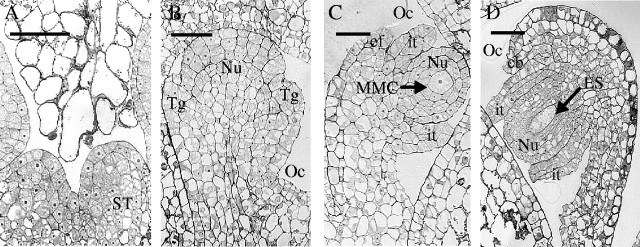
Development of female flower parts in ‘Gewurztraminer’ cuttings: (A) stage 12, sporogenous tissue; (B) stage 15, archespore; (C) stage 17, macrospore mother cell; and (D) stage 23, embryo sac. Arrows in C and D indicate starch grains. ES, Embryo sac; et, external tegument; f, funicle; it, internal tegument; MMC, macrospore mother cell; Nu, nucellus; Oc, ovarian cavity; ST, sporogenous tissue; Tg, tegument. Scale bars = 250 μm.
Female development was faster in PN than in GW (Table 2). In vines, the archespore appeared at stage 15 and meiosis occurred between stages 15 + 2 d and 15 + 8 d. Afterwards, the embryo sac developed between stages 15 + 8 d and 21. In cuttings, meiosis was slightly delayed between stages 15 + 3 d and 17, but the other steps of development were similar to those in vines.
Location of starch in the anther and the ovule
Changes of starch reserves were similar in the two plant types. Starch was found in all the sporophytic tissues of the anther (epidermis, endothecium and tapetum) in the vines (Table 3). In the gametophytic tissue, starch could be seen in PN microspores at all stages, but not at stages 12 and 15+8 d in GW (Table 3). Later on, amylogenesis/amylolysis occurred in the young pollen grains. In the cuttings, starch grains were not detected in sporophytic tissues, except in the endothecium at stages 15 + 3 d and 17 in PN, and only in stage 17 in GW (Table 3). No starch was detected in the gametophytic tissue (Table 3). The anther sporophytic tissues influence the concentrations of sugars in the anther by storing/mobilizing starch and regulate the volume of sugar reaching the pollen grain during development (Clément and Audran, 1995). The difference reported here between vines and cuttings may correspond, in terms of starch in the anther sporophytic tissues, to such buffering action of the sporophytic tissues and obviously does interfere with pollen development.
Table 3.
Presence (+) or absence (0) of starch in Gewurztraminer (GW) and Pinot Noir (PN) vines and cuttings
| Gewurztraminer (vine/cutting) |
Pinot Noir (vine/cutting) |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Developmental Stages |
||||||||||||||||||||||||
| 15 + 2 | 15 + 8 | 15 + 2 | 15 + 8 | |||||||||||||||||||||
| 12 |
15 |
15 + 1 |
15 + 3 |
17 |
21 |
12 |
15 |
15 + 1 |
15 + 3 |
17 |
21 |
|||||||||||||
| Male structures | ||||||||||||||||||||||||
| Epidermis | +/0 | +/0 | +/0 | +/0 | +/0 | +/0 | +/0 | +/0 | +/0 | +/0 | ||||||||||||||
| Endothecium | +/0 | +/0 | +/0 | +/0 | +/0 | 0/0 | +/0 | +/0 | +/+ | +/+ | ||||||||||||||
| Tapetum | +/0 | +/0 | +/0 | +/0 | +/0 | +/0 | +/0 | +/0 | +/0 | +/0 | ||||||||||||||
| Pollen | 0/0 | +/0 | +/0 | 0/0 | +/0 | +/0 | +/0 | +/0 | +/0 | +/0 | ||||||||||||||
| Female structures | ||||||||||||||||||||||||
| Ovary | 0/0 | 0/0 | +/0 | +/0 | +/+ | +/+ | +/0 | +/0 | +/0 | +/+ | +/+ | +/+ | ||||||||||||
| Nucellus | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | +/0 | 0/0 | 0/0 | ||||||||||||
| Tegument | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | +/0 | 0/0 | 0/0 | ||||||||||||
| Embryo sac | 0/0 | 0/0 | 0/0 | |||||||||||||||||||||
For vine and cutting, ten flowers from five different inflorescences were analysed to study the presence of starch, and each inflorescence was sampled from a different vine or cutting. For each stage of development, the ten flowers considered show similar results for starch content. Structures are scored a ‘+’ if the ten inflorescences had starch, and a ‘0’ if the ten inflorescences had no starch.
In the ovules of the vines, concentrations of starch were low except at stage 15 + 8 d in the teguments and nucellus of the PN ovules (not in GW) (Table 3). Nevertheless, amylaceous reserves were detected in ovaries of both cultivars from stage 12 and 15 + 2 d in PN and GW, respectively, to anthesis. In contrast, starch was not detected in the nucellus and teguments of the cuttings during flower development, except at stage 15+3 d for PN (Table 3), same as in the vines. In the cuttings, starch accumulation in the ovaries was delayed until stage 17 in GW and stage 15 + 3 d in PN. In grapevine (Lebon et al., 2004), as in other fruit trees (Rodrigo and Herrero, 1998; Rodrigo et al., 2000), the presence or absence of starch in the ovule at meiosis has a key influence on fertilization and fruit set. In the cuttings and vines, the variations in concentrations of starch in the inflorescences and ovules were similar.
There were differences in reproduction between GW and PN grown in the vineyard; GW exhibits faster inflorescence development (Fig. 2), more flowers per inflorescence (Table 1), but a lower flower abscission rate, percentage of fruit set and fewer seeds per fruit (Table 1). Moreover, starch has a greater incidence in PN ovules (Lebon et al., 2004). These differences were also detected in the cuttings grown under the conditions described here, further showing that the protocol used is reliable.
In grapevine, uncommitted primordium in the latent bud developed into inflorescence primordium and a potential fully developed inflorescence next spring, depending on environmental conditions (Boss et al., 2002). Rapid shoot growth can produce tendrils rather than inflorescences. Further development of the flowers is influenced by variety and environment (Boss et al., 2003). Flower abscission is poorly described in grapevine but can dramatically diminish the yield (Huglin and Schneider, 1998). The supply of carbohydrates to the flower is a key factor in abscission (Gu et al., 1996). A model of flower development in woody species should assist efforts to understand physiological traits that favour inflorescence initiation and flower development.
Cuttings can be used to develop doubled haploid grapevines. Until recently, attempts to obtain in vitro microspore embryogenesis from field plants generally resulted in diploid callus from the sporophytic tissues of the anther (Mauro et al., 1986). In apple, the success of microspore embryogenesis is dependent on the donor material. Anthers collected from the field did not produce haploid embryos (Höfer and Lespinasse, 1996), whereas those from cuttings gave doubled haploids (Höfer et al., 1999; Höfer, 2004).
LITERATURE CITED
- Boss PK, Bastow RM, Mylne JS, Dean D. 2004. Multiple pathways in the decision to flower: enabling, promoting, and resetting. Plant Cell 16: S18–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss PK, Buckeridge EJ, Poole A, Thomas MR. 2003. New insights into grapevine flowering. Functional Plant Biology 30: 593–606. [DOI] [PubMed] [Google Scholar]
- Boss PK, Sensi E, Hua C, Davies C, Thomas MR. 2002. Cloning and characterisation of grapevine (Vitis vinifera L.) MADS-box genes expressed during inflorescence and berry development. Plant Science 162: 887–895. [Google Scholar]
- Clément C, Audran JC. 1995. Anther wall layers control pollen sugar nutrition in Lilium Protoplasma 187: 172–181. [Google Scholar]
- Clément C, Chavant L, Burrus M, Audran JC. 1994. Anther starch variations in Lilium during pollen development. Sexual Plant Reproduction 7: 347–356. [Google Scholar]
- Coïc F, Lesaint L. 1971. Comment assurer une bonne nutrition en eau et en ions minéraux en horticulture. Horticulture Française 8: 11–14. [Google Scholar]
- Ebadi A, May P, Sedgley M, Coombe BG. 1995. Effect of low temperature near flowering time on ovule development and pollen tube growth in the grapevine (Vitis vinifera L.), cvs Chardonnay and Shiraz. Australian Jornal of Grape and Wine Research 1: 11–18. [Google Scholar]
- Eichhorn KW, Lorenz DH. 1977. Phöenologische Entwicklungsstadie. Der rebe. Nachrichtenb. Deutsch Pflanzenschutzd (Braunschweig) 29: 119–120. [Google Scholar]
- Gu S, Lombard P, Price S. 1996. Effect of shading and nitrogen source on growth, tissue ammonium and nitrate status and inflorescence necrosis in Pinot noir grapevine. American Journal of Enology and Viticulture 47: 173–180. [Google Scholar]
- Höfer M. 2004.In vitro androgenesis in apple—involvement of the induction phase. Plant Cell Reports 22: 365–370. [DOI] [PubMed] [Google Scholar]
- Höfer M, Lespinasse Y. 1996. Haploidy in apple. In: Jain SM, Sopory SK, Veilleux RE, eds. In vitro haploid production in higher plants, Vol. 3. Dordrecht: Kluwer, 259–274. [Google Scholar]
- Höfer M, Touraev A, Heberle-Bors E. 1999. Induction of embryogenesis from isolated microspores. Plant Cell Reports 18: 1012–1017. [Google Scholar]
- Huglin P, Schneider C. 1998.Biologie et écologie de la vigne. Paris: Tech and Doc, Lavoisier eds. [Google Scholar]
- Iglesias DJ, Tadeo FR, Primo-Millo E, Talon M. 2003. Fruit set dependence on carbohydrate availability in citrus trees. Tree Physiology 23: 199–204. [DOI] [PubMed] [Google Scholar]
- Jack T. 2004. Molecular and genetic mechanisms of floral control. Plant Cell 16: S1–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DI. 1991. Environmental and hormonal effects on development of early bunch stem necrosis. American Journal of Enology and Viticulture 42: 290–294. [Google Scholar]
- Jean D, Lapointe L. 2001. Limited carbohydrate availability as a potential cause of fruit abortion in Rubus chamaemorus. Physiologia Plantarum 112: 379–387. [DOI] [PubMed] [Google Scholar]
- Lalonde S, Beebe DU, Saini HS. 1997. Early signs of disruption of wheat anther development associated with the induction of male sterility by meiotic-stage water deficit. Sexual Plant Reproduction 10: 40–48. [Google Scholar]
- Lebon G, Duchêne E, Brun O, Magné C, Clément C. 2004. Flower abscission and inflorescence carbohydrates in sensitive and non-sensitive cultivars of grapevine. Sexual Plant Reproduction 17: 71–79. [Google Scholar]
- Mauro MC, Nef C, Fallot J. 1986. Stimulation of somatic embryogenesis and plant regeneration from anther culture of Vitis vinifera cv. Cabernet-Sauvignon. Plant Cell Reports 5: 377–380. [DOI] [PubMed] [Google Scholar]
- Mullins MG. 1966. Test plants for investigations of the physiology of fruiting in Vitis vinifera L. Nature 209: 419–420. [Google Scholar]
- Mullins MG, Rajasekaran K. 1981. Fruiting cuttings: revised method for producing test-plants of grapevine cultivars. American Journal of Enology and Viticulture 32: 35–40. [Google Scholar]
- Rieu I, Wolters-Arts M, Derksen J, Mariani C, Weterings K. 2003. Ethylene regulates the timing of anther dehiscence in tobacco. Planta 217: 131–137. [DOI] [PubMed] [Google Scholar]
- Rodrigo J, Herrero M. 1998. Influence of intraovular reserves on ovule fate in apricot (Prunus armeniaca L.). Sexual Plant Reproduction 11: 86–93. [Google Scholar]
- Rodrigo J, Hormaza JI, Herrero M. 2000. Ovary starch reserves and flower development in apricot (Prunus armeniaca). Physiologia Plantarum 108: 35–41. [Google Scholar]
- Ruiz R, Garcia-Luis A, Honerri C, Guardiola JL. 2001. Carbohydrate availability in relation to fruitlet abscission in Citrus. Annals of Botany 87: 805–812. [Google Scholar]
- Young LW, Wilen RW, Bonham-Smith PC. 2004. High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. Journal of Experimental Botany 55: 485–495. [DOI] [PubMed] [Google Scholar]
- Zapata C, Déléens E, Chaillou S, Magné C. 2004. Partitioning and mobilization of starch and N reserves in grapevine (Vitis vinifera L.). Journal of Plant Physiology 161: 1031–1040. [DOI] [PubMed] [Google Scholar]