Abstract
• Background and Aims Understanding the effects of the environment on the morphology and shoot growth activities of plants is crucial to identifying plant ecological strategies. This study analysed the bud morphology, bud activity, shoot growth dynamics and shoot water content at full hydration (WCh) of two species of Mediterranean sub-shrubs, Lepidium subulatum and Linum suffruticosum, co-existing in gypsum outcrops in north-east Spain.
• Methods Sampling was conducted monthly over 2 years in one population per species. Buds were dissected under a stereo-microscope. Shoot growth was measured as the mean increase in shoot length of 15 marked individuals between two consecutive samplings. Bud activity was studied following the variations in the number of leaf primordia shorter than 1 mm and longer than 0·025 mm in the buds.
• Key Results Both species bore naked buds and displayed discontinuous seasonal patterns of shoot growth, leaf primordia formation and WCh. The number of leaf primordia in the bud peaked before the beginning of shoot expansion. In both species, organogenesis and expansion were uncoupled throughout the year. The time lapse between these two processes varied throughout the year, and was greatest for those elements differentiated in autumn. WCh was more closely related to shoot expansion than to organogenesis.
• Conclusions Both species displayed similar bud morphology and similar seasonal patterns of bud and shoot growth, and WCh as a result of the strong seasonality of the Mediterranean climate in gypsum outcrops. The beginning of the spring period of expansion of long branches coincided with maximum values of WCh, while the rest period of summer matched minimum values. These results support the hypothesis that the growth of long branches is strongly related to WCh.
Keywords: Shoot growth, bud morphology, Mediterranean sub-shrubs, gypsum, Linum suffruticosum, Lepidium subulatum
INTRODUCTION
Plants growing in highly seasonal environments must adjust their morphology and the activity of their renewal structures and shoots to maximize survival and productivity (Meloche and Diggle, 2001). An understanding of these features is crucial to identifying the ecological strategies of plants (Nitta and Ohsawa, 1999). Mediterranean trees and shrubs normally bear cataphyllary or hipsophyllary buds in which meristems are protected during unfavourable seasons by specialized protective organs (Hoffmann, 1972; Ginocchio and Montenegro, 1996; but see Hoffmann and Hoffmann, 1976). Conversely, the buds of some species of Mediterranean sub-shrubs lack these protective organs (Montserrat-Martí et al., 2004; Palacio-Blasco et al., 2004). Most Mediterranean sub-shrubs bear their naked buds in the apices of two distinct types of branches: long and short branches. The latter usually develop in the axils of the leaves of long branches (Orshan, 1989). Few studies have addressed the morphology and growth dynamics of buds and shoots of Mediterranean sub-shrubs (but see Orshan, 1972; Gray and Schlesinger, 1981; Montserrat-Martí et al., 2004; Palacio-Blasco et al., 2004). In particular, very little is known about bud and shoot growth dynamics during the unfavourable seasons of winter and summer, or about the relationship between the growth of these organs. The growth of axillary short branches is maintained throughout the year, at least in some species growing in semi-arid environments (Orshan, 1972). However, it is unlikely that a continuous pattern of shoot growth and morphogenesis occurs under the seasonal conditions of a Mediterranean climate. Furthermore, shoot expansion may be rapid in Mediterranean sub-shrubs, thereby taking advantage of the short favourable periods of spring and autumn. Therefore, the shoots of these sub-shrubs may be at least partly preformed, with buds containing many leaf primordia prior to shoot expansion (Kozlowski and Clausen, 1966).
For shoot growth to occur, plants require increased bud and shoot hydration (Bradford and Hsiao, 1982; De Faÿ et al., 2000). The potential to hydrate might therefore vary through the year, and might be related to the growth activity of these two organs (De Faÿ et al., 2000). Several authors have studied the relationship between growth processes and the water content of buds and stems of woody plants (Jones and Laude, 1960; Cottignies, 1983, 1990; Essiamah and Eschrich, 1986; Tousignant et al., 2003). However, water content is highly dependent on the weather conditions at the sampling time (Tousignant et al., 2003) and is therefore not a good predictor of plant hydration capacity. In contrast, the water content at full hydration (WCh, %) is a measurement of the maximum amount of water a given organ can hold, expressed as the percentage of the fresh weight of the fully hydrated organ. In a previous study, both water content and water content at full hydration of the shoots of four species of Mediterranean shrubs were analysed monthly over one year (G. Montserrat-Martí, unpubl. data). According to these results, water content varied following rainfall, while the seasonality of water content at full hydration appeared to be related with shoot growth. Few studies have addressed WCh in relation to shoot growth. Davis and Mooney (1986) reported a sharp increase in WCh of the shoots of two co-occurring chaparral species during the growing season and concluded that this increase was related to organogenetical processes. More recently, the growth peaks in Cistus laurifolius shoots and leaves have been reported to coincide with maximum values of WCh (Montserrat-Martí et al., 2004). No studies relating WCh to shoot growth processes have been conducted in Mediterranean sub-shrubs.
Mediterranean gypsum outcrops such as the ones considered here are suitable to study the relationship between climatic seasonality and shoot growth processes as they are subjected to sharp environmental variations during the year (Rivas-Martínez and Costa, 1970; Nelson and Harper, 1991). Pure gypsum soils have a very low water retention capacity, and therefore do not buffer the effects of drought on plant performance (Guerrero Campo et al., 1999b). These stress factors are more intense in the ridges of pure gypsum hills, such as those considered in this study (Guerrero Campo et al., 1999a).
Here we describe the bud morphology and shoot growth dynamics of two species of Mediterranean sub-shrubs co-existing in gypsum outcrops. The relationship between shoot growth processes and the potential of shoots to become fully hydrated is also analysed. Between September 2002 and August 2004, a study was made of the phenology, bud morphology and activity, shoot growth dynamics and WCh of Lepidium subulatum and Linum suffruticosum plants co-existing in pure gypsum outcrops in north-east Spain.
MATERIALS AND METHODS
Species and study site
Linum suffruticosum L. (Linaceae) is an evergreen sub-shrub with a maximum height of 70 cm that inhabits low scrublands on gypsum soils and limestones in south-west Europe (Ockendon and Walters, 1968). Lepidium subulatum L. (Brassicaceae) is an evergreen sub-shrub up to 60 cm in height that is restricted to open scrublands on gypsum soils in eastern Spain and north-west Africa (De Carvalho e Vasconcellos, 1964). For ease of presentation, from here on L. suffruticosum and L. subulatum will be referred to as Linum and Lepidium, respectively.
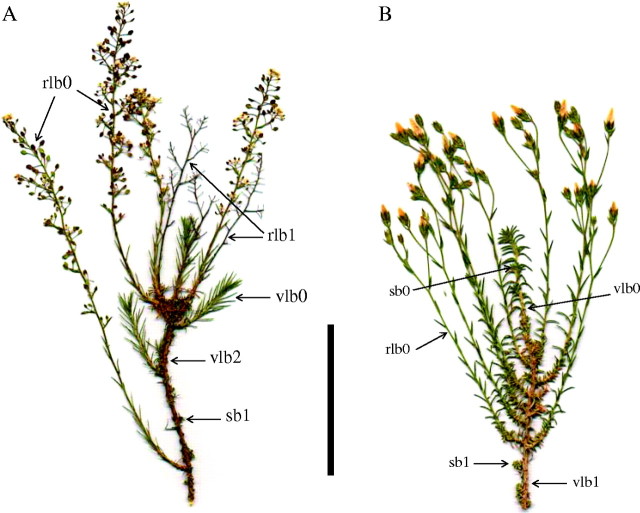
Both species were selected for a comparative analysis of shoot growth processes due to their similar crown morphology and architecture. Their leaves are linear, rigid and helicoidally arranged. Both species bear two distinct types of branches: long and short branches (Fig. 1). In the present study, short branches are those shorter than 2 cm in length, while long branches are those longer than 2 cm in length. Long branches either bear terminal inflorescences or remain vegetative, while short branches develop mainly in the axils of the leaves of vegetative long branches, but also grow at the base of some reproductive long branches, or on older stems (epicormic short branches), especially in Lepidium.
Fig. 1.
General morphology of a 3-year-old branch of (A) Lepidium subulatum and a 2-year-old branch of (B) Linum suffruticosum on 12 and 5 May 2003, respectively. vlb0, vlb1 and vlb2: vegetative long branches from 2003, 2002 and 2001, respectively; rlb0, rlb1: reproductive long branches from 2003 and 2002, respectively; sb0, sb1: short branches from 2003 and 2002, respectively. Scale bar = 5 cm.
The study site was located on the slope of a nearly pure gypsum hill in the gypsum outcrops of Villamayor, near Zaragoza, in north-east Spain (UTM: 30TXM8820; 320 m a.s.l; Fig. 2). The dominant substratum in this area is almost pure gypsum, with a few thin inserted outcrops of marls and clays (Quirantes, 1977). The climate at this site is semi-arid and highly seasonal, with a mean annual temperature of 14·6 °C, and an average annual rainfall of 334·5 mm, which falls mainly during spring and autumn (Rivas-Martínez, 1987). Summers are hot and dry and the mean maximum temperature of the warmest month (July) is 31·4 °C. Winters are cool and dry and the mean minimum temperature of the coldest month (January) is 1·6 °C (De León et al., 1987). At this site, these two species co-exist and display a similar degree of abundance. The area is covered by open low scrubland (termed tomillar) dominated by Ononis tridentata, Helianthemum syriacum, Helianthemum squamatum, Thymus zygis, Herniaria fruticosa and Gypsophila struthium subsp. hispanica, and the two sub-shrubs studied here (Braun-Blanquet and Bolòs, 1957). The area was partly ploughed for Pinus halepensis reforestation 15 years ago, although only some isolated pines survive on the upper part of the hill. Since then, the site has remained abandoned.
Fig. 2.
General view of the study area on 3 February 2003. Gypsum outcrops of Villamayor, near Zaragoza, in north-east Spain (UTM: 30TXM8820; 320 m a.s.l).
Phenology
Above-ground phenology was studied monthly from September 2002 to August 2004 on 15 marked plants of each species. Phenological processes such as flowering, fruiting, shoot expansion and leaf shedding were assessed by visual inspection. Estimations of the approximate amount of green biomass were also recorded for each individual. To do this, one standard 3-year-old branch was examined in each of the 15 marked individuals, and the percentage of its green biomass was visually estimated. At each sampling date, representative plant material was collected, pressed and stored in a herbarium for future verification.
Bud morphology and composition
Sampling was conducted monthly during an 18-month period, from September 2002 to February 2004. Ten 2-year-old branches were collected randomly from ten non-marked individuals within each population at each sampling date. Samples were kept at 4 °C until bud dissection, which was performed within the following 48 h. To avoid within-branch variability, only those buds located in standard short branches were dissected, thereby excluding extremely large or small short branches or those that were apparently malformed or damaged. Buds were examined under a stereo-microscope fitted with an ocular micrometer (MS5 Leica Microsystems, Heerbrugg, Switzerland) at ×10 or ×40. The properties (colour, vigour and developmental stage) of most leaf primordia were recorded. Because of the large number of leaf primordia in the buds of these two species (n > 20 in some months), only those shorter than 1 mm and longer than 0·025 mm were counted (hereafter Np) and measured. The resolution limit of the stereo-microscope was approximately 0·025 mm. Given that the maximum length of leaf primordia varied through the year (see below), we set an arbitrary upper limit of 1 mm on the basis of the lowest value of the maximum lengths of leaf primordia recorded. Adult leaves were distinguished from leaf primordia by the increased toughness of their epidermis, which indicated the cessation of their expansive growth. The length of leaf primordia was measured from their insertion point on the immature stem to their distal end.
Shoot growth
In September 2002, we selected and marked 15 well-developed adult individuals of each species. Sampling was conducted monthly over two years, from September 2002 to August 2004. At each sampling date, three 2-year-old branches were collected from distinct positions within the canopy of each plant. Repeated cuttings from the same branch were avoided so that the effect of the sampling method on plant growth was minimized. To check for possible interactions between sampling method and plant performance, 15 plants of each species, similar to those used for shoot growth analyses, were marked and left uncut. No differences were found in the survival and shoot vigour of sampled and control plants at the end of the sampling period. Therefore, the effect of sampling on plant performance was negligible. Samples were pressed at constant pressure and stored in a herbarium until measurements of shoot length were conducted under the stereo-microscope. For each branch, the lengths of the longest shoot and its three closest shoots were measured. Shoot length was measured from the insertion point on the stem to the tangent line between the apices of the most apical green leaves. Leaves were considered green when more than 50 % of their lamina was green. Destructive analyses were required because of the small size of undeveloped short branches. The relative growth rate (RGR) of the shoots of marked plants at each sampling date was calculated using the following formula:
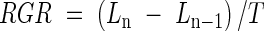 |
where Ln (mm) is the mean shoot length of month n, Ln−1 (mm) is the mean shoot length of month (n−1), and T (d) is the period between (n−1) and n.
Water content at full hydration
Sampling was conducted monthly between October 2002 and December 2003. Ten branches over 3 years old were randomly collected from ten non-marked individuals at each sampling date. Branches were placed in individual plastic bags and taken to the laboratory in a cooler. Once in the laboratory, branches were set at full hydration. To do this, the three most proximal centimeters of the stem of each branch were cut under water, and the remaining material was kept at 4 °C, with the first 3–4 cm of the stem immersed in distilled water, and covered by a wet plastic bag for 24 h. Full hydration weights of samples of whole short branches (including leaves and stems) and stems of the long branches were obtained for each hydrated branch. Subsequently, samples were oven-dried at 60 °C to a constant weight and dry weights were obtained. All weighing was conducted using a precision scale (MC1, Sartorius AG, Goettingen, Germany). Water content at full hydration (WCh, %) was calculated using the following formula:
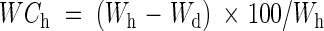 |
where Wh(mg) is the weight of a given sample at full hydration and Wd (mg) is the dry weight of the same sample.
Statistical analysis
All data were checked for normality, homocedasticity and outlier detection. RGR for each sampling date was analysed using a Student t-test in which the mean RGR was compared with a theoretical mean value equal to zero. In cases in which normality and homocedasticity were not attained, data were analysed using the non-parametrical Wilcoxon t-test for paired samples. Differences in Np and WCh between distinct sampling dates were analysed using one-way ANOVA. To study the relationship between WCh and growth, WCh data from short and long branches were sorted in two groups: ‘growth’ and ‘no growth’. The former included those WCh values of dates with mean RGR significantly greater than zero (α = 0·05); while the latter comprised the remaining WCh values. All data except those from the short branches of Linum lacked normality and/or homocedasticity. Therefore, to detect differences in WCh between ‘growth’ and ‘no growth’ groups, a Student t-test was run in the short branches of Linum, while the remaining data were analysed using the non-parametrical Mann–Whitney U-test for unpaired samples. All statistical analyses were conducted using SPSS 11·0 (SPSS Inc., Chicago, USA).
RESULTS
Phenology
Lepidium and Linum flowered in spring. The former began flowering before mid April and continued up to May/June, while the latter began flowering slightly later, in late April, and concluded by early June. Fruiting began by late April in Lepidium and finished by late June. Linum began fruiting by late May, ending by early July. Lepidium dispersed its seeds during late spring and summer, beginning in late May/June. In contrast, Linum began seed dispersal by late September. Both species showed protracted seed dispersal over several months. For both species, dry leaves tended to remain attached to the stems for several months and were gradually shed throughout the year. Lepidium plants were apparently dry in summer, with few green leaves in many cases. In contrast, Linum kept many green leaves through the summer. In early autumn, short branches restarted growth, slowly increasing the amount of green biomass of both species, which reached maximum values in spring.
Morphology of the renewal structures
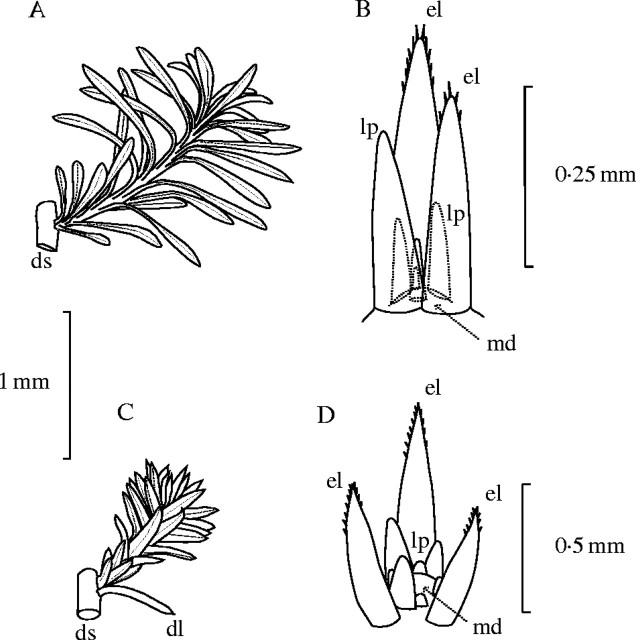
The meristematic tissues that gave rise to the long branches of Linum and Lepidium were the apical buds of axillary short branches and, less frequently, those of vegetative long branches (Fig. 3A, C). In Linum, buds consisted of a dome-shaped meristem surrounded by six to >30 helicoidally arranged leaf primordia in different stages of development (Fig. 3D). In Lepidium, buds comprised a dome-shaped meristem surrounded by two to >28 helicoidally arranged leaf primordia at different stages of development (Fig. 3B). No specialized protective organs, such as cataphylls or scales, were found in either species, indicating that bud formation is attained simply by the arrest of apical growth of short branches. Therefore, in Linum and Lepidium, leaf primordia are protected only by the expanded leaves of short branches, and hence these buds should be considered as being naked (Nitta and Ohsawa, 1998).
Fig. 3.
Morphology of the short branches and apical meristems of Lepidium subulatum, (A) and (B) respectively, and Linum suffruticosum, (C) and (D) respectively, in December 2003. For clarity, only the inner structures of the buds are represented. ls, long branch stem; ll, long branch leaf; lp, leaf primordia; md, meristematic dome.
Shoot growth
Linum and Lepidium showed very similar shoot growth dynamics (Fig. 4). Two periods of shoot extension were recorded that lasted for most of the year. The first occurred in spring and comprised long branch expansion from the apical buds of axillary short branches formed the previous year, and the concurrent growth of a new cohort of axillary short branches. The second period occurred in late summer and autumn and involved a slight expansion of short branches. During mid-summer and winter, the two species showed arrested shoot growth. In both species, long-branch expansion began by mid-March and finished around June (July in the case of Linum in 2004) with the development of terminal inflorescences in reproductive long branches and the formation of apical buds in vegetative ones. Not all short branches elongated to long branches in spring. Some remained unexpanded, increasing just some millimeters in length and drying out in early summer. The RGR of long branches peaked in May for the two species in both years of study, reaching 0·88 mm d−1 and 0·63 mm d−1 in 2003 and 2004, respectively, for Lepidium, and 1·45 mm d−1 and 1·05 mm d−1 for Linum. The mean RGR for the period of long-branch expansion was 0·40 mm d−1 and 0·25 mm d−1 in 2003 and 2004, respectively, for Lepidium, and 0·64 mm d−1 and 0·45 mm d−1 for Linum.
Fig. 4.
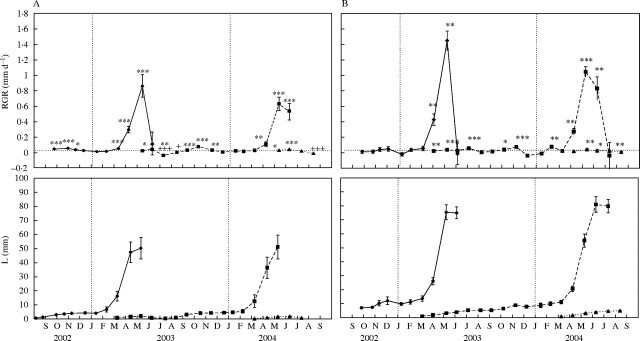
Relative growth rate dynamics (top) and mean length of the shoots (bottom) of (A) Lepidium subulatum and (B) Linum suffruticosum. Values are means of 15 shoots ± s.e. Solid lines represent shoots recorded in 2002, dashed lines are those from 2003, and dotted lines are those from 2004. Asterisks indicate RGR values significantly higher than zero (*P < 0·05, ** P < 0·01, ***P < 0·001) and crosses indicate RGR values significantly lower than zero (+ P < 0·05, ++ P < 0·01, +++ P < 0·001), after a Student's t-test, in case of normal data, or by Wilcoxon t-test otherwise.
The expansion of the new cohort of axillary short branches overlapped that of long branches in both species. This expansion was first detected by mid-March and was arrested by the end of June in Lepidium and by late July in Linum. Subsequently, the RGR of short branches reached close to zero in Linum during August and September, while in Lepidium it attained significant negative values during July (P < 0·01) and August (P < 0·05) because of the drying of most leaves caused by summer drought. The expansive growth of short branches was resumed in September in Lepidium and in October in Linum, thereby coinciding with late-summer and autumn rainfall, and was arrested again in December. During winter (from December to the end of February), both species maintained short branch RGR values close to zero, with the exception of Linum, which had an isolated pulse of short-branch growth in February (P < 0·01). The expansion of short branches that gave rise to the next cohort of long branches was resumed by mid-March.
Leaf primordia formation
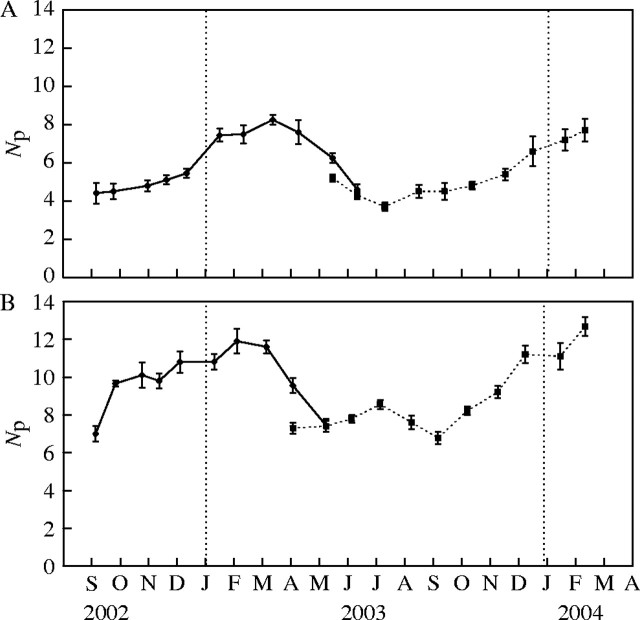
The number of leaf primordia shorter than 1 mm and longer than 0·025 mm (Np) in buds varied significantly between dates (P < 0·001), and showed similar patterns in the two species (Fig. 5). The maximum length of leaf primordia also varied through the year, peaking in spring and reaching minimum values in summer (Fig. 6). During autumn and winter, the apical meristem produced leaf primordia almost continuously. The older ones grew into adult leaves. However, most of these went through a slow period of development that resulted in an accumulation of leaf primordia at the end of winter (early March), when maximum Np values were reached in both species (Fig. 5). During early spring, the apical meristem continued to produce new primordia. The development of these into adult leaves was then very quick, and the sizes of leaf primordia and adult leaves were at their greatest (Fig. 6). The number of leaf primordia present in buds diminished steadily. At the end of spring, the formation of new structures by the meristem slowed down. Leaf primordia matured quickly, giving rise to shorter adult leaves. With the onset of summer drought, the formation of leaf primordia was apparently arrested. The tissues of most of the remaining leaf primordia within the bud hardened quickly, giving rise to tiny adult leaves. By this time, Np and the maximum length of leaf primordia reached minimum values in both species (Figs 5, 6).
Fig. 5.
Number of leaf primordia smaller than 1 mm and longer than 0·025 mm in the renewal buds (Np) of (A) Lepidium subulatum and (B) Linum suffruticosum. Solid lines represent buds from shoots recorded in 2002 and dotted lines are those from 2003. Values are means of ten buds ± s.e.
Fig. 6.
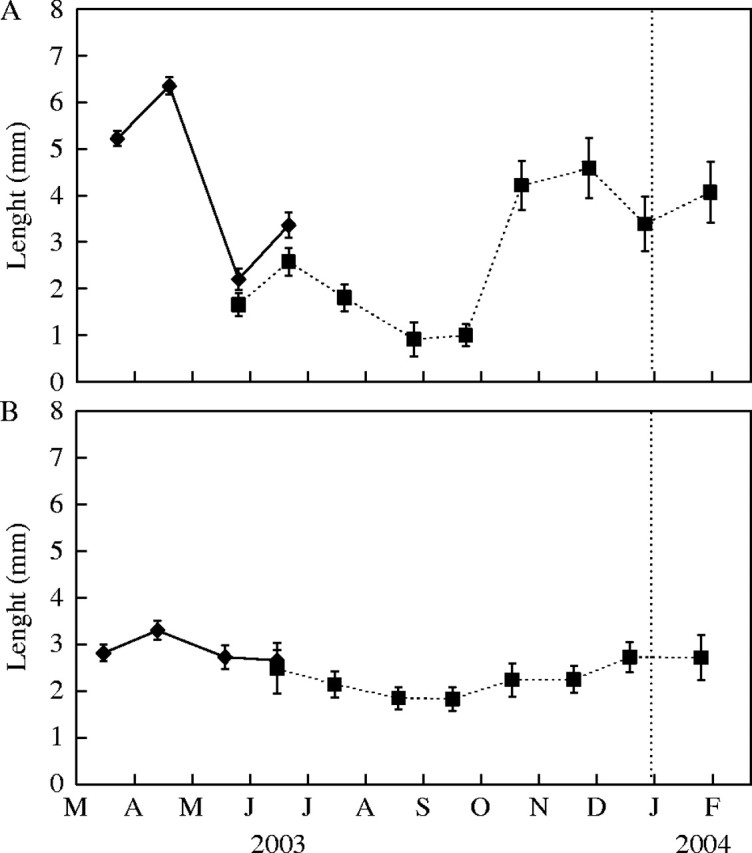
Maximum length of the leaf primordia of (A) Lepidium subulatum and (B) Linum suffruticosum. Solid lines represent shoots recorded in 2002 and dotted lines are those from 2003. Values are means of ten shoots ± s.e.
In spite of the similarities in their general patterns, Linum and Lepidium differed in the dynamics of Np during the first six months of short-branch expansion. In the former, the Np of the newly formed short branches increased during early summer, reaching a peak in July, while in the latter it decreased from the initial value after short-branch formation, reaching a minimum also in July (Fig. 5). From then on, Np values increased steadily in Lepidium until late winter, while in Linum they declined again to reach a new minimum in September and then rose again progressively until late winter. In both species, Np values peaked before the onset of the rapid expansion of long branches, while they were low before the slow autumn growth of short branches.
Water content at full hydration
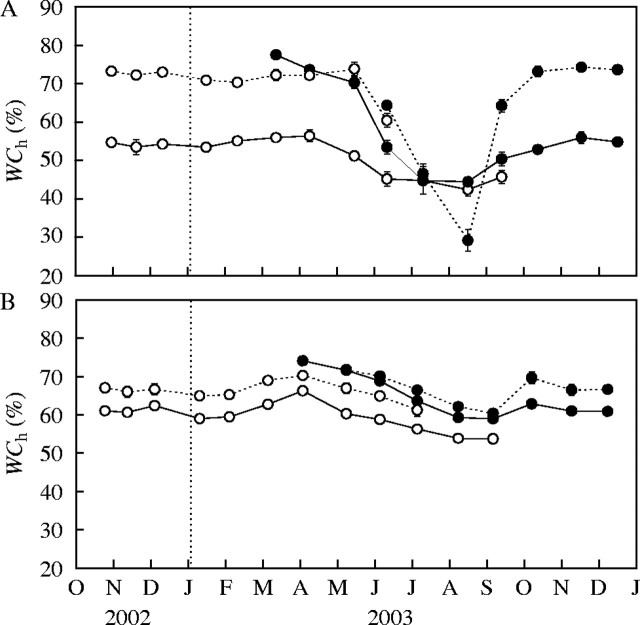
The variations in WCh were greater in Lepidium than in Linum (Fig. 7). However, both species displayed similar patterns of variation in this parameter during the course of the year, with significant differences between dates (P < 0·001; Fig. 7). Minimum values of WCh were reached in August and September for Lepidium and Linum, respectively. The extremely low WCh values of the short branches of Lepidium during the summer might be due to the presence of dry leaves and the difficulty of excluding them prior to weighing due to their small size. Dry leaves are not re-hydrated when the branches are set at full hydration, but they are included in the dry weight of short branches, resulting in abnormally low WCh values. Maximum values of WCh were attained in early May and November in Lepidium, while in Linum they were reached in April and October. During the autumn–winter period between these two maxima, WCh displayed nearly constant values in Lepidium, while in Linum WCh diminished to reach a second minimum. Therefore, the WCh values closely followed those of shoot RGR in both species; attaining maximum values in spring and minimum values in summer (Figs 3, 6). In Linum, minimum autumn and winter WCh values matched the reduced autumn and winter growth of short branches. In the short and long branches of Linum, WCh was significantly higher in months with significant positive growth compared with those months with negative or no growth (Table 1). This positive relationship between shoot growth and WCh was also significant in the short branches of Lepidium, but not in its long branches (Table 1).
Fig. 7.
Water content at full hydration (WCh) of the short and long branches of (A) Lepidium subulatum and (B) Linum suffruticosum. Dotted lines represent short branches while solid lines show the stems of long branches. Open circles are shoots recorded in 2002 while closed circles are those from 2003. Values are means from ten shoots ± s.e.
Table 1.
Relationship between shoot growth and water content at full hydration (WCh, %) after comparison of the WCh values of the ‘growth’ and ‘no growth’ groups in the long and short branches of Lepidium subulatum and Linum suffruticosum by Mann–Whitney U-test or Student's t-test
| Species and Branches |
WCh (%), ‘growth’ |
WCh (%), ‘no growth’ |
U (or t) |
P |
||||
|---|---|---|---|---|---|---|---|---|
| Lepidium subulatum | ||||||||
| Long branches | 54·0 ± 0·7 | 50·3 ± 2·0 | 21·0 | 0·126 | ||||
| Short branches | 73·0 ± 0·3 | 60·6 ± 6·4 | 8·0 | 0·038 | ||||
| Linum suffruticosum | ||||||||
| Long branches | 65·7 ± 2·0 | 60·5 ± 0·4 | 10·0 | 0·008 | ||||
| Short branches | 68·6 ± 0·9 | 65·3 ± 0·8 | (−2·5) | 0·022 | ||||
Values of WCh are means ± s.e. U, Mann–Whitney U-statistic; t, Student's t-statistic (Linum short branches only).
DISCUSSION
Bud morphology
Linum and Lepidium bore naked buds with no specialized protective organs. In this type of bud, protection is given by the surrounding leaves of the shoot, which are arranged compactly around the meristem (Nitta and Ohsawa, 1998). Naked buds have been reported in other species of Mediterranean sub-shrubs, and also in several tree species from other climates (Hallé et al., 1978; Nitta and Ohsawa, 1998, 1999). However, within woody Mediterranean species, trees and shrubs normally bear cataphyllary or hipsophyllary buds (Hoffmann, 1972; Ginocchio and Montenegro, 1996), while naked buds are more frequent in sub-shrubs. This observation opens up questions as to the adaptive significance of naked buds and their phylogenetic origin. Some authors have interpreted these buds as less specialized structures than scaled ones (Puntieri et al., 2002a). Cataphylls and scales might have evolved from foliar structures (Goffinet and Larson, 1981) to increase protection under harsh environmental conditions (Nitta and Ohsawa, 1998; Puntieri et al., 2002a). However, this hypothesis does not explain the presence of naked buds in Mediterranean sub-shrubs, which are frequently exposed to harder environmental conditions than Mediterranean trees or shrubs (Shmida and Burgess, 1988; Orshan, 1989).
Shoot growth and organogenesis
The growth activity of Linum and Lepidium shoots varied widely throughout the year, following the strong seasonality imposed by the Mediterranean climate on gypsum substrates. Most vegetative and reproductive activities of these two species occurred in the favourable periods of spring and autumn, hence avoiding summer drought and winter cold. These results contrast with the observations of maintenance of short-branch growth for other sub-shrubs of semi-arid environments (Orshan, 1972). These discrepancies could be attributed to the different methodologies applied to assess shoot growth in the two studies. The visual estimations of shoot growth used by Orshan might be inappropriate to distinguish low autumn growth rates from the inactivity of winter and summer.
Branch growth is the result of two processes: the differentiation of organ primordia from meristems, i.e. organogenesis, and the extension of these primordia into fully developed organs (Champagnat et al., 1986; Puntieri et al., 2002b). In Lepidium and Linum, the timing of these two processes was uncoupled. Hence, most leaf primordia that were differentiated in autumn and winter expanded next spring, giving rise to the leaves of long branches. This phenomenon of leaf primordia accumulation is similar to the preformation of leaves reported in the winter buds of temperate woody species (Kozlowski and Clausen, 1966; Marks, 1975; Inouye, 1986). In these plants, organogenesis and expansion of leaf primordia are separated by a period that normally coincides with winter dormancy (Puntieri et al., 2002b). During dormancy, the activity of the meristem is normally arrested (Owens and Molder, 1973; Gregory, 1980; Jordy, 2004). However, in Lepidium and Linum organogenesis was protracted throughout the year, including autumn and winter, although the rate of primordia differentiation might vary throughout the year. Therefore, the period of time between organogenesis and extension of leaf primordia varied widely through the year. The leaves differentiated in autumn remained in an immature state for longer than those formed in early spring or summer.
Water content at full hydration and its relation to shoot growth processes
The capacity of Lepidium and Linum shoots to become fully hydrated was closely related to shoot expansion. This relationship could be attributed to the necessity of expanding cells to maintain an adequate turgor pressure during the growth process (Bradford and Hsiao, 1982). The mechanisms used by cells to maintain this pressure, such as increasing osmotic potential, will also lead to an increased capacity of the organ to reach high WCh values. In their study on the WCh of two co-occurring chaparral species, Davis and Mooney (1986) ascribed the high spring WCh values to increased organogenetical activity of shoots. However, in our study maximum values of Np preceded maximum values of both RGR and WCh, and hence the values of WCh paralleled better the RGR values than those of Np. Therefore, in the shoots of Lepidium and Linum, WCh may be more closely related to expansion growth than to organogenetical processes.
Shoot growth differences between Lepidium and Linum
In spite of the general similarities of Lepidium and Linum, these two species presented differences in the performance of their phenological and growth activities, which might explain their distinct ecological strategies. Lepidium finished flowering, fruiting and shoot expansion earlier than Linum, hence avoiding summer drought more efficiently. Furthermore, Lepidium dried most of its transpiring body during summer, while Linum maintained many of its green leaves alive. The capacity of Mediterranean sub-shrubs to dry out part of their transpiring biomass with the onset of summer has been interpreted as a strategy to reduce water loss during summer drought (Orshan and Zand, 1962; Orshan, 1963, 1972). However, this strategy might entail several drawbacks, like reduced nutrient retention efficiency (Reich and Borchet, 1982), or reduced net growth at the end of the growing season. Therefore, with the onset of autumn, Lepidium must rebuild more green biomass than Linum. This is accomplished by increasing relative growth rate or by extending shoot growth. Given the lower RGR of Lepidium shoots, this shrub can attain a similar shoot development to Linum by the end of autumn only by extending the period of autumn shoot growth. This might explain why Lepidium resumes autumn growth of its short branches in September, while this growth is resumed in October in Linum. In addition, Lepidium seems also to avoid potential freezing damage to newly initiated tissues more efficiently than Linum. While the short-branch growth of the former is arrested during winter (from January to March), the latter is prone to isolated pulses, as recorded in February 2004. Therefore, Lepidium seems to follow a stress-avoiding strategy, while Linum is more stress-tolerant (sensu Grime, 2001). This stress-tolerant strategy might explain the presence of Linum in the stressful gypsum outcrops.
Concluding remarks
The seasonality of the Mediterranean climate in gypsum outcrops leads to a similar phenology, bud morphology and similar patterns of WCh and bud and shoot growth in Lepidium subulatum and Linum suffruticosum. These two species bear naked buds and undergo shoot expansion during the favourable periods of spring and autumn. In both species, the reduced duration of the suitable period for long-branch expansion leads to an accumulation of leaf primordia in late winter. Organogenesis and expansion are uncoupled in both species, and the lapse of time between these two processes varies throughout the year, being greatest for leaf primordia initiated in autumn. Finally, long-branch expansion coincides with maximum WCh values in both species. This observation supports the hypothesis of a strong relationship between high WCh and shoot expansion.
Acknowledgments
We thank Dr Rubén Milla, Professor Nancy Dengler, Dr Javier Puntieri and an anonymous referee for their valuable comments on earlier versions of the manuscript, and the Diputación General de Aragón for granting permission to collect plant material for this study. Robin Rycroft edited the English. S. Palacio was funded by a FPU scholarship (AP-2001–2311, MECD, Spain). This study is part of the research projects REN2002–02635/GLO and P-024/2001 supported by the Ministerio de Educación y Ciencia (Spain) and the Regional Government of Aragón (Spain), respectively.
LITERATURE CITED
- Bradford KJ, Hsiao TC. 1982. Physiological responses to moderate water stress. In: Lange OR, Novel PS, Osmond CB, Ziegler H, eds. Encyclopaedia of plant physiology new series, volume 12B. Physiological plant ecology II. Berlin, Heidelberg: Springer-Verlag, 264–324. [Google Scholar]
- Braun-Blanquet J, Bolòs O. 1957.Les groupements végétaux du Bassin Moyen de l'Ebre et leur dynamisme, 1st edn. Zaragoza: Anales de la Estación Experimental de Aula Dei. [Google Scholar]
- Champagnat P, Barnola P, Lavarenne S. 1986. Quelques modalités de la croissance rythmique endogène des tiges chez les végétaux ligneux. Naturalia Monspeliensia (supplément no. h.s.): 279–302. [Google Scholar]
- Cottignies A. 1983. Teneur en eau et dormance dans le bourgeon de Frêne. Zeitschrift für Pflanzenphysiologie 111: 133–139. [Google Scholar]
- Cottignies A. 1990. Potentiel osmotique et potentiel hydrique du bourgeon terminal de Frêne, au cours du cycle annuel. Comptes rendus du l'Académie des Sciences, Paris, série III 310: 211–216. [Google Scholar]
- Davis SD, Mooney HA. 1986. Tissue water relations of four co-occurring chaparral shrubs. Oecologia 70: 527–535. [DOI] [PubMed] [Google Scholar]
- De Carvalho e Vasconcellos J. 1964. Lepidium. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA, eds. Flora Europaea, Vol. 1, Lycopodiaceae to Platanaceae. Cambridge: Cambridge University Press, 330–333. [Google Scholar]
- De Faÿ E, Vacher V, Humbert F. 2000. Water-related phenomena in winter buds and twigs of Picea abies L. (Karst.) until bud-burst: a biological, histological and NMR study. Annals of Botany 86: 1097–1107. [Google Scholar]
- De León A, Arriba A, De la Plaza MC. 1987.Caracterización agroclimática de la provincia de Zaragoza, 1st edn. Madrid: Dirección General de la Producción Agraria. Ministerio de Agricultura, Pesca y Alimentación. [Google Scholar]
- Essiamah S, Eschrich W. 1986. Water uptake of deciduous trees during winter and the role of conducting tissues in spring reactivation. IAWA Bulletin n.s. 7: 31–38. [Google Scholar]
- Ginocchio R, Montenegro G. 1996. On the structural organization of the renewal buds and their implication for the survival of embryonic structures in central Chilean matorral shrubs. Revista Chilena de Historia Natural 69: 171–181. [Google Scholar]
- Goffinet MC, Larson PR. 1981. Structural changes in Populus deltoides terminal buds and in the vascular transition zone of the stems during dormancy induction. American Journal of Botany 68: 118–129. [Google Scholar]
- Gray JT, Schlesinger WH. 1981. Biomass, production and litterfall in the coastal sage scrub of Southern California. American Journal of Botany 68: 24–33. [Google Scholar]
- Gregory RA. 1980. Annual cycle of shoot development in sugar maple. Canadian Journal of Forest Research 10: 316–326. [Google Scholar]
- Grime JP. 2001.Plant strategies, vegetation processes, and ecosystem properties. 2nd edn. Chichester, UK: John Wiley & Sons. [Google Scholar]
- Guerrero Campo J, Alberto F, Hodgson J, García Ruiz JM, Montserrat Martí G. 1999. Plant community patterns in a gypsum area of NE Spain. I. Interactions with topographic factors and soil erosion. Journal of Arid Environments 41: 401–410. [Google Scholar]
- Guerrero Campo J, Alberto F, Maestro Martínez M, Hodgson J, Montserrat Martí G. 1999. Plant community patterns in a gypsum area of NE Spain. II. Effects of ion washing on topographic distribution of vegetation. Journal of Arid Environments 41: 411–419. [Google Scholar]
- Hallé F, Oldeman RAA, Tomlinson PB. 1978.Tropical trees and forests. An architectural analysis, 1st edn. Berlin: Springer-Verlag. [Google Scholar]
- Hoffmann A. 1972. Morphology and histology of Trevoa trinervis (Rhamnaceae), a drought deciduous shrub from the Chilean matorral. Flora 161: 527–538. [Google Scholar]
- Hoffmann AJ, Hoffmann AE. 1976. Growth pattern and seasonal behaviour of buds of Colliguaya odorifera, a shrub from the Chilean Mediterranean vegetation. Canadian Journal of Botany 54: 1767–1774. [Google Scholar]
- Inouye DW. 1986. Long-term preformation of leaves and inflorescences by a long-lived perennial monocarp, Frasera speciosa (Gentianaceae). American Journal of Botany 73: 1535–1540. [Google Scholar]
- Jones MB, Laude HM. 1960. Relationships between sprouting in chamise and the physiological condition of the plant. Journal of Range Management 13: 210–214. [Google Scholar]
- Jordy MN. 2004. Seasonal variation of organogenetic activity and reserves allocation in the shoot apex of Pinus pinaster Ait. Annals of Botany 93: 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski TT, Clausen JJ. 1966. Shoot growth characteristics of heterophyllous woody plants. Canadian Journal of Botany 44: 827–843. [Google Scholar]
- Marks PL. 1975. On the relation between extension growth and successional status of deciduous trees of the northeastern United States. Bulletin of the Torrey Botanical Club 102: 172–177. [Google Scholar]
- Meloche CG, Diggle PK. 2001. Preformation, architectural complexity, and developmental flexibility in Acomastylis rossii (Rosaceae). American Journal of Botany 88: 980–991. [PubMed] [Google Scholar]
- Montserrat-Martí G, Palacio-Blasco S, Milla-Gutiérrez R. 2004. Fenología y características funcionales de las plantas leñosas mediterráneas. In: Valladares F ed. Ecología del bosque mediterráneo en un mundo cambiante. Madrid: Ministerio de Medio Ambiente, Organismo Autónomo Parques Nacionales, 129–162. [Google Scholar]
- Nelson DR, Harper KT. 1991. Site characteristics and habitat requirements of the endangered dwarf bear-claw poppy (Arctomecon humilis Coville, Papaveraceae). Great Basin Naturalist 2: 167–175. [Google Scholar]
- Nitta I, Ohsawa M. 1998. Bud structure and shoot architecture of canopy and understorey evergreen broad-leaved trees at their northern limit in East Asia. Annals of Botany 81: 115–129. [Google Scholar]
- Nitta I, Ohsawa M. 1999. Bud and module of evergreen broad-leaved trees in Anaga cloud forests. In: Ohsawa M, Wildpret W, del Arco M, eds. Anaga Cloud Forest. A comparative study on evergreen broad-leaved forests and trees of the Canary Islands and Japan. Chiba: Laboratory of Ecology, Chiba University, 139–146. [Google Scholar]
- Ockendon DJ, Walters SM. 1968. Linum. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA, eds. Flora Europaea, Vol. 2, Rosaceae to Umbelliferae. Cambridge: Cambridge University Press, 206–211. [Google Scholar]
- Orshan G. 1963. Seasonal dimorphism of desert and Mediterranean chamephytes and its significance as a factor in their water economy. In: Rutter AJ, Whitehead FH, eds. The water relations of plants. London: Blackwell Scientific Publications, 207–222. [Google Scholar]
- Orshan G. 1972. Morphological and physiological plasticity in relation to drought. In: Proceedings of the International Conference on Wildland Shrub Biology and Utilization. Utah: Utah State University, 245–254. [Google Scholar]
- Orshan G. 1989.Plant pheno-morphological studies in Mediterranean type ecosystems. 1st edn. Dordrecht: Kluwer Academic Publishers. [Google Scholar]
- Orshan G, Zand G. 1962. Seasonal body reduction of certain desert halfshrubs. Bulletin of the Research Council of Israel 11: 35–42. [Google Scholar]
- Owens JN, Molder M. 1973. Bud development in western hemlock. I. Annual growth cycle of vegetative buds. Canadian Journal of Botany 51: 2223–2231. [Google Scholar]
- Palacio-Blasco S, Milla R, Montserrat-Martí G. 2004. Renewal structures and shoot growth of three species of Mediterranean dwarf shrubs growing along an altitudinal gradient. In: Arianoutsou M, Papanastasis V, eds. Proceedings of the 10th MEDECOS Conference. Rhodes, Greece. Rotterdam, The Netherlands: Millpress, Science Publishers. [Google Scholar]
- Puntieri JG, Barthélémy D, Mazzini C, Brion C. 2002. Periods of organogenesis in shoots of Nothofagus dombeyi (Mirb.) oersted (Nothofagaceae). Annals of Botany 89: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntieri JG, Stecconi M, Barthélémy D. 2002. Preformation and neoformation in shoots of Nothofagus antarctica (G. Forster) Oerst. (Nothofagaceae) shrubs from northern Patagonia. Annals of Botany 89: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirantes J. 1977.Estudio sedimentológico y estratigráfico del Terciario continental de los Monegros, 1st edn. Zaragoza: Institución Fernando el Católico, CSIC. [Google Scholar]
- Reich PB, Borchet R. 1982. Phenology and ecophysioloy of the tropical tree, Tabebuia neochrysantha (Bignoniaceae). Ecology 63: 294–299. [Google Scholar]
- Rivas-Martínez S. 1987.Memoria del mapa de series de vegetación de España, 1st edn. Madrid: Ministerio de Agricultura, Pesca y Alimentación, ICONA. [Google Scholar]
- Rivas-Martínez S, Costa M. 1970. Comunidades gipsícolas del centro de España. Anales del Instituto Botánico Cavanilles 27: 193–224. [Google Scholar]
- Shmida A, Burgess L. 1988. Plant growth-form strategies and vegetation types in arid environments. In: Werger MJA, Aart PJMvd, During HJ, Verhoeven JTA, eds. Plant form and vegetation structure, 1st edn. The Hague: SPB Academic Publishers, 211–241. [Google Scholar]
- Tousignant D, Richer C, Rioux J-A, Brassard N, Mottard J-P. 2003. Vegetative propagation of sugar maple: relating stem water content and terminal bud developmental stage to adventitious rooting of stem cuttings. Canadian Journal of Plant Sciences 83: 859–867. [Google Scholar]