Abstract
• Background and Aims The differentiation of terminal latewood tracheids of silver fir (Abies alba) trees grown in Slovenia was investigated in autumn/winter 2001/2002.
• Methods The experimental trees were divided into three groups: one with narrow annual rings, width less than 1 mm; one with annual ring widths between 1 and 4 mm; and one group with broad rings larger than 4 mm. The differentiation of terminal latewood tracheids was investigated by light-, electron- and UV-microscopy in tissues sampled in October and November 2001 and March 2002.
• Key Results In the middle of October, cambial divisions did not occur any more in any of the trees. In trees with narrow annual rings, cell wall deposition as well as lignification were completed in terminal latewood tracheids at this date, whereas in trees with annual ring widths of more than 1 mm these processes still continued. Electron microscopy as well as UV microscopy revealed an unlignified inner S2 layer and the absence of S3 and warty layers. With increasing distance from the cambium, wall formation and lignification gradually appeared to be completed. Samples of all trees taken in the middle of November only contained differentiated terminal latewood tracheids. At the structural and lignin topochemical level, November and March samples showed completed differentiation of walls of terminal latewood tracheids.
• Conclusions In trees with broader annual rings, the final steps of differentiation of the youngest latewood tracheids near the cambium still continued during autumn, but were finished prior to winter. It was concluded from structural observations that duration of cambial activity is longer in trees with broad annual rings than in trees with narrow rings.
Keywords: Silver fir (Abies alba), latewood tracheids, cell wall structure, autumn differentiation, lignification, light microscopy, transmission electron microscopy, UV-microspectrophotometry
INTRODUCTION
Xylem formation is to a great degree regulated by genetic and environmental factors. The process of differentiation of tracheary elements and fibres can be divided into four successive stages: (1) postcambial enlargement, which determines cell dimensions and shape, (2) formation of multilayered cell walls, (3) deposition of lignin within the cell wall polysaccharide matrix, and (4) cell death and autolysis of the protoplasm (Wardrop, 1965; Denne and Dodd, 1981; Fujita and Harada, 1991; Savidge, 1996, 2000; Plomion et al., 2001; Wodzicki, 2001). In gymnosperms, more than 90 % of the newly formed cells differentiate into axially orientated tracheids responsible for both water conduction and mechanical support. At the end of the growing season the cambium becomes dormant, although its most recently produced derivatives continue differentiating for some time (Murmanis and Sachs, 1969; Nix and Villiers, 1985; Donaldson, 1991, 1992, 2001; Gindl et al., 2001; Gričar et al., 2003; Schmitt et al., 2003).
The aim of this study with fir trees (Abies alba) of the Dinaric region in Slovenia was to add information at the ultrastructural level on the final steps of cell wall differentiation. Special attention was paid to the question as to how long wall formation continues after cessation of cambial activity. Samples taken in the autumn and winter of 2001/2002 were examined by light- and electron-microscopy and for topochemical aspects of lignin distribution within cell walls by UV-microspectrophotometry.
MATERIAL AND METHODS
This study was conducted in a permanently monitored forest stand located at Ravnik/Slovenia (elevation 500–700 m), about 50 km south-west of Ljubljana (approx. 46°N, 14°E ). The site is in the Dinaric region and is typical of silver fir/beech forests in Slovenia. Samples were taken from ten apparently healthy silver fir (Abies alba Mill.) trees, 150–180 years old with a mean stem diameter at breast height of approx. 50 cm. Samples containing inner phloem, cambium and outer xylem (30 × 10 × 10 mm3) were taken from all trees at breast height on 17 October 2001 (early autumn) and 14 November 2001 (late autumn), and on 8 March 2002 (late winter). They were immediately immersed in FEA (formaldehyde–ethanol–acetic acid) and stored for 1 week in a refrigerator. For light microscopy, sample blocks were then dehydrated using a graded ethanol series. Transverse sections (20 μm thick) were prepared without further processing using a Leica SM 2000R sliding microtome and double-stained with safranin and astra blue. Sections were mounted on glass slides using Euparal and examined with a Nikon Eclipse 800 light microscope. Two October samples containing cambium and outer xylem were chosen for detailed ultrastructural analyses by means of transmission electron microscopy (TEM) and UV-microscopy. These samples were dehydrated in a graded series of ethanol and water-free acetone and embedded in Spurr's epoxy resin (Spurr, 1969). For TEM, ultrathin transverse sections (80–100 nm thick) were stained with potassium permanganate according to Donaldson (1992). Examination was carried out with a Philips CM 12 TEM at accelerating voltages of 40 or 60 kV to enhance contrast. For UV spectroscopic measurements, unstained transverse sections (1 μm thick) were prepared from the epoxy resin-embedded samples, transferred to quartz slides, immersed in a drop of non-UV absorbing glycerine and covered with quartz cover-slips. UV-absorbance spectra were recorded using a Zeiss UMSP 80 micro-spectrophotometer. The specimens were investigated by point measurements with a spot size of 1 μm2 using the programme LAMWIN® (Zeiss International). The spectra were taken at wavelengths from 240 to 400 nm in 2 nm steps. For each point analysis, the measurements were automatically repeated 50 times. Spectra from October samples were taken from the first, second, fourth, sixth, eighth and tenth cell row from the cambium. For the November and March samples, spectra were taken from the first and second cell row from the cambium. UV absorbance was determined for the outer (near compound middle lamella), middle and inner (near cell lumen) regions of tangential secondary cell walls.
RESULTS AND DISCUSSION
Light microscopy revealed that the cambium in all samples taken on 17 October 2001 consisted of only three to six cells with a very small radial diameter, indicating dormancy. In addition, no developing xylem cells in early stages of postcambial growth were present. Staining with safranin and astra blue revealed that in trees with an outermost annual ring comprising more than 100 cells radially (typically giving a ring width of more than 4 mm), more than five cells were still actively differentiating. Staining also indicated that the inner regions of their secondary walls were incompletely lignified and that cytoplasm was still present in the cell lumina (Fig. 1). Trees with an outermost annual ring composed of 20–100 cells per radial row (corresponding ring width of 1–4 mm) also contained differentiating tracheids adjacent to the cambium, although in most cases this was limited to one or two cells per radial row. However, in trees with a narrow outermost annual ring, i.e. fewer than 20 cells per radial row and a ring width less than 1 mm, differentiation of terminal latewood tracheids was complete. Generally, the number of incompletely differentiated tracheids per radial row was related to the width of the outermost annual ring (Table 1). Four weeks later, on 14 November 2001, the fact that the entire wall of the terminal latewood tracheids stained red indicated that the cells were completely differentiated and fully lignified in all trees (Fig. 1). Samples collected in March were similar to those collected in November in that they contained completely differentiated latewood tracheids with thickened and lignified secondary walls. From this it may be concluded that the differentiation of the terminal latewood tracheids was completed by the middle of November.
Fig. 1.
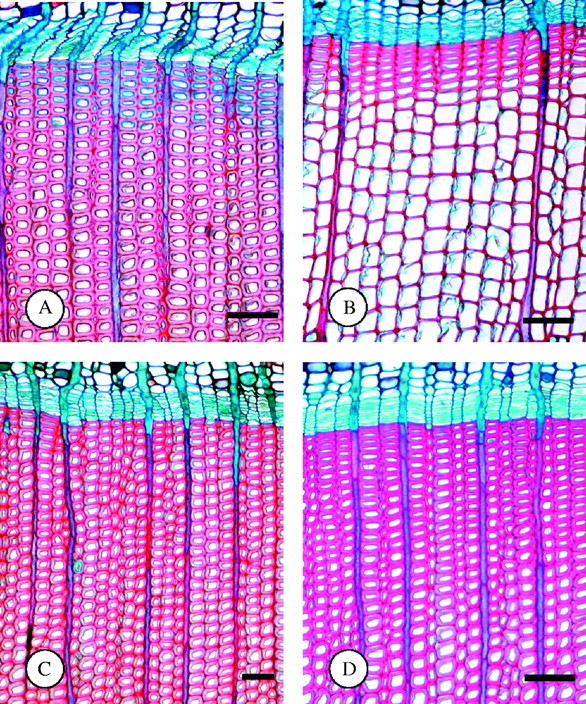
Light micrographs of transverse sections through cambium adjacent xylem. (A) Sampling on 17 October (early autumn): trees with wide annual growth rings show incompletely differentiated terminal latewood tracheids, as indicated by blue stained inner wall parts. (B) Sampling on 17 October: trees with narrow annual growth rings show completed differentiation of terminal latewood tracheids, as indicated by red stained cell walls. (C) Sampling on 14 November (late autumn): trees with wide annual growth rings show completed differentiation of last-formed tracheids, as indicated by red wall staining. (D) Sampling in March (late winter): no differences to November samples in trees with broader annual growth rings. Scale bars = 100 μm.
Table 1.
Relationship between total number of cells per radial row (total width) of the annual ring and number of incompletely differentiated latewood tracheids per radial row (width of incompletely differentiated tracheids) in silver fir trees in October
| Tree number |
Number of xylem cells per radial row in outer growth ring |
Number of incompletely differentiated xylem cells per radial row |
Width of annual growth ring (mm) |
Width of zone of incompletely differentiated xylem cells (μm) |
|---|---|---|---|---|
| 1 | 11·2 | 0·0 | 0·3 | 0·0 |
| 2 | 15·6 | 0·0 | 0·5 | 0·0 |
| 3 | 36·3 | 0·2 | 1·3 | 1·9 |
| 4 | 40·6 | 0·4 | 1·6 | 4·4 |
| 5 | 48·8 | 0·4 | 2·0 | 4·9 |
| 6 | 59·0 | 2·2 | 2·2 | 7·9 |
| 7 | 69·2 | 1·0 | 2·4 | 26·0 |
| 8 | 108·0 | 7·0 | 4·0 | 90·6 |
| 9 | 117·3 | 5·6 | 4·3 | 96·1 |
| 10 | 124·8 | 5·7 | 4·7 | 140·7 |
In all cases, number of measurements = 5.
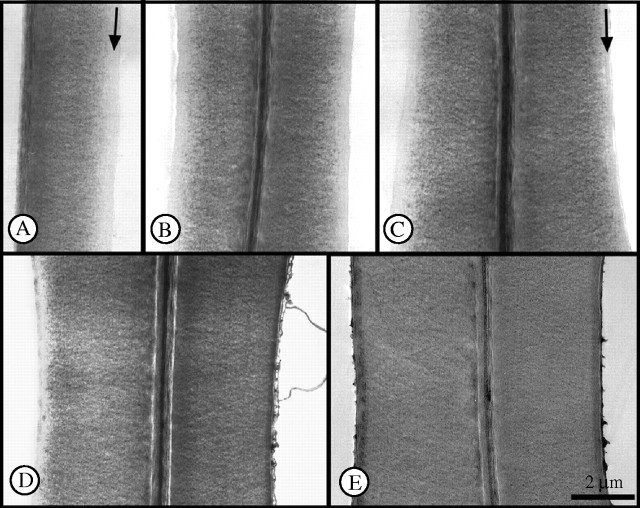
In recent years, potassium permanganate staining has been widely used in electron microscopy as an indicator for lignin distribution in different morphological regions of a woody cell wall (e.g. Maurer and Fengel, 1991; Donaldson, 1992; Singh and Donaldson, 1999; Schmitt and Melcher, 2004). In this study, sections stained with potassium permanganate revealed that the youngest latewood tracheids adjacent to the cambium of trees with broad annual rings were still in late stages of differentiation in the middle of October. Deposition of the polysaccharides of the S1 and S2 layers was evident in the cell walls, while the S3 and warty layer were not yet present. The compound middle lamella, S1 layer and outer S2 layer were stained with potassium permanganate, but staining decreased from the outer to the inner S2 layer (towards the lumen). From this it may be concluded that lignification had not yet taken place in the inner part of the S2 layer (Fig. 2). These results on the distribution of lignin within developing cell walls are in good agreement with the frequently described delay between polysaccharide deposition and lignin incorporation (Wardrop, 1965; Kutscha and Schwarzmann, 1975; Saka and Thomas, 1982; Terashima and Fukushima, 1988; Donaldson, 1991, 1992, 2001; Fukushima and Terashima, 1991; Terashima, 2000). In October samples, secondary wall formation appeared complete at a distance of about eight cells from the cambium. The S3 layer and the warty layer were clearly lignified, as demonstrated by their intense staining with potassium permanganate. By the middle of November, in all samples both secondary wall formation and lignification were completed also in the last-formed latewood tracheids. These observations confirm the conclusions drawn from light microscopy.
Fig. 2.
Electron micrographs of tangential tracheid walls of the outermost growth ring of a tree with broad rings; samples taken on 17 October. (A) Cambium-adjacent tracheid; inner S2 layer unlignified (arrow), S3 and warty layer not deposited. (B) Second (left) and third (right) tracheid row, inner S2 less lignified, S3 and warty layer not yet deposited. (C) Fourth (left) and fifth (right) tracheid row, fifth with S3 layer (arrow), S2 layer still less lignified. (D) Sixth (left) and seventh (right) tracheid row; sixth without warty layer, inner S2 and S3 less lignified; seventh with warty layer under formation, inner S2 and S3 lignified. (E) Tenth (left) and eleventh (right) tracheid row, both with differentiated walls and distinctly visible warty layer.
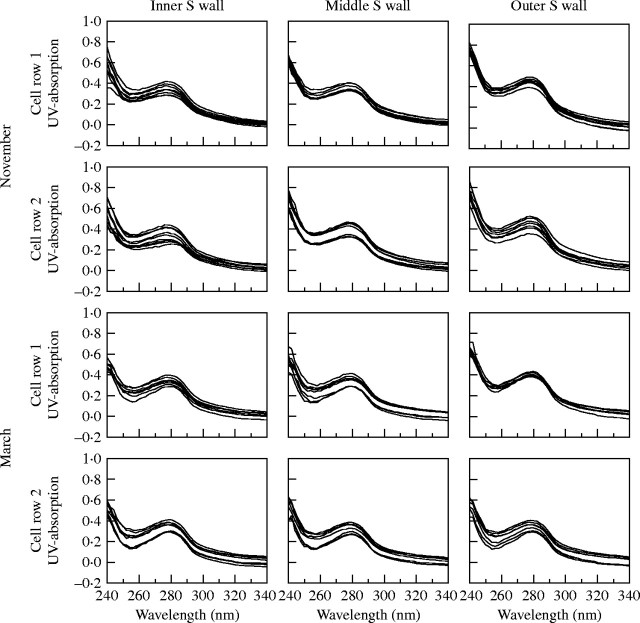
UV-microspectrophotometry was used for a semi-quantitative determination of lignin within individual cell wall layers. Softwood lignin is mainly composed of guaiacylpropane units and gives an absorption maximum at a wavelength of 280 nm, whereas hardwood lignin is a mixture of guaiacylpropane and syringylpropane units in varying ratios resulting in a shifted peak maximum towards 278–270 nm (Fergus and Goring, 1970; Fukazawa and Imagawa, 1981; Fengel and Wegener, 1989; Takabe et al., 1992; Koch and Kleist, 2001; Koch and Grünwald, 2004). In this study, UV microscopy of tangential secondary walls of differentiating latewood tracheids in trees with broad annual rings sampled in October showed an increasing absorbance at 280 nm with increasing distance from the cambium. This indicated lower lignin content in immature cell walls. Figure 3 shows representative spectra of developing secondary walls adjacent to the cambium and up to a distance of ten cell rows from it. In each tracheid, spectra were taken from the outer, middle and inner region of secondary walls. Absorbance values between log abs280nm 0·2 and 0·3 were recorded in the outer secondary walls of tracheids adjacent to the cambium. Structurally, such an outer secondary wall area represents S1 and outer S2. These values were lower than typically measured for the S2 layers of spruce tracheids (Koch and Kleist, 2001), where absorption is around log abs280nm 0·35 and 0·54. Extremely low absorption values between log abs280nm 0 and 0·2 were found in the middle region of secondary walls of the last-formed tracheids (mainly the central S2 layer). Spectra from the innermost secondary wall, mainly representing the inner S2 layer, showed an absorbance of less than log abs280nm 0·1. At about four cell rows from the cambium, the outer secondary wall reached an absorbance maximum of log abs280nm 0·35 to 0·45, and at about the sixth cell row the middle region showed a log abs280nm 0·3 to 0·4. The UV spectra taken from the inner secondary wall had their maximum in the eighth cell row with log abs280nm 0·3 to 0·4. It can be concluded from these measurements that in trees with broad annual rings, the differentiation of the latewood tracheids was not completed within eight cell rows from the cambium, although cambial activity had ceased. Corresponding absorbance values of spectra taken from November samples already showed maximum values for all three secondary wall regions in last-formed tracheids. This was also true for March samples (Fig. 4), clearly indicating that lignification was completed in the period between the middle of October and middle of November.
Fig. 3.
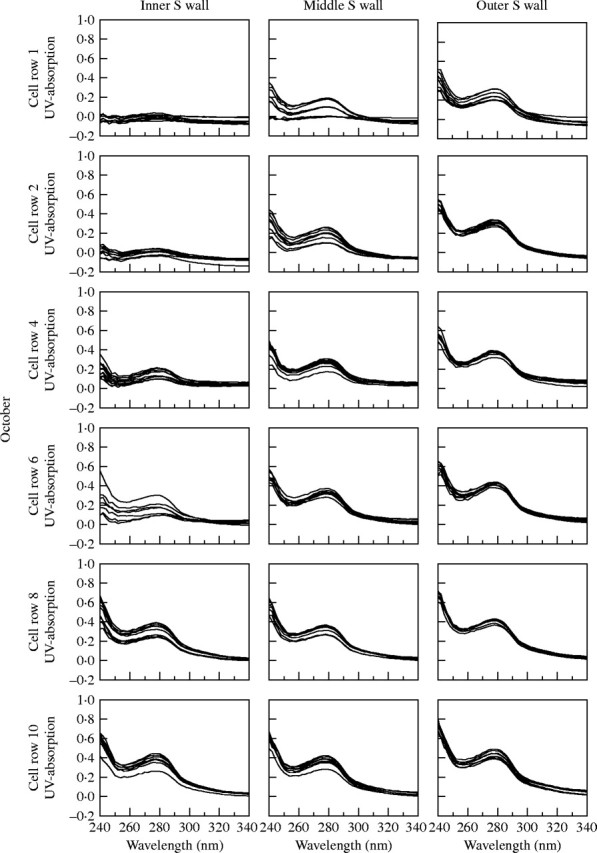
UV-absorption spectra measured in outer (near compound middle lamella), mid- and inner (near lumen) secondary wall of latewood tracheids. Absorption at wavelength 280 nm increases with increasing distance from the cambium in October.
Fig. 4.
UV-absorption spectra measured in outer (near compound middle lamella), mid- and inner (near lumen) secondary wall of latewood tracheids at the cambium in November and March. Absorption values at wavelength 280 nm are similar.
It has been shown by several authors that differentiation of secondary xylem produced late in the year is not completed simultaneously with the onset of cambium dormancy. Murmanis and Sachs (1969) investigated seasonal development of secondary xylem in Pinus strobus, and found cytoplasm still present in one-to-three tangential rows of latewood tracheids in samples taken in September, October and even in January. Nix and Villiers (1985) observed differentiation of the last-formed tracheids of loblolly (Pinus taeda) and slash (Pinus elliotti) pine during winter dormancy in South Carolina. Increasing wall thicknesses measured between November and March led them to conclude that the last-formed latewood cells of the current annual ring continued to deposit cell wall material until the following spring. Donaldson (1991) also reported incomplete lignification of secondary walls of latewood tracheids in Pinus radiata sampled in late autumn in New Zealand. In another study, Donaldson (1992) found that latewood was only partially lignified in most Pinus radiata trees sampled in late winter. Donaldson (2001) suggested from various observations that lignification is sensitive to environmental conditions, especially temperature, which seems to play an important role in slowing down this process. In support of our observations, Gindl et al. (2001) found the time course of cell wall thickening and lignification of tracheids in Norway spruce continued throughout the autumn. In the case of a valley-grown tree (elevation 580 m), cell wall thickening of latewood tracheids was completed on 20 October, but lignification was still taking place. However, at the end of the year, by 28 December, lignification of latewood tracheids was completed. All these observations confirm that lignification of latewood tracheids may be considerably delayed when compared with deposition of secondary wall polysaccharides.
In conclusion, the results obtained by light-, electron- and UV-microscopy for silver fir trees growing in mountainous regions of Slovenia clearly demonstrate that in early autumn (October) lignin deposition was still progressing in trees with broad annual rings. Similarly, we observed incompletely differentiated terminal latewood tracheids in trees with wide growth rings in the middle of October 1999 (Schmitt et al., 2003) and in the middle of October 2002 as well (J. Gričar, unpubl. res.). It is suggested that the date of completion of differentiation in the last-formed latewood tracheids is partly related to the duration of cambial activity. The cambium of more productive trees remains active longer and therefore produces more cells. On the other hand, the cambium of less productive trees stops earlier with its cell division activity leading to narrower annual rings. As a consequence of these differences, differentiation of tracheids laid down late in the season continues in October and perhaps even in the first days of November. Differentiation of the latewood tracheids was completed in all investigated trees before the middle of November.
Acknowledgments
The authors gratefully acknowledge the help of Martin Zupančič from the Department of Wood Science and Technology, University of Ljubljana, Prof. Dr. Jasna Štrus and her team from the Department of Biology, University of Ljubljana, and Tanja Potsch from the Institute for Wood Biology and Wood Protection, Federal Research Centre for Forestry and Forest Products, Hamburg. We are indebted to the Farmland and Forest Fund of the Republic of Slovenia, which enabled experimental work in the field. Financial support was obtained from the Ministry of Education, Science and Sport of the Republic of Slovenia.
LITERATURE CITED
- Denne MP, Dodd RS. 1981. The environmental control of xylem differentiation. In: Barnett JR, ed. Xylem cell development. Tunbridge Wells, UK: Castle House Publications Ltd, 236–255. [Google Scholar]
- Donaldson LA. 1991. Seasonal changes in lignin distribution during tracheid development in Pinus radiata Wood Science and Technology 25: 15–24. [Google Scholar]
- Donaldson LA. 1992. Lignin distribution during latewood formation in Pinus radiata IAWA Bulletin n.s. 12: 381–387. [Google Scholar]
- Donaldson LA. 2001. Lignification and lignin topochemistry—an ultrastructural view. Phytochemistry 57: 859–873. [DOI] [PubMed] [Google Scholar]
- Fengel D, Wegener G. 1989.Wood: chemistry, ultrastructure, reactions. Berlin: Walter de Gruyter. [Google Scholar]
- Fergus BJ, Goring DAI. 1970. The location of guaiacyl and syringyl lignins in birch xylem tissue. Holzforschung 24: 113–117. [Google Scholar]
- Fujita M, Harada H. 1991. Ultrastructure and formation of wood cell wall. In: David N, Hon S, Shiraishi N, eds. Wood and cellulosic chemistry. New York: Marcel Dekker Inc., 3–58. [Google Scholar]
- Fukazawa K, Imagawa H. 1981. Quantitative analysis of lignin using an UV microscopic image analyser. Variation within one growth increment. Wood Science and Technology 15: 45–55. [Google Scholar]
- Fukushima K, Terashima N. 1991. Heterogeneity in formation of lignin. XIV: formation and structure of lignin in differentiating xylem of Gingko biloba Holzforschung 45: 87–94. [Google Scholar]
- Gindl W, Grabner M, Wimmer R. 2001. Effects of altitude on tracheid differentiation and lignification of Norway spruce. Canadian Journal of Botany 79: 815–821. [Google Scholar]
- Gričar J, Straže A, Čufar K. 2003. Differentiaton of the last formed tracheids in wood of silver firs (Abies alba) having various cambial productivity. Zbornik gozdarstva in lesarstva (Research Reports Forestry and Wood Science and Technology) 70: 87–100. [Google Scholar]
- Koch G, Kleist G. 2001. Application of scanning UV microspectrophotometry to localise lignins and phenolic extractives in plant cell walls. Holzforschung 55: 563–567. [Google Scholar]
- Koch G, Grünwald C. 2004. Application of UV microspectrophotometry for the topochemical detection of lignin and phenolic extractives in wood fibre cell walls. In: Schmitt U, et al, eds. Wood fibre cell walls: methods to study their formation, structure and properties. Uppsala, Sweden: Swedish University of Agricultural Sciences, 119–130. [Google Scholar]
- Kutscha NP, Schwarzmann JM. 1975. The lignification sequence in normal wood of balzam fir. Holzforschung 29: 79–84. [Google Scholar]
- Maurer A, Fengel D. 1991. Elektronenmikroskopische Darstellung von strukturellen Einzelheiten in Nadelholz-Zellwänden anhand sehr dünner Ultramikrotomschnitte. Holz Roh-Werkstoff 49: 53–56. [Google Scholar]
- Murmanis L, Sachs I. 1969. Seasonal development of secondary xylem in Pinus strobus L. Wood Science and Technology 3: 177–193. [Google Scholar]
- Nix LE, Villiers K. 1985. Tracheid differentiation in southern pines during the dormant season. Wood and Fibre Science 17: 397–403. [Google Scholar]
- Plomion C, Leprovost G, Stokes A. 2001. Wood formation in trees. Plant Physiology 127: 1513–1523. [PMC free article] [PubMed] [Google Scholar]
- Saka S, Thomas RJ. 1982. A study of lignification in loblolly pine tracheids by the SEM-EDXA technique. Wood Science and Technology 16: 167–179. [Google Scholar]
- Savidge RA. 1996. Xylogenesis, genetic and environmental regulation—a review. IAWA Journal 17: 269–310. [Google Scholar]
- Savidge RA. 2000. Intristic regulation of cambial growth. Journal of Plant Growth Regulation 20: 52–77. [Google Scholar]
- Schmitt U, Grünwald C, Gričar J, Koch G, Čufar K. 2003. Wall structure of terminal latewood tracheids of healthy and declining silver fir trees in the Dinaric region, Slovenia. IAWA Journal 24: 41–51. [Google Scholar]
- Schmitt U, Melcher E. 2004. Section staining with potassium permanganate for transmission electron microscopy: a useful tool for lignin localisation. In: Schmitt U, et al, eds. Wood fibre cell walls: methods to study their formation, structure and properties. Uppsala, Sweden: Swedish University of Agricultural Sciences, 105–118. [Google Scholar]
- Singh AP, Donaldson LA. 1999. Ultrastructure of tracheid cell walls in radiata pine (Pinus radiata) mild compression wood. Canadian Journal of Botany 77: 32–40. [Google Scholar]
- Spurr AR. 1969. A low viscosity embedding medium for electron microscopy. Journal of Ultrastructural Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- Takabe K, Miyauchi S, Tsunoda R, Fukazawa K. 1992. Distribution of guaiacyl and syringyl lignins in Japanese beech (Fagus crenata): variation within an annual ring. IAWA Bulletin n.s. 13: 105–112. [Google Scholar]
- Terashima N. 2000. Formation and ultrastructure of lignified plant cell walls. In: Kim YS, ed. New horizons in wood anatomy. Proceedings of the 4th Pacific Regional Wood Anatomy Conference. Kwangju, Korea South: Chonnam National University Press, 169–180. [Google Scholar]
- Terashima N, Fukushima K. 1988. Heterogeneity in formation of lignin. XI. An autographic study of heterogeneous formation and structure of pine lignin. Wood Science and Technology 22: 259–270. [Google Scholar]
- Wardrop AB. 1965. Cellular diferentiation in xylem. In: Cote WA, ed. Cellular ultrastructure of woody plants Proceedings of the Advanced Science Seminar Pinebrook Conference. New York: Syracuse University Press, 61–97. [Google Scholar]
- Wodzicki TJ. 2001. Natural factors affecting wood structure. Wood Science and Technology 35: 5–26. [Google Scholar]