Abstract
• Background and Aims Respiration is an important component of plant carbon balance, but it remains uncertain how respiration will respond to increases in atmospheric carbon dioxide concentration, and there are few measurements of respiration for crop plants grown at elevated [CO2] under field conditions. The hypothesis that respiration of leaves of soybeans grown at elevated [CO2] is increased is tested; and the effects of photosynthesis and acclimation to temperature examined.
• Methods Net rates of carbon dioxide exchange were recorded every 10 min, 24 h per day for mature upper canopy leaves of soybeans grown in field plots at the current ambient [CO2] and at ambient plus 350 µmol mol−1 [CO2] in open top chambers. Measurements were made on pairs of leaves from both [CO2] treatments on a total of 16 d during the middle of the growing seasons of two years.
• Key Results Elevated [CO2] increased daytime net carbon dioxide fixation rates per unit of leaf area by an average of 48 %, but had no effect on night-time respiration expressed per unit of area, which averaged 53 mmol m−2 d−1 (1·4 µmol m−2 s−1) for both the ambient and elevated [CO2] treatments. Leaf dry mass per unit of area was increased on average by 23 % by elevated [CO2], and respiration per unit of mass was significantly lower at elevated [CO2]. Respiration increased by a factor of 2·5 between 18 and 26 °C average night temperature, for both [CO2] treatments.
• Conclusions These results do not support predictions that elevated [CO2] would increase respiration per unit of area by increasing photosynthesis or by increasing leaf mass per unit of area, nor the idea that acclimation of respiration to temperature would be rapid enough to make dark respiration insensitive to variation in temperature between nights.
Keywords: Glycine max Merrill., carbon dioxide, respiration, temperature, acclimation
INTRODUCTION
Respiration is an important component of plant carbon balance, with rapidly growing plants daily losing about one third of the carbon fixed in photosynthesis (McCree and Amthor, 1982). Predicting how plant carbon balance may be affected by the rising concentration of carbon dioxide in the atmosphere ([Ca]) is of importance both from a global carbon budget perspective and in predicting plant growth. While there are many measurements of responses of photosynthesis of plants to simulated increases in [Ca], and biochemical models that often provide a reasonable approximation of the observed photosynthetic responses, how respiration will respond to increases in [Ca] remains uncertain (Drake et al., 1999; Gonzalez-Meler et al., 2004).
Rising [Ca] is expected to increase leaf respiration in C3 species by increasing photosynthesis (Amthor, 2000), because increased photosynthesis at elevated [CO2] usually results in the accumulation of large amounts of non-structural carbohydrates, and these provide the respiratory substrates. Small carbohydrate concentrations have been found to decrease rates of dark respiration (e.g. Azcon-Bieto and Osmond, 1983; Hrubec et al., 1985; Noguchiand Terashima, 1997; Grimmer and Komor, 1999), but this is generally only apparent near the end of the night, when carbohydrates are depleted (Mullen and Koller, 1988; Verklei and Challa, 1988). Because photosynthetic rates of mature leaves greatly exceed the plant's capacity to utilize carbohydrates (except when stresses strongly limit photosynthesis), higher photosynthesis at elevated [CO2] also requires increased export of carbohydrates, which requires energy supplied by respiration. The energy required for translocation may represent a significant component of that produced by respiration of mature leaves (Boumaet al., 1995; Noguchi et al., 2001). In this study, respiration rates were measured on field-grown plants at the growth [CO2], to avoid complications of potential short-term effects of measurement [CO2] on respiration rates (Drakeet al., 1999) and to maintain normal plant source–sink balance. It was hypothesized that growth at elevated [CO2] would increase rates of respiration per unit of area by increasing photosynthesis.
There have been numerous measurements of respiration of plants grown at ambient and elevated [CO2] in controlled environment chambers (e.g. Gifford et al., 1985; Poorter et al., 1988; Bunce and Caulfield, 1991; Baker et al., 1992; Thomas et al., 1993; Grimmer and Komor, 1999; Sakai et al., 2001), including soybean (e.g. Thomas and Griffin, 1994; Bunce, 1995; Bunce and Ziska, 1996; Griffin et al., 2001b), but diverse responses have been found. In most studies in controlled environment chambers, light and temperature were kept constant from day to day and often during days; the relevance of these observations to respiration rates under field conditions is uncertain. One purpose of this study was to determine if the responses of respiration of soybean leaves to elevated [CO2] in the field were similar to those found under controlled environment conditions. While leaf respiration of a few tree species grown outdoors at ambient and elevated [CO2] has been studied (e.g. Wullschleger and Norby, 1992; Wullschleger et al., 1992; Mitchell et al., 1995; Kellomaki and Wang, 1998; Jach and Ceulemans, 2000; Hamilton et al., 2001; Griffin et al., 2001a; Zha et al., 2001), there are few measurements of respiration of crop plants grown at elevated [CO2] under field conditions. In summarizing responses for woody plants, Curtis and Wang (1998) found that elevated [CO2] reduced respiration rates per unit of mass, although that conclusion has been questioned (Gonzalez-Meler et al., 2004). Many of the studies on tree species have focused on expanding leaves to allow separation of growth and maintenance respiration, and therefore provide only limited data on responses of mature leaves, which in most systems will contribute the majority of leaf respiration. This study was restricted to fully expanded leaves.
Respiration of leaves often acclimates to temperature, such that after prolonged exposure to a range of temperatures, rates of respiration become nearly or completely independent of the exposure temperature (Atkin et al., 2001). The rate of acclimation of respiration relative to the rate of change in temperature would control rates of respiration in natural environments. However, there have been few studies concerning rates of acclimation of respiration to temperature. Atkin et al. (2001) found that acclimation in eucalyptus was sufficiently rapid that rates of respiration per unit of mass were relatively independent of seasonal changes in night temperature. In two deciduous tree species, leaf respiration acclimated within a day or two to large changes in temperature (Bolstad et al., 2003).
While measurements of whole-plant respiration rates would be desirable from a modelling or productivity perspective, experimental separation of plant from soil respiration remains problematic, and with regard to temperature acclimation, roots and shoots do not experience the same temperatures in the field. Therefore, the more specific hypotheses concerning responses of respiration to [CO2] and temperature were addressed at the single-leaf rather than at the whole-plant level. Both Atkin et al. (2001) and Griffin et al. (2002) found that leaf respiration rates differed depending on whether diurnal changes in temperature occurred for the whole shoot or just for the measured leaf, and emphasized that accurate estimates of leaf respiration required that natural temperature patterns occurred for the whole shoot. Therefore, in this study, the measured leaf was held at the ambient air temperature to which the whole shoot was exposed. It was hypothesized that acclimation of respiration to temperature in leaves of soybean would be sufficiently rapid that day-to-day variation in night-time temperature would not affect respiration rates.
MATERIALS AND METHODS
With the recognized need to conduct respiration measurements throughout whole dark periods, and to include nights with a range of temperatures and prior photosynthesis, it was considered more important to have replication over dates than to measure multiple leaves per [CO2] treatment on a given night. The potential drawback of this sampling design is that if the [CO2] treatment effects on respiration were strongly affected by temperature or daily photosynthesis, it might be difficult to detect [CO2] treatment effects using replication over time. The advantage is that any [CO2] treatment effects detected would be relatively robust against variation in temperature, plant age, daily photosynthesis, and time of day. Furthermore, the data could be used to examine effects of temperature and prior photosynthesis on respiration more effectively than if measurements were confined to a few dates.
Soybeans, Glycine max Merrill. ‘Kent’ were grown at the South Farm of the Beltsville Agricultural Research Center in open top chambers, as previously described (Bunce and Sicher, 2001). Two chambers were flushed with ambient air, and two were flushed with ambient air to which pure carbon dioxide was added at a rate sufficient to increase the concentration to 350 ± 50 µmol mol−1 above that of outside air. The [CO2] of the ambient air averaged 370 µmol mol−1 during the day and 450 µmol mol−1 at night during this study. Soybeans were planted in June of 2000 and 2003 in rows 30 cm apart and thinned to a final density of 25 plants m−2. Net carbon dioxide exchange rate measurements were made on a total of 16 d between July 18 and September 11. All plants were in the flowering to early pod-filling stages, and were producing new leaves throughout the measurement period. Thus all leaves measured were approximately the same age (about 2 weeks from unfolding) and none were senescent.
Net rates of carbon dioxide exchange were recorded every 10 min for 24 h for mature upper canopy leaves for one leaf from each [CO2] treatment on each measurement date. Leaf cuvettes were made of clear acrylic, with water jackets on upper and lower surfaces, and contained a mixing fan. Entire terminal leaflets of mature upper canopy leaves were placed in the cuvettes and sealed around the petiole with caulk. Thermocouples were pressed against the undersides of leaflets to measure temperature, and a quantum sensor was mounted beside the leaflets inside each cuvette. Leaves were held nearly horizontal, and were not shaded by other leaves. Water kept at the temperature of the outside air was circulated through the water jackets of the cuvettes. Each cuvette was used as part of an open gas exchange system. A gas blending system provided air at 370 and 720 µmol mol−1 [CO2] to the two cuvettes. The dew point of air entering the cuvettes was controlled to a few degrees below the expected minimum night temperature in order to prevent condensation in spite of transpiration. Flow rates of air entering the cuvettes were measured using a mass flow meter. The difference in [CO2] between air streams entering and leaving each cuvette and the flow rate entering each cuvette was determined by a differential CO2 analyser (Li-6252, LI-Cor, Lincoln, Nebraska) and a mass flow meter switched sequentially between cuvette airstreams. The airstreams were dried before entering the differential CO2 analyser. The sensitivity of the differential analyser was corrected for the absolute [CO2], which was measured by an absolute CO2 analyser (LI-6252). The zero drift of the differential analyser was automatically checked after each individual measurement, and calibration of the differential analyser at each background [CO2] was checked daily. Similar leaves of randomly selected plants from either of two chambers per [CO2] treatment were placed in the cuvettes early in the morning, and harvested to determine area and dry mass 24 h later. The rate of respiration of each leaf was summed for the entire night. ‘Night’ was defined as the time when the photosynthetic photon flux density was less than 5 µmol m−2 s−1.
Statistical analysis
Using the leaves measured at ambient and elevated [CO2] on the same day, paired t-tests were used to test for effects of [CO2] treatment on photosynthesis and respiration rates per unit of leaf area, leaf mass per unit of area, and respiration rates per unit of mass. Linear regressions were used to test for relationships between daily photosynthesis and daily PAR, and between respiration and daily photosynthesis. Linear and exponential regressions were used to test for relationships between respiration and average night-time temperature. Multiple regressions were used to test for effects of daily photosynthesis on respiration after accounting for the response of respiration to temperature. Separate regressions were developed for the two [CO2] treatments. When significant correlations between physiological parameters and environmental factors occurred, analysis of covariance was used to test for [CO2] effects, using the environmental factor as a covariable. Statistical tests were implemented using JMP v. 5, SAS Institute, Cary, NC.
RESULTS
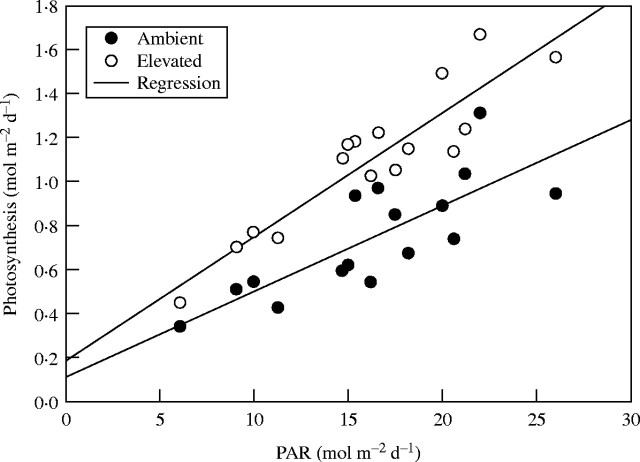
A typical daily pattern of net carbon dioxide exchange rate (NCE) is shown in Fig. 1 for a mostly clear day. Most of the scatter in NCE is in the daytime, and is related to intermittent cloudiness. NCE was higher at elevated [CO2] throughout the daytime, although the difference was much less at low than at high PPFD. Averaged over all 16 measurement days, the elevated [CO2] increased daytime NCE per unit of leaf area by an average of 48 % (Table 1). Daily photosynthesis per unit of area increased linearly with daily PAR for both [CO2] treatments, and was greater at elevated [CO2] at all values of daily PAR (Fig. 2). Respiration expressed per unit of area did not differ significantly between [CO2] treatments (Table 1). Because leaf dry mass per unit of area was increased by an average of 23 % by elevated [CO2], from 34 to 42 g m−2, respiration per unit of mass was significantly lower at elevated [CO2] (Table 1). There was no significant correlation between respiration and daily photosynthesis at either [CO2] (not shown).
Fig. 1.
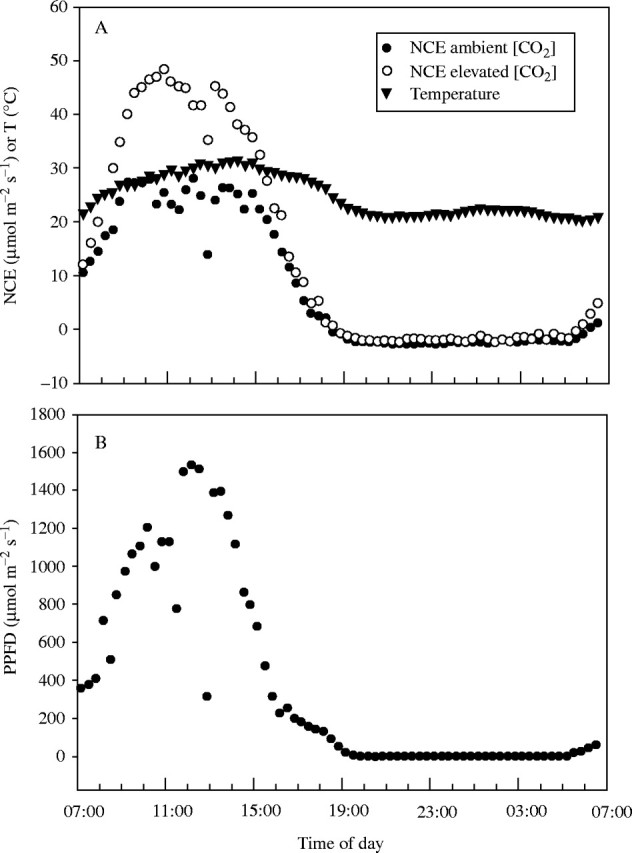
(A) Rates of net carbon dioxide exchange and leaf temperature, and (B) PPFD for a 24 h period beginning on day 221, 2000, for soybeans leaves grown and measured at ambient and ambient +350 μmol mol−1 [CO2]. Leaf temperature and PPFD are averaged across leaves, for clarity.
Table 1.
Mean values and ranges of daily photosynthesis and respiration of soybean leaves grown and measured at ambient and elevated [CO2]
| Ambient |
+350 µmol mol−1 |
||||||
|---|---|---|---|---|---|---|---|
| Variable |
Mean |
Range |
Mean |
Range |
Probability |
||
| Photosynthesis per area (mmol m−2 d−1) | 745 | 34–1310 | 1103 | 45–1670 | 0·001 | ||
| Respiration per area (mmol m−2 d−1) | 53 | 22–87 | 53 | 23–75 | 0·751 | ||
| Respiration per mass (mmol g−1 d−1) | 1·55 | 0·63–2·81 | 1·25 | 0·51–2·24 | 0·002 | ||
Means are for 24 h measurements on 16 days over two years, with a pair of leaves measured at both [CO2] on a given day. Ranges indicate day-to-day differences in average values. ‘Probability’ is the probability of obtaining a larger t-value under the hypothesis of no [CO2] treatment effect, using paired t-tests.
Fig. 2.
Relationships between daily integrals of photosynthesis and the integral of photosynthetically active radiation on different measurement dates, for leaves of soybean grown and measured at ambient and ambient +350 μmol mol−1 [CO2]. Linear regressions are shown for each [CO2] treatment, and had r2 values of 0·62 and 0·84 for ambient and elevated [CO2], respectively.
Respiration increased with average night temperature, increasing by a factor of 2·5 between 18 and 26 °C for both carbon dioxide treatments (Fig. 3). Exponential and linear regressions gave nearly the same r2 values (Fig. 3), with higher r2 values for respiration rates per unit of mass than for rates per unit of area. Analysis of covariance using temperature as a covariable indicated significantly lower respiration per unit of mass at elevated [CO2] (not shown) as did the paired t-test (Table 1), with no differences for respiration rates per unit of area.
Fig. 3.
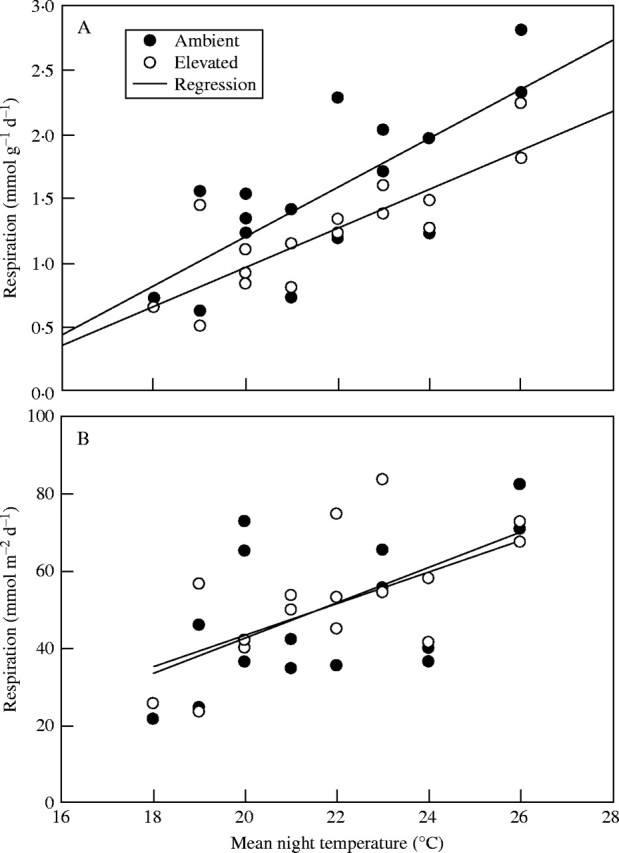
Relationships between daily integrals of respiration per unit of mass (A) and per unit of area (B), and mean night-time temperature on different measurement dates, for soybeans leaves grown and measured at ambient and ambient +350 μmol mol−1 [CO2]. For respiration rates per unit of mass, linear regressions had r2 values of 0·57 and 0·69 for ambient and elevated [CO2], respectively, and exponential relationships had r2 values of 0·59 and 0·68 for ambient and elevated [CO2], respectively. For respiration rates per unit of area, linear regressions had r2 values of 0·27 and 0·50 for ambient and elevated [CO2], respectively, and exponential relationships had r2 values of 0·27 and 0·51, respectively.
Seasonal patterns of mean daily temperature are shown in Fig. 4, with the dates of NCE measurement indicated. Three of the measurement dates (day of year 214 and 221 in 2000 and day of year 218 in 2003) followed at least two days when mean temperatures were consistently substantially above average, and three measurement dates (day of year 208 and 234 in 2000 and day of year 255 in 2003) followed lower than normal temperatures (Fig. 4). These measurement dates were examined for evidence of acclimation of respiration to temperature by determining whether respiration rates on those dates were consistently higher or lower than predicted by linear regressions relating respiration to temperature with those six dates excluded. If warmer temperatures resulted in acclimation of respiration to a lower rate for a given temperature, then respiration rates measured after above average temperatures would be lower than predicted from the regression. Similarly, exposure to cooler temperatures for a few days would result in respiration rates higher than predicted from the regression. This did not occur for either the higher or lower temperatures, for either [CO2] treatment, as rates for those dates were nearly identical to the rates predicted by the regressions, which excluded those dates (Table 2).
Fig. 4.
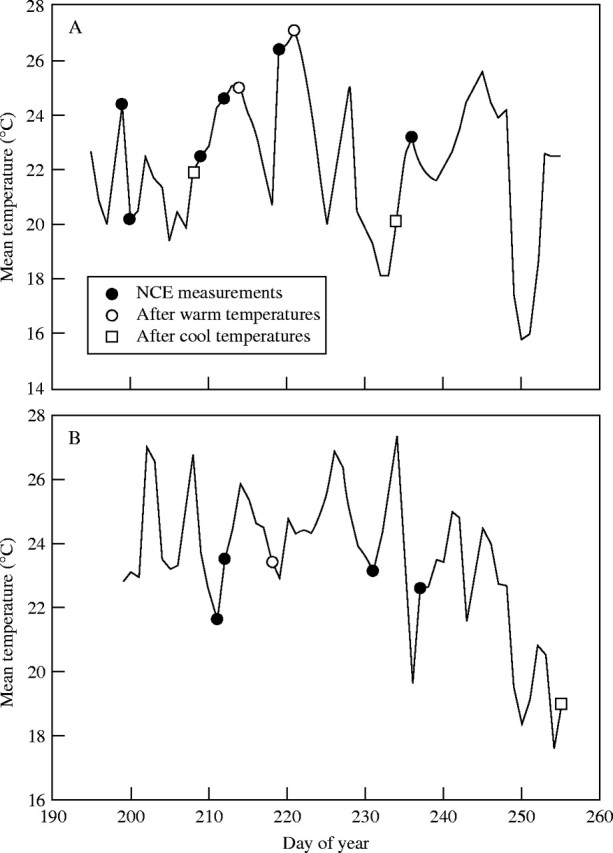
Mean daily air temperatures for (A) 2000 and (B) 2003 at the South Farm of the Beltsville Agricultural Research Center, Beltsville, Maryland. Symbols indicate dates when leaf net carbon dioxide exchange was measured. Measurement dates when previous temperatures had been warmer or cooler than average are indicated by special symbols.
Table 2.
Respiration of soybeans leaves grown and measured at ambient and ambient +350 μmol mol−1 [CO2] for six measurement dates which followed at least two days when mean temperatures were consistently substantially above or below average
| Following higher than average temperatures |
Following lower than average temperatures |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ambient [CO2] |
Elevated [CO2] |
Ambient [CO2] |
Elevated [CO2] |
||||||||||
| Actual |
Predicted |
Actual |
Predicted |
Actual |
Predicted |
Actual |
Predicted |
||||||
| 1·74 | 1·82 | 1·37 | 1·43 | 1·13 | 1·13 | 0·93 | 0·94 | ||||||
Actual rates were compared with rates predicted from the regressions relating respiration to temperature with the data for those six dates excluded. Each value is a mean for three measurement dates identified in the text. All values are mmol g−1 d−1.
Despite 2·5 fold ranges in daily photosynthesis between days for both carbon dioxide regimes, caused by cloudiness, multiple regression did not detect significant relationships (at P = 0·05) between daily photosynthesis and respiration expressed per unit of mass (Table 3) or per unit of area (not shown) for either carbon dioxide treatment, when temperature was also included in the regression.
Table 3.
Multiple linear regressions relating respiration rate per unit of mass to temperature and photosynthesis of the previous day for soybean leaves grown and measured at ambient and ambient +350 μmol mol−1 [CO2]
| Ambient |
Ambient + 350 µmol mol−1 |
|||||
|---|---|---|---|---|---|---|
| Parameter |
Coefficient |
Probability |
Coefficient |
Probability |
||
| Intercept | −2·52 ± 1·10 | 0·039 | −1·76 ± 0·61 | 0·017 | ||
| Temperature | 0·182 ± 0·061 | 0·011 | 0·119 ± 0·035 | 0·004 | ||
| Photosynthesis | 0·139 ± 0·573 | 0·812 | 0·358 ± 0·262 | 0·195 | ||
DISCUSSION
The equal rates of respiration per unit of area and lower rates per unit of mass for plants grown and measured at elevated [CO2] in this study are in agreement with one study of soybean leaf respiration in controlled environment chambers (Griffin et al., 2001b). Two other studies have found lower rates per unit of area at elevated [CO2] (Bunce, 1995; Griffin et al., 1999) and one found higher rates (Thomas and Griffin, 1994). Rates per unit of mass have either been lower or not different at elevated [CO2] in these studies. The lack of higher rates of respiration per unit of area at elevated [CO2] in this study contradicts the hypothesis that higher photosynthetic rates would increase respiration by increasing translocation rates, or by providing more non-structural carbohydrates as substrates for respiration. Of these, only higher photosynthesis was actually measured in this study, but increased photosynthesis must result in either more rapid translocation of carbohydrates or an increased content of carbohydrates, or both. It is conceivable that more rapid translocation at elevated [CO2] occurred during the day but not at night. However, this seems unlikely, because both Grimmer and Komor (1999) and Grodzinski et al. (1998) found that elevated [CO2] did not increase daytime translocation rates in C3 species. Furthermore, elevated [CO2] increased daytime starch and sucrose per unit of area by 86 % and 67 %, respectively, in another study using the same variety of soybeans in the same location (Bunce and Sicher, 2001).
A compensating reduction in maintenance respiration in leaves grown at elevated [CO2] would be a possible explanation for the lack increase in respiration despite increased photosynthesis. Lower maintenance respiration at elevated [CO2] has been suggested by a few other studies. In some cases lower maintenance respiration in leaves grown at elevated [CO2] could be attributed to lower protein content (e.g. Baker et al., 1992; Wullschleger et al., 1992; Drake et al., 1996; Kellomaki and Wang, 1998), but in other cases [CO2] treatment effects on leaf protein content were either non-existent or insufficient to explain the observed lower respiration rates (e.g. Bunce, 1995; Jach and Ceulemans, 2000; Griffin et al., 2001a). In the present study it is unlikely that growth at elevated [CO2] affected nitrogen content, because previous studies with this variety of soybean under similar conditions found no reduction in chlorophyll, ribulose bisphosphate carboxylase, or soluble protein per unit of area at elevated [CO2] (Bunce and Sicher, 2001). Bunce (1995) and Griffin et al. (2001b) suggested that more mitochondrial respiration and maintenance processes might be shifted to the light period at elevated [CO2]. However, because recent work has questioned the interpretation of estimates of daytime respiration (Pinelli and Loreto, 2003), this question is difficult to address experimentally. Clearly, growth at elevated [CO2] reduced respiration in these mature soybean leaves compared with our expectations based on current understanding of respiration.
In this study, there was no detectable effect on respiration rates of even the 2·5 fold range of daily photosynthesis caused by cloudiness. Whitehead et al. (2004) found a dependence of respiration on daily photosynthesis in oak when comparing fully exposed upper canopy leaves with leaves deliberately shaded or lower in the canopy, but such a relationship was not evident for day-to-day variation in daily photosynthesis among fully exposed upper leaves. Thus, even within a [CO2] treatment, relationships between respiration and daily photosynthesis are not always evident. When large changes in daily photosynthesis due to cloudiness have no detectable effect on respiration rates, perhaps it is not surprising that the smaller changes in photosynthesis caused by the elevated [CO2] treatment also did not increase respiration.
The results indicating a large response of respiration rate averaged over the night to the mean temperature of the night do not support the idea that acclimation of respiration to temperature would be rapid enough to make dark respiration insensitive to variation in temperature between nights. In fact, the 2·5 fold change in respiration for the 8 °C range in mean night-time temperatures is at least as large as that typically observed for short-term temperature changes (reviewed in Tjolker et al., 2001). Neither the rate of acclimation of respiration to temperature, nor the signal controlling acclimation (e.g. night-time temperature, daily mean temperature, carbohydrate status or demand for respiration) is known for soybean, or for any species. The observed response of respiration to night temperature might indicate either a slow rate of acclimation or an inconsistent environmental cue. The latter is quite likely, because, at this time of year in this climate, there is often little correlation between the mean temperature of a given night and the temperature of the preceding day. In this data set, for example, the mean temperature of a given night was correlated with the prior 24 h mean temperature, and with the prior night temperature, with r2 values of less than 0·25. However, even in examining days proceeded by at least two days with temperatures consistently above or below average, there was no evidence of acclimation of respiration to temperature. It is also possible that soybean has only limited potential for acclimation of respiration to temperature, since none was found in soybean cotyledons by Gonzalez-Meler et al. (1999). Possibly, acclimation of respiration to temperature in soybean is slower than in trees (Bolstad et al., 1993). For whatever reason, night-to-night variation in mean temperature strongly affected respiration rates of mature soybean leaves in the field.
In summary, the hypothesis that respiration rates would be increased by growth at elevated [CO2], and the hypothesis that rates would be independent of night-time temperature were both rejected. The results for soybean were consistent with the generalization developed for woody species that growth at elevated [CO2] does not affect leaf respiration rates per unit of area, but decreases respiration per unit of leaf mass by about 20 % (Curtis and Wang, 1998), in spite of increased photosynthesis. No influence of day-to-day variation in light or daily photosynthesis on respiration was detected, and respiration did not acclimate to variation in mean night temperature.
LITERATURE CITED
- Amthor JS. 2000. The McCree–de Wit-Penning de Vries-Thornley respiration paradigms: 30 years later. Annals of Botany 86: 1–20. [Google Scholar]
- Atkin OA, Holly C, Ball MC. 2001. Acclimation of snow gum (Eucalyptus pauciflora) leaf respiration to seasonal and diurnal variations in temperature: the importance of changes in the capacity and temperature sensitivity of respiration. Plant, Cell and Environment 23: 15–26. [Google Scholar]
- Azcon-Bieto J, Osmond CB. 1983. Relationship between photosynthesis and respiration. The effect of carbohydrate status on the rate of CO2 production by respiration in darkened and illuminated wheat leaves. Plant Physiology 71: 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JT, Laugel F, Boote KJ, Pickering NB. 1992. Effects of daytime carbon dioxide concentration on dark respiration of rice. Plant, Cell and Environment 15: 231–239. [Google Scholar]
- Bolstad PV, Reich P, Lee T. 2003. Rapid temperature acclimation of leaf respiration rates in Quercus alba and Quercus rubra Tree Physiology 23: 969–976. [DOI] [PubMed] [Google Scholar]
- Bouma TJ, De Visser R, Van Leeuwen H, De Kock MJ, Lambers H. 1995. The respiratory energy requirements involved in nocturnal carbohydrate export from starch-storing mature source leaves and their contribution to leaf dark respiration. Journal of Experimental Botany 46: 1185–1194. [Google Scholar]
- Bunce JA. 1995. The effect of carbon dioxide concentration on respiration of growing and mature soybean leaves. Plant, Cell and Environment 18: 575–581. [Google Scholar]
- Bunce JA, Caulfield F. 1991. Reduced respiratory carbon dioxide efflux during growth at elevated carbon dioxide in three herbaceous perennial species. Annals of Botany 67: 325–330. [Google Scholar]
- Bunce JA, Sicher RC. 2001. Water stress and day-to-day variation in apparent photosynthetic acclimation of field-grown soybeans to elevated carbon dioxide concentration. Photosynthetica 39: 95–101. [Google Scholar]
- Bunce JA, Ziska LH 1996. Responses of respiration to increases in carbon dioxide concentration and temperature in three soybean cultivars. Annals of Botany 77: 507–514. [Google Scholar]
- Curtis PS, Wang X. 1998. A meta-analysis of elevated CO2 effects on woody plant mass, form and physiology. Oecologia 113: 299–313. [DOI] [PubMed] [Google Scholar]
- Drake BG, Azcon-Bieto J, Berry J, Bunce J, Dijkstra J, Farrar J, Gifford RM, Gonzalez-Meler MA, Kock G, Lambers H. 1999. Does elevated atomspheric CO2 concentration inhibit mitrochondrial respiration in green plants? Plant, Cell and Environment 22: 649–657. [Google Scholar]
- Drake BG, Muehe MS, Peresta G, Gonzalez-Meler MA, Matamala R. 1996. Acclimation of photosynthesis, respiration and ecosystem carbon flux of a wetland on Chesapeake Bay, Maryland to elevated atmospheric CO2 concentration. Plant and Soil 187: 111–118. [Google Scholar]
- Gifford RM, Lambers H, Morison JIL. 1985. Respiration of crop species under CO2 enrichment. Physiologia Plantarum 63: 351–356. [Google Scholar]
- Gonzalez-Meler MA, Ribas-Carbo M, Giles L, Siedow JN. 1999. The effect of growth and measurement temperature on the activity of the alternative respiratory pathway. Plant Physiology 120: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Meler MA, Taneva L, Trueman RJ. 2004. Plant respiration and elevated atmospheric CO2 concentration: cellular responses and global significance. Annals of Botany 94: 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin KL, Tissue DT, Turnbull MH, Schuster W, Whitehead DW. 2001. Leaf dark respiration as a function of canopy position in Nothofagus fusca trees grown at ambient and elevated CO2 partial pressures for 5 years. Functional Ecology 15: 497–505. [Google Scholar]
- Griffin KL, Anderson OR, Gastrich MD, Lewis JD, Lin G, Schuster W, Seemann JR, Tissue DT, Turnbull MH, Whitehead D. 2001. Plant growth in elevated CO2 alters mitochondrial number and chloroplast fine structure. Proceedings of the National Academy of Science 98: 2473–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin KL, Sims DA, Seeman JR. 1999. Altered night-time CO2 concentration affects the growth, physiology and biochemistry of soybean. Plant, Cell and Environment 22: 91–99. [Google Scholar]
- Griffin KL, Turnbull M, Murthy R, Lin G, Adams J, Farnsworth B, Madato T, Bazin G, Potasnak M, Berry JA. 2002. Leaf respiration is differentially affected by leaf vs. stand-level nighttime warming. Global Change Biology 8: 479–485. [Google Scholar]
- Grimmer C, Komor E. 1999. Assimilate export by leaves of Ricinus communis L. growing under normal and elevated carbon dioxide concentrations: the same rate during the day, a different rate at night. Planta 209: 275–281. [DOI] [PubMed] [Google Scholar]
- Grodzinski B, Jiao J, Leonardos ED. 1998. Estimating photosynthesis and concurrent export rates in C3 and C4 species at ambient and elevated CO2 Plant Physiology 117: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JG, Thomas RB, DeLucia EL. 2001. Direct and indirect effects of elevated CO2 on leaf respiration in a forest ecosystem. Plant, Cell and Environment 24: 975–982. [Google Scholar]
- Hrubec TC, Robinson JM, Donaldson RP. 1985. Effects of CO2 enrichment and carbohydrate content on the dark respiration of soybeans. Plant Physiology 79: 684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jach ME, Ceulemans R. 2000. Short- versus long-term effects of elevated CO2 on nighttime respiration of needles of Scots pine (Pinus sylvestrus L.). Photosynthetica 38: 57–67. [Google Scholar]
- Kellomaki S, Wang KY. 1998. Growth, respiration and nitrogen content in needles of Scots pine exposed to elevated ozone and carbon dioxide in the field. Environmental Pollution 101: 263–274. [DOI] [PubMed] [Google Scholar]
- McCree KJ, Amthor ME. 1982. Effects of diurnal variation in temperature on the carbon balances of white clover plants. Crop Science 22: 822–827. [Google Scholar]
- Mitchell RJ, Runion GB, Prior SA, Rogers HH, Amthor JS, Henning FP. 1995. Effects of nitrogen on Pinus palustris foliar respiratory responses to elevated atmospheric carbon dioxide concentration. Journal of Experimental Botany 46: 1561–1567. [Google Scholar]
- Mullen JA, Koller HR. 1988. Trends in carbohydrate depletion, respiratory carbon loss, and assimilate export from soybean leaves at night. Plant Physiology 86: 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Terashima I. 1997. Different regulation of leaf respiration between Spinacia oleracea, a sun species, and Alocasia odore, a shade species. Physiologia Plantarum 101: 1–7. [Google Scholar]
- Noguchi K, Go C-S, Miyazawa S-I, Terashima I, Ueda S, Yoshinari T. 2001. Costs of protein turnover and carbohydrate export in leaves of sun and shade species. Australian Journal of Plant Physiology 28: 37–47. [Google Scholar]
- Pinelli P, Loreto F. 2003.12CO2 emission from different metabolic pathways measured in illuminated and darkened C3 and C4 leaves at low, atmospheric and elevated CO2 concentration. Journal of Experimental Botany 54: 1761–1769. [DOI] [PubMed] [Google Scholar]
- Poorter H, Pot S, Lambers H. 1988. The effect of an elevated atmospheric CO2 concentration on growth, photosynthesis and respiration of Plantago major Physiologia Plantarum 73: 553–589. [Google Scholar]
- Sakai H, Yagi K, Kobayashi K, Kawashima S. 2001. Rice carbon balance under elevated CO2 New Phytologist 150: 241–249. [Google Scholar]
- Thomas RB, Griffin KL. 1994. Direct and indirect effects of atmospheric carbon dioxide nenrichment of leaf respiraiton of Glycine max (L.) Merr. Plant Physiology 104: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RB, Reid CD, Ybema R, Strain BR. 1993. Growth and maintenance components of leaf respiration of cotton grown in elevated carbon dioxide partial pressure. Plant, Cell and Environment 16: 539–546. [Google Scholar]
- Tjolker MG, Oleksyn J, Reich PB. 2001. Modeling respiration of vegetation: evidence for a general temperature-dependent Q(10). Global Change Biology 7: 223–230. [Google Scholar]
- Verkleij FN, Challa H. 1988. Diurnal export and carbon economy in an expanding source leaf of cucumber at contrasting source and sink temperature. Physiologia Plantarum 74: 284–293. [Google Scholar]
- Whitehead D, Griffin KL, Turnbull MH, Tissue DT, Engel VC, Brown KJ, Schuster WSF, Walcroft AS. 2004. Response of total night-time respiration to difference in total daily photosynthesis for leaves in a Quercus rubra L. Canopy: implications for modelling canopy CO2 exchange. Global Change Biology 10: 925–938. [Google Scholar]
- Wullschleger SD, Norby RJ. 1992. Respiratory cost of leaf growth and maintenance in white oak saplings exposed to atmospheric CO2 enrichment. Canadian Journal of Forest Research 22: 1717–1721. [Google Scholar]
- Wullschleger SD, Norby RJ, Gunderson CA. 1992. Growth and maintenance respiration in leaves of Liriodendron tulipifera L. Exposed to long-term carbon dioxide enrichment in the field. New Phytologist 121: 515–523. [Google Scholar]
- Zha T, Ryyppo A, Wang K-Y, Kellomaki S. 2001. Effects of elevated carbon dioxide concentration and temperature on needle growth, respiration and carbohydrate status in field-grown Scots pines during the needle expansion period. Tree Physiology 21: 1279–1287. [DOI] [PubMed] [Google Scholar]