Abstract
• Background In angiosperms the seed is the outcome of double fertilization, a process leading to the formation of the embryo and the endosperm. The development of the two seed compartments goes through three main phases: polarization, differentiation of the main tissues and organs and maturation.
• Scope This review focuses on the maize kernel as a model system for developmental and genetic studies of seed development in angiosperms. An overview of what is known about the genetic and molecular aspects underlying embryo and endosperm formation and maturation is presented. The role played by embryonic meristems in laying down the plant architecture is discussed. The acquisition of the different endosperm domains are presented together with the use of molecular markers available for the detection of these domains. Finally the role of programmed cell death in embryo and endosperm development is considered.
• Conclusions The sequence of events occurring in the developing maize seed appears to be strictly regulated. Proper seed development requires the co-ordinated expression of embryo and endosperm genes and relies on the interaction between the two seed components and between the seed and the maternal tissues. Mutant analysis is instrumental in unravelling the genetic control underlying the formation of each compartment as well as the molecular signals interplaying between the two compartments.
Keywords: Zea mays, seed, mutants, development, embryo, embryogenesis, SAM, RAM, embryo maturation, endosperm, endosperm domains, PCD
INTRODUCTION
Seeds are a crucial step in the plant life cycle, representing the initiation of a new sporophytic generation. A seed has to achieve successful embryo development and germination, and it has to integrate embryo and seedling development with the environment. Angiosperm seeds undergo desiccation and dormancy. The maintenance of a viable embryo in this condition has required the development of some remarkable mechanisms, among which is the accumulation of osmoprotectants and other solutes, i.e. sugars and proteins. Seeds have also evolved protection against biotic stresses. To this purpose, they contain proteins with anti-fungal properties or molecules that discourage insects or other animals from eating the seeds or seedlings. Thus seeds represent a highly successful adaptation that ensures the survival and spread of higher plants.
Seeds are important for human life as the main means of propagation of crop species and as a source of food, feed and raw material. Recent renewed interest in seed biology has led to a better knowledge of the mechanisms underlying seed development, which is a prerequisite for new biotechnological as well as conventional breeding procedures.
Genetic analysis as a means of investigating the molecular mechanisms involved in seed development
Genetic analysis provides the opportunity to perform the dissection of complex developmental processes. In particular, monogenic mutants are instrumental in identifying single steps of these processes, as well as their molecular basis. Maize is well suited for this purpose since many mutants, induced by chemical or transpositional mutagenesis, are available and the two basic compartments of the seed, the embryo and the endosperm, are large structures easily amenable to experimental analysis. Mutants exhibiting small or incompletely developed endosperms and embryos or, less frequently, impaired in their endosperm development but with normal embryos, are generally defined as dek (defective kernel) (Neuffer and Sheridan, 1980; Scanlon et al., 1994). emp (empty pericarp) mutants represent the class of dek mutants with the most severe reduction in endosperm development. They are easily recognizable in segregating mature ears because they are devoid of endosperm material and are flattened by compression from the surrounding normal seeds (Scanlon et al., 1994; Fu et al., 2002).
Large numbers of mutants exhibiting phenotypes suggestive of specific defects in embryogenesis have been isolated and analysed (Clark and Sheridan, 1991; Heckel et al., 1999; Elster et al., 2000; Consonni et al., 2003). These mutants, characterized by impaired or arrested embryo development but normal endosperm, are commonly classified as emb (embryo-specific).
This review focuses on the maize kernel as a model for developmental and genetic studies of seed development in angiosperms. It presents an overview of what is known on the genetic and molecular aspects underlying embryo and endosperm formation and maturation. Specific attention is given to the role of the programmed cell death (PCD) process in embryo and endosperm development. The concerted development of the two seed components as well as the interactions between seed and maternal tissues are fundamental for proper seed development. These aspects are analysed in the light of recent data.
MAIZE SEED AND ITS ORIGIN
The two seed components
The maize seed comprises two major compartments, the embryo and the endosperm, both originating from the double fertilization events. The embryogenetic process allows the formation of a well-differentiated embryonic axis, surrounded by a single massive cotyledon, the scutellum. At maturity the embryo axis comprises all tissues that will give rise to the seedling structure. It consists of root and shoot primordia, which are located at opposite poles and of a stem with five or six internodes bearing a leaf at each node. The growth of the embryo occurs inside the endosperm. A great variation in endosperm size is observed within angiosperms. In a number of angiosperm species, mostly dicots, the endosperm is a transient structure that may be absorbed in the later stages of seed development. In most cereal species, the endosperm constitutes the bulk of the seed and persists at maturity. It comprises specific regions containing reserve proteins, complex carbohydrates and oils.
In maize, as in all flowering plants, the seed develops inside a coat of maternal origin. The pericarp, an adhering tissue deriving from the ovary wall, forms the outer covering of the kernel. A thin membrane deriving from the outer epidermal wall of the nucellus persists and forms a continuous layer between the pericarp and the endosperm (for a detailed description of maize seed anatomy, see Kiesselbach, 1949).
Embryo and endosperm origin
A century ago, Nawaschin (1898) in Russia and Guignard (1899) in France independently illustrated the origin of the seed from the double fertilization event, which is unique among living organisms. This process is achieved by two genetically identical sperm cells present in a single pollen tube. Upon fertilization the two generative sperm nuclei are released into the female gametophyte. The nucleus of one sperm fuses with the egg and produces the diploid embryo while the nucleus of a second sperm fuses with the polar nuclei of the embryo sac and gives rise to the triploid endosperm. The term ‘double fertilization’ implies that the endosperm originates from a sexual event. However, the triple fusion nucleus does not enter a process that results in an organism, but in a tissue with a closed developmental programme. The endosperm's evolutionary origin is still controversial (reviewed by Friedman, 1998, 2001). A widely accepted view states that the endosperm is derived from a supernumerary embryo that became an embryo-nourishing structure. Alternatively it is proposed that it represents a female gametophyte that was later sexualized.
The acquisition of double fertilization is considered a fundamental component of the evolutionary success of angiosperms (Stebbins, 1974). This success relies on two factors. The first one is the presence of the endosperm itself. In gymnosperms, the enlarged female gametophyte functions as a nutritive tissue. In angiosperms, the endosperm is a highly specialized tissue with nutritive function. The second factor is the establishment of specific ratios between maternal and paternal genomes in the embryo and in the endosperm, respectively. Disruption of this balance can lead to seed abortion. Embryo and endosperm are genetically identical, except for ploidy level. The embryo is diploid and the endosperm is triploid, so the ratio between the maternal and the paternal contribution differs in each compartment. In maize, the mutation ig (indeterminate gametophyte) provided a tool to manipulate the ploidy level (Lin, 1984). From this study, evidence was obtained that what is critical for proper seed development is not the overall ploidy level, but the relative contribution from the male and female parents. A 2:1 ratio of maternal to paternal genomes is necessary for proper endosperm development and, as a consequence, for correct seed development.
Moreover, angiosperm seeds develop within an ovary (whereas gymnosperms produce ‘naked’ seed). Thus the three seed components, embryo, endosperm and seed coat, are genetically distinct. It is believed that the ontogeny of the seed relies on the interplay of different genome dosages (Lopes and Larkins, 1993).
EMBRYOGENESIS
The zygote, one of the two outcomes of double fertilization, divides repeatedly to become an embryo. During this process it goes through different morphologically distinguishable stages corresponding to the acquisition and elaboration of new functions (for a detailed description of these phases, see Clark, 1996). Following repeated rounds of cell division, the embryo acquires a globular structure with radial symmetry; it then develops bilateral symmetry and goes through an intensive morphogenetic programme leading to the elaboration of a root and a shoot primordium at the two poles of the embryonic axis. At the end of this period the embryo initiates a maturation phase. This transition is correlated with an increase in abscisic acid (ABA) content and acquisition of desiccation tolerance.
A widely accepted view holds that embryogenesis proceeds through modules or domains, a concept originating from the analysis of mutants that disrupt the embryonic developmental pattern in arabidopsis (Meyer et al., 1991). In an alternative view, embryogenesis is visualized not as an isolated event but as the first step of a continuous developmental process interrupted by a stage of quiescence and resuming with germination (Kaplan and Cooke, 1997). However, efforts to isolate genes strictly expressed during embryogenesis (emb), i.e. exhibiting aberrant embryo development without deleterious effects on endosperm development, have unexpectedly led to the identification of genes controlling more general and fundamental plant processes (Meinke, 1995; Magnard et al., 2003; Ma and Dooner, 2004). It should be noted that the definition of the emb mutants as specifically affecting the embryo is not completely appropriate, since expression in tissues other than the seed has not been feasible because of their early lethality. This difficulty could be overcome by clonal analysis of mutant tissues induced in leaves of heterozygous +/emb seedlings following X-ray treatment to seeds, as recently reported with empty pericarp2 (emp2), a lethal mutant causing almost total suppression of endosperm formation (Fu and Scanlon, 2004). The analysis of emb mutants indicates that most frequently the embryo arrest takes place early in embryogenesis at the proembryo, transition or coleoptilar stage. A common feature emerging from histological analysis is that suppression of morphogenesis is accompanied by an uncontrolled pattern of cell division (Consonni et al., 2003). A link between defective cell division and suppression of morphogenesis has been also reported as the possible reason for the lack of pattern formation in arabidopsis seed mutants (Sollner et al., 2002).
Shoot and root apical meristems
The morphogenetic potential of the embryo is mediated by two meristematic regions, the shoot and root apical meristems, referred to as SAM and RAM, respectively, which are formed at the opposite poles of the embryo. They represent the sites at which organs are initiated and the patterns of the shoot and root systems are established (Fletcher, 2002). Contrary to what has been observed in arabidopsis, few mutants suppressing SAM formation have been reported in maize. The shootless mutant phenotype recently reported by Pilu et al. (2002) is the result of the interaction of two independent gene mutants. Genetic redundancy in the control of the establishment of the SAM could explain why mutants disrupting the SAM have escaped observation so far. Once formed, the SAM appears to be regulated by transcriptional activators of the homeobox gene family. Knotted1 (kn1) was the first homeobox gene to be identified in plants (Vollbrecht et al., 1991). The dominant mutant knotted phenotype consists of ‘knots’ on the leaf surface, representing groups of cells along the veins that continue to divide. By selecting for the loss of dominant mutant phenotype, Kerstetter et al. (1997) isolated recessive alleles of the gene, causing a reduced capacity for the self-renewal of meristematic cell populations. A recently isolated loss-of-function kn1 allele revealed a novel embryonic shoot phenotype resulting in plants that arrested as seedlings and are referred to as ‘limited shoot’ (Vollbrecht et al., 2000). Penetrance of this phenotype is background-dependent and correlates with meristem size. The variable background-dependent phenotype of this allele could be explained by assuming that the loss of kn1 function is compensated by the activity of a duplicated locus or by related class 1 knox genes or unrelated genes, again implying genetic redundancy in the control of SAM establishment or maintenance, as was suggested for the shootless phenotype previously mentioned.
Analysis of kn1 expression in the shoot meristem indicates that kn1 is down-regulated in a region where the first leaf primordium will initiate. Thus kn1 is likely to be playing a role in maintenance of the morphogenetic zone of the SAM (Vollbrecht et al., 2000). KN1 is a member of a class of related homeodomain (KNOX) proteins in maize that are expressed in shoot meristems and not in leaves (Reiser et al., 2000). Their corresponding genes are referred to as class 1 knotted-like homeobox (knox). They are expressed in SAMs and not in lateral organ primordia, an observation suggesting a mechanism of negative regulation in the meristem prior to organ initiation. Several genes have been identified that repress knox gene expression in leaf primordia, such as rough sheath2 (rs2), narrow sheath (ns) and leaf bladeless (lbl) (Reiser et al., 2000).
Some evidence is available that these genes act in a separate pathway to restrict knox gene expression in the leaf primordia (Schneeberger et al., 1998). Recently a new mutant was described and named semaphore 1 (sem1) (Scanlon et al., 2002). Its gene product is required for the negative regulation of a subset of maize knox genes. KNOX down-regulation is normal in the founder cells of sem1 mutant leaves and only in the later primordial stages of leaf development is KNOX accumulation observed. This suggests that SEMAPHORE is required to maintain knox transcriptional repression during later stages of lateral organ development, whereas the initial down-regulation of KNOX accumulation in young leaf primordia is controlled by separate gene functions (Scanlon et al., 2002).
Less is known on the downstream targets of KNOX function. Recent evidence, however, suggests that growth regulators may mediate KNOX activity in arabidopsis (Hay et al., 2002). In maize, the observation that both rs2 and sem1 mutants are correlated with defective auxin transport in the shoot provides further evidence that there is a link between ectopic knox gene expression and defective regulation of hormone concentrations in the plant shoot (Hay et al., 2002).
The RAM generates cells above the centre to make the main body and below it to make the root cap. There is a group of non-dividing cells representing the quiescent center (QC) surrounded by the mitotically active cells. The QC appears to function as an ‘organizer’ of the root architecture, inhibiting the differentiation of surrounding initial cells and regulating tissue-specific gene expression by means of cell-to-cell communication (Ponce et al., 2000). The unravelling of the RAM organization could be achieved through an analysis of mutants affecting the root apparatus. Unfortunately the detection of single gene mutants is made difficult by the fact that roots are not easily amenable to phenotypic screening, they are greatly affected in their architecture by environmental changes and several root traits are polygenically controlled. Despite these difficulties, the application of screening systems specifically devised for their detection has led to the isolation of several mutants that influence root architecture and that are transmitted as single-gene mutants. They can be grouped into four classes based on their influence on shoot-borne roots, lateral roots, root elongation and root hairs (Hochholdinger et al., 2004). At the molecular level, evidence has been obtained for genes specifically involved in root formation. Examples include several glycine-rich putative cell wall proteins that are produced in the columella and epidermis of the root tip (Ponce et al., 2000). It is expected that the availability of such mutants and similar ones impairing the SAM, as well as the advent of new techniques allowing the isolation of specific cell types, such as laser capture microdissection, together with transcriptome analysis, will allow deeper knowledge on the origin of meristems to be gained.
The embryonic maturation phase
Maturation, beginning when embryos cease cell division and start growing by cell enlargement, is characterized by the deposition of storage products and the acquisition of desiccation tolerance along with water removal from the maturing seed. During this phase there is an increased production of ABA that is involved in the control of the accumulation of storage proteins, desiccation tolerance and germination. ABA then returns to lower levels in the dry seed. The embryo then enters into a resting stage known as quiescence in which metabolic activity is suppressed. The role played by ABA has been established through the characterization of mutants with a reduced level of endogenous ABA or insensitive to ABA.
Those interested in the genetics of the late embryogenetic phase and germination are referred to excellent reviews covering this topic (McCarty, 1995; Finkelstein et al., 2002).
SPECIFICATION OF ENDOSPERM CELLS AND ENDOSPERM DOMAIN FORMATION
The endosperm of maize is a nuclear-type endosperm. In this type of endosperm, which is the most common in cereals, development starts with several rounds of divisions of the triploid nucleus without cytokinesis (Olsen, 2001). The primary endosperm nucleus is positioned at the micropylar end of the embryo sac and the first mitosis occurs in a plane perpendicular to the longitudinal axis of the embryo sac. At this stage, basal (chalazal) and distal (micropylar) domains are already distinguishable. Succeeding divisions occur synchronously and following a precise pattern. The resulting eight nuclei are ordered in a single plane at the chalazal pole of the endosperm. After this stage, the centrally placed nuclei migrate to the periphery of the syncytium where they continue to proliferate. Nuclei are regularly distributed in the cytoplasm that surrounds the central part of the cell, which is occupied by a large vacuole. Depending on genotype, 256–512 nuclei are produced by continued and synchronous divisions (Walbot, 1994; Olsen, 2004). By 3 d after pollination (DAP), cellularization begins following a precise scheme of divisions, in which nuclei at the periphery become cellularized and a new layer of syncitial nuclei is formed toward the centre of the endosperm (Walbot, 1994; Olsen, 2004). Cellularization continues in a centripetal manner until the endosperm becomes fully cellular. This period, between 8 and 12 DAP, is the most rapid period of endosperm growth.
The patterns of cell divisions have been traced back by sector analysis. The waxy (Wx) locus controls the accumulation of amylose, and excision of the Ac transposon from Wx generates sectors that can be visualized by iodine staining. Sector analysis illustrates that early divisions establish the left and right half of the endosperm, and later divisions generate conical sectors (McClintock, 1978).
Cell divisions cease in the central region of the endosperm after 12 DAP, whereas in the subaleurone region they continue until approx. 20 DAP. At this stage nuclei of the central region begin to endoreduplicate their DNA. Endoreduplication extends from the crown to the basal transfer cells. The number of endocycles and degree of endoreduplications depends on the different genotypes (Larkins et al., 2001). It is generally believed that endoreduplications provide high levels of gene expression in a tissue where intense gene activity is required and where there are strong limitations in terms of space and of time. Alternatively, Leiva-Neto et al. (2004) proposed that endoreduplication in the maize endosperm functions primarily to provide a store of nucleotides during embryogenesis and/or germination.
Mutants with suppressed endoreduplication have not yet been isolated. dek mutants have often been considered good candidates for genes involved in the mitotic and endoreduplication cell cycle. In this context, the study of 35 dek mutants has revealed a reduced level of endoreduplication in all cases except one (Kowles et al., 1992). Their molecular analysis should allow the characterization of defects in the mechanism controlling endoreduplication.
Description of different domains at the cellular level
Specification of different cell types inside the endosperm leads to the formation of four distinct domains. The largest part of endosperm is the central part, consisting of the starchy endosperm, a tissue made of large vacuolized cells in which starch and proteins accumulate. Storage product accumulation begins in this region around 14 DAP and continues until desiccation at seed maturity (after 40 DAP). In the latest phases, starchy endosperm development is characterized by DNA endoreduplication and programmed cell death. This latter process will be discussed in a separate section.
The aleurone is the outer layer of the endosperm and accumulates proteins and oil to high concentrations. The boundary between these two tissues is delimited by the first periclinal cell divisions that give rise to the external aleurone initials and the internal starchy initials. Aleurone expansion is then achieved by anticlinal divisions (Walbot, 1994). In maize, unlike other cereals, the aleurone is composed of a single layer of cells that appear small and isodiametric. Aleurone cells remain viable and, following hormone stimulation from the embryo, they will synthesize hydrolytic enzymes to mobilize storage products during germination.
At the posterior or chalazal pole, the aleurone layer is replaced by the basal endosperm transfer layer (BETL) that forms the interface between the sporophytic and the seed tissues. Cells in this domain facilitate nutrient import into the maize kernel, as evident by the presence of cell wall ingrowths which increase the surface area of the associated plasmalemma (Thompson et al., 2001; Offler et al., 2003).
The embryo surrounding region (ESR) is a small zone located at the micropylar pole, around the suspensor and basal half of the embryo. It is characterized by the presence of small cells with dense cytoplasm and may have a role in embryo nutrition or in establishing a physical barrier between the embryo and the endosperm during seed development (Opsahl-Ferstad et al., 1997).
This pattern of organization is indicative of the dual endosperm function, i.e. the uptake of nutrients from the maternal tissue and the synthesis and storage of reserves. It is remarkable that among different angiosperms the domain organization is conserved, as discussed by Costa et al. (2004).
Molecular markers in different domains
The existence in the differentiated endosperm of four distinct domains has become evident from the spatial distribution of specific molecular markers.
ESR has been characterized on the basis of the early expression of ZmEsr1, ZmEsr2, ZmEsr3, ZmAE1 (Zea mays androgenic embryo1) and ZmAE3 (Opsahl-Ferstad et al., 1997; Magnard et al., 2000) genes and by the expression of a recently isolated gene encoding for an invertase inhibitor (Bate et al., 2004).
The co-ordinated and spatially regulated activity of Esr genes was further demonstrated by promoter analysis (Bonello et al., 2000). The lack of Esr transcripts in endosperm of embryo-less mutants suggests that their activity is modulated by a signal originating from the embryo (Opsahl-Ferstad et al., 1997).
Genes involved in starch (reviewed in Smith, 1999) and prolamin storage protein (Muentz, 1998) biosynthesis are expressed in a co-ordinate and tissue-specific manner in the starchy endosperm. Highly conserved cis-regulatory sequences have been identified in the promoter of prolamin genes and corresponding trans-activating factors described (Foerde, 1985; Vincente-Carbajosa et al., 1997).
Several BETL genes have been identified: their expression is detected early during development at the start of the cellularization phase. They are Betl1, Betl2, Betl3 and Betl4 (Hueros et al., 1995, 1999), Bap1 (Basal layer-type antifungal protein1) and Bap2 (Serna et al., 2001). In some cases their characteristics suggest that they may play a role in the defence against pathogen entry into the seed (Hueros et al., 1999; Serna et al., 2001). More recently a maternally expressed gene1 (meg1) has been discovered, the product of which is localized to the labyrinthine ingrowths of the transfer cell walls (Gutierrez-Marcos et al., 2004).
The most useful markers for the aleurone are structural (C2, A1, A2, Bz and Bz2) and regulatory (C1, R and Vp1) genes involved in anthocyanin biosynthesis (reviewed by Cone, 1994).
Acquisition of different endosperm domains
Endosperm differentiation relies on two major phases: cell fate specification and cell specialization inside each domain, the latter occurring later during endosperm maturation. It has been proposed that the endosperm cell specification in cereals occurs in an early developmental stage during the free-nuclear to cellularization stage of development (Olsen, 2001).
For instance, signals for ESR formation must be elaborated during the first phases of development, since it is established starting from 4–5 DAP. Moreover the observation that embryo-less mutant kernels retain a cavity in the ESR region suggests that the endosperm has an intrinsic programme for the formation of this domain (Heckel et al., 1999).
The myb-domain protein ZmMRP-1, recently isolated as a BETL-specific gene, is a good candidate for determining specifically the differentiation of this region (Gomez et al., 2002). It acts as a positive regulator of BETL gene transcription, as shown in transient assays, and its expression precedes that of other BETL genes. glo1-1 (globby1-1), a recently isolated monogenic recessive mutant, causes aberrant nuclear divisions and cell proliferation in the early stages of endosperm development (Costa et al., 2003). BETL is disrupted by the mutation and the expression of BETL-specific transcripts is reduced. Study of the globby mutation indicates that the basis for cell specification at the chalazal pole occurs in a narrow window of syncitial endosperm development and that the transfer cell specification is an irreversible event. Cell identity is subsequently inherited in a cell lineage-dependent manner (Costa et al., 2003).
Further studies are needed to unravel the genetic control of cell specification and the nature of the molecular signals involved. Among emp and dek mutants, those with altered BETL should provide the opportunity to improve investigations on BETL specification and the molecular mechanisms underlying the transfer of nutrients and/or signals from the maternal sporophytic tissue to the seed. emp mutants exhibit a severe reduction of endosperm growth. However, as is evident in emp mutants characterized in the authors' laboratory, the four main endosperm domains are detectable, even though only partially developed. It is thus possible that emp genes represents a class of genes required for cell specialization and/or may be involved in controlling the grain-filling process.
Several mutants affecting aleurone development have been characterized (Becraft et al., 1996; Gavazzi et al., 1997; Becraft and Asuncion-Crabb, 2000; Shen et al., 2003; Lid et al., 2004). Their studies have been valuable in providing information on the mechanisms of aleurone cell determination and specialization. For a discussion of this subject see Olsen et al. (1998), Olsen (2004) and Costa et al. (2004). It is remarkable to observe that aleurone cell fate and starchy endosperm cell fate are not fixed, but remain interchangeable. This observation has been interpreted as if positional cues are required to specify and maintain aleurone cells (Becraft and Asuncion-Crabb, 2000).
THE ROLE OF PROGRAMMED CELL DEATH EMBRYO AND ENDOSPERM DEVELOPMENT
Programmed cell death (PCD) is a genetically regulated process of cell suicide occurring in multicellular organisms in response to developmental and environmental signals. In plants, as in animals, the programmed destruction of cells is relevant for morphogenesis and may be envisaged as the necessary counterpart of cell division in determining the shape and morphology of tissues and organs during differentiation (Greenberg, 1996; Pennell and Lamb, 1997; Lam and Greenberg, 2000; Wu and Cheung, 2000; Lam, 2004). In plants, in addition, PCD occurs as a defence mechanism, known as the hypersensitive response, to remove infected cells (Heath, 2000; Greenberg and Yao, 2004). The process is characterized by a succession of events, the most relevant being cytoplasm vacuolization, chromatin condensation and DNA fragmentation.
The parallel between animal and plant cell death has been much discussed and similarities and differences have been described. Dead plant cells, unlike animal cells, are not removed through phagocytosis by other cells, but are held in place. Apoptosis in animals is well characterized at the biochemical and molecular level. Less is known about PCD mechanisms in plants, where no orthologues for proteolytic enzymes called caspases involved in the apoptosis process are known, even though a ‘caspase-like’ activity is carried out by metacaspase proteins (van der Hoorn and Jones, 2004).
In maize, cell death events are known to occur throughout normal development both in the sporophyte and in the gametophyte (Buckner et al., 1998, 2000). In the developing caryopsis, cells die both in embryo and endosperm at predictable times and places.
During embryogenesis, PCD events mainly occur in structures or organs (scutellum and suspensor), having a transient function and not contributing to the body of the adult plant.
TUNEL-positive (terminal deoxyribonucleotidyl transferase-mediated dUTDP-fluorescein nick end-labelling) cells, are evident in the scutellum at 14 DAP in cell layers around the shoot primordium in a gradient from the embryo axis to the internal scutellum (Giuliani et al., 2002), even though the only evidence of TUNEL-positive nuclei is not enough to unquestionably demonstrate PCD. Fluorescent nuclei are also visible in the coleoptile and in the root cap, both tissues having a function limited to the embryogenetic stage (Fig. 1; Giuliani et al., 2002). The suspensor follows the same fate, since its function of transferring nutrients to the embryo from maternal tissues is accomplished during early embryogenesis. Starting from 14 DAP, a degeneration process proceeds from the top towards the bottom until the complete disappearance of this organ (Fig. 2; Giuliani et al., 2002).
Fig. 1.
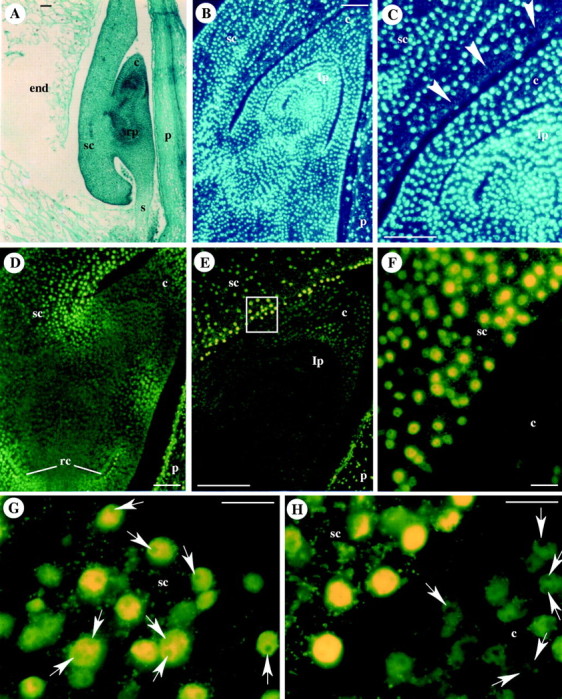
Programmed cell death in maize embryos at early developmental stages (stages L1–L2). (A) Safranin-fast green staining of a longitudinal section of an embryo at 14 DAP. (B, C) DAPI staining of longitudinal sections at 17 DAP showing evidence of nuclear loss (arrowheads) in scutellum layers surrounding the shoot. (D–H) In situ detection of DNA fragmentation by the TUNEL procedure (yellow fluorescence on nuclei). TUNEL-positive nuclei are evident in the scutellum layers surrounding the coleoptile (D–F), in the coleoptile and in the root cap (D). (F, enlargement of E) shows the difference between TUNEL-positive (yellow) and TUNEL-negative (dark green) nuclei. (G) One or two nucleoli (arrows) are present in TUNEL-positive nuclei of the scutellum at 14 DAP. (H) Nucleoli are absent in TUNEL-positive nuclei and present (arrows) in TUNEL-negative nuclei at 16 DAP. c, coleoptile; end, endosperm; lp, leaf primordium; p, pericarp; rc, root cap; rp, root primordium; s, suspensor; sc, scutellum. Scale bars: (A–E) = 100 μm, and (F–H) = 20 μm for. Reproduced from Giuliani et al., 2002.
Fig. 2.
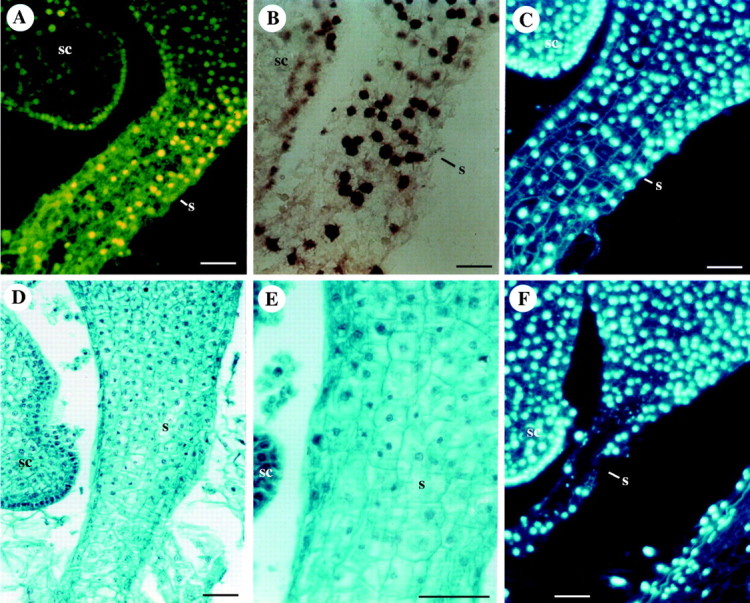
Programmed cell death in the suspensor of the maize embryo. (A, B) TUNEL-positive nuclei in the suspensor at 14 DAP visualized (A) directly by fluorescein fluorescence, and (B) indirectly by the secondary anti-fluorescein-AP conjugate. (C, F) DAPI staining of the suspensor nuclei in longitudinal sections at (C) 14 DAP, and at (F) 16 DAP. (D, E) Safranin-fast green staining of a longitudinal section at 15 DAP. sc, scutellum; s, suspensor. Scale bars = 50 μm. Reproduced from Giuliani et al. (2002).
In emb mutants, arrested at an early stage of development (late proembryo or transition) before differentiation of embryonic structures, no evidence of TUNEL-positive nuclei was found (Consonni et al., 2003). However, these mutants show abnormal proliferation of the suspensor. This was attributed to the absence of the cell death process in suspensor cells at a specific developmental time. It is thus conceivable that a signal is required for triggering PCD in those cells and that this signal is elaborated by the embryo proper (Consonni et al., 2003).
The placento-chalazal cells, in close contact with the lower portion of the endosperm, early in development (6–12 DAP) undergo a process of enucleation and degradation of cell content that is related to the transport of nutrients to the endosperm and cannot be considered a true PCD process. Later, this first cell death event is followed by a second PCD phase, related to the abscission process, visible only in a few cell layers below the enucleated cells and confirmed by TUNEL positiveness (Kladnik et al., 2004).
Studies have demonstrated that during endosperm development a programmed cell death process occurs related to the mobilization of stored products (Young and Gallie, 2000a).
Viability staining indicated that the first cells undergoing death at approx. 16 DAP are cells in the central endosperm. A second wave follows at 20 DAP, starting from the crown of the seed towards the base. Characteristic internucleosomal degradation, resulting in the appearance of a ladder of DNA fragments, was first detected at 28 DAP and continued to increase throughout development (Young et al., 1997). The DNA fragments are therefore the end products of a degradation process initiated some days previously.
The timing of cell death must be exactly regulated, since cells are programmed to die after the synthesis of starch and storage proteins and before dehydration. The progression of the cell death in developing maize endosperm therefore follows a highly organized pattern, whereas in wheat endosperm PCD initiates stochastically (Young and Gallie, 1999).
A genetic regulation of the entire process is confirmed by the analysis of mutants affecting endosperm development. In general, dek (defective kernel) mutations disrupting normal seed development lead to premature induction of PCD in the endosperm (Young and Gallie, 2000a).
In shrunken2 (sh2) mutant endosperms, cell death initiates earlier and progresses more rapidly compared with the wild type. It was demonstrated that ethylene and ABA are crucial elements to trigger PCD and that an elevated ethylene production in sh2 compared with wild-type kernels is responsible for a premature PCD that interferes with reserve deposition (Young et al., 1997; Young and Gallie, 2000b; Gallie and Young, 2004).
A contrasting result was obtained by the analysis of a series of emp mutants, where it appears that PCD is delayed if compared with the corresponding wild type (S. Dolfini et al., unpubl. res.). In these mutants, cell fate specification and specialization are only partially achieved and the endosperm is drastically reduced. This study may provide an indication that the progression of PCD is uncoupled to previous developmental phases (S. Dolfini et al., unpubl. res.).
The arrest of cell divisions and the progression of nuclear endoreduplication are related, as well as the completion of DNA amplification in the central endosperm, which is followed by the induction of cell death (Schweizer et al., 1995). It is still to be demonstrated whether endoreduplication is required for the entry of endosperm cells into PCD.
CONCLUSIONS
Polarization is the most obvious feature shared by both embryo and endosperm. In both cases the presence of distinct domains along the posterior–anterior axis is established early during development. In arabidopsis it has been shown that endosperm polarity is controlled maternally by a chromatin-remodelling complex (reviewed by Berger, 2003).
Genetic, molecular and cellular studies indicate that both embryo and endosperm formation require a distinct series of morphogenetic events leading to the acquisition of a proper structure. It is also becoming increasingly clear that the progression of their development relies on the interaction between the two compartments and on the interaction of the seed forming with the maternal tissue. In particular, the presence of a proper endosperm is required for successful embryo development. The cereal seed that is known today is the product of thousands of years of domestication and decades of controlled breeding, which have led to the elimination of some characteristics, i.e. the seed dispersal mechanism, on the one hand, and to the selection of useful traits on the other. Greater seed size with its accompanying increase in stored reserves is considered the most significant selected trait. In the case of maize, teosinte is believed to be the wild progenitor. It is possible that the genes that played the more significant roles in this process are those that currently show the highest degrees of polymorphism in comparison with their corresponding orthologous genes in the ancestral species.
LITERATURE CITED
- Bate NJ, Niu X, Wang Y, Reimann KS, Helentjaris TG. 2004. An invertase inhibitor from maize localizes to the embryo surrounding region during early kernel development. Plant Physiology 134: 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becraft PW, Stinard PS, McCarty D. 1996. CRINKLY4: A TNFR-like receptor kinase involved in maize epidermal differentiation. Science 273: 1406–1409. [DOI] [PubMed] [Google Scholar]
- Becraft PW, Asuncion-Crabb Y. 2000. Positional clues specify and maintain aleurone cell fate in endosperm development. Development 127: 4039–4048. [DOI] [PubMed] [Google Scholar]
- Berger F. 2003. Endosperm: the crossroad of seed development. Current Opinion on Plant Biology 6: 42–50. [DOI] [PubMed] [Google Scholar]
- Bonello JF, Opsahl-Ferstad HG, Perez P, Dumas C, Rogowsky PM. 2000.Esr genes show different pattern of expression in the same region of maize endosperm. Gene 246: 219–227. [DOI] [PubMed] [Google Scholar]
- Buckner B, Janick-Buckner D, Gray J, Johal GS. 1998. Cell-death mechanisms in maize. Trends in Plant Science 3: 218–223. [Google Scholar]
- Buckner B, Johal GS, Janick-Buckner D. 2000. Cell death in maize. Physiologia Plantarum 108: 231–239. [Google Scholar]
- Clark JK. 1996. Maize embryogenesis mutants. In: Wang TI, Cuming A, eds. Embryogenesis the generation of a plant. Oxford: BIOS Scientific Publications, 89–112. [Google Scholar]
- Clark JK, Sheridan WF. 1991. Isolation and characterization of 51 embryo-specific mutations of maize. Plant Cell 3: 935–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone K. 1994. Cloned anthocyanin genes and their regulation. In: Freeling M, Walbot, V eds. The maize handbook. New York, NY: Springer-Verlag, 282–285. [Google Scholar]
- Consonni G, Aspesi C, Barbante A, Dolfini S, Giuliani C, Giulini A, et al. 2003. Analysis of four maize mutants arrested in early embryogenesis reveals an irregular pattern of cell division. Sexual Plant Reproduction 15: 281–290. [Google Scholar]
- Costa LM, Gutierrez-Marcos JF, Brutnell TP, Greenland AJ, Dickinson HG. 2003. The globby1-1 (glo1-1) mutation disrupts nuclear and cell division in the developing maize seed causing alterations in endosperm cell fate and tissue differentiation. Development 130: 5009–5017. [DOI] [PubMed] [Google Scholar]
- Costa LM, Gutierrez-Marcos JF, Dickinson HG. 2004. More than a yolk: the short life and complex times of the plant endosperm. Trends in Plant Science 5: 507–514. [DOI] [PubMed] [Google Scholar]
- Elster R, Bommert P, Sheridan WF, Werr W. 2000. Analysis of four embryo-specific mutants in Zea mays reveals that incomplete radial organization of the proembryo interferes with subsequent development. Development Genes Evolution 210: 300–331. [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SSL, Rock CD. 2002. Abscisic acid signalling in seeds and seedling. Plant Cell 14 (Suppl): S15–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JC. 2002. Coordination of cell proliferation and cell fate decisions in the angiosperm shoot apical meristem. BioEssays 24: 27–37. [DOI] [PubMed] [Google Scholar]
- Foerde BG, Heyworth A, Pywell J, Kreiss M. 1985. Nucleotide sequences of a B1 hordein gene and the identification of possible upstream regulatory sequences in endosperm storage protein genes from barley, wheat and maize. Nucleic Acid Research 13: 7327–7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman WE. 1998. The evolution of double fertilization and endosperm: an historical perspective. Sexual Plant Reproduction 11: 6–16. [Google Scholar]
- Friedman WE. 2001. Developmental and evolutionary hypotheses for the origin of double fertilization in endosperm. Comptes Rendus de l'Académie des Sciences de Paris 324: 559–567. [DOI] [PubMed] [Google Scholar]
- Fu S, Scanlon MJ. 2004. Clonal mosaic analysis of EMPTY PERICARP 2 reveals non redundant functions of the duplicated HEAT SHOCK FACTOR BINDING PROTEIN during maize shoot development. Genetics 167: 1381–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Meeley R, Scanlon MJ. 2002.empty-pericarp2 encodes a negative regulator of the heat shock response and is required for maize embryogenesis. Plant Cell 14: 3119–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR, Young TE. 2004. The ethylene biosynthetic and perception machinery is differentially expressed during endosperm and embryo development in maize. Molecular Genetics and Genomics 271: 267–281. [DOI] [PubMed] [Google Scholar]
- Gavazzi G, Dolfini S, Allegra D, Castiglioni P, Todesco G, Hoxha M. 1997.Dap (Defective aleurone pigmentation) mutations affect maize aleurone development. Molecular General Genetics 256: 223–230. [DOI] [PubMed] [Google Scholar]
- Giuliani C, Consonni G, Gavazzi G, Colombo M, Dolfini S. 2002. Programmed cell death during embryogenesis in maize. Annals of Botany 90: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez E, Royo J, Guo Y, Thompson R, Hueros G. 2002. Establishment of cereal endosperm expression domains: identification and properties of a maize transfer cell-specific transcription factor, ZmMRP. Plant Cell 14: 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JT. 1996. Programmed cell death: a way of life for plants. Proceedings of the National Academy of Sciences of the USA 93: 12094–12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JT, Yao N. 2004. The role and regulation of programmed cell death in plant–pathogen interactions. Cellular Microbiology 6: 201–211. [DOI] [PubMed] [Google Scholar]
- Guignard L. 1899. Sur les anthéroziodes et la double copulation sexuelle chez les végétaux angiospermes. Comptes Rendus de l'Académie des Sciences de Paris 128: 864–871. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Marcos JF, Costa L, Biderre-Petit C, Khbaya B, O'Sullivan DM, Wormald M, et al. 2004.maternally expressed gene1 is a novel maize endosperm transfer cell-specific gene with a maternal parent-of-origin pattern of expression. Plant Cell 16: 1288–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A, Hardip K, Phillips A, Hedden P, Hake S, Tsiantis M. 2002. The gibberellin KNOTTED1-type homeobox function in plants with different body plans. Current Biology 12: 1557–1565. [DOI] [PubMed] [Google Scholar]
- Heath MC. 2000. Hypersensitive response-related death. Plant Molecular Biology 44: 321–334. [DOI] [PubMed] [Google Scholar]
- Heckel T, Werner K, Sheridan WF, Dumas C, Rogowsky PM. 1999. Novel phenotypes and developmental arrest in early embryo specific mutants of maize. Planta 210: 1–8. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Park WJ, Sauer M, Woll K. 2004. From weeds to crops: genetic analysis of root development in cereals Trends in Plant Science 9: 42–48. [DOI] [PubMed] [Google Scholar]
- van der Hoorn RA, Jones JD. 2004. The plant proteolytic machinery and its role in defence. Current Opinion in Plant Biology 7: 400–407. [DOI] [PubMed] [Google Scholar]
- Hueros G, Varotto S, Salamini F, Thompson RD. 1995. Molecular characterzation of BET1, a gene expressed in the endosperm transfer cells of maize. Plant Cell 7: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueros G, Royo J, Maitz M, Salamini F, Thompson RD. 1999. Evidence for factors regulating transfer cell-specific expression in maize endosperm. Plant Molecular Biology 41: 403–414. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Cooke TJ. 1997. Fundamental concepts in the embryogenesis of dicotyledons: a morphological interpretation of embryo mutants. Plant Cell 9: 1903–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter RA, Laudencia-Chingcuanco D, Smith LG, Hake S. 1997. Loss-of-function mutations in the maize homeobox gene, knotted-1, are defective in shoot meristem maintenance. Development 124: 3045–3054. [DOI] [PubMed] [Google Scholar]
- Kiesselbach TA. 1949.The structure and reproduction of corn. Lincoln, NE: University of Nebraska Press. [Google Scholar]
- Kladnik A, Chamusco K, Dermastia M, Chourey P. 2004. Evidence of programmed cell death in post-phloem transport cells of the maternal pedicel tissue in developing caryopsis of maize. Plant Physiology 136: 3572–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowles RV, McMullen MD, Yerk G, Phillips RL, Kraemer S, Srienc F. 1992. Endosperm mitotic activity and endoreduplication in maize affected by defective kernel mutations. Genome 35: 68–77. [Google Scholar]
- Lam E, Greenberg J. 2000. Cell death: the ‘Yin’ path in the balancing of the life cycle. Plant Molecular Biology 44: vii–viii. [DOI] [PubMed] [Google Scholar]
- Lam E. 2004. Controlled cell death, plant survival and development. Nature Reviews/Molecular Biology 5: 305–315. [DOI] [PubMed] [Google Scholar]
- Larkins BA, Dilkes BP, Dante RA, Coelho CM, Woo Y-M, Liu Y. 2001. Investigating hows and whys of DNA endoreduplication. Journal of Experimental Botany 52: 183–192. [PubMed] [Google Scholar]
- Leiva-Neto JT, Grafi G, Sabelli PA, Dante RA, Woo Y-M, Maddock S, et al. 2004. A dominant negative mutant of cyclin-dependent kinase a reduces endoreduplication but not cell size or gene expression in maize endosperm. Plant Cell 16: 1854–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lid SE, Al RH, Krekling T, Meeley RB, Ranch J, Opsahl-Ferstad HG, et al. 2004. The maize disorganized aleurone layer1 and 2 (dil1, dil2) mutants lack control of mitotic division plane in the aleurone layer of developing endosperm. Planta 218: 370–378. [DOI] [PubMed] [Google Scholar]
- Lin B-Y. 1984. Ploidy barrier in endosperm development in maize. Genetics 107: 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes MA, Larkins BA. 1993. Endosperm origin, development, and function. Plant Cell 5: 1383–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Dooner HK. 2004. A mutation in the nuclear-encoded plastid ribosomal protein S9 leads to early embryo lethality in maize. The Plant Journal 37: 92–103. [DOI] [PubMed] [Google Scholar]
- Magnard JL, Le Deunff E, Domenech J, Rogowsky PM, Testillano PS, Rougier M, et al. 2000. Endosperm-specific features at the onset of microspore embryogenesis in maize. Plant Molecular Biology 44: 559–574. [DOI] [PubMed] [Google Scholar]
- Magnard JL, Lehouque G, Massonneau A, Frangne N, Heckel T, Gutierrez-Marcos JF, et al. 2003. ZmEBE genes show a novel, continuous expression pattern in the central cell before fertilization and in specific domains resulting endosperm after fertilization. Plant Molecular Biology 53: 821–836. [DOI] [PubMed] [Google Scholar]
- McCarty DR. 1995. Genetic control and integration of maturation and germination pathways in seed development. Annual Review of Plant Physiology and Plant Molecular Biology 46: 71–93. [Google Scholar]
- McClintock B. 1978. Development of the maize endosperm as revealed by clones. In: Subtelny S, Sussex I, eds. The clonal basis of development. New York, NY: Academic Press, 217–237. [Google Scholar]
- Meinke DW. 1995. Molecular genetics of plant embryogenesis. Annual Review of Plant Physiology 46: 36–94. [Google Scholar]
- Meyer U, Torres Ruiz RA, Berleth T, Misera S, Jurgens G. 1991. Mutations affecting body organization in the Arabidopsis embryo. Nature 353: 402–407. [Google Scholar]
- Muentz K. 1998. Deposition of storage proteins. Plant Molecular Biology 38: 77–99. [PubMed] [Google Scholar]
- Nawaschin SG. 1898. Resultate einer Revision der Befruchtungsvogänge bei Lilium martagon und Fritillaria tenulla Bulletin de l'Académie des Sciences de Saint Petersbourg 9: 377–382. [Google Scholar]
- Neuffer MG, Sheridan F. 1980. Defective kernel mutants of maize. I. Genetic and lethality studies. Genetics 95: 929–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offler CE, McCurdy DW, Patrick JW, Talbot MJ. 2003. Transfer cells: cells specialized for a special purpose. Annual Review of Plant Biology 54: 431–454. [DOI] [PubMed] [Google Scholar]
- Olsen OA. 2001. Endosperm development: cellularization and cell fate specification. Annual Review of Plant Physiology and Plant Molecular Biology 52: 233–267. [DOI] [PubMed] [Google Scholar]
- Olsen OA. 2004. Nuclear endosperm development in cereals and Arabidopsis thaliana Plant Cell 16: S214–S227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen OA, Lemmon B, Brown R. 1998. A model for aleurone development. Trends in Plant Science 3: 168–169. [Google Scholar]
- Opsahl-Ferstad HG, Le Deunff E, Dumas C, Rogowsky PM. 1997.ZmESR, a novel endosperm specific gene expressed in a restricted region around the maize embryo. The Plant Journal 12: 235–246. [DOI] [PubMed] [Google Scholar]
- Pennell RJ, Lamb C. 1997. Programmed cell death in plants. Plant Cell 9: 1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilu R, Consonni G, Busti E, MacCabe AP, Giulini A, Dolfini S, et al. 2002. Mutations in two independent loci lead to suppression of the shoot apical meristem in maize. Plant Physiology 128: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce G, Lujan R, Campos ME, Reyes A, Nieto-Sotelo J, Feldman LJ, et al. 2000. Three maize root-specific genes are not correctly expressed in regenerated caps in the absence of the quiescent center. Planta 211: 23–33. [DOI] [PubMed] [Google Scholar]
- Reiser L, Sanchez-Baracaldo P, Hake S. 2000. Knots in the family tree: evolutionary relationships and functions of knox homeobox genes. Plant Molecular Biology 42: 151–166. [PubMed] [Google Scholar]
- Scanlon MJ, Stinard PS, James MG, Myers AM, Robertson DS. 1994. Genetic analysis of 63 mutations affecting maize kernel development isolated from Mutator stock Genetics 136: 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon MJ, Henderson DC, Bernstein B. 2002. SEMAPHORE1 functions during the regulation of ancestrally duplicated knox genes and polar auxin transport in maize. Development 129: 2663–2673. [DOI] [PubMed] [Google Scholar]
- Schneeberger R, Tsiantis M, Freeling M, Langdale JA. 1998. The rough sheath2 gene negatively regulates homeobox gene expression during maize leaf development. Development 125: 2857–2865. [DOI] [PubMed] [Google Scholar]
- Schweizer L, Yerk-Davis GL, Phillips RL, Srienc F, Jones RJ. 1995. Dynamics of maize endosperm development and DNA endoreduplication. Proceedings of the National Academy of Sciences of the USA 92: 7070–7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serna A, Maitz M, O'Connell T, Santandrea G, Thevissen K, Tienens K, et al. 2001. Maize endosperm secretes a novel antifungal protein into adjacent maternal tissue. The Plant Journal 25: 687–698. [DOI] [PubMed] [Google Scholar]
- Shen B, Li C, Min Z, Meeley RB, Tarcynsky MC, Olsen O-A. 2003.Sal1 determines the number of aleurone cell layers in maize endosperm and encodes a class E vacuolar sorting protein. Proceedings of the National Academy of Sciences of the USA 100: 6552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM. 1999. Making starch. Current Opinion on Plant Biology 2: 223–229. [DOI] [PubMed] [Google Scholar]
- Sollner R, Glasser G, Wanner G, Sommerville GR, Jurgens G, Assaad FF. 2002. Cytokinesis-defective mutants of Arabidopsis Plant Physiology 129: 678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. 1974.Flowering plants: evolution above the species level. Cambridge, MA: Harvard University Press. [Google Scholar]
- Thompson RD, Hueros G, Becker HA, Maitz M. 2001. Development and functions of seed transfer cells. Plant Science 160: 775–783. [DOI] [PubMed] [Google Scholar]
- Vincente-Carbajosa VJ, Moose SP, Parsons RL, Schmidt RJ. 1997. A maize zinc finger protein binds the prolamin box in zein gene promoters and interacts with the basic leucine zipper transcriptional activator Opaque2 Proceedings of the National Academy of Sciences of the USA 94: 7685–7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbrecht E, Veit B, Sinha N, Hake S. 1991. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature 350: 241–243. [DOI] [PubMed] [Google Scholar]
- Vollbrecht E, Reiser L, Hake S. 2000. Shoot meristem size is dependent on inbred background and presence of the maize homeobox gene, knotted 1 Development 127: 3161–3172. [DOI] [PubMed] [Google Scholar]
- Walbot V. 1994. Overview of key steps in aleurone development. In: Freeling M, Walbot V, eds. The maize handbook. New York, NY: Springer-Verlag, 78–80. [Google Scholar]
- Wu HM, Cheung AY. 2000. Programmed cell death in plant reproduction. Plant Molecular Biology 44: 267–281. [DOI] [PubMed] [Google Scholar]
- Young TE, Gallie DR. 1999. Analysis of programmed cell in wheat endosperm reveals differences in endosperm development between cereals. Plant Molecular Biology 39: 915–926. [DOI] [PubMed] [Google Scholar]
- Young TE, Gallie DR. 2000. Programmed cell death during endosperm development. Plant Molecular Biology 44: 283–301. [DOI] [PubMed] [Google Scholar]
- Young TE, Gallie DR. 2000. Regulation of programmed cell death in maize endosperm by abscisic acid. Plant Molecular Biology 42: 397–414. [DOI] [PubMed] [Google Scholar]
- Young TE, Gallie DR, DeMason DA. 1997. Ethylene-mediated programmed cell death during maize endosperm development of wild-type and shrunken2 genotypes. Plant Physiology 115: 737–751. [DOI] [PMC free article] [PubMed] [Google Scholar]


