Abstract
• Background and Aims The systematic position of the genus Metagentiana and its phylogenetic relationships with Crawfurdia, Gentiana and Tripterospermum have not been explicitly addressed. These four genera belong to one of two subtribes (Gentianinae) of Gentianeae. The aim of this paper is to examine the systematic position of Crawfurdia, Metagentiana and Tripterospermum and to clarify their phylogenetic affinities more clearly using ITS and trnL intron sequences.
• Methods Nucleotide sequences from the internal transcribed spacers (ITS) of nuclear ribosomal DNA and the plastid DNA trnL (UAA) intron were analysed phylogenetically. Ten of fourteen Metagentiana species were sampled, together with 40 species of other genera in the subtribe Gentianinae.
• Key Results The data support several previously published conclusions relating to the separation of Metagentiana from Gentiana and its closer relationships to Crawfurdia and Tripterospermum based on studies of gross morphology, floral anatomy, chromosomes, palynology, embryology and previous molecular data. The molecular clock hypothesis for the tested sequences in subtribe Gentianinae was not supported by the data (P < 0·05), so the clock-independent non-parametric rate smoothing method was used to estimate divergence time. This indicates that the separation of Crawfurdia, Metagentiana and Tripterospermum from Gentiana occurred about 11·4–21·4 Mya (million years ago), and the current species of these three genera diverged at times ranging from 0·4 to 6·2 Mya.
• Conclusions The molecular analyses revealed that Crawfurdia, Metagentiana and Tripterospermum do not merit status as three separate genera, because sampled species of Crawfurdia and Tripterospermum are embedded within Metagentiana. The speciation and rapid radiation of these three genera is likely to have occurred in western China as a result of upthrust of the Himalayas during the late Miocene and the Pleistocene.
Keywords: Asia, biogeography, Crawfurdia, Gentiana, Gentianeae, Gentianaceae, Metagentiana, molecular systematics, ITS, trnL (UAA) intron, Tripterospermum
INTRODUCTION
Crawfurdia was established by Wallich in 1826 (Wallich, 1826). In the same year, Blume (1826) described a new genus Tripterospermum based on T. trinerve from Java. These two genera were re-examined by Marquand (1931, 1937), who did not accept Crawfurdia and Tripterospermum and merged them into Gentiana. However, the two have been retained as separate genera by many botanists (Smith, 1965; Ho and Liu, 1990; Struwe et al., 2002). Metagentiana was separated from Gentiana on the basis of observations related to its gross morphology, floral anatomy, chromosomes, palynology, embryology and molecular data (Yuan et al., 1996; Ho et al., 2002a; Struwe et al., 2002). It was previously treated as Gentiana section Stenogyne, established by Franchet (1884) and revised by Kusnezov (1894). Prior to Ho et al. (2002a), most botanists followed Kusnezov (1894) and considered this group as a section of Gentiana (Pringle, 1978; Ho and Liu, 1990; Struwe et al., 2002). Metagentiana consists of 14 species, two of which are endemic to Thailand and Myanmar, and the rest are concentrated in south-west China. Most species of Metagentiana are herbaceous local endemics growing in alpine scrub, meadows and coniferous forests (Ho and Pringle, 1995; Ho et al., 2002a). The systematic position of Metagentiana and its phylogenetic relationships with Crawfurdia, Gentiana and Tripterospermum have not been explicitly addressed. These four genera belong to one of two subtribes (Gentianinae) of Gentianeae (Struwe et al., 2002). The other, larger subtribe is Swertiinae, which includes Bartonia, Comastoma, Frasera, Gentianella, Gentianopsis, Halenia, Jaeschkea, Latouchea, Lomatogonium, Megacodon, Obolaria, Pterygocalyx, Swertia and Veratrilla (Struwe et al., 2002). Smith (1965) suggested that Gentiana section Stenogyne (Metagentiana) had a closer affinity with Tripterospermum and Crawfurdia than with any other section of Gentiana, and Löve and Löve (1976) recommended that it should be transferred to the genus Tripterospermum, tentatively as a subgenus (Tripterospermum subgenus Stenogyne) based on morphological characters. Halda (1995) treated section Stenogyne as a subgenus of Gentiana. Karyological studies in Gentiana have been reviewed by several authors (Yuan, 1993; Yuan and Küpfer, 1993a, b; Küpfer and Yuan, 1996). Yuan and Küpfer (1993a) reported chromosome numbers and karyotype asymmetry for six species of Metagentiana for the first time and suggested that it had a unique and isolated position in the genus Gentiana because of its higher basic chromosome numbers (x = 17, 21 and 23). Ho et al. (2002b) also reported chromosome numbers for two other species (M. souliei, 2n = 46; M. serra, 2n = 34). Yuan et al. (1996) suggested that section Stenogyne (Metagentiana) should be excluded from Gentiana because inclusion of this section in Gentiana makes the genus paraphyletic based on a molecular phylogeny reconstructed from nucleotide sequences of the internal transcribed spacers (ITS) of nuclear ribosomal DNA; this and the plastid trnL (UAA) intron (trnL) have been shown to be suitable markers for phylogenetic reconstruction within a genus or closely related genera (Baldwin et al., 1995; Wang et al., 1999; Bain and Golden, 2000; Liu et al., 2002). Moreover, previous studies at the generic level of subtribe Gentianinae and the sectional level of Gentiana have shown the phylogenetic utility of ITS and trnL sequences (Yuan and Küpfer, 1995; Gielly and Taberlet, 1996; Yuan et al., 1996; Yuan and Küpfer, 1997). Phylogenetic trees generated by parsimony analysis of these data are principally congruent with morphological observations, and they have improved or clarified some morphological misinterpretations and conflicts (Yuan and Küpfer, 1995; Gielly and Taberlet, 1996; Yuan et al., 1996, 2003; Yuan and Küpfer, 1997). However, only four species representing the three genera Crawfurdia, Metagentiana and Tripterospermum have been studied in previous phylogenetic analyses using ITS sequences (Yuan et al., 1996). Although it has been suggested that the section Stenogyne should be excluded to maintain the monophyly of Gentiana, there were insufficient samples in the cited studies (with regards to Metagentiana) to provide deeper insights into the relationships among these genera. Neither divergence dating nor biogeographic analyses of these groups were performed in any previous studies. In addition, major goals of modern biogeography are to reconstruct the phylogenies of genera and evaluate their origin and evolution against the geological and palaeoclimatic histories of their distribution areas (Avise, 2000).
Applying a molecular phylogenetic approach based on ITS and trnL sequences, the present study is focused on the new genus Metagentiana as well as Crawfurdia and Tripterospermum, allowing their systematic position to be examined and their phylogenetic affinities clarified. An attempt is also made to infer the divergence time of these three genera using a molecular clock hypothesis and to assess putative correlations between the origin of the three genera and geological events.
MATERIALS AND METHODS
Plant species and material
Sequence data were acquired for 22 species of Crawfurdia, Gentiana, Metagentiana and Tripterospermum as indicated by asterisks in Table 1. The voucher specimens were deposited in the Herbarium of the Northwest Plateau Institute of Biology, Xining, Qinghai Province. Sequences for 28 additional species were retrieved from GenBank. The origins of samples, voucher information, GenBank accession numbers and chromosome numbers of species studied are listed in Table 1. Representatives of subtribe Swertiinae, the sister clade of subtribe Gentianinae, Gentianella moorcroftiana, Gentianopsis barbata (Gentianopsis crinita for trnL), Megacodon stylophorus and Swertia bimaculata were used as outgroups according to the results of previous studies (Yuan and Küpfer, 1995; Gielly and Taberlet, 1996; Yuan et al., 1996).
Table 1.
Origin of samples, voucher information, chromosome numbers and GenBank accession numbers of DNA sequences of Crawfurdia, Gentiana, Metagentiana and Tripterospermum
| GenBank accesssion no. |
|||||||
|---|---|---|---|---|---|---|---|
| Genus/section |
Species |
Locality |
Voucher |
Chromosome number: 2n (n)/x |
ITS or ITS1, ITS2 |
trnL (UAA) intron |
|
| Crawfurdia Wall. | x = 23 | ||||||
| C. delavayi Franch. | Mt Cangshan, Yunnan, 3620 m | Chen03140 | 46 (23) | AY562176* | AY563391* | ||
| C. speciosa Wall. | Cuona, Tibet, 3000 m | 751592 | 46 (23) | AY858675* | AY858682* | ||
| C. tibetica Franch. | Mt Gongga, Sichuan, 2300 m | Chen03097 | 46 (23) | AY563383* | AY563393* | ||
| Gentiana (Tourn.) L. | x = ? | ||||||
| Calathianae Froelich | G. terglouensis Hacquet | Hochobir, Austria | – | 42, 38 or 40 | – | X77897 | |
| G. verna L. | St Cergue, Switzerland | – | 28 (14) | – | X75704 | ||
| Chondrophylla Bunge | G. aristata Maxim. | Maqu, Gansu, China; 3500 m | Y92-328 | 14 (7) | Z48100/Z48116 | – | |
| G. boryi Boissier | Sierra Nevada, Spain; 2300 m | Z93-S1 | 20 or 26 | – | X77874 | ||
| G. flexicaulis H. Smith ex Marq. | Mt Taibai, Shaanxi, China; 3400 m | Y92-264 | 14 (7) | Z71937/Z71938 | – | ||
| G. heleonastes H. Smith ex Marq. | Maqu, Gansu, China, 3650 m | G032 | 12 (6) | Z71939/Z71940 | – | ||
| G. piasezkii Maxim. | Mingxian, Gansu, China; 2900 m | Y92-272 | 36 (18) | Z71955/Z71956 | – | ||
| G. squarrosa Ledeb. | Xiahe, Gansu, China; 3000 m | G046 | 38 (19) | Z71965/Z71966 | – | ||
| Ciminalis (Adanson) | G. alpine Villars | Grand Chavalard, Switzerland | Y93-09 | 36 (18) | – | X77868 | |
| Dumorti | G. angustifolia Villars | Hautes, Franch | Kadereit 95/24 | 36 | – | X75699 | |
| G. clusii Perrier and Songeon | Grand Chavalard, Switzerland | Y93-13 | 36 (18) | – | X77879 | ||
| G. dinarica G. Beck | Abruzzi, Italy | Hungerer | 36 | – | X77882 | ||
| G. ligustica R. de Vilmorin and Chopinet | Alpes Maritimes, France | Merxmuller | 36 | – | X77886 | ||
| Cruciata Gaudin | G. crassicaulis Duthie ex Burk. | Mangkang, Tibet, 3800 m | Ge015 | 26 (13) | AY858676* | AY858684* | |
| G. cruciata L. | Botanic Garden, Geneva, Switzerland | – | 52 | – | AF102434 | ||
| G. macrophylla Pall. | Dangchang, Gansu, China | Y92-271 | 26 (13) | Z48067/Z48086 | – | ||
| G. straminea Maxim. | Maqu, Gansu, China | Y92-313 | 52 (13) | AF346015 | – | ||
| Dolichocarpa T. N. Ho | G. tetrasticha Marq. | Dangxiong, Tibet, China; 4500 m | Y92-128 | 24 (12) | Z71967/Z71968 | – | |
| Frigida Kusnez. | G. algida Pall. | Rocky Mt, Colorado, USA | Y91-S10 | 24 (12) | Z48142/Z48117 | AJ490239 | |
| G. frigida Haenke | Mt Rila, Bulgaria | NEU93-17 | 24 (12) | – | X77883 | ||
| Isomeria Kusnez. | G. depressa D. Don | Zhangmu, Tibet, China | Y92-118 | 24 (12) | Z48062/Z48081 | – | |
| Gentiana | G. montserratii Vivant ex Greuter | Castanesa, Spain | – | – | – | X77887 | |
| Monopodiae T. N. Ho | G. callistantha Diels et Gilg | Luqu, Gansu, China | Y92-298 | 26 (13) | Z48095/Z48078 | – | |
| G. futtereri Diels et Gilg | Dingqing, Tibet, China, 4160 m | Liu1056 | – | – | AY858685* | ||
| G. veitchiorum Hemsl. | Leiwuqi, Tibet, China, 4280 m | Liu1042 | 24 (12) | AY858677* | |||
| Microsperma T. N. Ho | G. atropurpurea T. N. Ho | Dangxiong, Tibet, China, 4620 m | Liu1095 | – | AY858678* | AY858686* | |
| G. delavayi Franch. | Lijiang, Yunnan, China; 2850 m | Y92-229 | 26 (13) | Z48099/Z48080 | – | ||
| Phyllocalyx T. N. Ho | G. phyllocalyx C. B. Clarke | Makalu, Nepal | Neu97-30 | 26 (13) | AJ318537/ | AJ315189 | |
| Gentianella Moench. | AJ410316 | ||||||
| G. moorcroftiana (Wall. Ex Griseb.) | – | R. McBeath 2093 | 26 | AJ294615/ | AJ408007 | ||
| Airy-Shaw | AJ294675 | ||||||
| Gentianopsis Ma | G. barbata (FrÖel.) Ma | Mengyuan, Qinghai, China | Liu J.Q.654 | 26 (13) | AF346007 | – | |
| G. crinita (Froelich) Ma | Mainz Botanic Garden, Germany | – | 78 | – | AF102433 | ||
| Megacodon (Hemsl.) | x = 7 | ||||||
| H. Smith | M. stylophorus (C. B. Clarke) H. Smith | Lijiang, Yunnan, China | Ge106 | x = ? | AY858679* | AY858687* | |
| Metagentiana T. N. Ho and S. W. Liu | x = 17, 21, 23 | ||||||
| M. eurycolpa (Marquand) T. N. Ho and S. W. Liu | Luquan, Yunnan, 2600 m | Zhang0274 | – | AY858673* | – | ||
| M. gentilis (Franchet) T. N. Ho and S. W. Liu | Kunming, Yunnan, 2220 m | Chen03122 | 42 (21) | AY562177* | AY563386* | ||
| M. leptoclada (I. B. Balfour and Forrest) T. N. Ho and S. W. Liu | Dali, Yunnan, 3500 m | Liu22798 | – | AY858674* | AY858681* | ||
| M. primuliflora (Franchet) T. N. Ho and S. W. Liu | Kunming, Yunnan, 2290 m | Chen03124 | 42 (21) | AY562178* | AY563385* | ||
| M. pterocalyx (Franchet) T. N. Ho and S. W. Liu | Heqing, Yunnan, 3620 m | Chen03158 | 34 (17) | AY562171* | AY563384* | ||
| M. rhodantha (Franchet) T. N. Ho and S. W. Liu | Kunming, Yunnan, 2220 m | Chen03123 | 46 (23) | AY562174* | AY563390* | ||
| M. serra (Franchet) T. N. Ho, S. W. Liu and S. L. Chen | Lijiang, Yunnan, 2450 m | Chen03141 | 34 (17) | AY562175* | AY563387* | ||
| M. souliei (Franchet) T. N. Ho, S. W. Liu and S. L. Chen | Lijiang, Yunnan, 3320 m | Chen03146 | 46 (23) | AY562170* | AY563388* | ||
| M. striata (Maximowicz) T. N. Ho, S. W. Liu and S. L. Chen | Daofu, Sichuan, 3510 m | Chen03081 | 46 (23) | AY562173* | AY563389* | ||
| M. villifera (H. W. Li ex T. N. Ho) T. N. Ho and S. W. Liu | Yunlian, Sichuan, 800 m | Chuan0018 | – | AY858672* | AY858680* | ||
| Swertia L. | S. bimaculata (Sieb. Et Zucc.) Hook. f. et Thoms ex C. B. Clarke | Makalu, Nepal | NEU97Z-22 | x = 11 | AJ318552/AJ410331 | AJ315204 | |
| Tripterospermum Blume | x = 23 or 10 | ||||||
| Tripterospermum | T. cordatum (Marq.) H. Smith | Kunming, Yunnan, 2210 m | Chen03112 | 46 (23) | AY562172* | AY563392* | |
| T. filicaule (Hemsl.) H. Smith | Mt Gongga, Sichuan, 1990 m | Chen03098 | – | – | AY858683* | ||
| T. volubile (D. Don) Hara | Nielamu, Tibet, 2300 m | 1279 | – | AY858667* | – | ||
| Platyspermum C. J. Wu | T. chinense (Migo) H. Smith | Mt Tianmu, Zhejiang, 1506 m | Liu160810 | – | AY858668* | – | |
Indicates sequences that were registered in the present study (?, not affirmative; –, not available).
Chromosomal numbers reported by Yuan and Küpfer (1993a, b), Chen et al. (1997) and Ho et al. (2002a, b).
DNA extraction and PCR amplification
DNA was extracted from silica-gel-dried leaf material (Chase and Hills, 1991) or from leaf tissue taken from herbarium sheets. Total genomic DNA was extracted using the 2× CTAB procedure of Doyle and Doyle (1987) or the CASsuper Plant Genomic DNA Isolation Kit (CASarray, Shanghai, China). The ITS region was amplified with universal primers 1 and 4 (White et al., 1990), and primers ‘c’ and ‘d’ were used to amplify the trnL intron (Taberlet et al., 1991) in 25-μL reactions. PCRs were performed in a Biometra thermal cycler programmed for 4 min at 94 °C, followed by 36 cycles of 94 °C for 50 s, 53 °C (46 °C for trnL) for 50 s and 72 °C for 50 s, with a final extension of 72 °C for 7 min.
PCR purification and sequencing
All successfully amplified DNA fragments were purified using a CASpure PCR Purification Kit following the manufacturer's protocol (CASarray) prior to sequencing. The primers used for sequencing were the same as those used for PCR. The sequencing reactions were carried out in a Biometra thermal cycler (Tpersonal 48) using a DYEnamic Dye Terminator Cycle Sequencing Kit (Amersham) following the recommended protocol, but with the reaction volumes scaled down to 10 μL. The cycle sequencing products were cleaned using Autoseq 96 plates (Amersham) and then analysed with a MegaBACE DNA Analysis System (Amersham Biosciences Corp.). Both strands of DNA were sequenced.
Sequence alignment
The ITS and trnL sequences were aligned using ClustalX (Thompson et al., 1997), with additional minor manual adjustments. Potentially informative and unambiguously assessable indels were scored as binary characters regardless of their length, and added to the sequence data matrix (Simmons and Ochoterena, 2000). The boundaries of the sequences in the studied material were made by comparison with the published sequences of the genera of subtribe Gentianinae retrieved from GenBank (Yuan and Küpfer, 1995; Yuan et al., 1996).
Phylogenetic analysis and molecular clock test
Phylogenetic analyses were performed using PAUP* 4.0b10 (Swofford, 2003). In maximum parsimony (MP) analysis, characters were equally weighted and unordered (Fitch, 1971), with all gaps treated as missing data. Heuristic searches with 100 random additions of sequence replicates, in combination with ACCTRAN character optimization, MULPARS, tree-bisection-reconnection branch-swapping and STEEPEST DESCENT on were utilized to search for possible multiple islands of most-parsimonious trees (Maddison, 1991). The relative support for individual clades was evaluated by bootstrap (BS) analysis (Felsenstein, 1985). BS values were calculated using 1000 replicates of heuristic searches, each with 10 random addition sequence replicates using tree-bisection-reconnection and MULPARS on options.
Support for each branch was assessed using both BS and Bayesian analyses. BPP were estimated as the proportion of trees sampled after burn-in that contained each of the observed bipartitions (Larget and Simon, 1999). Analyses were performed with MrBayes v2.01 (Huelsenbeck and Ronquist, 2001), with GTR + Γ + PINVAR parameters being estimated during the run, and using the default value of four Markov chains. Multiple chains can assist in more easily traversing tree-space and help avoid entrapment in local topological optima. The Monte Carlo Markov chain length was 1 000 000 generations, and the chain was sampled every 100 generations. Log-likelihood values for sampled trees stabilized after approx. 200 000 generations. Therefore, the last 8000 sampled trees were used to estimate BPP, also called Bayesian support values. If >95 % of the sampled trees contained a given clade, it was considered to be significantly supported by the data produced.
A maximum likelihood (ML) analysis was also conducted, using PAUP* 4.0b10. For the ML analyses, among-site rate variations were modelled using a gamma distribution and the shortest trees from the MP analyses as starting points for ML estimation of transitions : transversion (ti/tv) ratios and the alpha parameter of the gamma distribution for among-site rate variation. Then an iterative procedure as described in Swofford et al. (1996), in which the most likely tree from each heuristic search was used to re-estimate the ti/tv ratio and alpha parameter, was followed. This procedure was repeated until essentially no change occurred in the likelihood estimate between iterations. The program Modeltest, Version 3.06 (Posada and Crandall, 1998) was used to find the model of sequence evolution that best fits the data set according to hierarchical likelihood ratio (LR) tests (P = 0·05). The GTR + I + G model (GTR = general time reversible; I = proportion of invariable sites; G = gamma distributed among-site rate variation) and TVM + G model (TVM = transversion model) in the ITS and trnL data, respectively, and parameter settings (gamma shape, base frequencies) were selected through the Hierarchical Likelihood Ratio Tests procedure implemented in Modeltest. The LR test statistic is distributed as χ2 with degrees of freedom equal to the number of free parameters between the two models (Goldman, 1993) when the models of sequence evolution are nested. A molecular clock hypothesis was also tested using the LR test, based on twice the difference between the log-likelihoods for trees generated from clock and clock-free analyses (Muse and Weir, 1992; Baldwin and Sanderson, 1998; Wang et al., 2000), using a χ2 distribution with N – 2 degrees of freedom (where N is the number of terminal taxa in the tree). When the LR test rejected a molecular clock hypothesis, ML trees based on the ITS and trnL intron sequences were subjected to non-parametric rate smoothing (NPRS) (Sanderson, 1997) using the default settings in TreeEdit v.1.0a8 (Rambaut and Charleston, 2000; Richardson et al., 2001) to estimate divergence times.
RESULTS
The lengths of the unaligned ITS and trnL sequences varied from 689 to 692 base pairs (bp) and from 252 to 271 bp, respectively, among species of Crawfurdia, Metagentiana and Tripterospermum. The aligned ITS and trnL sequences were 699 bp and 327 bp long, respectively. Mean pairwise distances ranged from 0·96 % (M. euryolapa vs. M. leptoclada) to 7·72 % (M. pterocalyx vs. M. rhodantha) for ITS and from 0·37 % (M. gentiles vs. M. serra and M. leptoclada vs. M. serra) to 7·91 % (M. pterocalyx vs. M. primuliflora) for trnL within Metagentiana. The highest pairwise distance found between Metagentiana and other genera in subtribe Gentianinae was 17·37 % (G. aristata vs. M. rhodantha) and 15·23 % (G. boryi vs. M. primuliflora) for ITS and trnL, respectively (distance matrix not shown).
When gaps were excluded, the ITS sequences used for analyses contained 193 potentially informative changes. The strict consensus tree was generated from 387 most-parsimonious trees in one island each with 740 steps, a consistency index of 0·57 and a retention index of 0·72 (not shown). The Bayesian majority rule consensus tree pooled from the Bayesian trees is shown in Fig. 1. Many of the nodes along the spine of this tree have strong or moderate BPP and BS support. One of the ML trees is also shown (Fig. 2A). The topology of the ML trees is similar to that of the Bayesian majority rule consensus tree with only minor differences between them. The present analysis strongly supports the placement of Crawfurdia, Metagentiana and Tripterospermum (grey frame), as sister to the Gentiana clade (BPP = 100 %; BS = 94 %), as previously reported by Yuan et al. (1996). In the Crawfurdia, Metagentiana and Tripterospermum clade (Fig. 1), M. eurycolpa and M. leptoclada form a clade (BPP = 64 %; BS = 85 %) sister to the rest. Five species of Metagentiana (M. gentiles, M. primuliflora, M. rhodantha, M. serra and M. villifera) form a second clade (BPP = 93 %; BS = 93 %). Three species of Metagentiana (M. pterocalyx, M. striata and M. souliei) form a third clade placed with species of Crawfurdia and Tripteropermum. In this third clade, Crawfurdia tibetica forms a clade with Tripterospermum cordatum and T. volubile; thus the recognition of Metagentiana, Tripterospermum and Crawfurdia as three distinct genera is not supported.
Fig. 1.
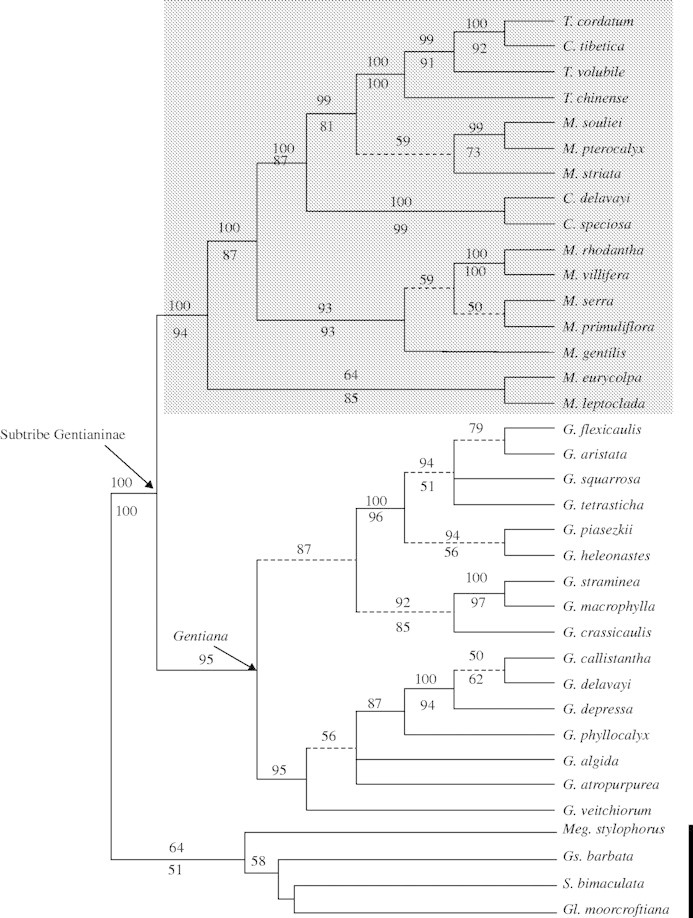
Fifty per cent Bayesian majority rule consensus tree based on ITS sequence data. Numbers above and below the branches indicate the Bayesian posterior probabilities and the MP bootstrap values based on 1000 replicates, respectively. Dashed lines denote branches that collapse in the strict consensus tree from the parsimony analysis. The species of Crawfurdia, Metagentiana and Tripterospermum are indicated by grey shading. The thick black line indicates the four outgroups.
Fig. 2.
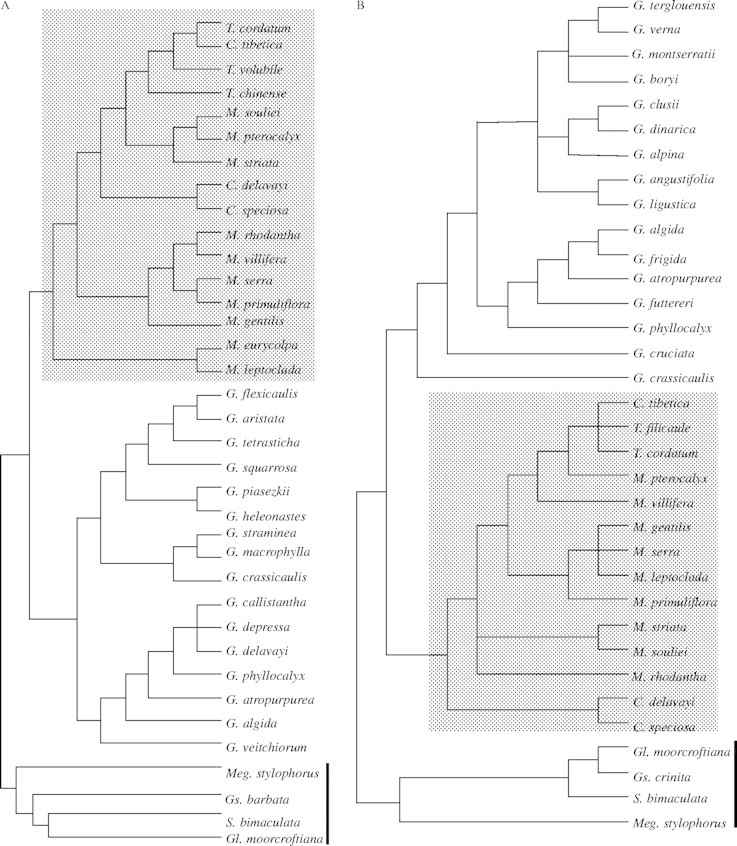
(A) The best maximum likelihood tree based on the ITS matrix (likelihood score, –ln 4997·54138). (B) The the best maximum likelihood tree based on the trnL matrix (likelihood score, –ln 1598·70584). The species of Crawfurdia, Metagentiana and Tripterospermum are indicated by grey shading. The thick black line indicates the four outgroups.
The aligned trnL data included 75 potentially informative characters when gaps were excluded. The Bayesian majority rule consensus tree is shown in Fig. 3. Parsimony analysis identified 3321 most parsimonious trees with 212 steps, a consistency index of 0·72 and a retention index of 0·85. The ML analysis resulted in a tree with a likelihood score of –ln 1598·70548 (Fig. 2B). Because the trnL sequence of M. eurycolpa was not obtained, this species was omitted in the phylogenetic analyses of trnL. These analyses also support the placement of Crawfurdia, Metagentiana and Tripterospermum (grey frame) together as sister to the Gentiana clade (BPP = 100 %; BS = 93 %). In agreement with the results based on ITS data, Crawfurdia, Metagentiana and Tripterospermum were polyphyletic.
Fig. 3.
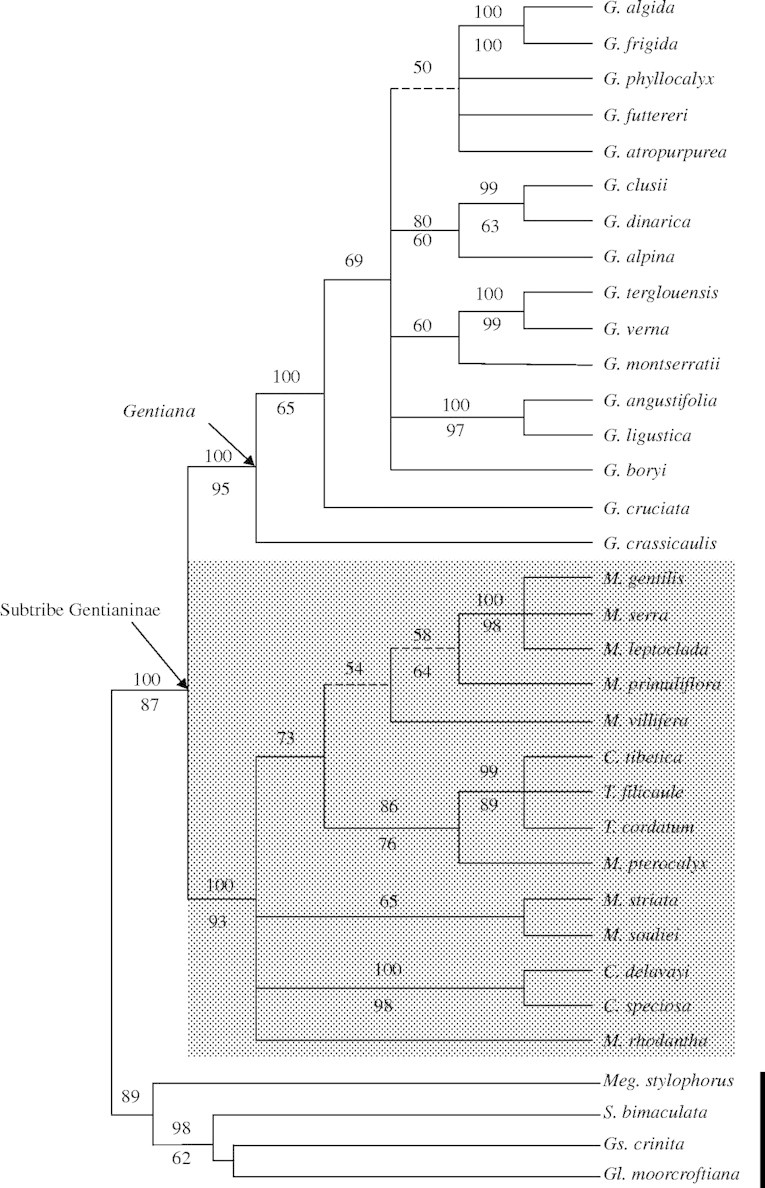
Fifty per cent Bayesian majority rule consensus tree based on trnL sequence data. See Fig. 1 for further details.
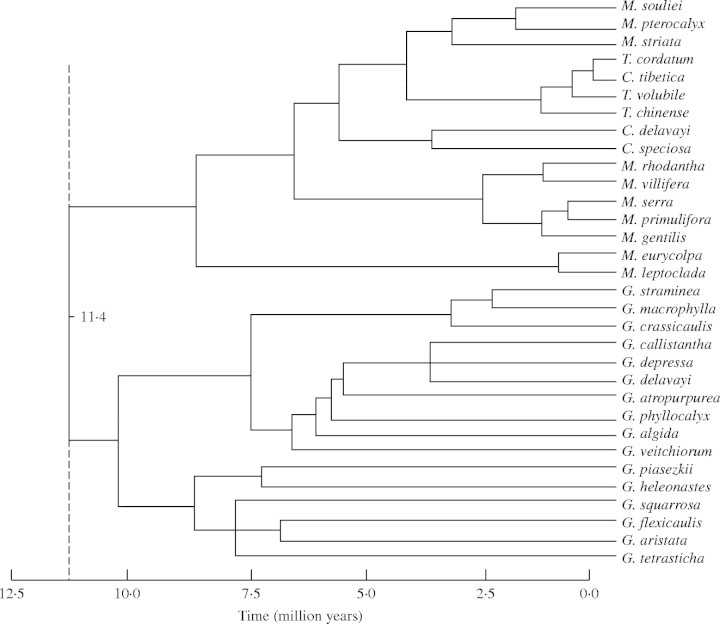
The LR test rejected a molecular clock hypothesis based on their models and correlative parameters for both ITS and trnL (P < 0·01). The ML trees based on ITS and trnL were subjected to clock-independent NPRS to obtain homogenized rates. There is no fossil record for the tribe Gentianeae to calibrate the molecular phylogeny of Crawfurdia, Metagentiana and Tripterospermum. The lowest rate of 4·48 × 10−9 substitutions per site per year (s/s/y) and the highest rate of 8·41 × 10−9 s/s/y previously reported for ITS data of Gentianella (Hagen and Kadereit, 2001) were used to estimate divergence times within the clade consisting of Crawfurdia, Metagentiana and Tripterospermum. The inferred divergence times were plotted on the ITS phylogenetic tree obtained with ML (Fig. 4). According to the rates and ITS sequence divergence, the clade formed by Crawfurdia, Metagentiana and Tripterospermum diverged from Gentiana about 11·4–21·4 Mya (million years ago). The divergence times between extant species range from 0·4 to 6·2 Mya.
Fig. 4.
The maximum likelihood tree with estimated ages based on NPRS of ITS (8·41 × 10−9 s/s/y of the evolutionary rates). The dashed line denotes the divergence time of Crawfurdia, Metagentiana and Tripterospermum from Gentiana.
DISCUSSION
Polyphyly of Crawfurdia, Metagentiana and Tripterospermum
Crawfurdia, Metagentiana and Tripterospermum are not monophyletic according to the molecular data presented here. In the phylogenetic tree based on ITS, ten species of Metagentiana fell mainly in two clades: M. pterocalyx, M. striata and M. souliei grouped with the six sampled species of Crawfurdia and Tripteropsermum, whereas M. gentiles, M. primuliflora, M. rhodantha, M. serra and M. villifera grouped together as a strongly supported clade (Figs 1, 2A). With trnL, nine species of Metagentiana formed four clades in the Bayesian majority rule consensus tree (Fig. 3) and three clades in the ML tree (Fig. 2B). Metagentiana villifera formed a clade with M. gentiles, M. leptoclada, M. primuliflora and M. serra in the Bayesian majority rule consensus tree and grouped with M. pterocalyx and the three sampled species of Crawfurdia and Tripterospermum as a clade in the ML tree. These findings indicate that Crawfurdia, Metagentiana and Tripterospermum should not be treated as three distinct genera. However, the present study sampled only six species (out of about 41 defined by Ho and Pringle, 1995) of Crawfurdia and Tripterospermum. Analysis including more species of Crawfurdia and Tripterospermum is necessary to clarify the phylogenetic and taxonomic status of the three genera.
Relationships of Crawfurdia, Metagentiana and Tripterospermum
Phylogenetic trees for Crawfurdia, Metagentiana and Tripterospermum based on the ITS and trnL in this study agree to a certain extent with the phylogenetic hypothesis based on analyses of gross morphology, floral anatomy, chromosomes, palynology, embryology and other molecular data (Smith, 1965; Nilsson, 1967; Löve and Löve, 1976; Yuan et al., 1993a, b, 1996; Chen et al., 1999a, b, 2000; Ho et al., 2000, 2002b). The genus Metagentiana is easily recognized by its solitary, sessile flowers, sessile, broad-ovate to ovate-triangular leaves and large leaf-like bracts, which make this genus more similar to Crawfurdia and Tripterospermum than to Gentiana (Ho et al. 2002a). The stems of Gentiana and Metagentiana are erect and branched, whereas in Crawfurdia and Tripterospermum they are twining. The midveins of the calyx lobes are keeled and winged into the calyx tube in Crawfurdia, Metagentiana and Tripterospermum but are not keeled in Gentiana. The style is filiform and about as long as the ovary in these three genera, but is linear to cylindrical and shorter than the ovary in Gentiana (Ho et al., 2002a). Pollen morphology of subtribe Gentianinae was studied by Nilsson (1967), who suggested that there were close relationships between Crawfurdia, Metagentiana and Tripterospermum. Chromosome numbers have been reported for eight species of Metagentiana (Yuan and Küpfer, 1993a; Ho et al., 2002b). Their basic chromosomal numbers are x = 17, 21 and 23. The karyotypes of Metagentiana are 3A and 3B according to Stebbin's classification (Stebbins, 1971). The higher and apparently secondary basic numbers and asymmetrical karyotypes suggested that Metagentiana had an isolated position in the genus Gentiana (Yuan and Küpfer, 1993a; Table 1). Metagentiana is more similar to Crawfurdia and Tripterospermum than to Gentiana in karyological characters because Crawfurdia and Tripterospermum also have high basic numbers (x = 23) and asymmetrical karyotypes (S. L. Chen, unpubl. res.). In embryological characters, Metagentiana has a unitary original tapetum, uninucleate tapetal cells which do not protrude into the anther locule, one-celled middle layers, a typical parietal placenta, a hypertropous ovule type and ovules often arranged in four columns (Ho et al., 2000). Gentiana has a dual original tapetum, binucleate tapetal cells which elongate and protrude into the anther locule to form ‘trabeculae’ and ‘placentoids’, two-celled middle layers, a superficial parietal placenta, an anatropous ovule and ovules arranged in 10–30 columns (Ho and Liu, 1999). Crawfurdia and Tripterospermum share the same unitary original tapetal and typical parietal placenta with Metagentiana (Chen et al., 2000; Ho et al., 2000). Thus previous evidence indicates that Metagentiana has a closer relationship with Crawfurdia and Tripterospermum than with Gentiana. The molecular data in this study indicate that these three genera form a monophyletic group as sister group to Gentiana and that all three genera are polyphyletic as currently circumscribed.
Biogeographic considerations
Tribe Gentianeae is widely distributed, with the highest diversity occurring in the Old World. Of the two major clades of Gentianeae, subtribe Gentianinae is clearly centred in the Old World (Struwe et al., 2002). All members of Crawfurdia, Metagentiana and Tripterospermum grow in central to east Asia. Within Gentiana, some sections are primarily European, with a few species in north-west Africa, north-east North America and central Asia (Meusel et al., 1978). The widespread sections are generally most diverse in eastern Asia (Ho and Liu, 1990). An exception to this is Gentiana sect. Pneumonanthe which is most diverse in eastern North America. In the context of this distributional pattern and the inferred phylogenetic relationships, it seems most likely that the ancestor of Gentianinae occupied an alpine temperate range in the Old World, and that the New World and southern hemisphere were colonized secondarily (Yuan et al., 1996). The distribution of generic and specific diversity would suggest an eastern Asian origin for Gentianinae (Struwe et al., 2002). Virtually nothing is known about the timescale of this diversification, except that the European Gentiana sect. Ciminalis may have begun to radiate 2 Mya according to Hungerer and Kadereit (1998). However, geological and paleobotanical studies in south-east Asia, especially in south-western China, provide a good framework to develop a scenario regarding the divergence and radiation of Crawfurdia, Metagentiana and Tripterospermum. South-east Asia has a relatively high proportion of Tertiary relicts of vascular plants (Wu, 1980; Tiffney, 1985a, b; Qian et al., 2003). During the early Tertiary, a relatively uniform, warm climate covered the northern Hemisphere (Tiffney, 1985a). During this time, a relatively continuous, homogeneous flora with many tropical and subtropical elements, called ‘the boreotropical flora’ (Wolfe, 1975), spanned most of the current Arctic area (Latham and Ricklefs, 1993). This boreotropical flora was gradually shaped into a mixed mesic forest and became fragmented as cooling climates in the middle and late Tertiary towards the Pleistocene forced the flora southward (Wolfe, 1975; Tiffney, 1985a). During the climate cooling, cold-intolerant taxa at higher latitudes either migrated to lower latitudes or went extinct, giving way to cool-adapted taxa derived from the boreotropical flora or which evolved during the climate cooling (Leopold and MacGinitie, 1972; Wolfe, 1975; Tiffney, 1985a; Xiang and Soltis, 2001). The present data from the ITS analysis suggest that the three genera diverged from Gentiana about 11·4–21·4 Mya. This estimated time correlates well with the climate cooling in the Miocene (5·3–23 Mya). During the Miocene, south-western China was apparently occupied by mesic mixed deciduous hardwood forest with numerous broad-leaved evergreens (Hu and Chaney, 1940). Especially during the late Miocene, the drier climate had spread extensively in the western Himalayan region including south-western China, where evergreen forest was replaced gradually by semi-deciduous and dry deciduous forest with a rapid expansion of grasslands (Quade et al., 1989, 1995). A major reason is that the uplift of several thousand metres of the Himalayas with perhaps 2300–3000 m increase since the middle Miocene had resulted in drastic changes in the regional biota and dry climates in the Himalayan region. Gentiana, Primula and Rhododendron are among many genera that radiated widely in the mountains of China and underwent rapid radiation, probably driven by the Himalayan uplift since the late Miocene (Axelrod et al., 1996). Therefore, it is possible that the ancestors of these three genera grew in south-western China during the Miocene and produced more species and occupied a wider distribution in the subsequent radiation. The current species of these three genera are mainly distributed in western China and grow in coniferous forest, alpine shrub and alpine meadow habitats. The divergence time of the current species of three genera (about from 0·4 and 6·2 Mya) estimated from ITS sequences corresponds well with this hypothesis.
Acknowledgments
The authors thank the decision editor Dr Mike Fay and anonymous reviewers for critical comments, Dr Yuan Yongming for constructive comments on the manuscript, and Professor Ho Tingnong for identifying specimens. This work was supported by the key innovation project of the Chinese Academy of Sciences (KSCX2-SW-106) and the National Science Foundation of China (30170066).
LITERATURE CITED
- Avise JC. 2000.Phylogeography: the history and formation of species. Cambridge, MA: Harvard University Press. [Google Scholar]
- Axelrod DI, Al-Shehbaz I, Raven PH. 1996. History of the modern flora of China. In: Zhang A, Wu SG, eds. Floristic characteristics and diversity of East Asian plants. Beijing: China Higher Education Press, 43–55. [Google Scholar]
- Bain JF, Golden JL. 2000. A phylogeny of Packera (Senecioneae; Asteraceae) based on internal transcribed spacer region sequence data and a broad sampling of outgroups. Molecular Phylogenetics and Evolution 16: 331–338. [DOI] [PubMed] [Google Scholar]
- Baldwin BG, Sanderson MJ. 1998. Age and rate of diversification of the Hawaiian silversword alliance (Compositae). Proceedings of the National Academy of Sciences of the USA 95: 9402–9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin BG, Sanderson MJ, Porter JM, Wojciechowski MF, Campbell CS, Donoghue MJ. 1995. The ITS region of nuclear ribosomal DNA: a valuable source of evidence on angiosperm phylogeny. Annals of the Missouri Botanical Garden 82: 247–277. [Google Scholar]
- Blume CL. 1826.Tripterospermum. In: Bijdrangen tot de Flora van Nederlandsch Indië. Batavia, 849. [Google Scholar]
- Chase MW, Hills HH. 1991. Silica gel: an ideal material for field preservation of leaf samples for DNA studies. Taxon 40: 215–220. [Google Scholar]
- Chen SL, Ho TN, Liu JQ. 1997. The chromosome number of 8 species in Gentiana (Gentianaceae) from the alpine mountains of the western China. Acta Botanica Boreali-Occidentalia Sinica 17: 547–550. [Google Scholar]
- Chen SL, Ho TN, Liu JQ, Hong DY. 1999. Mega-microsporogeneis and female-melegametagenesis of Tripterospermum chinense (Gentianaceae). Acta Biologica Plateau Sinina 14: 26–34. [Google Scholar]
- Chen SL, Ho TN, Liu JQ, Hong DY. 1999. Floral anatomy of Crawfurdia and its related taxa (Gentianaceae). Acta Biologica Plateau Sinina 14: 35–46. [Google Scholar]
- Chen SL, Ho TN, Liu JQ, Hong DY. 2000. Embryology of Tripterospermum cordatum (Gentianaceae). Acta Botanica Yunnanica 22: 53–58. [Google Scholar]
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf material. Phytochemical Bulletin 19: 11–15. [Google Scholar]
- Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Fitch WM. 1971. Toward defining the course of evolution: minimum change for a specific tree topology. Systematic Zoology 20: 406–416. [Google Scholar]
- Franchet A. 1884. Description de quelques especes de Gentiana de Yunnan. Bulletin de la Société Botanique de France 31: 373–378. [Google Scholar]
- Gielly L, Taberlet P. 1996. A phylogeny of the European gentianas inferred from plastid trnL (UAA) intron sequences. Botanical Journal of Linnean Society 120: 57–75. [Google Scholar]
- Goldman N. 1993. Statistical tests of models of DNA substitution. Journal of Molecular Evolution 36: 182–198. [DOI] [PubMed] [Google Scholar]
- Hagen KB, Kadereit JW. 2001. The phylogeny of Gentianella (Gentianaceae) and its colonization of the southern hemisphere as revealed by nuclear and plastid DNA sequence variation. Organisms Diversity and Evolution 1: 61–79 [Google Scholar]
- Halda JJ. 1995. Synopsis of the new system of the Gentiana. Acta Musei Richnoviensis Section Nature 3: 25–29. [Google Scholar]
- Ho TN, Liu JQ. 1999. Embryology of Gentiana lawrencei var. farreri Acta Botanica Boreali-Occidentalia Sinica 19: 234–240. [Google Scholar]
- Ho TN, Liu SW. 1990. The infrageneric classification of Gentiana (Gentianaceae). Bulletin of the British Museum (Natural History) Botany 20: 169–192. [Google Scholar]
- Ho TN, Pringle JS. 1995. Gentianaceae. In: Wu ZY, Raven PH, eds. Flora of China, Vol. 16. Beijing/St Louis, MO: Science Press/Missouri Botanical Garden, 1–140. [Google Scholar]
- Ho TN, Chen SL, Liu JQ, Hong DY. 2000. Embryology of Gentiana striata (Gentianaceae). Acta Botanica Boreali-Occidentalia Sinica 20: 960–967. [Google Scholar]
- Ho TN, Chen SL, Liu SW. 2002.Metagentiana, a new genus of Gentianaceae. Botanical Bulletin of Academia Sinica 43: 83–91. [Google Scholar]
- Ho TN, Liu JQ, Chen SL. 2002. Contribution to the karyomorphology of seven species in Gentiana (Gentianaceae). Acta Biologica Plateau Sinina 15: 67–73. [Google Scholar]
- Hu HH, Chaney RW. 1940. A Miocene flora from Shantung Province, China. In: Whyte RO, ed. The evolution of East Asian environments Vol. 2. Palaeobotany, palaeozoology and palaeoanthropology, Publications of the Carnegie Institution of Washington, 507: 1. Hong Kong: Center of Asian Studies, 472–481. [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogeny. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Hungerer KB, Kadereit JW. 1998. The phylogeny and biogeography of Gentiana L. sect. Ciminalis (Adans.) Dumort.: a historical interpretation of distribution ranges in the European high mountains. Perspectives in Plant Ecology, Evolution and Systematics 1: 121–135. [Google Scholar]
- Küpfer P, Yuan YM. 1996. Karyological studies on Gentiana sect. Chondrophyllae (Gentianaceae) from China. Plant Systematics and Evolution 200: 161–176. [Google Scholar]
- Kusnezov NI. 1894. Subgenus Eugentiana of genus Gentiana Trudy S.-Peterburghskago Obščestvah. Estesttvoispytatelej, Otde lenie Botaniki 24: 1–507. [Google Scholar]
- Larget B, Simon DL. 1999. Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Molecular Biology and Evolution 16: 750–759. [Google Scholar]
- Latham RE, Ricklefs RE. 1993. Global patterns of tree species richness in moist forests: energy-diversity theory does not account for variation in species richness. Oikos 67: 325–333. [Google Scholar]
- Leopold EB, MacGinitie HD. 1972. Development and affinities of Tertiary floras in the Rocky Mountains. In: Graham A, ed. Floristics and paleofloristics of Asia and eastern North America. Amsterdam: Elsevier, 147–200. [Google Scholar]
- Liu JQ, Gao TG, Chen ZD, Lu AM. 2002. Molecular phylogeny and biogeography of the Qinghai-Tibet Plateau endemic Nannoglottis (Asteraceae). Molecular Phylogenetics and Evolution 23: 307–325. [DOI] [PubMed] [Google Scholar]
- Löve A, Löve D. 1976. The natural genera of Gentianaceae. In: Kachroo P, ed. Recent advances in botany. Delhi: Dehra Dun, 205–222. [Google Scholar]
- Maddison DR. 1991. The discovery and importance of multiple islands of most-parsimonious trees. Systematic Zoology 40: 315–318. [Google Scholar]
- Marquand CVB. 1931. New Asiatic gentianas: II. Bulletin of Miscellaneous Information, Royal Gardens, Kew 1931: 68–88. [Google Scholar]
- Marquand CVB. 1937. The gentianas of China. Bulletin of Miscellaneous Information, Royal Gardens, Kew 1937: 134–180. [Google Scholar]
- Meusel H, Jäger EJ, Rauschert S, Weinert E. 1978. Vergleichende chorologie der zentraleuropäischen Flora I-II. Jena: Fischer Verlag. [Google Scholar]
- Muse SV, Weir BS. 1992. Testing for equality of evolutionary rates. Genetics 132: 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S. 1967. Pollen morphological studies in the Gentianaceae-Gentianinae. Grana Palynologica 7: 46–143 [Google Scholar]
- Posada D, Crandall KA. 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- Pringle JS. 1978. Sectional and subgeneric names in Gentiana (Gentianaceae). Sida 7: 232–247. [Google Scholar]
- Qian H, Song JS, Krestov P, Guo QF, Wu ZM, Shen XS, Guo XS. 2003. Large-scale phytogeographical patterns in East Asia in relation to latitudinal and climatic gradients. Journal of Biogeography 30: 129–141. [Google Scholar]
- Quade J, Cerling TE, Bowman JR. 1989. Development of Asian monsoon revealed by marked ecological shift during the latest Miocene in northern Pakistan. Nature 342: 163–165. [Google Scholar]
- Quade JJ, Cater ML, Ojha TP, Adam J, Harrison TM. 1995. Late Miocene environmental change in Nepal and the northern Indian subcontinent: stable isotopic evidence from paleosols. Geological Society of America Bulletin 107: 1381–1397. [Google Scholar]
- Rambaut A, Charleston M. 2000. TreeEdit: Phylogenetic Tree Editor. Oxford: University of Oxford. [Google Scholar]
- Richardson JE, Weitz FM, Fay MF, Cronk QC, Linder HP, Reeves G, Chase MW. 2001. Rapid and recent origin of species richness in the Cape flora of South Africa. Nature 412: 181–183. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ. 1997. A nonparametric approach to estimating divergence times in the absence of rate constancy. Molecular Biology and Evoution 14: 1218–1231. [Google Scholar]
- Simmons MP, Ochoterena H. 2000. Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology 49: 369–381. [PubMed] [Google Scholar]
- Smith H. 1965. Notes on Gentianaceae Notes from the Royal Botanic Garden, Edinburgh 26: 237–258. [Google Scholar]
- Stebbins GL. 1971.Chromosomal evolution in higher plants. London: Edward Arnold. [Google Scholar]
- Struwe L, Kadereit JW, Klackenberg J, Nilsson S, Thiv M, von Hagen KB, et al. 2002. Systematics, character evolution, and biogeography of Gentianaceae, including a new tribal and subtribal classification. In: Struwe L, Albert VA, eds. Gentianaceae—systematics and natural history. Cambridge: Cambridge University Press, 21–309. [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic analysis using parsimony (* and other methods). Version 4.0b10. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Swofford DL, Olsen GJ, Waddell PJ, Hillis DM. 1996. Phylogenetic inference. In: Hillis DM, Moritz C, Mable BK, eds. Molecular systematics. Sunderland, MA: Sinauer, 407–514. [Google Scholar]
- Taberlet P, Gielly L, Patou G, Bouvet J. 1991. Universal primers for amplification of three non-coding regions of plastid DNA. Plant Molecular Biology 17: 1105–1109. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffney BH. 1985. Perspectives on the origin of the floristic similarity between eastern Asia and eastern North America. Journal of the Arnold Arboretum 66: 73–94. [Google Scholar]
- Tiffney BH. 1985. The Eocene North Atlantic land bridge: its importance in Tertiary and modern phytogeography of the Northern Hemisphere. Journal of the Arnold Arboretum 66: 243–273. [Google Scholar]
- Wallich N. 1826.Tentamen Florae Napalensis Illustratae, Part 2. Calcutta, 37–64. [Google Scholar]
- Wang JB, Zhang WJ, Chen JK. 1999. Application of ITS sequences of nuclear DNA in phylogenetic and evolutionary studies of angiosperms. Acta Phytotaxonomica Sinica 37: 407–416. [Google Scholar]
- Wang XQ, David CT, Sang T. 2000. Phylogeny and divergence times in Pinaceae: evidence from three genomes. Molecular Biology and Evoution 17: 773–781. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds. PCR protocols: a guide to methods and application. San Diego, CA: Academic Press, 315–322. [Google Scholar]
- Wolfe JA. 1975. Some aspects of plant geography of the Northern Hemisphere during the Late Cretaceous and Tertiary. Annals of the Missouri Botanical Garden 62: 264–279. [Google Scholar]
- Wu CY. 1980.The vegetation of China. Beijing: Science Press. [Google Scholar]
- Xiang QY, Soltis DE. 2001. Dispersal-vicariance analyses of intercontinental disjuncts: historical biogeographical implications for angiosperms in the Northern Hemisphere. International Journal of Plant Sciences 162: S26–S39. [Google Scholar]
- Yuan YM. 1993. Karyological studies on Gentiana sect. Cruciata (Gentianaceae) from China. Cytologia 46: 99–114. [Google Scholar]
- Yuan YM, Küpfer P. 1993. Karyological studies on Gentiana sect. Frigida senso lato and sect. Stenogyne (Gentianaceae) from China. Bulletin de la Société entomologique de France 116: 65–78. [Google Scholar]
- Yuan YM, Küpfer P. 1993. Karyological studies of Gentianopsis Ma and some related genera of Gentianaceae from China. Cytologia 58: 115–123. [Google Scholar]
- Yuan YM, Küpfer P. 1995. Molecular phylogenetics of the subtribe Gentianinae (Gentianaceae) inferred from the sequences of internal transcribed spacers (ITS) of nuclear ribosomal DNA. Plant Systematics and Evolution 196: 207–226. [Google Scholar]
- Yuan YM, Küpfer P. 1997. The monophyly and rapid evolution of Gentiana sect. Chodronphyllae Bunge s.l. (Gentianaceae): evidence from the nucleotide sequences of the internal transcribed spacers of nuclear ribosomal DNA. Botanical Journal of the Linnean Society 123: 25–43. [Google Scholar]
- Yuan YM, Küpfer P, Doyle JJ. 1996. Infrageneric phylogeny of the Genus Gentiana (Gentianaceae) inferred from nucleotide sequences of the internal transcribed spacers (ITS) of nuclear ribosomal DNA. American Journal of Botany 83: 641–652. [Google Scholar]
- Yuan YM, Wohlhauser S, Möller M, Chassot P, Mansion G, Grant J, et al. 2003. Monophyly and relationships of the tribe Exaceae (Gentianaceae) inferred from nuclear ribosomal and plastid DNA sequences. Molecular Phylogenetics and Evolution 28: 500–517. [DOI] [PubMed] [Google Scholar]