Abstract
• Background and Aims The anatomical structure and development of adventitious roots were analysed in the basal monocotyledon, Acorus calamus, to determine to what extent those features are related to phylogenetic position.
• Methods Root specimens were harvested and sectioned, either with a hand microtome or freehand, at varying distances from the root tip and examined under the microscope using a variety of staining techniques.
• Key Results Roots of Acorus calamus possess a unique set of developmental characteristics that produce some traits similar to those of another basal angiosperm group, Nymphaeales. The root apical meristem organization seems to be intermediate between that of a closed and an open monocotyledonous root apical meristem organization. The open-type root apical meristem consists of a curved zone of cortical initials and epidermal initials overlying the vascular cylinder initials; the epidermal part of the meristem varies in its association with the cortical initials and columellar initials of the promeristem. The cortex develops an endodermis with only Casparian bands, a dimorphic exodermis with Casparian bands and suberin lamellae, and a polygonal aerenchyma by differential expansion, as also observed in the Nymphaeales and some dicotyledonous species. The stele has characteristics like those of members of the Nymphaeaceae.
• Conclusions Specific anatomical and developmental attributes of Acorus roots seem to be related to the phylogenetic position of this genus.
Keywords: Acorus, anatomy, apical meristem, cortex, phylogeny, root, stele
INTRODUCTION
While roots of many species and groups of flowering plants have recently been studied developmentally, the roots of the plants considered to be basal monocotyledons by the Angiosperm Phylogeny Group (Angiosperm Phylogeny Group, 1998, 2003) have received little attention with regard to root characteristics that may be relevant to their phylogenetic position. The basal monocotyledon, Acorus calamus (sweet flag, Acoraceae), has been determined to diverge quite early from the trunk of the phylogenetic tree of angiosperms. This species is phylogenetically closely associated with the basal dicotyledonous angiosperm group, the Nymphaeales (Angiosperm Phylogeny Group, 1998, 2003), which has been recognized to be an ancient group (Friis et al., 2001). The phylogenetic position of Acorus was determined mainly from nucleic acid sequencing analysis, but anatomical and morphological data supporting this position are rather scarce. While some features of its root apex and general root anatomy were reported long ago (e.g. Janczewski, 1874; Holle, 1876; Kroll, 1912), little consistent information has been found on the developmental and structural features of its roots (see Keating, 2003), except for the analysis of vessels by Carlquist and Schneider (1997). Therefore, attention was focused on the root apical meristem and the development of the cortex of adventitious roots in Acorus calamus, but also included are related observations on other root features, including stelar and epidermal characteristics.
MATERIALS AND METHODS
Plants of Acorus calamus were obtained from the Institute of Botany in Trebon, Czech Republic, and from various growers in eastern North America. The plants were either sampled directly or further cultivated in hydroponics in one-quarter strength Hoagland solution in cold greenhouses. Tips of adventitious roots of various lengths were sampled, fixed, dehydrated and embedded in paraplast wax (according to Jensen, 1964). Sections, microtomed at 6–10 µm, were stained with aqueous 1 % safranin and counter-stained with 0·5 % fast green or aniline blue in 95 % ethanol.
For examination of the stages of cortex development and structure, root specimens were harvested and sectioned, either with a hand microtome or freehand, at varying distances from the root tip. Specimens were examined unstained or stained with toluidine blue O (0·01 % in water) under bright field or epifluorescence (microscope Olympus BX 51). Most specimens were stained with berberine hemisulfate and counterstained with aniline blue (Brundrett et al., 1988) or toluidine blue O, or stained in fluorol yellow (Brundrett et al., 1987); these were examined under ultraviolet (330–385 nm) or blue (460–490 nm) excitation. Pictures were taken with digital camera Apogee KX32ME (Apogee Instruments Inc.)
RESULTS
The adventitious roots of Acorus calamus have an open type of root apical meristem, which might superficially appear to be closed (Fig. 1A, B; see Clowes, 1981, 2000). This is because multi-tiered, curved files of cells in the young cortex or ground meristem appear to emanate from near the centre of the apical initials (Fig. 1A). However, these are not continuous files of cells from the tip of the initials into the cortex. The young epidermis or protoderm abuts against either the edges of the zone of cortical and outer columellar initials (Fig. 1A) or more centrally within the zone of cortical and columellar initials, although there appears to be no characteristic pattern at any growth stage. There are no distinct boundaries between the cortical–epidermal initials and the initials of the central root cap (Fig. 1A), and cell divisions are found that produce cells bridging any such possible boundary. There are also no regular files of cells with distinct and regular periclinal divisions in the ground meristem that would produce radially aligned cells (Fig. 1E, F) across the cortex, although there are one to two (rarely three) periclinal divisions in the innermost part of cortex (Fig. 1E, F). There are periclinal to oblique cell divisions scattered throughout the ground meristem, as manifested by cell walls of recent divisions, just proximal to the cortical initials (Fig. 1E, F) that increase the width of the cortex, and there are one or more sets of regular periclinal cell divisions in the prohypodermis, outermost layer of the cortex adjacent to the protoderm, that produce the multiseriate hypodermis (Fig. 2C, D).
Fig. 1.
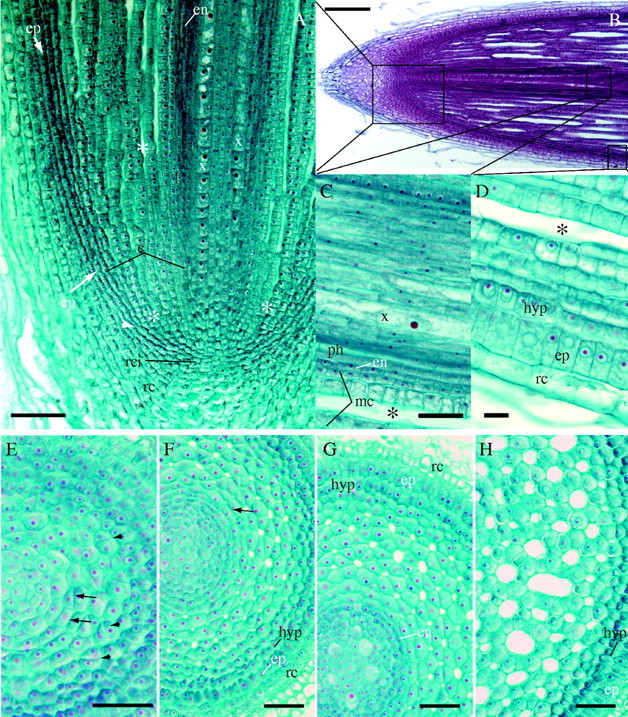
Longitudinal or cross-sections of root apex (paraplast sections stained with safranin–aniline blue or safranin–fast green FCF). (A) Detail of apical meristem arrangement: developing cortex (c) is marked by lines from epidermis to the central cylinder with developing xylem (x); proendodermis (en) becomes distinguishable in inner cortex farther from the tip. The origin of epidermis (arrows, ep) and outer cortex (c) occurs at the site of the periclinal divisions (arrowhead). The development of aerenchymatous intercellular spaces (asterisks) occurs close to the root tip. rc, lateral root cap; rci, root cap initials. Scale bar = 100 μm. (B) Overall view of root apex, stained with safranin–aniline blue. Scale bar = 200 μm. (C) The innermost layer of ground meristem forms the proendodermis (en) as well as several layers of adjacent middle cortex (mc); xylem (x); phloem (ph). Scale bar = 50 μm. (D) Epidermis (ep) and hypodermis (hyp) remain covered by root cap cells (rc) at considerable distance (about 3 m) from the root tip. Asterisks indicate aerenchymatous lacuna. Scale bar = 50 μm. (E–H) Series of cross-sections from the root apex showing the development of cortical tissues: 30 μm (E), 140 μm (F), 300 μm (G) and 750 μm (H) behind the root apical meristem. Arrowheads indicate oblique divisions in developing cortex; arrows indicate periclinal divisions in the inner cortex. en, proendodermis; ep, epidermis; hyp, hypodermis; rc, root cap cells. Scale bars = 50 μm.
Fig. 2.
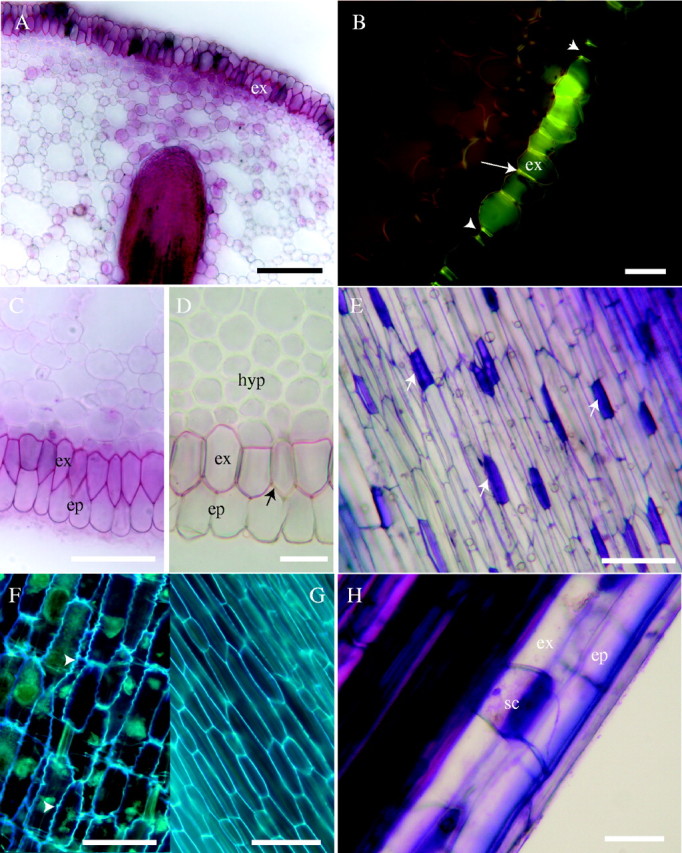
Structure and development of hypodermis; fresh sections. (A) Lateral root primordium growing towards suberized exodermis (ex) through the cortex with well-developed aerenchymatous channels: stained with sudan red 7B. Scale bar = 100 μm. (B) Casparian bands (arrow) in exodermis. The fluorescence in short cells (arrowheads) is, compared with outer tangential walls of surrounding cells of exodermis (ex), efficiently suppressed after staining with toluidine blue indicating absence of suberin lamella; 40 mm from the root tip of 280-mm-long root; fresh section, berberine–toluidine blue, blue excitation. Scale bar = 50 μm. (C) Exodermis (ex) and epidermis (ep) stained with sudan red 7B for lipids; 40 mm behind the tip. Scale bar = 50 μm. (D) Thickening of cell walls in inner layers of hypodermis (hyp) 250 mm from root tip. There is also an obvious absence of complete suberin lamella in a passage cell (arrow) of exodermis (ex); stained with sudan red 7B. ep, Epidermis. Scale bar = 50 μm. (E) Surface view of exodemis, with outer tangential short cell walls (arrows) intensely stained with toluidine blue. Scale bar = 50 μm. (F) Surface view of exodermis 50 mm from tip of root, 270 mm long, showing typical undulation of radial cell walls (arrowheads); autofluorescence in UV. Scale bar = 50 μm. (G) Surface view of exodermis at base of 270-mm-long root; autofluorescence in UV. Scale bar = 50 μm. (H) Detail of short cell (sc) of exodermis (ex); epidermis (ep); stained with toluidine blue. Scale bar = 25 μm.
Central cortex and aerenchyma
Intercellular spaces in the adventitious roots of A. calamus begin within the edge of the cortical initials and are surrounded by four to six cells (Fig. 1A, E, F). Within 50 µm of the cortical initials, there were abundant intercellular spaces (Fig. 1A, B, E). Oblique cell divisions present in ground meristem, except in the proendodermis and prohypodermis, increase the number of cells surrounding a space (Fig. 1E–G). The spaces grow into lacunae by expansion of the cell walls and addition of narrower new cells bordering the spaces (Fig. 1E, F), a process described as differential expansion by Seago et al. (2000). Eventually, the cortex consists of a large zone of aerenchyma with polygonal lacunae more or less uniformly distributed from the layer outside the endodermis to the inner edge of the hypodermis. The largest lacunae observed become bounded by 12–18 cells (Figs 1H, 2A). Lateral root primordia grow through fully developed aerenchyma of the cortex (Fig. 2A); there are no strands of intact parenchyma remaining opposite developing primordia nor windows of nonsuberized hypodermis as were observed in reed and some other monocots like members of the Poaceae (Justin and Armstrong, 1987; Soukup et al., 2002).
Development and structure of the hypodermis
The hypodermis is initiated by a separate layer of initials within the apical meristem (Fig. 1A) that divides periclinally at least once to produce a biseriate to multiseriate zone of cells under the epidermis (Figs 1F–H, 2D). Approximately 400 µm behind the tip of the cortex, the long and short cells of the exodermis are distinguishable. Initiation of wall modifications in the exodermis (the outermost layer of the hypodermis) starts at some distance behind the apex and, similar to the endodermis, was accompanied by protoplast autofluorescence. Maturation of Casparian bands (Fig. 2B) and suberin lamellae (Fig. 2C, D), which occurs more or less concomitantly, takes place farther (20–30 mm) behind the tip of growing roots. Not all the exodermal cells possess a complete suberin lamella. These are the short cells, which represent ‘passage cells’ of the dimorphic exodermis (Fig. 2D, E, H). The outer tangential walls of these cells were intensely stained by toluidine blue O, contrasting them to the surrounding long exodermal cells (Fig. 2H). Complete suberin lamellae were not found in the short cells (Fig. 2B, D).
The exodermal walls show a characteristic waviness (Fig. 2F), which became less obvious with development of the exodermis and elongation of its cells (Fig. 2G). There are also one to three additional layers of the nonsuberized hypodermis, internal to the exodermis, which do not produce Casparian bands and suberin lamellae, but which often have secondarily thickened walls in longer roots (Fig. 2D).
Development and structure of the endodermis
The proendodermis can be recognized by autofluorescence of its protoplasts before any cell wall modification is detectable (Fig. 3A). The autofluorescence is apparently caused by a high content of phenolic compounds being transported from vascular tissues (see Van Fleet, 1961). Distinct Casparian bands occur later on as dark dots of wavy cell wall strips expanding to occupy most of those walls, and they stain with sudan for lipid (a component of suberin) (Fig. 4D), and with HCl–phloroglucinol for lignin or lignin-like material (Fig. 4C). In spite of discernable autofluorescence in endodermal cell walls (Fig. 4A) no suberin deposition was detectable in tangential cell walls and thus the endodermis should be considered to stay in developmental state I (Clarkson and Robards, 1975), even in fully determinate, overwintering roots. The presence of Casparian bands was confirmed by band plasmolysis even in basal parts of old roots (Fig. 4D), which would not occur with suberin lamella deposition (Haas and Carothers, 1975).
Fig. 3.
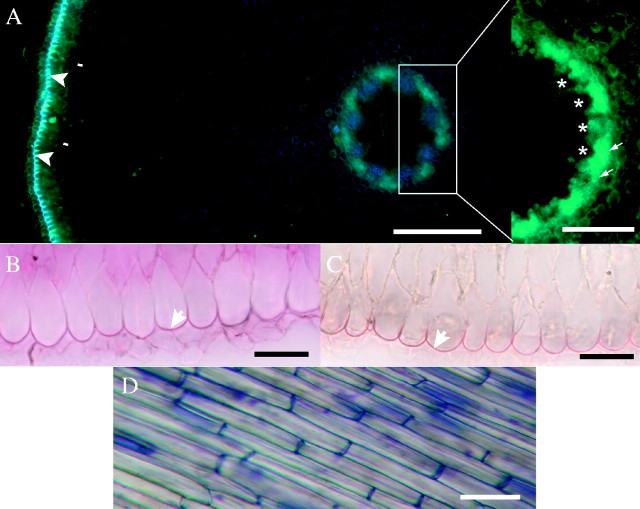
(A) Cross-section at 5 mm from the root tip shows autofluorescence of outer tangential epidermal cell walls (arrowheads). Protoplasts of endodermis (arrows) can be recognized on the outer edge of autofluorescent area external to vascular tissues (asterisks); not stained; fresh section. Scale bar = 50 μm. (B) Lipidic material was detected in the outer epidermal cell walls (arrow); fresh section (5 mm from root tip) stained with sudan red 7B. Scale bar = 25 μm. (C) Lignification of outer epidermal cell walls (arrow); fresh section; HCl–phloroglucinol; 5 mm from tip. Scale bar = 25 μm. (D) Surface view of epidermis; fresh longitudinal section stained with toluidine blue, about 30 mm from tip. Scale bar = 50 μm.
Fig. 4.
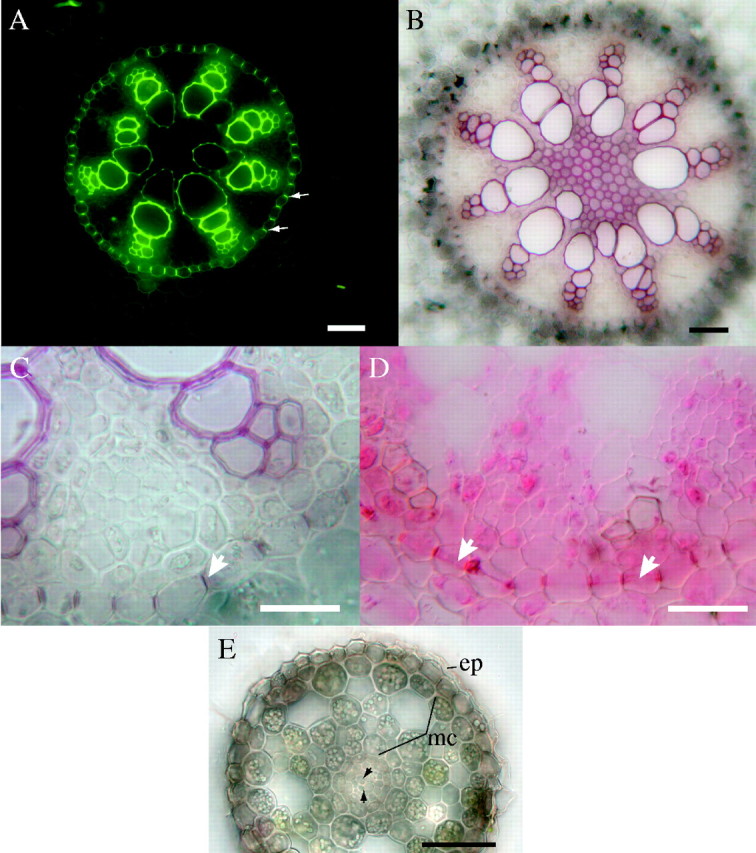
Central cylinders in cross-section in basal part of a well-established root (27 cm long); fresh sections. (A) Autofluorescence under blue excitation. Arrows indicate Casparian bands. Scale bar = 50 μm. (B) Lignified xylem with sclerenchyma in centre of stele; HCl–phloroglucinol. Scale bar = 50 μm. (C) Casparian bands (e.g. arrow) observed in endodermis; HCl–phloroglucinol. Scale bar = 50 μm. (D) Casparian bands observed in endodermis; arrows indicate protoplasts showing band plasmolysis; sudan red 7B. Scale bar = 50 μm. (E) Cross-section of determinate nodal root 30 mm from the root tip showing sloughing epidermis (-ep) and reduced middle cortex (mc). Central cylinder (cc) contains only reduced vascular tissues (arrowheads); differential interference contrast optics. Scale bar = 50 μm.
Stele and epidermis
The vascular cylinder or stele of A. calamus has an interesting configuration of protoxylem and protophloem elements; there are five (Fig. 1G) to ten strands or poles (Fig. 4B) of protoxylem and protophloem elements in basal parts of the roots, and six to eight strands (Fig. 4A) are more common. The centre of the cylinder is not occupied by any large metaxylem element, but there is often sclerenchymatous or parenchymatous pith 5–15 cells across (Fig. 4A, B) with sclerenchymatous cells between some late metaxylem elements. Stele structure is modified along the root. Near the tips of old, long, adventitious roots with terminated growth, the root is very narrow and has only one or two central xylem elements and a small cortex with few lacunae (Fig. 4E).
The epidermis can be distinguished from other cells very near the root tip at a stage where it is covered by several layers of root cap cells. Its outer tangential walls are autofluorescent (Fig. 3A), and give a positive response to Sudan and HCl–phloroglucinol staining (Fig. 3B and C). This was the first cell wall modification in tissues behind the root tip detected by the methods used. In longitudinal section these cells are elongate, narrow and uniform (Fig. 3D). In older, long roots, the epidermis may be dead and sloughed off.
DISCUSSION
As early as 1874, Janczewski classified Acorus calamus as having a closed meristem with three separate initials for the stele, cortex/epidermis, and rootcap (Janczewski, 1874). Clowes (2000) found both open and closed meristems in Acorus, but he did not present any images. It would be easy to describe the Acorus root apical meristem as closed because, on first examination of sections or examining non-median sections, the curving files of ground meristem and of protoderm seem to converge in the centre of the apical meristem, but closer examination shows that there are not many distinct files from the apical meristem into the ground meristem and cortex; the protoderm abuts either cortical or columellar initials. Transverse sections also revealed the lack of a regular radial organization in the cortex that would be a very distinct feature of a closed, one-tiered root apical meristem, often found in monocotyledons (Clowes, 2000). The adventitious roots of A. calamus (Monocotyledonae, Acorales, Acoraceae) have a unique set of developmental characteristics that produce root traits very similar to those of the basal angiosperm group, Nymphaeales. Both of the groups divided early from the phylogenetic tree and are considered sister groups to more advanced angiosperms (Angiosperm Phylogeny Group, 1998, 2003).
The root apical meristem organization of Acorus is close to that of Cabombaceae of the Nymphaeales. Similar to Nymphaeales, the epidermis originates from peripheral cells of cortex initials, features typical of monocots (see Clowes, 1981, 2000; Seago et al., 2000), but in Acorus there is no continuous layer of aligned cells from the tip of the cortical initials into the ground meristem. The organization might appear similar to intermediate-open pattern which was described as the ancestral type of dicotyledonous meristem arrangement (Groot et al., 2004). However, it is not quite the same as Acorus is a monocotyledon, and its meristems bear some features typical of monocots (Clowes, 2000).
The plants so far studied that have aerenchymatous development by differential expansion and its resultant characteristic polygonal organization (Justin and Armstrong, 1987; Laan et al., 1989) include members of the Nymphaeaceae (Seago et al., 2000; Seago, 2002) Acoraceae (Němec, 1907; see also Keating, 2003) and Rumex (Polygonaceae; Justin and Armstrong, 1987; Laan et al., 1989). The differences between members of the Nymphaeaceae and Acoraceae are the lack of cortical diaphragms and sclereids in the Acoraceae, but in this regard, it should be noted that members of the Cabombaceae, closely related to the Nymphaeaceae (Angiosperm Phylogeny Group, 1998; 2003), also lack these structures and thus their root anatomy is even more similar to the Acoraceae. Like members of the Nymphaeaceae (Seago et al., 2000), the endodermis in Acorus roots is derived by few regular periclinal divisions in the innermost layer of the ground meristem, and the multiseriate hypodermis is derived by one to few periclinal divisions in the outer portions of the ground meristem at the edge of the initials.
The endodermis possesses Casparian bands and does not form recognizable, complete suberin lamellae (see Maillefer, 1921). The uniseriate, dimorphic exodermis has Casparian bands and suberin lamellae, and is part of a multiseriate hypodermis, the inner layers (non-exodermal) of which have thickened walls. These general characteristics of endodermis and exodermis are similar to those found in the Nymphaeaceae (Seago, 2002). The presence of a dimorphic exodermis with long and short cells sets Acorus apart from the Nymphaeaceae, but it is consistent with the reports of Kroemer (1903), von Guttenberg (1968), Shishkoff (1987) and Clowes (2000), except that it was found that all cells produce Casparian bands. Shishkoff's study (Shishkoff, 1987) suggested that other early monocots in the closely related Alismatales (Angiosperm Phylogeny Group, 2003) also have a dimorphic exodermis.
Very early modification of outer periclinal cell walls of the root epidermis is described in the literature as cutinization (for a review, see von Guttenberg, 1968). However, no data on chemical composition of the lipidic impregnating compound were found. Impregnation of the epidermis in Acorus precedes any detectable modifications of exo- and endodermal cell walls. Its formation might be connected with protection of underlying tissues as suggested by von Guttenberg (1968). It can only be speculated whether it can temporarily form an apoplastic barrier, a role known for the exodermis that develops farther from the root tip.
While Acorus has been classified as a monocot, its unique root stelar structure has long been known to bridge the boundary between dicots and monocots; for example, Maillefer (1921) illustrated a pentarch arrangement of xylem and phloem and noted that six and seven poles of xylem were also common. He further noted that these characteristics represented an intermediate condition between monocots and dicots. Keating (2003) has recently characterized Acorus as having nine poles of xylem and phloem. While the polyarch condition is not absolute for monocots, it has long been used to describe the normal condition. Interestingly, Nymphaea and most other genera of the Nymphaeaceae are also characterized as having a variable number of poles, usually five to nine (Conard, 1905; Seago, 2002, see also Schneider et al., 1995). This is particularly intriguing because morphologists have sometimes characterized the dicotyledons as typically having two to five strands of primary xylem while the monocotyledons are polyarch with six to many strands (e.g. Haupt, 1953; Eames, 1961). Indeed, Eames (1961, p. 23) went further to state that the tetrarch situation was probably ‘the primitive type because it best fit structurally into the hypocotyl’ and that the polyarch situation seemed ‘highly specialized’; these speculations have rarely been challenged. Further, the characteristics of the vessel elements in Acorus (Carlquist and Schneider, 1997) and Nymphaea, as well as other members of the Nymphaeaceae (Schneider et al., 1995), have been determined to be remarkably similar. Obviously the findings described here and those of Seago et al. (2000), when combined with the aforementioned vessel studies, suggest that the steles of the Nymphaeaceae and Acoraceae are quite similar. Given the differential expansion in their aerenchymas (Seago et al., 2000) and their stelar organization of five to nine xylem (phloem) strands (Conard, 1905; Maillefer, 1921; Seago et al., 2000; Keating, 2003), it appears that Acorus and Nymphaea are very similar with regard to root structure, and more similar to each other than to other dicotyledons and monocotyledons, thus from an anatomical perspective agreeing with the phylogenetic approach of the Angiosperm Phylogeny Group (1998, 2003). Clearly, the stelar and cortical structural characteristics of Acorus and Nymphaea should be considered basal or primitive, not derived (see Carlquist and Schneider, 1997).
This intermediate position of some structural and development features bridging traits of typical monocots and dicotyledons was also observed in Acorus anthers (Duval, 2001). This also suits its above-mentioned phylogenetic position well that is otherwise based mostly on molecular sequence analysis (Angiosperm Phylogeny Group, 1998, 2003). The Nymphaeales and Acorales both branch very early (Angiosperm Phylogeny Group, 1998, 2003; Floyd and Friedmann, 2001; Soltis, 2003) from the phylogenetic tree and are closer to the phylogenetic origin of the tree. It is quite likely that some of the features of root development described above could be present in their ancestors. Some of the features remain in later separating phylogenetical branches of both monocotyledons and dicotyledons. Further research on the phylogenetic context of other early diverging lineages of flowering plants (extant plants: Amborellales and Illicialles, Floyd and Friedmann, 2001; extinct plants: Archefructaceae, Sun et al., 2002) might give further insight into this field. Further work is also being done to elucidate the determinate nature and structure of adventitious roots in Acorus.
Acknowledgments
The work was supported by project no. MSMT 113100003 of The Ministry of Education, Czech Republic. J.L.S. expresses appreciation to Carol A. Peterson and Daryl E. Enstone, University of Waterloo, Ontario, Canada, and to Jim Ireland, Richard Keating and Marilyn Seago.
LITERATURE CITED
- Angiosperm Phylogeny Group. 1998. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden 85: 531–553. [Google Scholar]
- Angiosperm Phylogeny Group. 2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society 141: 399–436. [Google Scholar]
- Brundrett MC, Enstone DE, Peterson CA. 1988. A berberine–aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma 1446: 133–142. [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA. 1987. Efficient lipid staining in plant material with sudan red 7B or fluorol yellow 088 in polyethylene glycol. Biotechnic & Histochemistry 66: 133–142. [DOI] [PubMed] [Google Scholar]
- Carlquist S, Schneider EL. 1997. Origins and nature of vessels in monocotyledons. I. Acorus. International Journal of Plant Sciences 158: 51–56. [Google Scholar]
- Clarkson DT, Robards K. 1975. The endodermis, its structural development and physiological role. In: Torrey JG, Clarkson DT, eds. Structure and function of roots. London: Academic Press, 415–436. [Google Scholar]
- Clowes FAL. 1981. The difference between open and closed meristems. Annals of Botany 48: 761–767. [Google Scholar]
- Clowes FAL. 2000. Pattern in root meristem development in angiosperms. New Phytologist 146: 83–94. [Google Scholar]
- Conard HS. 1905. The waterlilies: a monograph of the genus Nymphaea. Washington, DC: Carnegie Institution of Washington. [Google Scholar]
- Duvall MR. 2001. An anatomical study of anther development in Acorus L.: phylogenetic implications. Plant Systematics and Evolution 228: 143–152. [Google Scholar]
- Eames AJ. 1961.Morphology of the angiosperms. New York, NY: McGraw-Hill. [Google Scholar]
- Fleet van DS. 1961. Histochemistry and function of the endodermis. Botanical Review 27: 165–220. [Google Scholar]
- Floyd SK, Friedman WE. 2001. Evolution of endosperm developmental patterns among basal flowering plants. International Journal of Plant Sciences 161: S57–S81. [Google Scholar]
- Friis EM, Pedersen KR, Crane PR. 2001. Fossil evidence of water lilies (Nymphaeales) in the early cretaceous. Nature 410: 357–360. [DOI] [PubMed] [Google Scholar]
- Groot EP, Doyle JA, Nichol SA, Rost TL. 2004. Phylogenetic distribution and evolution of root apical meristem organization in dicotyledonous angiosperms. International Journal of Plant Sciences 165: 97–105. [Google Scholar]
- Guttenberg von H. 1968. Der primäre Bau der Angiospermenwurzel. In: Linsbauer K, Tischler G, Pascher A, eds. Handbuch der Pflanzenanatomie, Vol. VIII. Berlin: Gebrüder Borntraeger, 1–472. [Google Scholar]
- Haas DL, Carothers ZB. 1975. Some ultrastructural observations on endodermal cell development in Zea mays roots. American Journal of Botany 62: 336–348. [Google Scholar]
- Haupt AW. 1953.Plant morphology. New York, NY: McGraw-Hill. [Google Scholar]
- Holle HG. 1876. Über den Vegetationspunkt der Angiospermen Wurzeln, insbesondere die Haubenbildung. Botanische Zeitung 34: 241–255, 257–264. [Google Scholar]
- Janczewski E de. 1874. Recherches sur l'accroissement terminal des racines dans les Phanerogames. Annales des Sciences Naturelles Series 20: 162–201. [Google Scholar]
- Jensen WA. 1962.Botanical histochemistry. San Francisco, CA: W.H. Freeman and Co. [Google Scholar]
- Justin SHFW, Armstrong W. 1987. The anatomical characteristics of roots and plant response to soil flooding. New Phytologist 106: 465–405. [Google Scholar]
- Keating RC. 2003.The anatomy of the monocotyledons. Vol. IX. The Acoraceae and Araceae. Oxford: Oxford University Press. [Google Scholar]
- Kroemer K. 1903. Wurzelhaut Hypodermis und Endodermis der Angiospermwurzel. Bibliotheca Botanica 59: 1–151. [Google Scholar]
- Kroll GH. 1912. Kritische Studie über die Verwertbarkeit der Wurzelhaubentypen für Entwicklungsgeschicte. Beiheft zum Botanisches Zentralblatt 28: 134–158. [Google Scholar]
- Laan P, Berrovoets MJ, Lythe S, Armstrong W, Blom CWPM. 1989. Root morphology and aerenchyma formation as indicators of the flood-tolerance of Rumex species. Journal of Ecology 77: 693–703. [Google Scholar]
- Maillefer A. 1921. Sur la presence d'une assise dans la racine d'Acorus calamus Bulletin de la Societie vaudoise des sciences naturelles 53: 77–79. [Google Scholar]
- Nêmec B. 1907.Anatomie a fyziologie rostlin. Prague, Czech Republic: Nakladatelství České Akademie Císaře Františka Josefa pro Vědy, Slovesnost a Umění. [Google Scholar]
- Schneider EL, Carlquist S, Beamer K, Kohn A. 1995. Vessels in Nymphaeaceae: Nuphar, Nymphaea, and Ondinea International Journal of Plant Sciences 156: 857–862. [Google Scholar]
- Seago Jr JL. 2002. The root cortex of the Nymphaeaceae, Cabombaceae, and Nelumbonaceae. Journal of the Torrey Botanical Society 129: 1–9. [Google Scholar]
- Seago Jr JL, Peterson CA, Kinsley LJ, Broderick J. 2000. Development and structure of the root cortex in Caltha palustris L. and Nymphaea cordata Ait. Annals of Botany 86: 631–640. [Google Scholar]
- Shishkoff N. 1987. Distribution of the dimorphic hypodermis of roots in Angiosperm families. Annals of Botany 60: 1–15. [Google Scholar]
- Soltis DE, Soltis PS, Bennet MD, Leitch IJ. 2003. Evolution of genome size in the angiosperms. American Journal of Botany 90: 1596–1603. [DOI] [PubMed] [Google Scholar]
- Soukup A, Votrubova O, Cizkova H. 2002. Development of anatomical structure of roots of Phragmites australis New Phytologist 153: 277–287. [Google Scholar]
- Sun G, Ji Q, Dilcher DL, Zheng S, Nixon KC, Wang X. 2002. Archaefructaceae, a new Basal Angiosperm family. Science 296: 899–904. [DOI] [PubMed] [Google Scholar]