Abstract
• Background and Aims The reproductive biology of Syngonanthus mucugensis and S. curralensis (Eriocaulaceae) was studied in areas of ‘campo rupestre’ vegetation in the Chapada Diamantina, north-eastern Brazil. These species are herbaceous and the individuals have a grouped distribution. Their leaves are united in a rosette, and their inflorescence is monoecious, of the capitulum type. The staminate and pistillate rings mature in a centripetal manner on the capitulum.
• Methods A field study was conducted, including observations concerning the morphology and biology of the flowers, fruit development, insect visits and anemophily, in both S. mucugensis and S. curralensis. Experimental pollinations were also carried out to study the mating systems of S. mucugensis.
• Key Results Both species flower from June to August. The staminate cycle lasts approx. 7 d, and the pistillate cycle from 3 to 4 d, with no temporal overlap between them on the same capitulum. The pollen viability of S. mucugensis was 88·6%, and 92·5% for S. curralensis. The inflorescences of both species demonstrated ultraviolet absorbance, and a sweet odour was detected during both the staminate and pistillate phases. No nectar production was ever noted, although nectaries are present. Both species were visited by numerous groups of insects, with the Diptera being the principal pollinators, especially the species of Syrphidae and Bombyliidae. There were secondary pollinators among species of Coleoptera and Hymenoptera. There was no evidence of wind pollination. Syngonanthus mucugensis is a self-compatible species, and forms fruits by agamospermy at low frequencies.
• Conclusions This is apparently the first report for pollination biology and mating systems of Eriocaulaceae. Conversely to that stated by some authors, entomophily, mainly effected by species of Diptera but also by species of Coleoptera and Hymenoptera, is probably the only pollination system in these species. In spite of the monoecious inflorescences without overlap of the staminate and pistillate phases, geitonogamy may occur in S. mucugensis, as the species is self-compatible and different capitula in the same plant at different phases is common.
Keywords: Syngonanthus mucugensis, Syngonanthus curralensis, Eriocaulaceae, entomophily, myophily, mating systems, pollination, reproductive biology, campo rupestre, Chapada Diamantina
INTRODUCTION
The Eriocaulaceae comprises approx. 1200 species, grouped into ten genera. The family has a pantropical distribution, although the majority of the species occur in the Neotropics, especially in the mountains of Venezuela and Brazil (Giulietti and Pirani, 1988; Giulietti and Hensold, 1990; Lazzari, 2000). In Brazil, the majority of the species have a geographical distribution restricted to the country's central region (Giulietti, 1997). This family is typical of the Brazilian ‘campo rupestre’ vegetation, and is especially notable in the mountains of the Espinhaço mountain chain, in the states of Minas Gerais and Bahia (Lazzari, 2000). An especially large number of species are found in this region (Harley, 1995; Miranda and Giulietti, 2001) and it probably represents the principal centre of genetic diversity for Eriocaulaceae (Giulietti and Hensold, 1990).
The family belongs to the order Poales, which comprises 18 families and about 19 500 species (Judd et al., 2002; APG, 2003). The two early divergent families are wind (Typhaceae) and animal (Bromeliaceae) pollinated but the relationships among these families and the remainder of the order are unclear. The two largest clades of the Poales, namely the core Poales (Poaceae, Joinvilleaceae, Restionaceae, Flagellariaceae, and some other small families) and the sedge/rush clade (Cyperaceae, Juncaceae and Thurniaceae), comprises species typically anemophilous (Judd et al., 2002; APG, 2003), but some species of Poaceae and Cyperaceae have evolved pollination by insects several times (Adams et al., 1981; Judd et al., 2002). Mayacaceae, Rapateaceae and Xyridaceae, the latter the sister group of Eriocaulaceae, are probably entomophilous, based on morphological characters, but there are virtually no studies on this subject.
Studies on reproductive biology are still very incipient in the family Eriocaulaceae (Sano, 1996; Scatena et al., 1997; Castellani and d'Eça-Neves, 2000), and there is apparently no published work on reproductive systems for this group. Information concerning pollination systems in Eriocaulaceae has been based solely on morphological considerations and casual observations. Some authors recognize anemophily as the principal pollination system for this family (Kral, 1966; Cronquist, 1981; Judd et al., 2002), while others indicate entomophily (Dahlgren et al., 1985; Hensold, 1988; Sano, 1996, Stützel, 1998; Rosa and Scatena, 2003). Cronquist (1981), states that nectar glands occur only within the genus Eriocaulon. Judd et al. (2002) indicate the absence of nectaries as a synapomorphy for the clade formed after the early divergence of the Bromeliaceae and Typhaceae.
Appendices on the pistillate flowers of some Eriocaulaceae have been considered nectaries according to Hensold (1988), Stützel (1998), and Stützel and Gansser (1995), as they have a probable secretory function. The pistillodes in staminate flowers possibly perform this same function, reinforcing the possibility of entomophily for the family. Based on the occurrence and morphology of the appendices, recent floral anatomy studies carried out by Rosa and Scatena (2003) suggest the occurrence of insect-pollination in Eriocaulon elichrysoides and Syngonanthus caulescens, and the authors agree with the view of entomophily as the most common syndrome for the family.
Many species of Eriocaulaceae are popularly known as ‘everlasting flowers’ as they have a small, delicate, and whitish capitulum with a paleaceous texture that retains the appearance of being still fresh even many years after harvesting (Giulietti, 1997). The scapes and inflorescences are harvested before seed production, dried in the sun, and then sold for decorative purposes. The majority of ‘ever-living flowers’ belong to the genus Syngonanthus, especially S. sect. Eulepis, due to the fact that these species have capitula with showy involucral bracts (Lazzari, 2000), including S. mucugensis Giul. and S. curralensis Moldenke. These species are herbaceous, show a grouped distribution of individuals in the field, and are approx. 30–40 cm tall. Both have their leaves united into a rosette, with monoecious inflorescences in the form of a capitulum, with small whitish flowers.
Starting in the 1980s, the commercialization of these species as dried flowers generated a significant amount of income, employment and foreign trade for Brazil, with a large fraction of the harvested plants being exported to the United States and Europe (Giulietti et al., 1988). As these species demonstrate a grouped distribution of individuals, entire populations could be collected every year. This has compromised their reproduction and brought them near to extinction. In addition to this economic exploration, fire has been traditionally used to ‘manage’ these species, burning the taller Cyperaceae and Poaceae species, thus ‘opening’ the area and causing a massive flowering of the Syngonanthus species, and constitutes one more factor against their survival.
No work has yet been done on any aspect of the ecology of S. mucugensis or S. curralensis. The present work is part of a broader project with the goal of aiding the conservation of these and other threatened groups of plants of the Chapada Diamantina by managing their use, covering topics concerning the mechanisms of floral biology, pollination, reproduction, demography, ecology, population genetics, and propagation of these species. In this paper, the following questions are addressed: Are Syngonanthus mucugensis and S. curralensis pollinated either by insects or by wind or both? What are the morphological characters associated to the pollination system(s) presented in each species? Is S. mucugensis self-compatible?
MATERIALS AND METHODS
Study area
This study was carried out in areas of ‘campo rupestre’ vegetation in the municipalities of Mucugê (the Mucugê Municipal Park) and Morro do Chapéu, in the Chapada Diamantina mountain range, Bahia State, Brazil. ‘Campo rupestre’ occurs on outcrops of quartzite, sandstone and gneiss, which form sandy soils of variable depths. The vegetation is dominated by herbaceous and sub-shrubs in the open areas mixed with shrubs and herbs growing in rock outcrops. The families Poaceae, Cyperaceae, Velloziaceae and Eriocaulaceae are well represented, with several endemic taxa in this formation (Giulietti and Pirani, 1988; Harley, 1995; Borba and Semir, 1998). The Mucugê Municipal Park is located at approx. 1000 m a.s.l. The soils there are undeveloped, thin (rarely >50 cm deep), and have very low nutrient levels. The local climate is tropical semi-humid, with heaviest rainfall during the southern hemisphere spring and summer (from September to March). The temperatures reach a minimum of 13 °C in the winter and >30 °C in the summer, with an annual average of 19·8 °C (Stradmann, 1998). In Morro do Chapéu, the landforms are predominantly tabular, at altitudes varying from 480 to >1000 m a.s.l. The soils are acidic, with low fertility. The climate is classified as Cwb (Köppen, 1948): high-altitude tropical with mild summers. The average temperature for the coldest month (July) is <18 °C, while the average temperature for the warmest month (January) remains below 22 °C (CPRM, 1995). The average annual temperature is 19·7 °C. Average annual rainfall is approx. 800 mm (DNMET, 1992, in CPRM, 1995).
The two populations of Syngonanthus mucugensis studied were both located within the Mucugê Municipal Park (12°59′46″S; 41°20′40″W/12°59′44′S; 41°20′19″W), at altitudes of 959 and 989 m. The population of S. curralensis examined was within the municipal limits of the municipality of Morro do Chapéu, in a region known as Tabuleiro dos Tigres (11°36′04″S; 41°09′47″W; at 1094 m). Only the plants located within a 50 × 2 m transect in each of these three areas were examined.
Floral biology, pollination, and reproductive biology
Field observations were undertaken from June to August 2003, and from June to September 2004. The number of inflorescences produced by the plants within the three transects were recorded, as well as observations concerning the morphology and biology of the flowers, fruit development, insect visits, anemophily and their reproductive systems. Approximately 100 inflorescences were studied during 10 consecutive days in order to determine the duration of the pistillate and staminate phases, the occurrence of overlap between these phases, as well as the time and duration of anthesis.
The time during which pollen was available on the flowers was analysed by observing 20 inflorescences each hour between the daylight hours from 0600 to 1800 h, using a ×20 hand-held lens. Pollen from the anthers of approx. 20 flowers from seven inflorescences was collected approx. 30 min after total exposure of the anthers. Laboratory analysis of pollen viability was performed using the nitroblue–tetrazolium enzymatic test to measure dehydrogenase activity (Dafni, 1992). The possible presence of pollenkitt was determined by examining fresh pollen with an optical microscope. Stigma morphology, receptivity, and the presence of secretions on the stigma surface were observed in the field in approx. 30 flowers every hour during the period of anthesis. Alpha-naphthyl acetate was employed to detect esterase activity (Dafni, 1992). The presence of nectaries was determined by dissecting flowers under a stereomicroscope, and the presence of nectar was analysed in the field by direct examination of the flowers between 0800 and 1700 h. The presence of osmophores was checked by emerging the flowers in 1 % neutral-red for 10 min and then washing them in a 5 % solution of glacial acetic acid (modified from Vogel, 1990). The presence of pigments with absorption ranges within the ultra-violet spectrum was analysed by maintaining the inflorescences in an atmosphere of ammonium hydroxide for 5 min (Scogin et al., 1977).
To determine the occurrence of pollen dispersion by the wind, four pollen traps were mounted for 2 d along each transect during the period 0800–1700 h. The traps consisted of three stacked vertical wooden plates spaced approx. 15 cm from one another, and set near ground level. To the discs were affixed plastic plaques with approx. 1-cm-diameter holes backed with adhesive tape to trap pollen (P. G. Kevan, University of Guelph, Canada, pers. comm.). The exposed tape was examined under a microscope to check for the presence of pollen derived from either S. mucugensis or S. curralensis. A previous experiment, as described above, was carried out in a flowering population of Rhynchospora almensis (Cyperaceae) to test the efficiency of the method, resulting in capture of pollen grains in 10 % of the holes. Additionally, glass slides covered with phenol-containing gelatine were distributed along the transects (at the same height as capitula in the staminate phase, and approx. 20 cm from them) between 0800 and 1700 h for two consecutive days. Another experiment was carried out to test the wind velocity that might be necessary for pollen dispersion. In this experiment, a fan was used to generate wind velocities of 1, 2, 3 and 4 m s−1 (at approx. 15 cm distance from the anthers) in inflorescences (two for each wind velocity) collected in the field and moved to the laboratory. Pollen was collected on eight glass slides (two for each velocity) covered with phenol-containing gelatine. Reference slides with S. mucugensis and S. curralensis pollen were previously prepared to facilitate their identification. The natural wind velocity at the height of the capitulum was also measured during the course of the field work using a digital anemometer. Measurements were taken every hour (for 2 min) between 0800 and 1700 h for 8 d for the populations of S. mucugensis, and for 5 d for the population of S. curralensis.
Observations of inflorescences in both the staminate and pistillate phases were undertaken to monitor visits to flowers. Observations were initially carried out between 0600 and 1800 h, but this was later adjusted to between 0800 and 1700 h, as no visits were observed outside this time interval. For S. mucugensis, two groups with six and 36 inflorescences (five inflorescences at staminate phase and one at pistillate phase, and 29 at staminate phase and seven at pistillate phase, respectively) were subjected to a total of 81 h of observation, for a total of 1602 inflorescence-hours (1293 staminate and 309 pistillate). A visit was scored as the insect reached the inflorescence and contacted at least one receptive flower. For S. curralensis, two groups with 15 and 44 inflorescences (14 staminate and one pistillate inflorescence, and 36 staminate and eight pistillate inflorescences, respectively) were subjected to a total of 54 h of observation, for a total of 1854 inflorescence-hours (1503 staminate and 351 pistillate). The behaviour patterns of the floral visitors during the masculine and feminine phases of the inflorescences were analysed to determine the pollinators and the resources utilized by them. Due to the difficulty of distinguishing between congeneric species in the field, visitor numbers were sometimes pooled. Insect visitors were collected and sent to specialists for identification, and will be stored with the collections of the Entomology Laboratory at the Universidade Estadual de Feira de Santana, Bahia, Brazil.
Mating systems were studied in depth only in S. mucugensis. Sixty inflorescences at the flower bud stage were enclosed in open-weave cloth sacks designed to exclude insect pollinators. To test for self-compatibility, pollination experiments were undertaken after anthesis using pollen from different capitula of the same individual (geitonogamy) as well as pollen from different individual (xenogamy). Pollen transfer was affected with the aid of a ×20 hand-held lens by detaching an inflorescence in the staminate phase, after removing the involucral bracts, and using it to brush gently an inflorescence in the pistillate phase, putting the anthers in contact with the stigmas. After this manual pollination, the pollinated cycle was marked and the inflorescences were once again covered with cloth sacks. A control group of 20 covered inflorescences were prepared to check for spontaneous self-pollination and/or agamospermy. All experimental inflorescences were maintained covered for approx. 30 d, after which they were harvested and examined for fruit formation in all pistillate flowers present in the hand-pollinated cycle. Additionally, seven inflorescences were maintained without any manipulation to quantify natural fruit production.
At 24, 48 and 96 h after the initiation of the pollination experiments, flowers from three inflorescences in each treatment were harvested and placed in a 70 % solution of FAA. These flowers were subsequently immersed in a 10 n solution of NaOH at 60 °C, washed in distilled water, stained with 0·25 % aniline-blue, and examined for evidence of pollen tube growth and/or fertilization of the ovules using an epifluorescence microscope, following a modified version of the method in Martin (1959). Fruits and withered flowers present on the inflorescences used to verify agamospermy and natural self-pollination were also examined as above to check for pollen deposition, formation of pollen tubes and fertilization of the ovules.
RESULTS
Floral biology
Syngonanthus mucugensis and S. curralensis are herbaceous species (approx. 40 and 30 cm tall, respectively), have leaves united in a basal rosette, produce monoecious inflorescences in the form of a capitulum, and demonstrate centripetal maturation of the flowers (Figs 1 and 2). The capitula of S. mucugensis are approx. 2 cm in diameter, with from 1 to 24 (3·2 on average) flowering stalks per individual plant. There are 9–14 series of involucral bracts, surpassing the height of the flowers, the outermost 4·3–6·3 mm long, the innermost 7·2–9·5 mm long. Staminate flower have sepals 2·1–3·4 mm long, connate at the base, petals 0·8–1·8 mm long, connate up to the middle and pistillodes approx. 0·8 mm long. Pistillate flowers have sepals 2·5–3·7 mm long which are free, petals 1·6–2·3 mm long, connate up to the middle, styles 2·1–3·6 mm long and appendices 1–1·7 mm long. The capitula of S. curralensis are approx. 1 cm in diameter, with from one to 17 (3·4 in average) flowering stalks per individual plant. There are 8–13 series of involucral bracts, not surpassing the height of the flowers, the outermost 1·5–2·9 mm long, the innermost 4·7–5·8 mm long. Staminate flowers have sepals 2·1–2·9 mm long, connate at the base, petals 0·8–1·7 mm long, connate up to the middle and pistillodes approx. 0·5 mm long. Pistillate flowers have sepals 2·3–3·3 mm long which are free, petals 1·5–2 mm long, connate up to the middle, styles 1·5–3·2 mm long and appendices 0·7–1·2 mm long. Both species flower from July to August. Syngonanthus mucugensis demonstrates a flowering peak in July, and the capitulum show three cycles of staminate flowers alternating with three cycles of pistillate flowers. In some inflorescences, however, only two cycles of pistillate flowers were observed. The outer ring is always composed of masculine flowers. The temporal phases of staminate and pistillate flowers never overlap in the same capitulum, although different capitula of the same individual plant may be in different phases at any given moment.
Fig. 1.

Syngonanthus mucugensis: (A) habit; (B) central region of the capitulum in pistillate phase, indicating the yellow appendices (arrows); (C) individuals of Mythicomyia sp. (Diptera, Bombyliidae) visiting flowers of capitulum in staminate phase; (D) individual of Tenebrionidae (Coleoptera) visiting flowers of capitulum in staminate phase (in the detail, a individual of Crysomelidae sp); (E) individual of Halictidae (Hymenoptera) feeding on the nectar and collecting pollen in flowers of capitulum in stainate phase (notice the pollen load in the hind tybia indicated by an arrow). Scale bars: A = 5 cm; B–E = 2·5 mm.
Fig. 2.
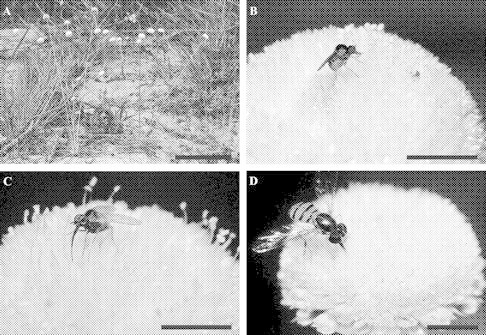
Syngonanthus curralensis: (A) habit; (B and C) individual of Bombyliidae (Diptera) visiting flowers of capitulum in staminate phase; (D) individual of Toxomerus sp. (Diptera, Syrphidae) visiting flowers of capitulum in staminate phase. Scale bars: A = 10 cm; B–D = 2·5 cm.
The staminate flowers undergo anthesis between 0830 and 0930 h in both species. Each day, between four and 15 staminate flowers in capitula of S. mucugensis, and between eight and 19 staminate flowers in capitula of S. curralensis, mature and open. These flowers last for only a single day. Each staminate cycle in the two species lasts for approx. 7 d. The pistillate flowers of both species undergo anthesis at approx. 0800 h. The 15–50 pistillate flowers in the pistillate ring of S. mucugensis and the approx. 30 pistillate flowers in S. curralensis almost all open on the same day. The flowers themselves last approx. 3 d, but the flower is available for pollination only on the first day as the stigmas and the appendices are withered by the second day. The entire pistillate cycle lasts approx. 4 d. A period of approx. 30 min passes between the initiation of floral opening (spreading of the sepals and petals) and total exposition of the stamens and petals in both flower types of S. mucugensis and S. curralensis. Both the anthers and stigmas are located above the perianth. The internal region of the flowers, holding either the pistillodes (in the staminate flowers) or the appendices (in the pistillate flowers), are noticeable due to their yellow coloration, in contrast to the other whitish flower parts (Figs 1, 2 and 3D).
Fig. 3.
Pistillate flowers of S. mucugensis in epifluorescence microscopy: (A) pollen grains germinating (arrow) and pollen tubes penetrating the stigma (xenogamy; 24 h); (B) ovules penetrated by pollen tubes—arrows indicate fertilization (xenogamy; 48 h); (C) seed beginning to develop—arrow indicates the proembryo (xenogamy; 4 d); (D) gynoecium, showing the styles and appendices—arrow indicates the phloem cells of the appendices (light fluorescence).
Anther dehiscence occurs near 0900 h, and pollen is available on the flowers until approx. 1500 h. Pollen viability was 88·6 % in S. mucugensis, and 92·5 % in S. curralensis. Neither pollenkitt nor stigmatic secretions were noted in either species. Stigmatic receptivity in S. mucugensis was initiated as soon as the stigmas were exposed above the extended perianth, in the later part of the afternoon. Receptivity lasted approx. 10 h. Syngonanthus curralensis demonstrated no enzymatic reaction that would indicate stigma receptivity, even though the flowers tested were apparently in the same morphological phase as those of S. mucugensis (stigmas erect, yellow appendices, perianth rigid).
Nectar production was not noted at any time in either species. The neutral-red test did not yield any colour differentiation in the floral parts that would indicate the presence of osmophores, possibly due to the paleaceous texture of the inflorescence. However, a sweet odour was detectable emanating from capitula in the staminate and pistillate phases of S. mucugensis. Occasionally, and with less intensity, odours were detected on S. curralensis during the hottest part of the day (1000–1300 h). Reaction with ammonium hydroxide demonstrated absorbance of ultraviolet light by the inflorescences of both species, in all regions of the capitulum.
Floral visitors
The staminate and pistillate flowers of S. mucugensis and S. curralensis were visited by insects belonging to three orders: Coleoptera, Diptera, and Hymenoptera (Figs 1 and 2). The flowers of S. mucugensis were visited by 19 insect species, while S. curralensis was visited by 11 insect species. A majority of the insect visitors belonged to the Diptera. The insect species of the families Syrphidae and Bombyliidae (Diptera) were responsible for the greatest number of visits to staminate and pistillate flowers of both plant species examined (Table 1). Insect visitors of the Syrphidae made 477 visits to inflorescences at the staminate phase and 43 to inflorescences at the pistillate phase of S. mucugensis during the 8 d of observation, while species of Bombyliidae made 51 visits to inflorescences at the staminate phases and 13 to inflorescences at the pistillate phase. The opposite was seen in S. curralensis, which was visited by a greater number of insects of the species of Bombyliidae (80 visits to inflorescences at the staminate phase and 17 visits to inflorescences at the pistillate phase), and lesser numbers of Syrphidae (seven visits to the staminate phase and three visits to the pistillate phase) during 5 d of observations. Insects of three species visited both S. mucugensis and S. curralensis: Mythichomyia sp.2 and sp.4 (Bombyliidae), as well as Toxomerus sp. (Syrphidae) (Table 1).
Table 1.
Insect visitors, number and duration of visits (in seconds) in inflorescences at staminate and pistillate phases of Syngonanthus curralensis and S. mucugensis, in Mucugê and Morro do Chapéu, Chapada Diamantina, Brazil
|
S. mucugensis |
S. curralensis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visitor (order/family/species) |
Staminate |
Pistillate |
Staminate |
Pistillate |
Duration of the visits (s) |
Classification of visitors |
||||||
| Coleoptera | ||||||||||||
| Crysomelidae | ||||||||||||
| Crysomelidae sp. | 4 | 0 | – | – | 10–38 | V | ||||||
| Curculionidae | ||||||||||||
| Curculionidae sp. 1 | 2 | 0 | – | – | 5–128 | V | ||||||
| Curculionidae sp. 2 | 2 | 0 | – | – | 45–60 | V | ||||||
| Curculionidae sp. 3 | – | – | * | – | – | V | ||||||
| Histeridae | ||||||||||||
| Histeridae sp. | – | – | * | – | – | V | ||||||
| Tenebrionidae | ||||||||||||
| Tenebrionidae sp. | 24 | 12 | – | – | 12–149 | P | ||||||
| Diptera | ||||||||||||
| Bombyliidae | ||||||||||||
| Apolysis sp., Mythichomyia sp. 2 and 4 | – | – | 80 | 17 | 2–198 | P | ||||||
| Mythichomyia sp. 1, 2, 3 and 4 | 51 | 13 | – | – | 5–182 | P | ||||||
| Sarcophagidae | ||||||||||||
| Mantidophaga sp. | * | – | – | – | – | V | ||||||
| Microcerella sp.1 | – | – | 4 | 1 | 2–39 | OP | ||||||
| Microcerella sp.2 | * | – | – | – | – | V | ||||||
| Trichopoda sp. | 24 | 8 | – | – | 5–47 | P | ||||||
| Sarcophagula sp. | – | – | 1 | 1 | 12–46 | OP | ||||||
| Syrphidae | ||||||||||||
| Chrysotoxum sp. | 184 | 17 | – | – | 1–156 | P | ||||||
| Ocyptamus sp. 1 | 7 | 2 | – | – | 5–95 | OP | ||||||
| Ocyptamus sp. 2 | * | – | – | – | – | V | ||||||
| Toxomerus sp. | 286 | 24 | 7 | 3 | 1–180 | P | ||||||
| Syrphidae sp. | * | – | – | – | – | V | ||||||
| Tachinidae | ||||||||||||
| Epalpus sp. | * | – | – | – | – | V | ||||||
| Peleteria sp. 1 | – | – | * | – | – | V | ||||||
| Peleteria sp. 2 | * | – | – | – | – | V | ||||||
| Peleteria sp. 3 | – | – | 0 | 3 | 9–26 | V | ||||||
| Hymenoptera | ||||||||||||
| Halictidae sp. 1, 2 | 4 | 1 | – | – | 15–42 | OP | ||||||
Classification of the visitors according to morphology, behaviour and frequency of visits (see text): V, visitor; OP, occasional pollinator; P, effective pollinator.
Visits not occurring in the observation period.
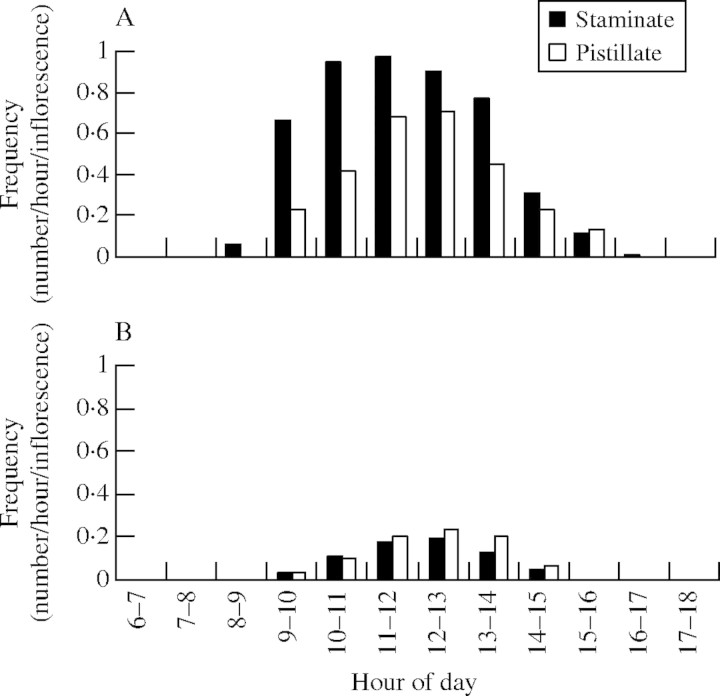
Insect visits occurred during the period from 0800 to 1700 h with S. mucugensis, but only from 0900 to 1500 h with S. curralensis. There was a visitation peak near 1200 h for both species (Fig. 4). Visitation frequency was greater for individuals of S. mucugensis than for S. curralensis. The frequency of visitation to inflorescences at the staminate phase was greater than that observed to inflorescences at the pistillate phase for S. mucugensis. On the other hand, S. curralensis demonstrated a similar frequency of visitation to both inflorescence phases (Fig. 3). The duration of effective floral visits (resulting in the removal or deposition of pollen) ranged from 2 to 198 s (e.g. Syrphidae 5–180 s, Bombyliidae 2–198 s, Tenebrionidae 12–149 s, Halictidae 5–120 s) (Table 1).
Fig. 5.
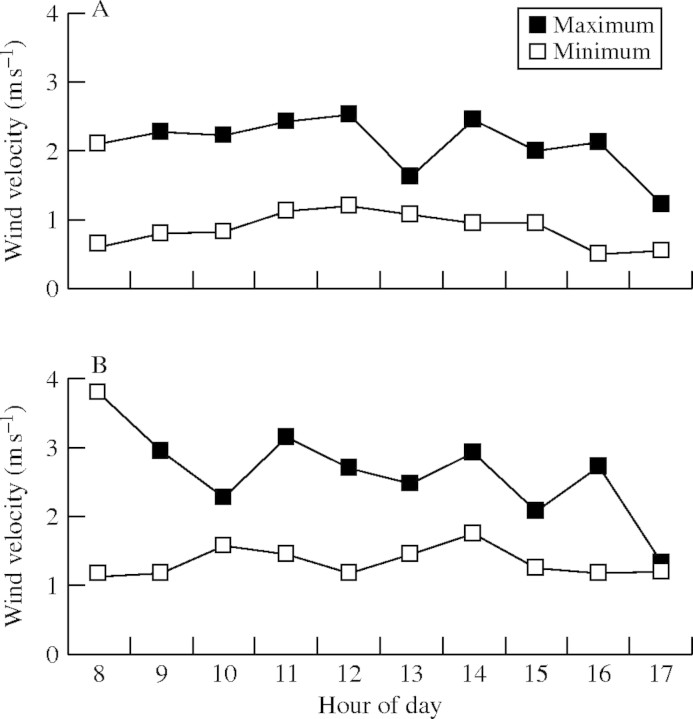
Minimum and maximum wind velocity in the study areas in a period of (A) 8 d for Syngonanthus mucugensis and (B) 5 d for S. curralensis, during the flowering period.
Fig. 4.
Frequency of visits per hour per inflorescence in staminate and pistillate phases of (A) Syngonanthus mucugensis and (B) S. curralensis.
Similar behaviour patterns were observed on flowers of S. mucugensis and S. curralensis by insect visitors belonging to the families Bombyliidae, Sarcophagidae, Syrphidae and Tachinidae (Diptera) (Figs 1C and 2B–D). When visiting staminate or pistillate flowers, the fly bends over the flower, lowering its head towards the interior of the same flower (where the pistillodes and appendices are located), and then introduces its proboscis. When visiting staminate flowers, these visitors sometimes perambulate over the entire inflorescence, apparently visiting essentially all of the flowers, and moving about the inflorescence, the insect's legs and practically all of its ventral region touches the anthers, resulting in the attachment of pollen grains. During visits to pistillate flowers, pollen grains are transferred to the stigmas when the insect visitors bend forward towards the appendices, or while moving about the inflorescence. Individuals of Syrphidae were not observed feeding on pollen grains.
Individuals of the family Tenebrionidae (Coleoptera) were observed visiting only flowers of S. mucugensis (Fig. 1D). These insects land on the staminate-phase inflorescence and move about the flowers, apparently seeking food. They eat exposed pollen on the anthers, while other pollen grains adhere to their legs. When visiting both staminate and pistillate flowers, they will incline their bodies towards the floral regions with the pistillodes and appendices in a manner similar to the Diptera, but appear to be chewing those plant structures. Pollen grains are transferred to the stigmas while they are feeding on the appendices. Representatives of the families Chrysomelidae, Curculionidae and Histeridae (Coleoptera) were observed only of staminate flowers on S. mucugensis and S. curralensis, feeding on the pollen.
Individuals of Halictidae (Hymenoptera) were observed visiting only S. mucugensis (both floral types), although they normally visit only a single flower on any inflorescence. Upon landing, they lean forward towards the central region of the staminate or pistillate flowers, and then extend their proboscis to collect liquids from the pistillodes and appendices and pollen with their legs (Fig. 1E). Pollen grains adhere to all parts of the insect, and apparently are easily transferred to the stigmas.
Eight insect species were considered effective pollinators of S. mucugensis due to their observed behaviour, the frequency of their visits to staminate and pistillate flowers, and their body morphology: Mythicomyia sp.1, Mythicomyia sp.2, Mythicomyia sp.3, Mythicomyia sp.4 (Bombyliidae), Halictidae sp., Trichopoda sp. (Sarcophagidae), Toxomerus sp. (Syrphidae) and Tenebrionidae sp. Likewise, four insect species were considered effective pollinators of S. curralensis: Apolysis sp., Mythicomyia sp.2, Mythicomyia sp.4 (Bombyliidae) and Toxomerus sp. (Syrphidae).
Ocyptamus sp.1 (Syrphidae), Halictidae sp.1 and Halictidae sp.2 were considered only occasional pollinators of S. mucugensis, while Microcerella sp.1 and Sarcophagula sp. (Sarcophagidae) were considered only occasional pollinators of S. curralensis as these insects were rarely seen during the observation period. Thirteen insect species were considered non-pollinating visitors (nine on S. mucugensis and four on S. curralensis) as they were not observed transferring pollen to pistillate flowers, or were only observed visiting staminate flowers (Table 1).
Anemophily
No pollen grains from either S. mucugensis or S. curralensis (or any other Eriocaulaceae species) were captured on the gelatine-coated slides or adhesive tape traps set out to collect wind-blown pollen. Twenty-three and 52 pollen grains of species belonging to other families were captured on the gelatine-coated slides in Mucugê (S. mucugensis population) and Morro do Chapéu (S. curralensis population), respectively. Pollen grains (n = 84) of species belonging to other families were captured on adhesive traps only in the experiment in Morro Chapéu. Wind velocity in the study area of S. mucugensis registered a minimum of 0·86 m s−1 and a maximum of 2·09 m s−1. Wind velocity in the study area of S. curralensis was slightly greater, registering a minimum of 1·33 m s−1 and maximum of 2·64 m s−1.
Mating system of S. mucugensis
Both cross-pollination and self-pollination demonstrated essentially equivalent success in terms of fruit formation. Natural pollination yielded a mean of 92·2 % of fruit formation, with all inflorescences demonstrating a fruiting percentage above 84 % (Table 2). Four out of 15 inflorescences demonstrated fruit formation by apomixy at a low frequency (2–14·9%), with a mean of 3·2%. The possibility of geitonogamy was rejected as no germinating pollen grains were encountered, nor was there any evidence of pollen tube formation in any of these fruits.
Table 2.
Percentage of fruit set in experimental pollinations of xenogamy, geitonogamy, experiments of agamospermy and spontaneous geitonogamy, and natural pollination in Syngonanthus mucugensis, in Mucugê, Chapada Diamantina, Brazil
| Treatment |
Inflorescences (n) |
Flower (n) |
Fruit set (%) |
|---|---|---|---|
| Xenogamy | 15 | 676 | 42·5 |
| Geitonogamy | 12 | 552 | 41·1 |
| Agamospermy/spontaneous geitonogamy | 15 | 562 | 3·2 |
| Natural pollination | 7 | 294 | 92·2 |
No differences were noted in pollen germination, pollen tube growth or fertilization between experimental tests of xenogamy and geitonogamy in S. mucugensis. After 24 h, pollen tube germination was confirmed, and the pollen tubes had travelled almost the entire length of the style (Fig. 3A). Fertilization was observed after 48 h (Fig. 3B), with the initiation of embryo and seed growth after 4 d (Fig. 3C).
Both species have dark-brown three-locular loculicidal capsules, measuring 1·5 mm. Three light seeds measuring nearly 0·5 mm are produced in each fruit. Fruit ripens after nearly 45 d, during the dry season, and seed dispersal is anemocoric and/or barochoric.
The appendices of the pistillate flowers demonstrated strong fluorescence near their terminal region due to large concentrations of phloem cells (Fig. 3D), suggesting their function as nectaries.
DISCUSSION
According to some authors, Eriocaulaceae may demonstrate anemophily (Kral, 1966; Cronquist, 1981; Judd et al., 2002) as well as entomophily (Cronquist, 1981; Dahlgren et al., 1985; Hensold, 1988; Sano, 1996; Stützel, 1998; Rosa and Scatena, 2003). However, these conclusions were based solely on morphological characteristics of the group, and/or occasional observations of insect visitation. No experimental studies or systematic observations seem to have been published concerning the family Eriocaulaceae, and certainly not for Syngonanthus.
The presence of pistillodes on the staminate flowers and appendices on the pistillate flowers of some species of Eriocaulaceae (Giulietti, 1984; Lazzari, 2000) has generated a series of questions concerning their function. According to Stützel (1998), Stützel and Gansser (1995) and Hensold (1988), these appendices, located between the styles, could be considered nectaries because of their probable secretory functions. This would strengthen the idea of entomophily within the family. The large quantity of phloem cells observed in the appendices of S. mucugensis suggests their role as nectaries.
The flowers of S. mucugensis and S. curralensis demonstrate characteristics frequently associated with both wind pollination and entomophily. Small flowers of separate sexes, placement of the anthers and stigmas above the perianth, small and numerous pollen grains, and a large number of staminate flowers in relation to pistillate flowers are common characteristics of anemophily. As all these traits are seen in both species studied, anemophily would be a plausible pollination system. However, these characteristics are also encountered in flowers with low levels of specialization that are pollinated by small insects, such as small Diptera and Coleoptera (Faegri and van der Pijl, 1979; Endress, 1998). The presence of odours, pigments on the flowers that absorb ultra-violet light, as well as pistillodes and nectariferous appendices, all seen in both species studied, favour the hypothesis of entomophily.
The inflorescences of S. mucugensis and S. curralensis have a flat profile, their sexual organs are exposed, insect rewards are easily accessible, and there are no apparent morphological adaptations to specific visitors. These flowers are easily visited by non-specialized insects with short mouthparts (allophylics) (Faegri and van der Pijl, 1979). Both plant species have morphological characteristics seen in plants that are pollinated by various groups of small insects (polyphylic), by dystrophic visitors (not having adaptations to specific floral morphologies), by insects that may often be destructive (such as the Coleoptera), as well as allotrophic visitors that are poorly adapted to the flowers and have a mixed diet (such as the Diptera). The frequency of visitation and the observation of the transfer of pollen to the pistillate flowers by individuals of the families Tenebrionidae (Coleoptera), Bombyliidae, Sarcophagidae, Syrphidae (Diptera) and Halictidae (Hymenoptera) suggest that biotic pollination is the principal form of pollination in S. mucugensis and S. curralensis. This conclusion is in accordance with the findings of Rosa and Scatena (2003), suggesting entomophily in some species of the family, including Syngonanthus (S. caulescens).
Pollination rarely occurs by anemophily in Arecaceae, and entomophily is most common within the family, which has cantarophilous, mellitophilous and myophilous species. This is the opposite of what was first imagined in the 19th century, based solely on the morphological characteristics of this family (Henderson, 1986). This seems similar to that which occurred in Eriocaulaceae, this family being regarded as anemophilous in some studies of the group based on morphological grounds.
A number of species of Arecaceae and Euphorbiaceae demonstrate general reproductive morphology similar to S. mucugensis and S. curralensis, such as monoecious inflorescences and grouped reduced flowers, which are related to pollination by wind and/or by small insects. The presence of an odour, such as noted for the species of Syngonanthus studied here, constitutes a strong indicator of entomophily for a number of species of Piperaceae, although their inflorescences could favour pollination by wind as well as by insects (Figueiredo and Sazima, 2000). Small, relatively open flowers, that are grouped in dense inflorescences favour cantarophily (Endress, 1998), as seen in Arecaceae, in which beetles are very important pollinators of a number of species (Listabarth, 2001; Voeks, 2002). However, coleopterans do not appear to have an important role in pollination in other groups with roughly similar inflorescences, as in Croton floribundus and C. priscus (Euphorbiaceae), for these insects will remain for a long time on a single inflorescence, often copulating there (Passos, 1995). The coleopterans that visit S. mucugensis and S. curralensis exhibit essentially the same behaviour as that observed for these two species of Croton. Nonetheless, the Tenebrionidae species was considered an effective pollinator of S. mucugensis as it entered in contact with the reproductive parts of both floral types.
The presence of characteristics related to both anemophily and entomophily (such as the presence of nectar) indicate a mixed system of pollination (ambophily) for the above-mentioned Croton species; they can be pollinated either by flies or by the wind (Passos, 1995). Similarly, studies by Figueiredo and Sazima (2000) confirm pollination by both wind and by small insects in some species of Piperaceae, while other species of Piperaceae are exclusively entomophilic or anemophilic. Anemophily may be derived from entomophily among the Monocotyledonae, for in some anemophilous plants there are vestiges of nectaries on the flowers and specific odours (Faegri and van der Pijl, 1979). The strict relationship between coleopterans and palms suggests that cantarophilous pollination is an ancestral condition, with anemophily a derived characteristic (Henderson, 1986). However, recent evidence suggests that ambophily may be more common than would be supposed, and may represent either a transition or definitive phase in many species within the Monocotyledonae (Culley et al., 2002).
These conclusions concerning the occurrence of entomophily and the absence of anemophily in S. mucugensis and S. curralensis are extremely important in terms of their conservation management. This is especially true since the local plant gatherers collect a large fraction of all inflorescences, and believe that burning the areas where the plants occur will stimulate future growth and flowering. These two practices almost certainly affect the reproduction of both species due to the fact that many of their floral visitors are resident species, with short flight characteristics (such as the Diptera species), and any practices which lead to a decrease in the number of potential pollinators will necessarily affect the maintenance of the population of S. mucugensis and S. curralensis.
The occurrence of entomophily and anemophily in Eriocaulaceae will need to be more closely examined to find out how efficient insects and wind are as pollination vectors among the many species. It is possible that the family contains species that are exclusively anemophilous, exclusively entomophilous or have a mixed system (ambiphilic), as observed in Poaceae and Cyperaceae (Adams et al., 1981; Koshy et al., 2001; Judd et al., 2002). More detailed field studies will be needed to shed light on this subject.
The present study was apparently the first to closely examine reproductive systems in Eriocaulaceae. Results point to self-compatibility in S. mucugensis, as indicated by the closely equivalent reproductive success observed in both xenogamy and geitogamy. The pollination experiments to test for xenogamy and geitonogamy demonstrated very low values of fruit formation in relation to open pollination, suggesting experimental procedural errors (e.g. pistillate flowers not receptive, anthers of staminate flowers empty). The fruiting percentages were classified in five classes (of 20% range) and a bimodal distribution became evident (the first curve with values below 22·2 %, and the second with values above 49·1 %). If the lowest values (first bimodal curve) were then discarded, the average values of fruiting for xenogamy and geitonogamy increase to 73·2 % (excluding eight inflorescences of 15) and 62·5 % (excluding six inflorescences of 12), respectively. In any case (either inflorescences pooled or excluding inflorescences in which procedural errors may have occurred) both cross-pollination and self-pollination demonstrated essentially equivalent fruit set, supporting the conclusion of self-compatibility in S. mucugensis. Variability for self-compatibility, with some plants exhibiting high fruit set in the self-fertilized treatment while others being self-incompatible, probably is not a plausible explanation for those low values of fruit set in some inflorescences because in some cases discrepant results were found in different inflorescences of the same plant, and similar results were observed in xenogamous pollinations as well.
Genetic population studies in these populations have pointed to a high inbreeding in these plants (A. C. S. Pereira, E. L. Borba and A. M. Giulietti, submitted), a condition probably favoured by the clustered distribution and low distance seed dispersal of these plants, and by self-compatibility associated with possibility of geitonogamy between inflorescences on different phases in the same individual. All of the inflorescences of S. mucugensis utilized in the experiments of open pollination demonstrated fruiting successes above 84 %, demonstrating the efficiency of the natural pollination agents, and showing it not to be a pollinator-limited species. Studies of the Poaceae indicate that self-incompatibility is common in that group (Baumann et al., 2000), although many species do demonstrate self-compatibility (Guilherme and Ressel, 2001). In the same way, Eriocaulaceae may also have self-compatible and self-incompatible species (as seen in other Monocotyledonae). Further studies in this family, as well as in other groups, will be necessary to better define the evolution of the reproductive systems in the order Poales.
It is unlikely that spontaneous geitonogamy occurs within capitula of this species, as a result of the strong temporal separation of the pistillate and staminate phases (dicogamy). Contamination, or a failure of the cloth barrier, in the experiments described here were unlikely, for all the fruits that were formed were examined, and only one ungerminated pollen grain was encountered. Thus, agamospermy seems to occur in S. mucugensis, although at a very low frequency. Because the seeds for evaluation (30 d after pollination) were harvested and fixed before the fruits were mature (approx. 45 d after pollination) it is not known if the seeds were viable. However, those seeds did not present any morphological difference (either on shape or size) to seeds developed by xenogamy or geitonogamy. Nonetheless, the occurrence of agamospermy in S. mucugensis and Eriocaulaceae deserves more extensive investigation.
Acknowledgments
The authors thank Roy A. Funch, Delmar L. Alvim, Oremildes A. Oliveira for helping on field trips, Ivan F. Castro for identification of the insects, and Roy A. Funch, Robert Voeks and the anonymous reviewer for improvements to the manuscript. This work was supported by a grant from Fundo Nacional do Meio Ambiente to ELB (FNMA #75/2001). C.O.C.R. received a fellowship from the Fundação de Apoio à Pesquisa do Estado da Bahia (FAPESB). E.L.B. is supported by a grant (PQ2) from CNPq.
LITERATURE CITED
- Adams DE, Perkins WE, Estes JR. 1981. Pollination systems in Paspalum dilatatum Poir (Poaceae)—an example of insect pollination in a temperate grass. American Journal of Botany 68: 389–394. [Google Scholar]
- APG. 2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society 141: 399–436. [Google Scholar]
- Baumann U, Juttner J, Bian X, Langridge P. 2000. Self-incompatibility in the grasses. Annals of Botany 85 (Suppl. A): 203–209. [Google Scholar]
- Borba EL, Semir J. 1998. Wind-assisted fly pollination in three Bulbophyllum (Orchidaceae) species occurring in the Brazilian campos rupestres. Lindleyana 13: 203–218. [Google Scholar]
- Castellani TT, D'Eça-Neves FF. 2000. Population ecology of Paepalanthus polyanthus: predispersal hazards and seed production. Acta Botanica Brasilica 14: 317–326. [Google Scholar]
- CPRM. 1995.Projeto mapas municipais do município de Morro do Chapéu (BA). Salvador: Compahia de Pesquisa de Recursos Minerais. [Google Scholar]
- Cronquist A. 1981.An integrated system of classification of flowering plants. New York: Columbia University Press. [Google Scholar]
- Culley TM, Weller SG, Sakei AK. 2002. The evolution of wind pollination in angiosperms. Trends in Ecology and Evolution 17: 361–369. [Google Scholar]
- Dafni A. 1992.Pollination ecology. A practical approach. New York: Oxford University Press. [Google Scholar]
- Dahlgren RMT, Clifford HT, Yeo PF. 1985.The families of the Monocotyledons. Structure, evolution, and taxonomy. Berlin: Springer-Verlag. [Google Scholar]
- Endress PK. 1998.Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press. [Google Scholar]
- Faegri K, Pijl van der L. 1979.The principles of the pollination ecology, 3rd edn. Oxford: Pergamon Press. [Google Scholar]
- Figueiredo RA, Sazima M. 2000. Pollination biology of Piperaceae species in southeastern Brazil. Annals of Botany 85: 455–460. [Google Scholar]
- Giulietti AM. 1984.Estudos taxonômicos no gênero Leiothrix Ruhland (Eriocaulaceae) na Serra do Cipó, Minas Gerais, Brasil. Professor degree Thesis, Universidade de São Paulo, Brazil. [Google Scholar]
- Giulietti AM. 1997.Análise crítica da evolução da morfologia e da sistemática das Eriocaulaceae. Titular degree Thesis, Universidade Estadual de Feira de Santana, Brazil. [Google Scholar]
- Giulietti AM, Hensold N. 1990. Padrões de distribuição geográfica dos gêneros de Eriocaulaceae. Acta Botanica Brasilica 4: 133–158. [Google Scholar]
- Giulietti AM, Pirani JR. 1988. Patterns of geographic distribution of some plant species from the Espinhaço Range, Minas Gerais and Bahia, Brazil. In: Heyer WR, Vanzolini PE, eds. Proceedings of a Workshop on Neotropical Distribution Patterns. Rio de Janeiro: Academia Brasileira de Ciências, 39–69. [Google Scholar]
- Giulietti N, Giulietti AM, Pirani JR, Menezes NL. 1988. Estudos em sempre-vivas: importância econômica do extrativismo em Minas Gerais, Brasil. Acta Botanica Brasilica 1: 179–193. [Google Scholar]
- Guilherme FAG, Ressel K. 2001. Biologia floral e sistema de reprodução de Merostachys riedeliana (Poaceae: Bambusoideae). Revista Brasileira de Botânica 24: 205–211. [Google Scholar]
- Harley RM. 1995. Introdução. In: Stannard BL, ed. Flora of the Pico das Almas, Chapada Diamantina, Bahia, Brazil. Richmond: Royal Botanic Gardens, Kew, 43–78. [Google Scholar]
- Henderson A. 1986. A review of pollination studies in the Palmae. The Botanical Rewiew 52: 221–259. [Google Scholar]
- Hensold NC. 1988. Morphology and systematics of Paepalanthus subgenus Xeractis (Eriocaulaceae). Systematic Botanic Monographs 23: 1–150. [Google Scholar]
- Judd WS, Campbell CS, Kellogg EA, Stevens, PF, Donogue MJ. 2002.Plant systematics: a phylogenetic approach, 2nd edn. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Köppen W. 1948.Climatologia com un estudio de los climas de la Tierra (Transl. by Peres PRH). Mexico: Fondo de Cultura Economica. [Google Scholar]
- Koshy KC, Harikumar D, Narendran TC. 2001. Insect visits to some bamboos of the Western Ghats, India. Current Science 81: 833–838. [Google Scholar]
- Kral R. 1966. Eriocaulaceae of the continental North America north of Mexico. Sida 4: 285–332. [Google Scholar]
- Lazzari LRP. 2000.Redelimitação e revisão de Syngonanthus Sect. Eulepis (Bong. ex Koern.) Ruhland – Eriocaulaceae. PhD Thesis, Universidade de São Paulo, Brazil. [Google Scholar]
- Listabarth C. 2001. Palm pollination by bees, beetles and flies: why taxonomy does not matter. The case of Hyospathe elegans (Arecaceae, Arecoidae, Areceae, Euterpeinae). Plant Species Biology 16: 165–181. [Google Scholar]
- Martin FW. 1959. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technology 34: 125–128. [DOI] [PubMed] [Google Scholar]
- Miranda EB, Giulietti AM. 2001. Eriocaulaceae no Morro do Pai Inácio (Palmeiras) e Serra da Chapadinha (Lençóis), Chapada Diamantina, Bahia, Brasil. Sitientibus Série Ciências Biológicas 1: 15–32. [Google Scholar]
- Passos LM. 1995.Fenologia, polinização e reprodução de duas espécies de Croton (Euphorbiaceae) em mata semidecídua. Master thesis, Universidade Estadual de Campinas, Brazil. [Google Scholar]
- Rosa MM, Scatena VL. 2003. Floral anatomy of Eriocaulon elichrysoides and Syngonanthus caulescens (Eriocaulaceae). Flora 198: 188–199. [Google Scholar]
- Sano PT. 1996. Fenologia de Paepalanthus hilairei Koern., P. polyanthus (Bong.) Kunth e P. robustus Silveira: Paepalanthus sect. Actinocephalus Koern.—Eriocaulaceae. Acta Botanica Brasilica 10: 317–328. [Google Scholar]
- Scatena VL, Lima AAA, Lemos-Filho JP. 1997. Aspectos fenológicos de Syngonanthus elegans (Bong.) Ruhl. (Eriocaulaceae) da Serra do Cipó, MG, Brasil. Arquivos Biologicos Tecnologicos 40: 153–167. [Google Scholar]
- Scogin R, Young DA, Jones CE. 1977. Anthochlor pigments and pollination biology. II. The ultraviolet patterns of Coreopsis gigantea (Asteraceae). Bulletin of the Torrey Botanical Club 104: 155–159. [Google Scholar]
- Stradmann MTS. 1998.Plano de Manejo do Parque Municipal de Mucugê. Mucugê: Prefeitura Municipal de Mucugê. [Google Scholar]
- Stützel T. 1998. Monocotyledons: Alismatanae and Comelinanae (except Gramineae). In: Kubitzki K, ed. Flowering plants: the families and genera of vascular plants. Vol. IV. Berlin: Springer-Verlag, 197–207. [Google Scholar]
- Stutzel T, Gansser N. 1995. Floral morphology of North American Eriocaulaceae and its taxonomic implications. Feddes Repertorium 106: 495–502. [Google Scholar]
- Voeks RA. 2002. Reproductive ecology of the piassava palm (Attalea funifera) of Bahia, Brasil. Journal of Tropical Ecology 18: 121–136. [Google Scholar]
- Vogel S. 1990.The role of scent glands in pollination. On the structure and function of osmophores (Transl. by Bahatti JS). Washington: Smithsonian Institution Libraries. [Google Scholar]