Abstract
• Background and Aims The cotyledons of Lupinus angustifolius contain large amounts of cell wall storage polysaccharide (CWSP) composed mainly of (1→4)-β-linked d-galactose residues in the form of branches attached to a rhamnogalacturonan core molecule. An exo-(1→4)-β-galactanase with a very high specificity towards (1→4)-β-linked d-galactan has been isolated from L. angustifolius cotyledons, and shown to vary (activity and specific protein) in step with CWSP mobilization. This work aimed to confirm the hypothesis that galactan is the main polymer retrieved from the wall during mobilization at the ultrastructural level, using the purified exo-galactanase as a probe.
• Methods Storage mesophyll cell walls (‘ghosts’) were isolated from the cotyledons of imbibed but ungerminated lupin seeds, and also from cotyledons of seedlings after the mobilization of the CWSP. The pure exo-(1→4)-β-galactanase was coupled to colloidal gold particles and shown to be a specific probe for (1→4)-β-d-galactan. They were used to localize galactan in ultrathin sections of L. angustifolius cotyledonary mesophyll tissue during CWSP mobilization.
• Key Results On comparing the morphologies of isolated cell walls, the post-mobilization ‘ghosts’ did not have the massive wall-thickenings of pre-mobilization walls. Compositional analysis showed that the post-mobilization walls were depleted in galactose and, to a lesser extent, in arabinose. When pre-mobilization ghosts were treated with the pure exo-galactanase, they became morphologically similar to the post-mobilization ghosts. They were depleted of approximately 70% of the galactose residues that would have been mobilized in vivo, and retained all the other sugar residues originally present. Sharply defined electron-transparent wall zones or pockets are associated with CWSP mobilization, being totally free of galactan, whereas wall areas immediately adjacent to them were apparently undepleted.
• Conclusions The exo-(1→4)-β-galactanase is the principal enzyme involved in CWSP mobilization in lupin cotyledons in vivo. The storage walls dramatically change their texture during mobilization as most of the galactan is hydrolysed during seedling development.
Keywords: Galactan, Lupinus angustifolius, exo-β-galactanase, β-galactosidase, pectin, enzyme-gold cytochemistry, cell wall porosity
INTRODUCTION
The presence of galactose-containing material in seed cell walls was first noted in the late 19th Century. Schulze and Steiger (1892) identified the main sugar released on hydrolysis of the thickened cell walls of lupin cotyledonary tissues as galactose. Hirst et al. (1947) isolated a galactan from the seeds of the white lupin (Lupinus albus) and used methylation analysis to demonstrate that the linkage was (1→4). They concluded that the structure was (1→4)-β-linked d-galactan, and proposed that some arabinose was associated with it in the cell walls. Brillouet and Riochet (1983) analysed the compositions of the seed cell walls of several species of lupins and found that both the ratio of galactose to arabinose and the ratio of rhamnose to galacturonic acid correlated positively with the percentage of cell wall material in the seed cotyledons. These correlations were attributed to an increase in sites (rhamnose residues) for anchoring galactan side chains to a pectic core. Studies by Al-Kaisey and Wilkie (1992), involving methylation of the entire cell walls of Lupinus angustifolius cotyledons, have provided further evidence for the predominance of (1→4)-galactosyl linkages, and the attachment of galactan to a rhamnogalacturonan backbone.
The ultrastructural changes accompanying the mobilization of cell wall storage polysaccharides (CWSP) in lupin cell walls were documented by Parker (1984), who observed that electron transparent pockets of apparent degradation appeared in the storage cell walls near the plasmalemma, that degradation proceeded outwards towards the middle lamella and that thinner cell walls remained after CWSP mobilization was complete. The biochemistry of the post-germinative mobilization of CWSP from Lupinus angustifolius ‘Unicrop’ cotyledons was described by Crawshaw and Reid (1984). Before germination, the cotyledonary cell wall material is rich in galactose (76 %) and arabinose (13 %) residues, the remainder being composed of small amounts of uronic acid, xylose and glucose (rhamnose was not assayed). Following germination most of the galactose and arabinose residues were mobilized from the cell wall, leaving a small amount of residual wall material relatively enriched in the other monosaccharides (Crawshaw and Reid, 1984).
A β-galactosidase with a very high specificity towards lupin β-galactan, on which it acted as an exo-(1→4)-β-galactanase, was isolated by Buckeridge and Reid (1994). They showed that the exo-(1→4)-β-galactanase was synthesized de novo, that its activity and specific protein varied in step with cell wall mobilization, and that cotyledonary extracts contained no detectable endo-galactanase activity. This provided strong circumstantial evidence that the exo-galactanase was an important enzyme involved in galactan mobilization in vivo.
We now describe the isolation of whole cell walls (‘cell wall ghosts’) from lupin cotyledonary mesophyll cells before and after CWSP mobilization, and the action on the former of the purified exo-(1→4)-β-galactanase. We further report the preparation of a gold-conjugate of the pure enzyme, and its use to localize galactan in sections of cotyledons during CWSP mobilization.
METHODS
Plant material
Seeds of Lupinus angustifolius L. ‘New Zealand Bitter Blue’ were obtained from Royal Sluis Ltd, Leyland, Preston, UK. Seeds were soaked for 24 h in distilled water at 20°C, transferred to trays (30 × 57 × 3 cm deep; 300 seeds per tray) and allowed to germinate at 20 ± 2°C (photon fluence rate = 200 µmol m−2 s−1). The seedlings were grown at 20°C with a light intensity at the cotyledon surface of 850 µmol m−2 s−1 (12 h day, 12 h night).
Hydrolysis of polysaccharides
Polysaccharides were hydrolysed to their monosaccharide constituents using 72 % H2SO4/4% H2SO4 (Saeman et al., 1945). Polysaccharide (5 mg) was incubated for 45 min at 30°C in 72 % w/w H2SO4 (0·1 mL). The sample was diluted to 4 % w/w with distilled water and hydrolysed in an autoclave for 1 h at 120°C.
Analysis of monosaccharides by HPAEC (high performance anion exchange chromatography)
The neutral monosaccharides in the neutralized (NaOH) hydrolysates were identified by HPAEC. Samples were applied into a CarboPac PA1 anion-exchange column (250 × 4 mm; Dionex, Camberley, UK) which was eluted isocratically with a 20 mm NaOH solution for 2 min followed by water for 28 min at a flow rate of 1 mL min−1. Sugars were determined quantitatively using a pulsed amperometric detector (Dionex).
Determination of uronic acids
The content of uronic acids was determined in isolated galactan from L. angustifolius using the procedure described by Blumenkrantz and Asboe-Hansen (1973).
Isolation and analysis of galactan from Lupinus angustifolius cotyledons
Galactan was isolated from cotyledons of fully imbibed L. angustifolius seeds following exactly the same procedure described by Buckeridge and Reid (1994).
Galactose determination using galactose dehydrogenase
d-galactose was determined using β-d-galactose dehydrogenase (Sigma Chemicals; Kurz and Wallenfels, 1974) according to the procedure described in Buckeridge and Reid (1994).
Enzyme assays
β-galactosidases and/or β-galactanases were detected in extracts from the cotyledons of L. angustifolius using two substrates: p-nitrophenyl-β-d-galactopyranoside (PNPGal) and isolated lupin galactan. The conditions used were as described by Buckeridge and Reid (1994).
Preparation of the cell wall ghosts (CWG) and examination by scanning electron microscopy
Seeds of L. angustifolius imbibed for sixteen hours had their testae and embryonic axes removed. The dissected cotyledons were ground in a mortar with 3 volumes (v/w) of 100 mm potassium phosphate buffer pH 7·0. The suspension was further homogenized in an Ultra-turrax homogenizer (Divtech Equipment Co., Cincinnati, USA) with four pulses of 15 s each. The homogenate was centrifuged for 5 min in a bench centrifuge and the pellet re-suspended (1 : 1 v/v) in buffer. The suspension was sieved through a 250-µm pore nylon mesh, the filtrate centrifuged and the pellet re-suspended as before. The resuspended filtrate was sieved through a 53-µm pore nylon mesh. The residue was re-suspended in buffer, centrifuged as before and the pellet was washed three times with distilled water. The clean pellet was re-suspended in a minimum volume of water, dialysed against distilled water for 16 h at 4°C and freeze-dried.
For preparation of the CWG from 14 d-old cotyledons, a different procedure was used. Cotyledons were stripped of the epidermis, ground in a mortar with three volumes (v/w) of 100 mm potassium phosphate buffer pH 7·0 and centrifuged as above. The pellet was re-suspended and sieved through a 140-µm pore nylon mesh. The residue was frozen and thawed, centrifuged and the pellet re-suspended as above and the suspension was ground in glass homogenizer. After sieving (140-µm pore nylon mesh) the residue was subjected to the same procedure once more and a last filtration through 53-µm pore nylon mesh was performed. The residue was re-suspended in buffer, dialysed and freeze-dried as described for the 16-h cotyledons (see above).
The procedures described above were optimized for the two developmental stages of the tissue, by following the resultant isolated cell wall material using light microscopy.
The freeze-dried CWG were hydrolysed with sulphuric acid and analysed by thin layer chromatography and HPAEC as described above.
For enzyme assays, the dry CWG were re-suspended in 50 mm ammonium acetate buffer, pH 5·0, and incubated for various times at 30°C with or without enzyme. Release of galactose was monitored using the galactose dehydrogenase assay. When no more galactose was released (144 h), the hydrolysed (and control) CWG were dialysed against distilled water for 20 h (four changes) and freeze-dried. The dry CWG were coated with gold (Edwards Supercoater S150) and observed using a scanning electron microscope (ISI 60A).
Light and electron microscopy
Cotyledons were fixed using a solution of 2% paraformaldehyde : 2·5% glutaraldehyde in 0·1 m sodium cacodylate buffer, pH 7·4. Cubes of approximately 1 mm3 from the central part of the cotyledons were dehydrated in an alcohol series up to 100 %. The blocks were embedded in LR-White resin (Ted Pella, UK), following the manufacturer's instructions exactly. Polymerization of the resin was performed at 60°C overnight. Sections (1 mm) were cut in an ultramicrotome (LKB Ultratome III, 8800), from the fixed and embedded blocks.
For light microscopic observations, sections were placed on glass slides and stained with methylene blue. Sections that were fixed, embedded and cut as described above were covered with some drops of 0·1 % methylene blue and incubated for 1 min at 60°C. The excess of methylene blue was removed by blotting the slide on Whatman No. 3 paper. The sections were then observed and photographed directly using a camera-attached microscope (Zeiss, Oberkichen, FRG).
For electron microscopy observations, sections were collected on nickel grids and stained with 2% uranyl acetate (20 min), washed several times with distilled water, incubated with 4% lead citrate (10 min), washed with distilled water as before and observed using a transmission electron microscope (Jeol JEM 100C).
The sectional fractal dimension for the photographs displayed in Fig. 6 was calculated using the box counting method of high-resolution scanned photographs (Williams, 1997).
Preparation of the colloidal gold and estimation of the gold number
Fifty millilitres of a 0·01 % tetrachloroauric acid solution was boiled for 2–3 min and 2 mL of 2 % trisodium citrate was added rapidly. The solution was maintained in a boiling water bath (approx. 5 min) until it went from a very light yellow to purple and then wine-red in colour. With this procedure, 15 nm particles were formed (Roth, 1983).
In order to estimate the amount of pure enzyme necessary to stabilize the colloidal gold sol, 0, 5, 10, 20, 30, 40 and 50 mL of partially denatured (50°C for 5 min) pure exo-(1→4)-β-galactanase was incubated with 250 mL of gold solution. After 1 min, 25 mL of 10% NaCl was added to each tube. Blue, through violet up to wine-red colours developed, wine-red solutions being the ones in which the gold particles had been stabilized by the pure enzyme. In this experiment, stable red colour was observed in the solutions that had from 20 mL (0·35 mg of enzyme protein) to 50 mL (0·8 mg of enzyme protein) of enzyme for 270 mL of gold solution (Roland and Vian, 1991).
For the preparation of the enzyme–gold probe, 200 mL (3·5 mg of protein) of active, partially denatured (50°C for 10 min) or denatured (boiled for 5 min) enzyme protein dissolved in 20 mm Tris–HCl pH 8·6 was mixed with 0·8 mL of the colloidal gold solution at room temperature. The mixture was centrifuged (13000 g) and the red mobile pool was carefully removed. This procedure yielded approx. 50 mL of a dark-red enzyme–gold probe, which was used for localization of galactan on electron microscopic sections.
Ultrathin sections cut, fixed and embedded in LR-White as described above, were collected on nickel grids and incubated with a mixture (1:1) of the enzyme–gold complex and 0·1 m ammonium acetate buffer, pH 5·0, for 30 min in a moist chamber at room temperature. After incubation, the sections were washed thoroughly with distilled water and counter-stained with uranyl acetate and lead citrate as described above.
RESULTS
Characteristics of storage mesophyll ‘cell wall ghosts’ from L. angustifolius cotyledons before and after CWSP mobilization
Wet sieving procedures, involving tissue homogenization and filtration through nylon screens of various mesh sizes, were developed to obtain samples of cell walls from cotyledon storage parenchyma cell walls before and after CWSP mobilization. On examination under light and scanning electron microscopes the wall preparations were essentially free of cytoplasmic contamination. They also contained a high proportion of individual ‘cells’, the walls of which had been locally punctured allowing the contents to escape, whilst retaining the original cell and wall morphology. These wall preparations were termed ‘cell wall ghosts’ (CWG; Fig. 1A). In the CWG from cotyledons before CWSP mobilization, the contrast between the thickened areas of the storage cell walls and the thin-walled pit-fields areas was very obvious. Although the CWG from cotyledons after CWSP mobilization were similar in size, and their pit-fields could still be discerned, the walls seemed uniformly thin, and they had a ‘flimsy’ appearance (Fig. 1B). A comparison can be made with the light microscopy in Figs 6A, 7A and 7C: in the last ones the thickened storage wall reduces down so that the whole wall has become thin and practically uniform. The CWG from 16-h imbibed (before CWSP mobilization), and 14-d post-imbibition (after CWSP mobilization) were subjected to acid hydrolysis, and their monosaccharide compositions were determined. A comparison between the CWG before (16 h imbibition) and after mobilization in vivo (after 14 d; Table 1) shows that galactose is the principal component retrieved. Its proportion decreased from 66 to 30% of the neutral sugars in the wall, whereas the other neutral and also uronic acids increased several fold (4–5 times for rhamnose, glucose and uronic acids and 1·3 times for arabinose and xylose). These data suggest that a limited proportion of arabinose and xylose may also have been mobilized in vivo.
Fig. 1.
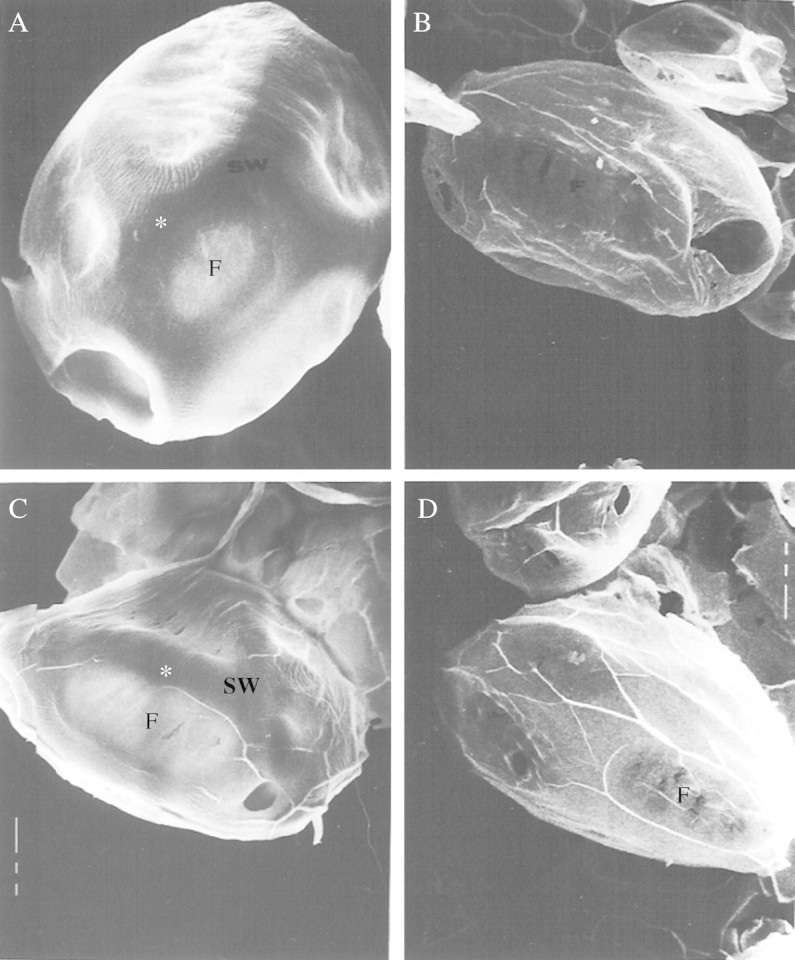
Appearance of ‘cell wall ghosts’ (CWG) by scanning electron microscopy. (A) CWG from 16-h imbibed cotyledons (×950); (B) CWG from 14-d-old cotyledons (×390); (C) 16-h CWG incubated for 144 h in 50 mm ammonium acetate buffer, pH 5·0, 30 °C (×607); (D) 16-h CWG incubated with pure exo-(1,4)-β-galactanase for 144 h in 50 mm ammonium acetate buffer, pH 5·0, 30 °C (×510). F, pit field; SW storage wall. White asterisks mark a storage thickened-wall area.
Table 1.
Composition (%) of storage mesophyll cell walls (‘cell wall ghosts’) from Lupinus angustifolius cotyledons
| Neutral monosaccharides* |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rha† |
Ara |
Gal |
Glc |
Xyl |
Uronic acids |
|||||
| CWG, 16-h imbibition | 2 | 12 | 66 | 4 | 15 | 83 | ||||
| CWG, 14-d | 11 | 16 | 30 | 23 | 20 | 35‡ | ||||
As % of total neutral monosaccharides.
Rha, rhamnose; Ara, arabinose; Gal, galactose; Xyl, xylose.
As % of cell wall material.
Digestion in vitro of pre-mobilization CWG with pure lupin exo-(1→4)-β-galactanase
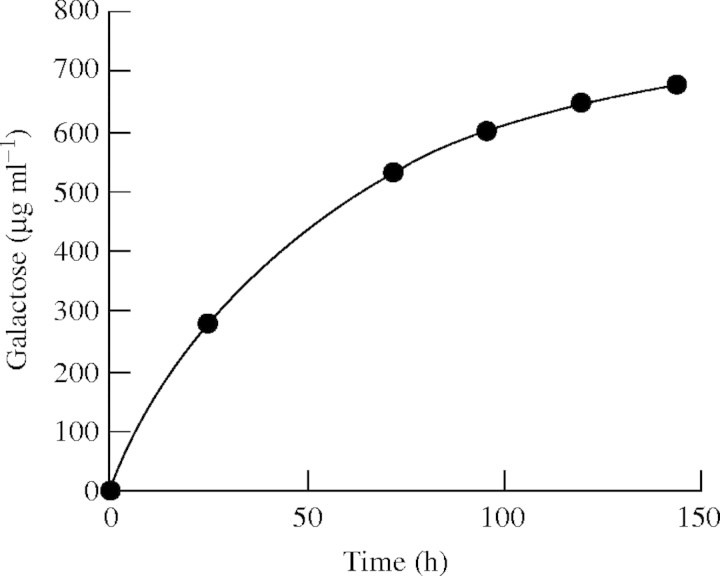
CWG from 16-h imbibed cotyledons were digested with the pure lupin exo-galactanase until no further release of galactose was detected (Fig. 2). No galactose was released from control CWG incubated under exactly the same conditions, but without the enzyme (not shown). The CWG were recovered after incubation, examined by scanning electron microscopy and hydrolysed prior to analysis of their neutral monosaccharide compositions. The 16-h CWG that had been incubated in buffer without enzyme were indistinguishable morphologically from 16-h CWG that had not been incubated (Fig. 1A, C) and the monosaccharide composition of the control CWG was the same after incubation as before (Table 1).
Fig. 2.
Cumulative release of galactose from CWGs incubated with pure exo-(1→4)-β-galactanase at 30 °C, pH 5·0. CWGs were washed and more enzyme was added at 50 and 96 h.
Table 2 shows the percentage of loss of each monosaccharide of the CWG after wall modification in vivo, treatment with a crude extract containing active hydrolases and digestion with pure exo-galactanase. Compositional data are normalized on the assumption that the glucose contents (arising presumably from cellulose) of the CWG do not change either during CWSP mobilization in vivo or during digestion with exo-galactanase. The CWG did not contain starch, as assessed by iodine staining.
Table 2.
Comparison of the percentage of loss of each monosaccharide from cell wall ghosts (CWG) after reserve mobilization in vivo (14 d), incubation with cotyledon enzyme extract (crude enzyme) and with purified exo-(1→4)-β-galactanase
| Monosaccharide |
CWG after 14 d |
CWG+crude enzyme |
CWG+exo-(1→4)- β-galactanase |
|---|---|---|---|
| Rhamnose | 45 | 10 | 0 |
| Arabinose | 57 | 56 | 0 |
| Galactose | 92 | 86 | 63 |
| Glucose | 0 | 0 | 0 |
| Xylose | 45 | 37 | 0 |
Galactose residues constitute almost 70 % of the neutral sugars in the storage cell walls (Table 1), and approximately 86% of the cotyledon wall material is removed during CWSP mobilization (Crawshaw and Reid, 1984). Thus the 92% depletion in galactose residues occurring in vivo (Table 2) represents the loss of most of the polysaccharide material originally present in the wall. The 57% depletion of arabinose residues (originally 12% of the wall) noted in vivo (Table 2) also represents a significant depletion in absolute terms, but the large percentage losses of arabinose, rhamnose and xylose (very minor components of the cotyledonary cell walls according to Crawshaw and Reid, 1984) represent the loss of only a very small percentage of the total wall material. When the 16-h CWG were treated with the purified exo-galactanase, the galactose residues of the wall were depleted to the extent of 63%, which represents 68% of the galactose residues which would have been removed during mobilization in vivo, and a major part of the total cell wall material originally present.
In appearance (Fig. 1D) the galactanase-treated CWG were very similar to the CWG from 14-d (post-mobilization)cotyledons (Fig. 1C), in that the walls were thin, and localized storage thickenings could scarcely be discerned.
Stabilization of a gold sol using the exo-galactanase, and investigation of the specificity of the gold complex as a cytochemical probe
Gold sols (particle diameter 15 nm) were stabilized successfully using the pure exo-galactanase, but no labelling of the cell wall was observed in sections from 16-h imbibed cotyledons (Fig. 3A). On the other hand, when exo-(1→4)-β-galactanase was partially denatured for 10 min at 50°C prior to complexing with the gold particles, labelling was observed on the cell walls (Fig. 3B). The protein bodies were also labelled with the exo-galactanase–gold complex, although much less strongly than the storage walls (Fig. 3A, B). Thus, only gold complexes formed from heat-treated enzyme were used in the controls described below.
Fig. 3.
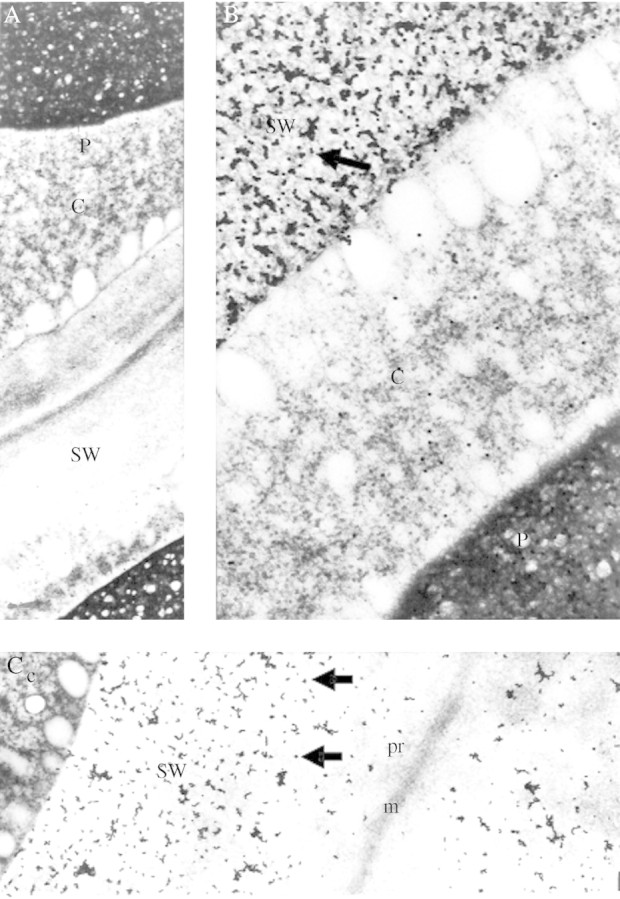
Labelling of 16-h imbibed lupin cotyledonary sections using gold complexes from native and heat-treated (50 °C for 10 min) exo-galactanase. (A) Staining with native exo-(1→4)-β-galactanase gold complex (×9200). No gold staining is observed in this micrograph. (B) Staining with heat treated exo-(1→4)-β-galactanase gold complex (×37 600). The arrow points to a cluster of gold particles. (C) Wall cross-section showing specific staining of the secondary cell wall thickenings (storage walls) labelled with heat-treated gold complex (×14 600). c, cytoplasm; m, middle lamella; P, protein body; pr, primary wall, SW, storage wall.
When sections were ‘blocked’ with protein (BSA) prior to treatment with the gold complex, it was found that protein concentrations greater than 5 mg mL−1 eliminated labelling of the protein bodies completely, without affecting labelling of cell walls (not shown). Inclusion of soluble lupinseed galactan (5 mg mL−1) during treatment with the gold complex suppressed labelling of the cell walls completely, but did not eliminate the labelling of the protein bodies (Fig. 4B). Pre-treatment of sections with active exo-(1→4)-β-galactanase greatly reduced labelling of both cell walls and protein bodies (compare Fig. 4C and D). In Fig. 4D, the dark aggregates in the cell walls are not gold particles. They may be wall material that has collapsed after removal of galactan. In contrast, gold particles are clearly visible in Fig. 4C (arrow).
Fig. 4.
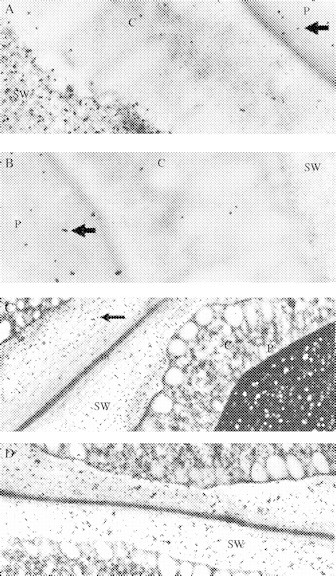
Cytochemical controls using sections of 16-h imbibed lupin cotyledons. (A) Enzyme boiled (5 min) before gold complexing (×32 000); (B) addition of lupin (1→4)-β-galactan during staining with the gold-complexed enzyme (×32 000). (C) Enzyme boiled (5 min) before gold complexing (×13 300); (D) Pre-incubated with active exo-(164)-β-galactanase before staining with heat-deactivated gold complex (×13 300). After in vitro hydrolysis with exo-galactanase clusters with higher density can be seen, but no gold particles stained them. We assumed that these clusters are probably due to the appearance of the fibrous material that is left after hydrolysis, as shown in Figure 7B. Arrows in (A), (B) and (C) point to gold particles. c, cytoplasm; P, protein body; SW, storage wall.
Although protein bodies were labelled with the galactanase–gold probe (Fig. 4A), labelling of cell walls was always restricted to the storage thickenings of mesophyll parenchyma cell walls. It did not occur in the middle lamella or primary walls layers (Fig. 3C), and was hardly found in the walls of the cells of vascular or epidermal tissues. These observations, the known action of the enzyme, and the control experiments described above indicated strongly that the galactanase–gold probe bound specifically to (1→4)-β-linked galactan in the cell wall.
Use of the exo-galactanase–gold complex as a cytochemical probe during CWSP mobilization
Sections from L. angustifolius cotyledons were screened using the gold probe between seed imbibition and the completion of CWSP mobilization 16 d later.
As the seeds take approximately 24 h to imbibe water, a distinct enlargement of the cell wall was observed from day 1 to day 2 (Fig. 5A, C). This is the period before the appearance of exo-galactanase (days 0 to 4, Buckeridge and Reid, 1994) and examination of the storage cell walls (but not middle lamellae and primary walls) showed strong staining with the gold complex (Fig. 5B, D).
Fig. 5.
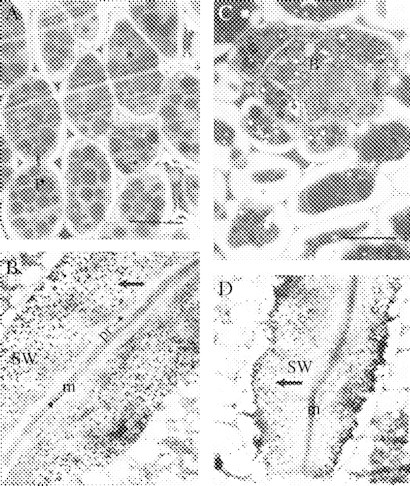
(A, B) Day 0 (16-h imbibed seeds). (A) Light microscopy of the storage mesophyll tissue of imbibed lupin cotyledons. Staining with methylene blue. Scale bar = 50 μm. (B) Ultrastructural view of a junction between two adjacent mesophyll cells from lupin cotyledons (×11 600). (C, D) Day 2. (C) Light microscopy of the storage mesophyll tissue, showing vascular bundle. Scale bar = 40 μm. (D) Ultrastructural view of a junction between two adjacent storage mesophyll cells (×11 600). Arrows in (B) and (D) point to gold particles. Labelling with the heat-treated, gold-complexed enzyme followed by staining with 2% uranyl acetate and 4% lead citrate. m, middle lamella; P, protein body; pr, primary wall; SW, storage wall; B, vascular bundle.
The period of rapid increase in exo-galactanase in this seed population (Buckeridge and Reid, 1994) begins at day 6 (radicle protrusion) and continues until day 11. Day 6 sections examined under the light microscope (Fig. 6A) showed for the first time altered reactivity with the enzyme gold probe. There were areas in the storage cell wall that were labelled as intensely as before, and zones from which gold-labelling was completely absent (e.g. the clear areas in Fig. 6B). The non-labelled areas all appeared to connect at some point with the inner edge of the wall, next to the plasma membrane. They resembled, and almost certainly correspond to, the ‘digestion pockets’ observed by Parker (1984). The boundaries between the labelled and non-labelled areas of the wall were very clear, but not smooth or regular. The impression was that the non-labelled areas were advancing outwards into the wall, expanding irregularly from the plasma membrane, and probably coalescing. The non-labelled areas were not empty, containing a network of electron-dense fibres superimposed on a paler mottled background (arrows in Fig. 6D). By days 8–10, when mobilization is still in course (Fig. 7A, C), the unlabelled areas had apparently advanced and coalesced (Fig. 7B, D), and very few labelled patches remained at days 10–12 (Fig. 7D).
Fig. 6.
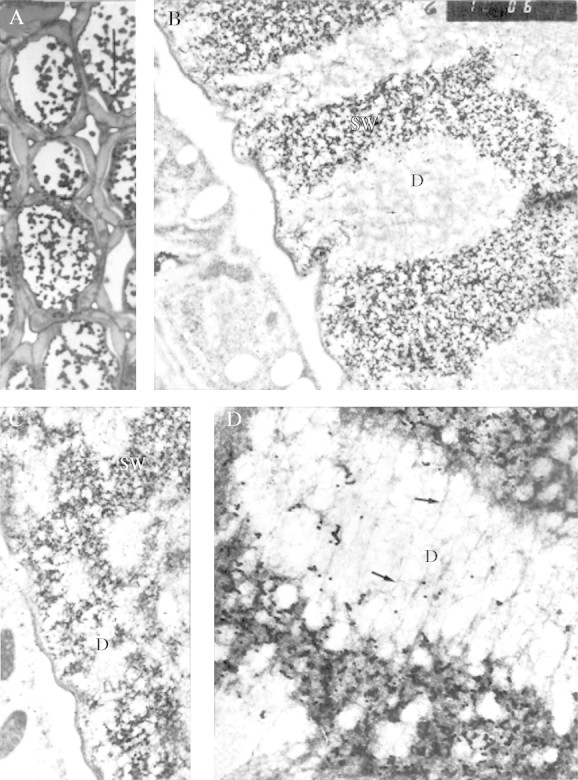
Day 6. (A) Light microscopy of the storage mesophyll tissue. Scale bar = 50 μm. (B) Ultrastructural details of cell wall degradation in storage mesophyll, which appear after degradation of galactan in vivo (×13 900). Fractal dimension, D = 1·62. (C) Detail of cytoplasm–cell wall junction showing digestion pockets, labelled ‘D’ (×13 900). Fractal dimension, D = 1·71. (D) Detail of a digestion pocket and surroundings (×34 600). Fractal dimension, D = 1·71. The arrows point to fibrous material (possibly clusters of rhamnogalacturonan core molecules). Labelling with the heat-treated, gold-complexed enzyme followed by staining with 2 % uranyl acetate and 4 % lead citrate. D, digestion pocket; SW, storage wall.
Fig. 7.
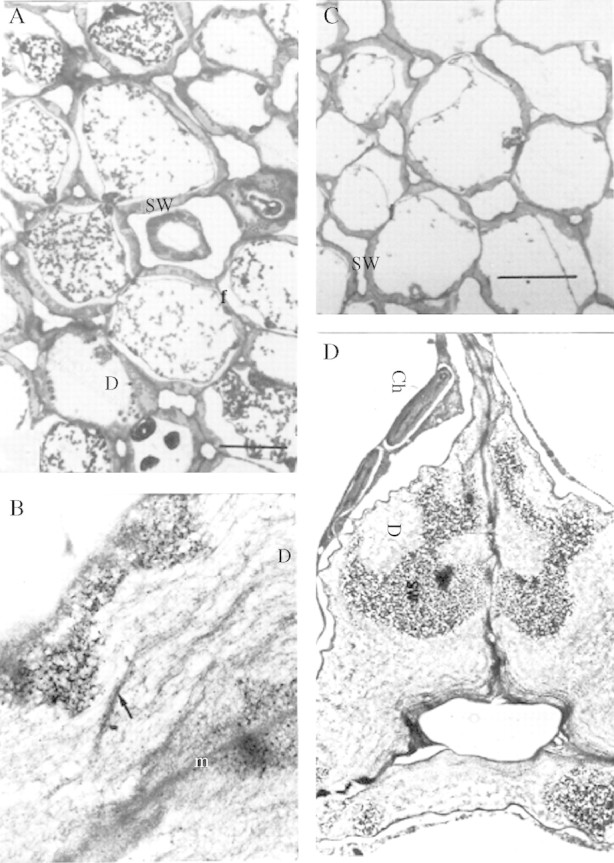
(A, B) Day 8. The dark regions in the wall are stained with galactanase–gold particles whereas the clear regions are not. (A) Storage mesophyll tissue during degradation of storage cell wall. (B) Ultrastructure of the storage cell wall during mobilization. The arrow points to fibrous material (possibly clusters of rhamnogalacturonan core molecules), which appears after degradation of galactan in vivo (×15 400). (C, D) Day 10. (C) Storage mesophyll tissue. Scale bar = 40 μm. (D) Ultrastructural view of a junction between three adjacent mesophyll cells (×4600). Labelling with the heat-deactivated gold-complexed enzyme followed by staining with 2% uranyl acetate and 4% lead citrate. Ch, chloroplasts; D, digestion pocket; SW, storage wall.
After day 12, cell walls became very thin and most of them were devoid of labelled areas. A few could, however, still be found, and retained their high staining intensity and sharp boundaries (not shown).
DISCUSSION
We have demonstrated that treatment in vitro of CWG from storage mesophyll parenchyma cells with lupin exo-galactanase results in the removal of the massive storage wall thickenings, giving a CWG that is scarcely distinguishable morphologically from those isolated cotyledons after CWSP mobilization in vivo. We have also shown that the enzyme treatment in vitro catalyses the removal from the wall of 68% of those galactose residues which would have been removed during CWSP mobilization in vivo, without the simultaneous removal of any other sugar residue. Since galactose residues comprise some 60% of the storage wall this represents the removal by a single enzyme of the major part of the carbohydrate mobilized in vitro. Given the known specificity of the galactanase (Buckeridge and Reid, 1994), we have clearly confirmed that (1→4)-β-d-galactan is a major structural feature of the storage cell walls, and that at least most of it is accessible to exo-hydrolysis without the need for any other facilitating enzyme. Given that endo-galactanase has been sought but not detected in lupin cotyledons, and that exo-galactanase activity and protein vary in step with CWSP mobilization, it may reasonably be concluded that the exo-galactanase is the main enzyme involved in CWSP mobilization in vivo. Other enzyme activities are, however, clearly involved and are necessary to allow the removal from the storage cell wall of quantitatively significant amounts of arabinose residues, minor amounts of other non-galactose sugar residues and a quantity of galactose residues corresponding to approx. 30% of the amount removed by exo-galactanase in vivo. The galactose residues that are not hydrolysed on treatment of CWG with the galactanase in vitro may not be (1→ 4)-β-linked, or may be (1→ 4)-β-linked but inaccessible to the enzyme either because of lateral branching (Al-Kaisey and Wilkie, 1992) or physical entanglements.
Our preparation and use of an exo-galactanase–gold complex to probe the distribution of galactan in the cell walls of lupin cotyledons before and after CWSP mobilization demonstrates that removal of galactan from the storage cell walls coincides exactly with the appearance and increase in exo-galactose activity and protein (Buckeridge and Reid, 1994). Apart from reinforcing the importance of this enzyme in galactan hydrolysis in vivo, these studies have confirmed that the electron-transparent wall zones observed earlier by Parker (1984) are indeed linked to CWSP mobilization. We have now shown that these electron-transparent pockets contain no material capable of binding the galactanase–gold probe, whereas the wall material immediately adjacent to them retains full reactivity with the complex (Fig. 6D). This remarkably sharp boundary between galactan-containing and fully galactan-depleted zones, together with the irregular pattern of the apparent expansion of the depleted zones, is strongly suggestive that the enzyme catalysing galactan digestion, the exo-galactanase, does not diffuse freely through the storage wall. The network of the wall may be too tight to allow the galactanase (Mr = 60 kDa) to diffuse rapidly through the wall, so that galactan hydrolysis by the action of the enzyme is necessary to allow the ‘front’ of mobilization to advance. Alternatively, diffusion of the enzyme into the wall matrix may be possible in theory, but limited in practice by affinity-binding of virtually all available enzyme molecules to galactan chains at the advancing dissolution front. This would, of course, require the front to have a very large surface area, but this may well be the case, as witnessed by the highly invaginated outlines of the digestion zones. We have calculated sectional fractal dimension for the photographs displayed in Fig. 6 and found D = 1·6–1·7. This indicates a degree of roughness that greatly increases the wall surface area (Williams, 1997). It would also require a high density at the advancing front of non-reducing galactan chain-ends, which would be available if the chains are short and anchored to a rhamnogalacturonan backbone.
Our data show very clearly that the massive deposits of galactan are restricted to the ‘storage’ thickenings of the cotyledon mesophyll cell walls. The middle lamellae and thin primary walls do not bind the exo-galactanase–gold complex, and they appear to remain relatively unchanged during CWSP mobilization, although we cannot say with certainty that their polysaccharide composition remains unaltered. Structural studies (Al-Kaisey and Wilkie, 1992) indicate that the cotyledonary storage cell walls of Lupinus angustifolius do contain small amounts of the primary cell wall constituents such as cellulose and xyloglucan in addition to pectin and overwhelming quantities of the ‘storage’ constituents. It seems reasonable that the cellulose and xyloglucan would be present predominantly in the primary cell walls. Our data also confirm and reinforce the existing hypothesis that the galactan chains in the storage cell walls are anchored to a rhamnogalacturonan core. The fibrous residue remaining in galactan-depleted areas of the cell walls (Figs 6, 7) could represent this backbone, stripped of all or most of its side-chains. Our results are fully consistent with the hypothesis (Buckeridge et al., 2000) that the CWSP represent the superdeposition of polysaccharides whose biological function has been transferred in the course of evolution from a mainly structural one in primary cell walls to a predominantly storage one in certain seed organs. Also, considering that the gene that encodes the purified lupin exo-galactanase has already been cloned and sequenced (access number AJ011047), the present work is an example of a clear relationship between gene–enzyme–polysaccharide function in a biological system.
Supplementary Material
Acknowledgments
We thank CNPq and FAPESP (BIOTA 98/05124–8) for funding to MSB. MSB thanks his colleague Heitor Monteiro Duarte from Techische Universität Darmstadt for the calculations of fractal dimensions of the partially degraded walls in Fig. 6.
LITERATURE CITED
- Al-Kaisey MT, Wilkie KCB. 1992. The polysaccharides of agricultural lupin seeds. Carbohydrate Research 227: 147–161. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. 1973. New method for quantitative determination of uronic acids. Analytical Biochemistry 54: 484–489. [DOI] [PubMed] [Google Scholar]
- Brillouet JM, Riochet D. 1983. Cell wall polysaccharides and lignin in cotyledons and hulls of seeds from various lupin (Lupinus L.) species. Journal of Food Science and Agriculture 34: 861–868. [Google Scholar]
- Buckeridge MS, Reid JSG. 1994. Purification and properties of a novel β-galactosidase or exo-β-(1→4)-galactanase from the cotyledons of germinated Lupinus angustifolius L. seeds. Planta 192: 502–511. [DOI] [PubMed] [Google Scholar]
- Buckeridge MS, Santos HP, Tiné MAS. 2000. Mobilization of storage cell wall polysaccharides in seeds. Plant Physiology and Biochemistry 38: 141–156. [Google Scholar]
- Crawshaw LA, Reid JSG. 1984. Changes in cell-wall polysaccharides in relation to seedling development and the mobilization of reserves in the cotyledons of Lupinus angustifolius cv. Unicrop. Planta 160: 449–454. [DOI] [PubMed] [Google Scholar]
- Hirst EL, Jones JKN, Walder WO. 1947. Pectic substances. Part 7: The constitution of the galactan from Lupinus albus Journal of the American Chemical Society, 1225–1229. [PubMed] [Google Scholar]
- Kurz G, Wallenfels K. 1974.d-galactose. U.V.-assay with galactose dehydrogenase. In: Bergmeyer HV, ed. Methods of enzymatic analysis. Volume 3, 2nd edn. Weinheim: Verlag Chemie, 1279–1282. [Google Scholar]
- Parker ML. 1984. Cell wall storage polysaccharides in cotyledons of Lupinus angustifolius L. II mobilization during germination and seedling development. Protoplasma 120: 233–241. [Google Scholar]
- Roland JC, Vian B. 1991. General preparation and staining of thin sections. In: Electron microscopy of plant cells. London: Academic Press, 1991, 2–66. [Google Scholar]
- Roth J. 1983. The colloidal gold marker system for light and electron microscopic cytochemistry. In: Bullock G, Petrusz P, eds. Techniques in immunochemistry. Volume 2. London: Academic Press, 217–284. [Google Scholar]
- Saeman JF, Buhl JL, Harris EE. 1945. Quantitative saccharification of wood and cellulose. Industrial and Engineering Chemistry. Analytical Edition 17: 35–37. [Google Scholar]
- Schulze E, Steiger E. 1892. Zur Kenntnis des Paragalaktans. Landwirtsch. Vers. Stn. 41: 223–220. [Google Scholar]
- Williams GP. 1997.Chaos theory tamed. Washington DC: Joseph Henry Press/National Academy Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.