Abstract
• Background and Aims Plathymenia reticulata (Leguminosae) is a Brazilian tree that occurs in two biomes: Cerrado, a woody savanna vegetation, and the Atlantic Forest, a tropical forest. In this study, phenological patterns and their variability within and among populations located in these biomes and in transitional zones between them were assessed.
• Methods During a 15-month period, individuals from two populations in Cerrado, two in the Atlantic Forest, and six in transitional zones (three in a cerrado-like environment and three in forest fragments) were evaluated in Minas Gerais State, Brazil. The individuals were evaluated monthly according to the proportion of the canopy in each vegetative phenophase (leaf fall, leaf flush and mature leaves) and each reproductive phenophase (floral buds, flowers, immature fruits and mature fruit/seed dispersal). In order to assess the phenological variability within and among populations, habitats and biomes, the Shannon–Wiener diversity index, the Morisita–Horn similarity index and genetic population approach of partitioning diversity were used.
• Key Results Populations of P. reticulata, in general, showed similar phenology; the main differences were related to leaf fall, a process that starts months earlier in the Cerrado than in transitional sites, and even later in forest areas. Considerable synchrony was observed for reproductive phenology among populations and between biomes. Most phenological diversity was due to differences among individuals within populations.
• Conclusion In spite of environmental differences, P. reticulata from the Atlantic Forest and Cerrado showed similar phenological behavior with only about 10 % of the total diversity being attributed to differences between biomes.
Keywords: Atlantic Forest, Cerrado, Morisita–Horn similarity index, leaf phenology, Plathymenia foliolosa, Plathymenia reticulata, reproductive phenology, Shannon–Wiener diversity index
INTRODUCTION
Flowering and fruiting phenology may have an important influence on reproductive success, as it determines reproductive synchrony among potential mates (Marquis, 1988) and may influence the attraction of pollinators and seed dispersers (Augspurger, 1981). Additionally, the phenology of vegetative stages is important as cycles of leaf flush and leaf fall are intimately related to processes such as growth, plant water status and gas exchange (Reich, 1995).
Augspurger (1983) noted that the understanding of tropical phenology was best developed at the community level and this view has not changed in the intervening years. Many studies have evaluated a single species' phenology, but, in general, they have not examined differences among or within populations (but see Marquis, 1988; Seghieri and Simier, 2002). Few studies have made comparisons among populations located at sites characterized by marked environmental differences (e.g. Frankie et al., 1974; Borchert, 1980). This approach is valuable as it may help to understand the extent of phenological variability as a survival strategy in different environments and how abiotic factors influence phenological patterns.
The genus Plathymenia (Leguminosae, Mimosoideae) is a good model to study in relation to this perspective as it is adapted to biomes with environmental differences, namely the Brazilian Cerrado, a woody savanna vegetation, and the Brazilian Atlantic Forest, a tropical forest. These habitat differences were considered in distinguishing two Plathymenia species, with P. foliolosa growing in forest areas and P. reticulata in Cerrado (Heringer, 1956). However, in a recent revision of this genus, Warwick and Lewis (2003) proposed it to be monospecific, containing only P. reticulata. This tree is known as ‘vinhático’ and occurs in at least 15 Brazilian states. It has been classified by the Brazilian Agricultural Research Corporation (EMBRAPA) as one of the most important and useful plant species from the Cerrado (Almeida et al., 1998) due to its high quality wood and potential use for the recovery of degraded areas (Heringer and Ferreira, 1972).
As noted by Adler and Kielpinski (2000), the first step in studying phenology is to identify spatial and temporal patterns, as such information is important in laying the foundation for identifying factors that underline those patterns. The main objective of this work was to describe the phenology of P. reticulata, evaluating populations from Cerrado, Atlantic Forest and transitional sites. Some studies on the phenology of Cerrado flora (e.g. Oliveira, 1998; Batalha and Mantovani, 2000; Bulhão and Figueiredo, 2002) and the Atlantic Forest flora (e.g. Morellato et al., 2000; Fisher and Santos, 2001) are available in the literature but, to our knowledge, this is the first comparison of phenological patterns among populations of a species that occurs in both biomes.
In a study evaluating populations of Plathymenia from Cerrado and Atlantic Forest, Lacerda et al. (2002) showed that 60 % of molecular variation was due to differences among plants from the different biomes. Furthermore, evaluating Cerrado populations of this species, Lacerda et al. (2001) reported that most of the genetic variation in this biome was attributable to differences among plants within populations. Considering that abiotic factors affect phenological patterns (Reich, 1995) and also that genetic components can influence these patterns (Murfet, 1977), we predicted that most of the phenological differences in Plathymenia would be found between biomes, and that within a biome most of the differences would be between individuals within populations. To assess phenological variability and its distribution within and among populations, habitats and biomes, an approach based on the Morisita–Horn similarity index, the Shannon–Wiener diversity index and genetic population methodology of partitioning diversity was applied.
MATERIAL AND METHODS
Study biomes
The phenology of natural populations of P. reticulata occurring in areas of the Atlantic Forest and Cerrado was evaluated. These Brazilian biomes are both considered priority ‘hot spots’ for conservation due to the high levels of biodiversity and endemic species, and the fact that they are among the most endangered eco-regions on Earth (Myers et al., 2000).
The Brazilian Atlantic Forest once covered more than 1 million km2, but human activities have reduced it toabout 5 % of the biome's original cover (Mittermeier et al., 1999). Different vegetation types of the Atlantic Forest are recognized; in Minas Gerais State semi-deciduous inland forest is the most common type and characterizes the forest sites studied here. Brazilian Cerrado covered almost 2 million km2, but today it is reduced to only 20 % of the original area (Fonseca et al., 1999). Several Cerrado vegetation types are recognized, and populations of P. reticulata are found particularly in the strict woodland/savanna and grassland formations (Lacerda et al., 2001) where the study took place.
The climate in both Cerrado and semi-deciduous Atlantic Forest is characterized by rainfall concentrated from November to March, making monthly variation in precipitation greater than the variation in temperature. The two biomes differ in the length and severity of the dry season. In the studied regions in Minas Gerais, semi-deciduous forest has a mean annual precipitation of about 1480 mm (Stallings et al., 1991), while Cerrado sites have 1200 mm of rain (data from the 5th Brazilian Meteorological District, Belo Horizonte). Data for temperature and rainfall for the regions for 2003 are shown in Fig. 1.
Fig. 1.
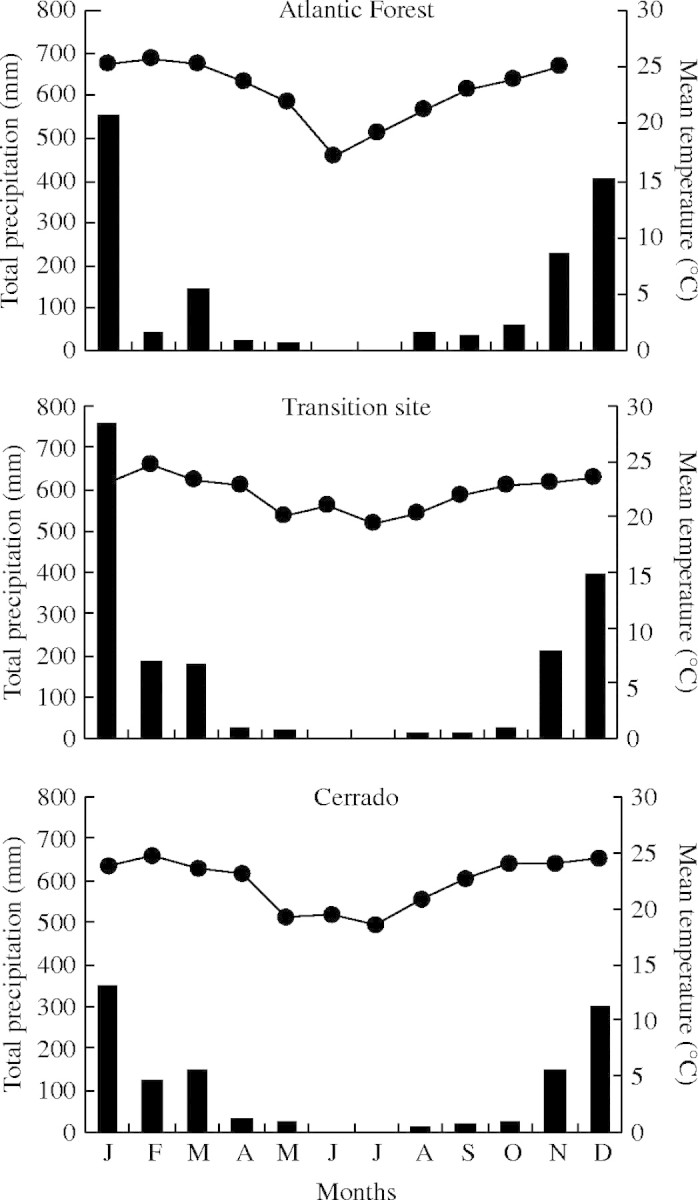
Meteorological data for the studied regions in Minas Gerais State during 2003 (precipitation = bars, temperature = lines): Atlantic Forest (data from meteorological stations located in Dionísio and João Monlevade), transition site between biomes (Belo Horizonte) and Cerrado (Pompéu and Sete Lagoas).
Study sites
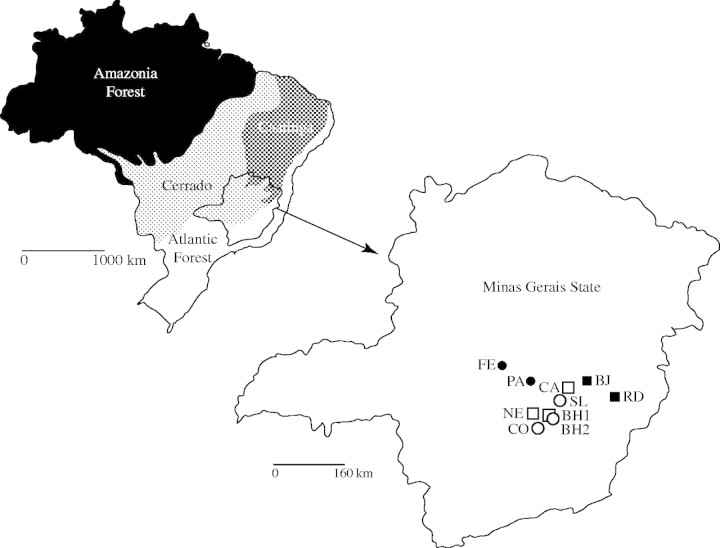
The study was performed following a three-level perspective: populations, habitats and biomes. Ten populations of P. reticulata were evaluated from Minas Gerais State, located in two different biomes (Atlantic Forest and Cerrado) and four different habitat types as follows. (1) Atlantic Forest core area: two populations located in the Rio Doce valley, Parque Estadual do Rio Doce (RD, 19°48′S 42°36′W) and Bom Jesus do Amparo (BJ, 19°45′S 43°31′W), where Plathymenia trees reach 20 m in height and until recently were classified as P. foliolosa. (2) Cerrado core area: two populations located in the central State area, Felixlândia (FE, 18°43′S 45°03′W) and Paraopeba (PA, 19°18′S 44°30′W), where P. reticulata trees are shorter and twisted. (3) Atlantic Forest in a transition site with Cerrado: three populations, Belo Horizonte (BH1, 19°56′S 46°56′W), Ribeirão das Neves (NE, 19°48′S 44°06′W) and Caeté (CA, 19°46′S 43°39′W), all showing forest characteristics with denser vegetation, higher trees and P. reticulata individuals being morphologically similar to forest individuals. (4) Cerrado in a transition site with Atlantic Forest: three populations, Belo Horizonte (BH2, 19°56′S 46°56′W), Santa Luzia (SL, 19°49′S 43°48′W) and Contagem (CO, 19°54′S 44°03′W), with P. reticulata trees showing Cerrado characteristics. Henceforth the habitats are identified by the letters F (forest), FT (forest in transition), C (Cerrado) and CT (Cerrado in transition) following the population name. Locations and other population characteristics are shown in Fig. 2.
Fig. 2.
Brazil's main biomes, with detail of Minas Gerais State and approximate locations of the P. reticulata populations studied: Atlantic Forest core area (black squares), Cerrado core area (black circles), in transition sites between biomes with forest characteristics (white squares), and transitional sites with Cerrado characteristics (white circles).
Phenological data sampling
Eleven to 20 individuals of P. reticulata in each population were sampled, totaling 182. Trees were chosen randomly, but in forest sites only trees with visible crowns were included. The distance among individuals within a population varied from 10 m to 1 km.
Trees were marked and observations were made monthly from October (BJ-F, CA-FT, NE-FT, BH1-FT, BH2-CT, SL-CT and CO-CT) or November 2002 (RD-F, PA-C and FE-C) through to December 2003. A single person made all observations of individual tree crowns using binoculars to evaluate the vegetative phenological phases (leaf fall, leaf flush and mature leaf) and reproductive phases (floral buds, open flowers, immature and mature fruit/seed dispersal; as the difference between closed mature pods and mature pods releasing seeds is very hard to determine under field conditions, both forms were considered as the same phase). Characteristics were graded relatively as follows: 0 = absence of the characteristic; 1 = presence of the characteristic in 1–25 % of the crown; 2 = 26–50 %; 3 = 51–75 %; and 4 = 76–100 %. For each group of phenological phases, individuals were characterized by the combination of assessed grades. For example: a tree evaluated as 1-2-1 for vegetative phenology would show grade 1 for leaf fall, grade 2 for leaf flush and grade 1 for mature leaf (the grades totalling 4, or 100 % of the crown). Note that these grades always follow the order specified in this example. For the reproductive phases, the combination 1-2-1-0 would mean that this individual was grade 1 for floral buds, grade 2 for open flowers, grade 1 for immature fruits and grade 0 for mature fruit/seed dispersal (again totaling 4, or 100 % of the crown). The grades henceforth always follow the order specified here. For both reproductive and vegetative phenology, each grade combination represented a specific phenological state.
Analysis of data
In order to describe monthly variability in phenology, for each individual a grade combination was attributed and three different analyses were performed separately for the vegetative and reproductive phases. For reproductive data, only individuals that flowered at least once during the observation period were considered. A diversity index was used to estimate synchrony among and within populations, habitats and biomes. Higher values for this index indicate higher phenological diversity, meaning lower phenological synchrony. This index was also used to estimate the partitioning of diversity; for example, which percentage of the total phenological diversity that was due to differences in behaviour among individuals within populations and among different populations. Finally, a similarity index was used to estimate synchrony among populations, with higher values indicating higher phenological synchrony. Details about each of the analyses are given below.
Diversity of phenological behaviour was estimated using the Shannon–Wiener diversity index. This index is widely used among community ecologists and is commonly adapted to other scientific areas (e.g. population genetics; Lacerda et al., 2001). According to Magurran (1988), the index is:
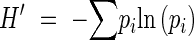 |
Frequencies of different phenological states (given by the grade combination) were used instead of frequencies of different species in a community. Accordingly, pi was considered as the proportion of individuals found in the ith combined grade, with pi = ni/N; ni being the number of individuals showing the phenological status i, and N the total number of individuals in the population. Populations with greater numbers of phenological states show higher values of this index, and less phenological synchrony.
Four different calculations were performed for each month. (1) Diversity index for each one of the ten populations, in which a mean value was attributed to the level of phenological diversity within populations (Hpop). (2) Mean values for each one of the four habitats (Hhab) without considering the population effect. (3) Mean values for the two biomes (Hbio), without considering population or habitat effect. (4) Total diversity using data from all evaluated individuals (Htotal). Using these indexes, partitioning of total diversity was determined. In a way similar to Lacerda et al. (2001), (Hpop/Htotal) × 100 was considered to be the percentage of diversity attributable to differences in phenology among individuals within populations, and [(Htotal – Hpop)/Htotal] × 100 the diversity attributable to differences among populations. (Hhab/Htotal) × 100 was considered the percentage of diversity attributable to differences among individuals within habitats, and [(Htotal – Hhab)/Htotal] × 100 the diversity attributable to differences among habitats. Finally, (Hbio/Htotal) × 100 was considered the percentage of diversity attributable to differences within biomes, and [(Htotal – Hbiol)/Htotal] × 100 the diversity attributable to differences between biomes.
Besides diversity, similarity among populations also was evaluated monthly by the Morisita–Horn similarity index (Magurran, 1988):
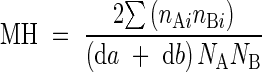 |
where 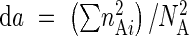 ,
, 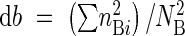 , NA is the total number of individuals in population A, NB the total number of individuals in population B, nAi is the number of individuals in the ith combined grade in A, and nBi is the number of individuals in the ith combined grade in B. Mean similarity indexes were used in cluster analysis (UPGMA; Ludwig and Reynolds, 1988) to group populations, both for vegetative and reproductive data.
, NA is the total number of individuals in population A, NB the total number of individuals in population B, nAi is the number of individuals in the ith combined grade in A, and nBi is the number of individuals in the ith combined grade in B. Mean similarity indexes were used in cluster analysis (UPGMA; Ludwig and Reynolds, 1988) to group populations, both for vegetative and reproductive data.
RESULTS
General phenological pattern
Individuals of P. reticulata showed marked drought-deciduousness (Fig. 3). In Atlantic Forest, leaf fall was minor in the months before June and became pronounced after that. In Cerrado, some level of leaf fall occurred in all months of the year, but was most pronounced in the dry season. In all ten populations, leaf fall peaked in August, when the majority of observed individuals were completely deciduous. In September leaf flush began and two months later the individuals showed mature leaves throughout the tree crown.
Fig. 3.
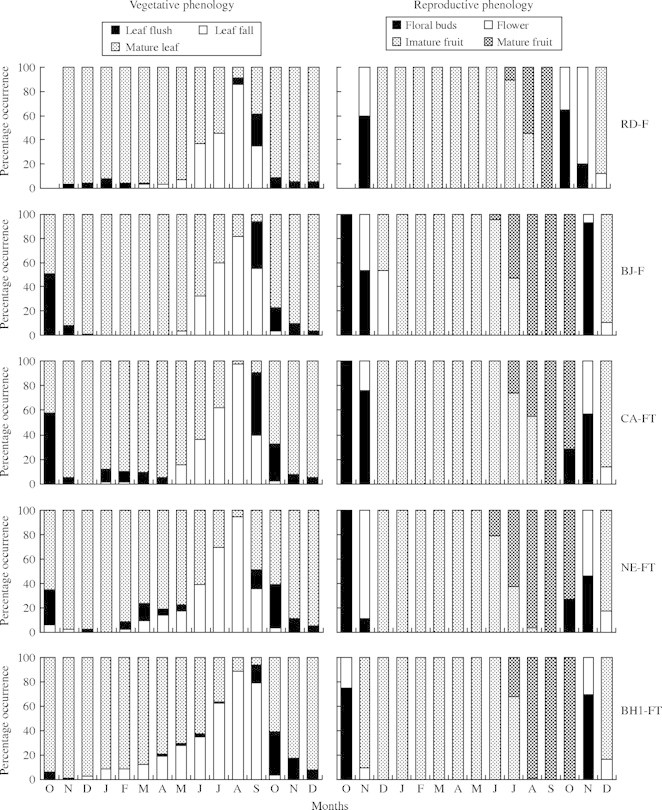
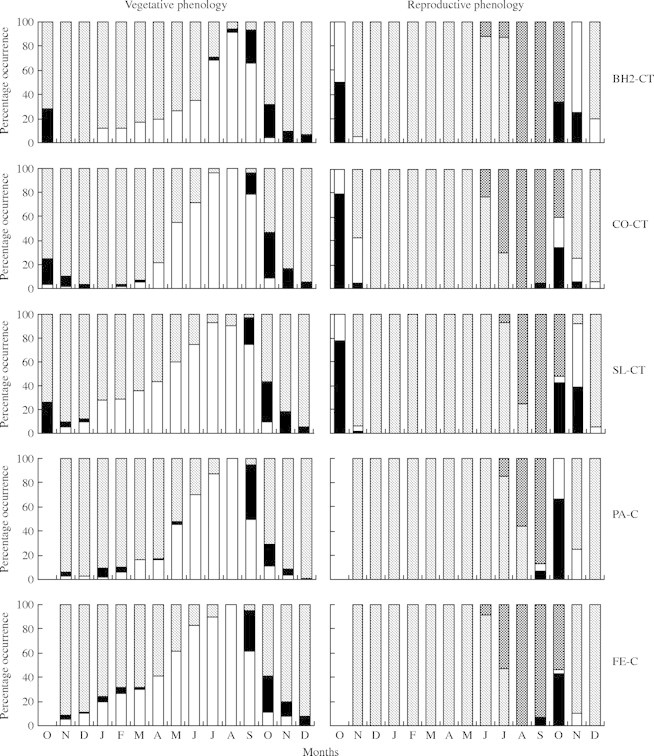
Monthly mean percentages of vegetative and reproductive phenologies found in individuals of P. reticulata from ten populations. Note that for reproductive data, in each month, only individuals that showed reproductive characteristics were considered.
Of the 182 evaluated individuals, 146 showed a reproductive event. In all populations most trees reproduced at least once (70–100 % of the individuals observed), except in RD-F where only six out of 20 did so. Flowering occurred in the beginning of the wet season (Fig. 3). Light-green floral buds started to develop in September in some individuals in Cerrado, and in October in most populations. After inflorescence development, flowering peaked in November in all populations and soon small, dark-green fruits were observed. Fruit development took several months and the appearance of big, brown fruits started to be observed in June. Mature pods split in two parts and released winged seeds. Seed dispersal peaked in August, but its duration was not easily determined as many empty pods remained on trees. In November, wherever possible, some remaining pods were collected and evaluated. They either had no seed or seeds that were damaged by fungi and insects. Therefore, October was considered the possible end of the seed dispersal period.
Variability in phenological pattern
Populations exhibited different levels of diversity in vegetative phenology (Table 1), with means for monthly diversity ranging from 0·61 to 0·98. BJ-F showed the lowest value of diversity, meaning it was the most synchronous population, while BH1-FT was the least synchronous. Mean values for habitats demonstrated that Cerrado and the two transitional habitats showed similar and higher diversity levels when compared to the Atlantic Forest core sites. Similar levels of total diversity were found in the two biomes.
Table 1.
Shannon–Wiener diversity index means (±s.d.) for vegetative and reproductive phenology of P. reticulata individuals within populations, habitats and biomes, for data collected from October 2002 to December 2003
| Vegetative |
Reproductive |
|||
|---|---|---|---|---|
| Population | ||||
| RD-F | 0·80 ± 0·44 | 0·50 ± 0·57 | ||
| BJ-F | 0·61 ± 0·53 | 0·56 ± 0·58 | ||
| CA-FT | 0·81 ± 0·45 | 0·50 ± 0·57 | ||
| NE-FT | 0·82 ± 0·44 | 0·48 ± 0·44 | ||
| BH1-FT | 0·98 ± 0·47 | 0·49 ± 0·51 | ||
| BH2-CT | 0·75 ± 0·43 | 0·41 ± 0·47 | ||
| CO-CT | 0·79 ± 0·47 | 0·50 ± 0·61 | ||
| SL-CT | 0·91 ± 0·25 | 0·52 ± 0·57 | ||
| PA-C | 0·78 ± 0·45 | 0·47 ± 0·56 | ||
| FE-C | 0·90 ± 0·37 | 0·47 ± 0·55 | ||
| Hpop | 0·82 ± 0·10 | 0·49 ± 0·04 | ||
| Habitat | ||||
| Forest core | 0·80 ± 0·50 | 0·70 ± 0·60 | ||
| Forest trans. | 1·06 ± 0·48 | 0·69 ± 0·62 | ||
| Cerrado core | 1·03 ± 0·31 | 0·69 ± 0·68 | ||
| Cerrado trans. | 1·02 ± 0·44 | 0·60 ± 0·65 | ||
| Hhab | 0·98 ± 0·12 | 0·67 ± 0·05 | ||
| Biome | ||||
| Atlantic Forest | 1·03 ± 0·48 | 0·76 ± 0·64 | ||
| Cerrado | 1·11 ± 0·37 | 0·74 ± 0·71 | ||
| Hbio | 1·08 ± 0·05 | 0·75 ± 0·01 | ||
Relative to vegetative phenology, lower levels of diversity for reproductive phases were found, indicating greater synchrony (Table 1). Monthly mean population diversity ranged from 0·41 to 0·56. BJ-F, the population with the lowest level of diversity for vegetative phenology, had the highest diversity for reproductive phenology. Again, similar levels were found when comparing biomes.
For both vegetative and reproductive phenology, mean values of diversity increased succesively from populations, to habitats and to biomes, indicating a structured diversity (Table 1).
The partitioning of phenological diversity, both for vegetative and reproductive phases, showed that more variation was due to differences in phenological behaviour among individuals in the same population than among populations (Table 2). A similar pattern was found when individuals within habitats (without considering population effects) and individuals within biomes (without considering population and habitat effects) were considered. Considerable variation in percentages of diversity within and bewteen populations, habitats and biomes was observed during the study. Diversity within populations, for example, ranged from 55·5 to 94·4 % for vegetative phenology and from 47·1 to 69·7 % for reproductive phenology (Table 2). Approximately 90 % of the total variation in phenology found in P. reticulata, for both vegetative and reproductive data, was due to differences in behaviour of individuals located in the same biome, while the remaining 10 % variation was attributable to the differences among plants from Cerrado and Atlantic Forest (Table 2).
Table 2.
Partitioning of phenological diversity (Shannon–Wiener index) in populations of P. reticulata. Data are percentages attributable to differences within and among populations, within and among habitats and within and between biomes. Values refer to 15-month means (October 2002 to December 2003)
| Vegetative |
Reproductive |
|
|---|---|---|
| Within populations | 69·7 ± 10·3 | 58·9 ± 7·8 |
| Among populations | 30·3 ± 10·3 | 41·1 ± 7·8 |
| Within habitats | 83·0 ± 6·6 | 82·3 ± 7·3 |
| Among habitats | 17·0 ± 6·6 | 17·7 ± 7·3 |
| Within biomes | 91·0 ± 5·8 | 90·1 ± 7·4 |
| Between biomes | 9·0 ± 5·2 | 9·9 ± 7·4 |
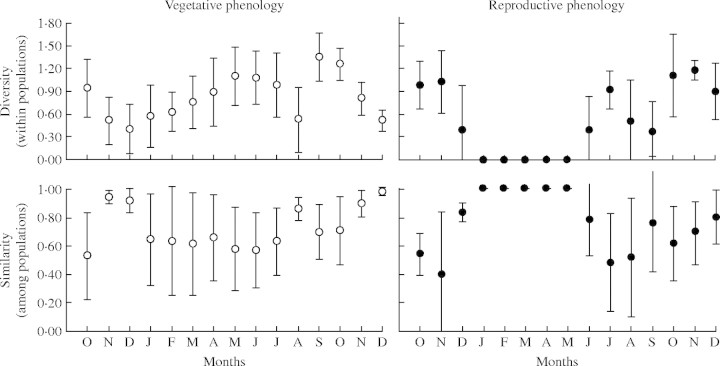
Indices for both similarity among populations and diversity within populations showed great monthly variation (Fig. 4). Vegetative phenology for April to July, the dry season, was marked by high diversity within populations and low similarity between them, indicating a low synchrony among trees both within and among populations. This result is due to the fact that some individuals start leaf fall earlier than others (Fig. 3). However, in August, the peak dry season, most trees had lost all leaves (Fig. 3), and therefore low diversity within populations and high similarity among them were observed. In the transition to the rainy season (September and October), the highest levels of diversity within populations were observed, as all vegetative phases occurred at the same time at the population level. Higher similarity among populations and lower diversity within populations occurred in November and December.
Fig. 4.
Monthly variation in the mean of phenological indexes of diversity within populations (Shannon–Wiener index), and similarity among populations (Morisita–Horn index) of P. reticulata. Bars indicate ± s.d.
For reproductive phenology, populations had relatively high diversity during the flowering period (October and November) and intermediate levels of similarity among them. In months when only immature fruit were present, individuals showed no diversity within populations, and hence similarity reached its maximum. This pattern changed when seed dispersal began (June and July) and was characterized by higher diversity and lower similarity. Similar to vegetative phenology, low diversity and high similarity were observed in August when seed dispersal characterized most individuals (Fig. 4).
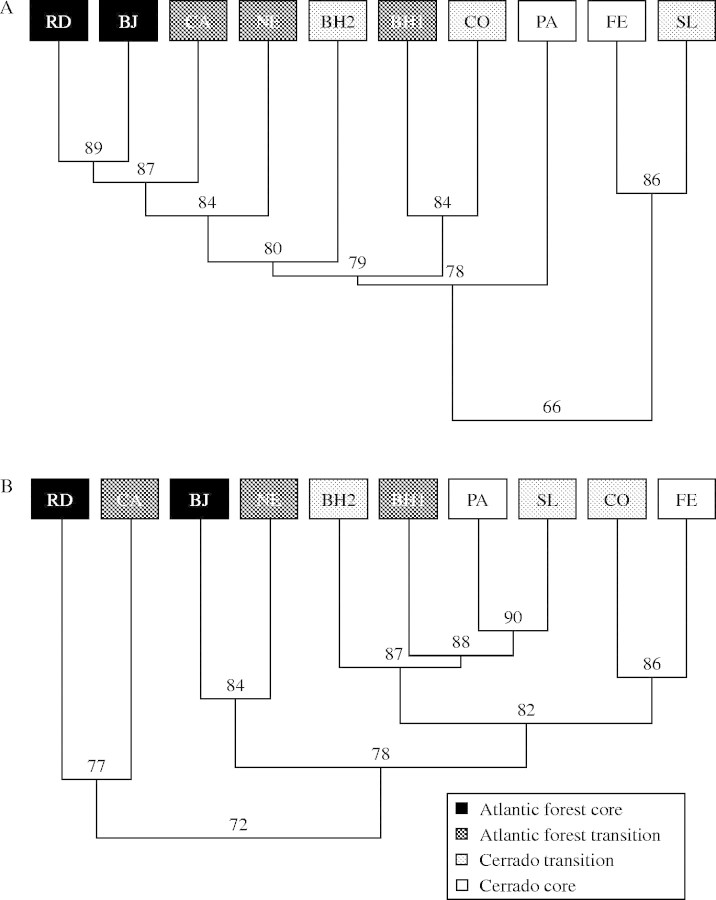
Similarities among populations differed within habitats both for vegetative phenology (Atlantic Forest = 0·89, forest in transitional sites = 0·82, Cerrado = 0·66, Cerrado in transitional sites = 0·66) and reproductive phenology (0·72, 0·79, 0·81 and 0·82, respectively). The vegetative phenologies of populations in Atlantic Forest were more similar to each other (0·84) than for populations in Cerrado (0·77), while the opposite was observed for reproductive phenology (0·77 and 0·83, respectively). The clusters generated by the similarity indices demonstrated that the observed patterns for vegetative and reproductive phenology were equivalent. Populations in core areas of Atlantic Forest and Cerrado were less similar, while populations in transitional sites showed a more intermediate position (Fig. 5).
Fig. 5.
Cluster analysis considering phenological similarity among populations (Morisita–Horn index) for (A) vegetative phases and (B) reproductive phases.
DISCUSSION
General phenological pattern
Some phenological aspects of P. reticulata reported here have also been described by other authors who have evaluated this species at different locations. Funch et al. (2002) classified Plathymenia individuals as showing episodic production and loss of leaves in a gallery forest in Cerrado in Bahia State. Flowering and fruiting patterns were annual, regular and of an intermediate duration. Similarly, Bulhão and Figueiredo (2002) emphasized the deciduousness found in P. reticulata in a marginal area of Cerrado in Maranhão State. The similar phenological patterns reported for P. reticulata populations at widely separated locations are in accordance with the results of Frankie et al. (1974), who showed that other tropical species occurring in both dry and wet sites exhibited a similar phenology in all populations. The tree species Erythrina poeppigiana, however, shows a transition from an evergreen to a deciduous habit with an increase in water stress (Borchert, 1980). Borchert (1980) suggested that this transition might be widespread in tropical trees, thus making P. reticulata an exception.
Reich et al. (1992) stated that deciduous species are probably favoured wherever annual variation in temperature or water availability results in marked favourable versus unfavourable periods of carbon gain. For P. reticulata, water availability could have been an environmental pressure promoting deciduousness, as this neotropical species occurs in an area characterized more by dry and wet seasons than by seasons of high and low temperature. However, most of the tree species co-occuring with Plathymenia in Cerrado or Atlantic Forest are not deciduous. In Cerrado, during the dry season the soil surface tends to dry but the zone located at 2 m depth remains moist (Oliveira, 1998). For this reason woody species are usually not severely affected by the dry season and deciduousness is more characteristic of herbs (Oliveira, 1998). In the part of the Atlantic Forest along the Brazilian coast, there is virtually no dry season and trees are evergreen (Morellato et al., 2000). The inland Atlantic Forest displays a rather seasonal climate but severe soil water limitation probably does not occur (Lemos Filho and Mendonça Filho, 2000). Co-occurring tree species showing different phenological behaviour have been shown in other tropical environments (e.g. Myers et al., 1997). The variation may be caused by differences in depth of the root system (Medina and Francisco, 1994; Myers et al., 1997) or may be due to different physiological strategies related to water use and accumulation (Myers et al., 1997).
The beginning of leaf fall represented the main differences found among populations from Cerrado and Atlantic Forest. In Cerrado, trees started to lose leaves earlier than in Atlantic Forest, while transitional regions exhibited an intermediate pattern. According to Reich (1995), the timing of leaf fall is usually a function of tree water status, and this is determined not only by the structural and functional state of the tree but also by the environmental water status, including soil water availability and air vapour pressure deficit (Lemos Filho and Mendonça Filho, 2000). Therefore, differences in timing of leaf fall observed in P. reticulata populations were probably related to differences in water status between sites, starting earlier in drier areas (Cerrado) and later where the drought period is less severe (Atlantic Forest).
Leaf flush began in September, when the wet period starts but rain is still minimal. Rain-induced flushing is possible but not very likely. On the other hand, the fact that flushing occurred around the spring equinox suggests flushing may be induced by increase in day length. This has been documented for several tropical species, including some from Cerrado (Rivera et al., 2002).
The P. reticulata flowering season started in September or October, soon after flushing. This indicates that flowers are formed in new shoots and are not directly induced by environmental signals (Borchert et al., 2004). The wet season flowering pattern observed in P. reticulata is in accordance with the findings of Morellato et al. (2000), who suggested that in Atlantic Forest flowering, in general, is highly seasonal and concentrated at the beginning of the wetter season. Phenological studies in Cerrado show that flowering is less seasonal, although it also tends to occur at the beginning of the wet season (Batalha and Mantovani, 2000) or in the late dry period (Oliveira, 1998).
In contrast to flowering, fruiting phenology is aseasonal in Atlantic Forest (Morellato et al., 2000) but more periodic in Cerrado (Oliveira, 1998). In Cerrado, the time of seed dispersal is related to dispersal syndromes: zoochoric species usually release seeds in the wet season, while anemochoric species disperse seeds in the late dry season (Oliveira, 1998). Plathymenia exhibits anemochory (Batalha and Mantovani, 2000; Funch et al., 2002) and its peak of dispersal in August is in accordance with the general pattern proposed by Oliveira (1998). In anemochorous fruit, the pericarp dehydrates before releasing seeds, making dry months a suitable period for this event (Batalha and Mantovani, 2000). Moreover, wind dispersal is more efficient in this season (Augspurger and Franson, 1987). After dispersal, P. reticulata seeds remain in the soil for a few months without germination, as the species shows dormancy imposed by a hard and impermeable seed coat (Lacerda et al., 2004). Seedlings start to appear in November or December (M. F. Goulart, pers. obs.).
In most populations, the majority of individuals reproduced at least once during the study period, but RD-F is clearly an exception as only six of 20 trees reproduced. Supra-annual reproduction is not an uncommon event in tropical forests (Frankie et al., 1974). Janzen (1969, 1970) proposed that the irregular reproduction in tropical species could be some kind of escape strategy against seed predators. In P. reticulata a considerable amount of seeds (35 %) were damaged by coleopterans (M. F. Goulart, pers. obs.), although this was not restricted just to forest sites, and reported data are insufficient to test Janzen's hypothesis.
Variability in phenological pattern
Variability among individuals within populations and among populations in vegetative phenology was more evident than variability in reproductive phenology. In vegetative phenology, in general, asynchrony among individuals and among populations was greater in the dry season. This pattern has also been documented in E. poeppigiana, in which trees were synchronous in the wet season but asynchronous in the dry season (Borchert, 1980). An explanation for this finding could be that genetic differences among individuals are more likely to be evident in a stressful situation, as shown by physiological data (Lemos Filho et al., 2004). This same explanation may also apply to the fact that, for P. reticulata, greater asynchrony was observed within populations from Cerrado or transitional regions than within populations from Atlantic Forest sites. Cerrado could be considered to be a more stressful environment than the Atlantic Forest as it is characterized by a stronger and more prolonged drought season. This data agrees with that of Seghieri and Simier (2002), who also observed greater individual variability in phenology under less favourable conditions. These authors hypothesized that asynchrony among individuals may reflect population flexibility, as the adaptation of individuals to a variety of conditions should contribute to population maintenance and expansion, certainly a hard task especially in a stressful environment.
Reproductive synchrony was not perfect among individuals and among populations of P. reticulata, as indicated by the diversity patterns found here. Similar patterns have been reported for other species (Murfet, 1977; Marquis, 1988). Rathcke and Lacey (1985) suggested that there should be some advantages in partial synchrony of flowering among individuals and populations, as it promotes cross-pollination among distant individuals, thus enhancing genetic diversity, and it also helps to avoid competition for pollinators. Variation in the initial date of flowering and the duration of this phase could be caused not only by genetic differentiation among individuals (e.g. Murfet, 1977) but also by microspacial variation in climatic conditions (e.g. Marquis, 1988; Borchert, 1994), plant size (e.g. Borchert, 1980) and herbivory (e.g. Marquis, 1988).
Partitioning of phenological diversity showed that the majority of the total diversity was due to differences among individuals within populations, and not among populations. This pattern is common in natural plant populations and has been reported both for phenological (e.g. Seghieri and Simier, 2002) and morphological data (e.g. Kocacic and Simic, 2001), and especially for genetic diversity in a great number of species (Hamrick and Godt, 1989; Nybom and Bartish, 2000), including P. reticulata (Lacerda et al., 2001). Populations of P. reticulata located in Atlantic Forest and in Cerrado have quite similar phenological behaviour, with only about 10 % of the total diversity being attributable to differences between the biomes. This level of differentiation between biomes is low when compared with genetic differentiation based on molecular data: Lacerda et al. (2002) reported that 60·5 % of genetic diversity in Plathymenia could be attributed to differences between biomes. However, it should be noted that in Lacerda's study only one population at a transitional site between biomes was evaluated. If more populations of this type had been included in the analysis, the proportion of the genetic diversity between biomes may have been lower. Nevertheless, comparisons between phenological diversity and genetic diversity suggest that the former is a more conserved characteristic, which is in agreement with Nicotra et al. (1997), who argued that physiological traits are under strong stabilizing pressures.
The diversity reported here in phenology is in accordance with other studies on the variability among populations of Plathymenia located in Cerrado, Atlantic forest and transitional sites. Lacerda et al. (2002) evaluated populations using RAPD markers and M. F. Goulart (unpubl. data) evaluated fruit and seed morphology. In both studies, differences among Atlantic Forest and Cerrado populations were found and individuals from transitional areas showed an intermediate pattern. All these results have some taxonomic value and the phenological data add to this pattern. Davies and Ashton (1999) reported that, when closely related species occur in sympatry, they usually flower at different times or show different flower morphologies and pollinators. In addition, Frankie et al. (1974) reported that congeneric species usually do not show strong synchrony in flowering phenology. Although there are exceptions to the reported patterns (e.g. Almeida and Alves, 2000), the fact that the flowering period is similar between Atlantic Forest and Cerrado populations of Plathymenia suggests, as pointed out by Stuessy (1990), that they may interbreed. Moreover, Plathymenia flower morphology is similar in both biomes and the same group of floral visitors was observed in different areas (mostly generalist bees and wasps; M. F. Goulart, pers. Obs.), reinforcing the possibility of gene flow. Gene flow along the cline could explain the intermediate pattern described for populations in transition zones between the biomes.
Acknowledgments
The authors thank Carol Augspurger for comments that greatly improved the manuscript. This work was supported by the Brazil Long Term Ecological Research Program—Conselho Nacional de Desenvolvimento Científico e Tecnológico (PELD—CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and the Post-graduate program in Ecology and Wildlife Conservation and Management (Universidade Federal de Minas Gerais). Maíra Figueiredo Goulart received an MSc fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). José Pires Lemos Filho received a research fellowship from the Conselho Nacional de Desenvolvimento Tecnológico (CNPq). CEMIG (Companhia Energética de Minas Gerais), V&M Florestal, Fundação Zoo-Botânica de Belo Horizonte, Instituto Estadual de Florestas de Minas Gerais, and Parque Estadual do Rio Doce provided facilities. The authors thank A. A. Azevedo and others for their volunteer help on field trips.
LITERATURE CITED
- Adler GH, Kielpinski KA. 2000. Reproductive phenology of a tropical canopy tree, Spondias mombin Biotropica 34: 686–692. [Google Scholar]
- Almeida EM, Alves MAS. 2000. Fenologia de Psychotria nuda e P. brasiliensis (Rubiaceae) em uma área de Floresta Atlântica no sudeste do Brasil. Acta Botanica Brasilica 14: 335–346. [Google Scholar]
- Almeida SP, Proença CEB, Sano SM. 1998.Cerrado: espécies vegetais úteis. Planaltina: EMBRAPA—CPAC. [Google Scholar]
- Augspurger CK. 1981. Reproductive synchrony of a tropical shrub: experimental studies on effects of pollinators and seed predators on Hybanthus prunifolius (Violaceae). Ecology 62: 775–788. [Google Scholar]
- Augspurger CK. 1983. Phenology, flowering synchrony and fruit set of six neotropical shrubs. Biotropica 15: 257–267. [Google Scholar]
- Augspurger CK, Franson SE. 1987. Wind dispersal of artificial fruits varying in mass, area and morphology. Ecology 68: 27–42. [Google Scholar]
- Batalha MA, Mantovani W. 2000. Reproductive phenological patterns of cerrado plant species at the Pé-de-Gigante reserve (Santa Rita do Passa Quatro, SP, Brazil): a comparison between the herbaceous and woody floras. Revista Brasileira de Biologia 60: 129–145. [DOI] [PubMed] [Google Scholar]
- Borchert R. 1980. Phenology and ecophysiology of tropical trees: Erythrina poeppigiana O. F. Cook. Ecology 61: 1065–1074. [Google Scholar]
- Borchert R. 1994. Soil and stem water strage determine phenology and distribution of tropical dry forest trees. Ecology 75: 1437–1449. [Google Scholar]
- Borchert R, Meyer SA, Felger RS, Porter-Bolland L. 2004. Environmental control of flowering periodicity in Costa Rican and Mexican tropical dry forests. Global Ecology and Biogeography 13: 409–425. [Google Scholar]
- Bulhão CF, Figueiredo PS. 2002. Fenologia das leguminosas arbóreas em uma área de cerrado marginal no nordeste do Maranhão. Revista Brasileira de Botânica 25: 361–369. [Google Scholar]
- Davies SJ, Ashton PS. 1999. Phenology and fecundity in 11 sympatric pioneer species of Macaranga (Euphorniaceae) in Borneo. American Journal of Botany 86: 1786–1795. [PubMed] [Google Scholar]
- Fisher E, Santos FAM. 2001. Demography, phenology and sex of Calophyllum brasiliense (Clusiaceae) trees in the Atlantic forest. Journal of Tropical Ecology 17: 903–909. [Google Scholar]
- Fonseca GAB, Mittermeier RA, Cavalcanti RB, Mittermeier CG. 1999. Brazilian Cerrado. In: Mittermeier RA, Myers N, Gil PR, Mittermeier CG, eds. Hot Spots: Earth's biologically richest and most endangered terrestrial ecoregions. Mexico City: CEMEX, 148–155. [Google Scholar]
- Frankie GW, Baker HG, Opler PA. 1974. Comparative phenological studies of trees in tropical wet and dry forests in the lowlands of Costa Rica. Journal of Ecology 62: 881–919. [Google Scholar]
- Funch LS, Funch R, Barroso GM. 2002. Phenology of gallery and montane forest in the Chapada Diamantina, Bahia, Brazil. Biotropica 34: 40–50. [Google Scholar]
- Hamrick JL, Godt MJW. 1989. Allozyme diversity in plant species. In: Brow HD, Clegg MT, Kahler AL, Weir BS, eds. Plant population genetics, breeding and genetic resources. Sunderland, MA: Sinauer Associates Inc., 43–36. [Google Scholar]
- Heringer EP. 1956. O gênero Plathymenia. Anais da Sociedade Botânica do Brasil 7: 55–64 [Google Scholar]
- Heringer EP, Ferreira MB. 1972. Árvores úteis no cerrado (I): Vinhático: o gênero Plathymenia Benth. P. foliolosa Benth. e P. reticulata Benth., vinhático da mata e vinhático do campo (par vicariante). Cerrado 5: 28–34. [Google Scholar]
- Janzen DH. 1969. Seed-eaters versus seed size, number, toxicity and dispersal. Evolution 23: 1–27. [DOI] [PubMed] [Google Scholar]
- Janzen DH. 1970. Herbivores and the number of tree species in tropical forest. American Naturalist 104: 501–528. [Google Scholar]
- Kocacic S, Simic D. 2001. Intrapopulational and interpopulational relations of Betula pendula Roth (Betulaceae) in Croatia, based on leaf morphometry. Acta Biologica Cracoviensia Series Botanica 43: 87–96. [Google Scholar]
- Lacerda DR, Acedo MDP, Lemos Filho JP, Lovato MB. 2001. Genetic diversity and structure of natural populations of Plathymenia reticulata (Mimosoideae), a tropical tree from the Brazilian Cerrado. Molecular Ecology 10: 1143–1152. [DOI] [PubMed] [Google Scholar]
- Lacerda DR, Lemos Filho JP, Acedo MDP, Lovato MB. 2002. Molecular differentiation of two vicariant neotropical tree species, Plathymenia foliolosa and P. reticulata (Mimosoideae), inferred using RAPD markers. Plant Systematic and Evolution 235: 67–77. [Google Scholar]
- Lacerda DR, Lemos Filho JP, Goulart MF, Ribeiro RA, Lovato MB. 2004. Seed dormancy variation in natural populations of two tropical leguminous tree species: Senna multijuga (Caesalpinoideae) and Plathymenia reticulata (Mimosoideae). Seed Science Research 14: 127–135. [Google Scholar]
- Lemos Filho JP, Mendonça Filho CV. 2000. Seasonal changes in the water status of three woody legumes from the Atlantic forest, Caratinga, Brazil. Journal of Tropical Ecology 16: 21–32. [Google Scholar]
- Lemos Filho JP, Goulart MF, Lovato MB. 2004. Chlorophyll fluorescence parameters in populations of two legume trees: Stryphnodendron adstringes (Mart.) Coville (Mimosoidea) and Cassia ferruginea (Schrad.) Schrad. Ex DC. (Caesalpinoideae). Revista Brasileira de Botânica 27: 527–532. [Google Scholar]
- Ludwig JL, Reynolds JF. 1988.Statistical ecology: a primer on methods and computing. New York: John Wiley & Sons. [Google Scholar]
- Magurran AE. 1988.Ecological diversity and its measurement. Cambridge: Cambridge University Press. [Google Scholar]
- Marquis RJ. 1988. Phenological variation in the neotropical understory shrub Piper arieianum: causes and consequences. Ecology 69: 1552–1565. [Google Scholar]
- Medina E, Francisco M. 1994. Photosynthesis and water relation of savanna tree species differing in leaf phenology. Tree Physiology 14: 1367–1381. [DOI] [PubMed] [Google Scholar]
- Mittermeier RA, Fonseca GAB, Rylands AB, Mittermeier CG. 1999. Atlantic Forest. In: Mittermeier RA, Myers N, Gil PR, Mittermeier CG, eds. Hot Spots: Earth's biologically richest and most endangered terrestrial ecoregions. Mexico City: CEMEX, 137–147. [Google Scholar]
- Morellato LPC, Talora DC, Takahasi A, Bencke CC, Romera EC, Zipparro VB. 2000. Phenology of the Atlantic rain forest trees: a comparative study. Biotropica 32: 811–823. [Google Scholar]
- Murfet IC. 1977. Environmental interaction and the genetics of flowering. Annual Review of Plant Physiology 28: 253–278. [Google Scholar]
- Myers BA, Duff GA, Eamus D, Fordyce IR, O'Grady A. 1997. Seasonal variation in water relations of trees of differing leaf phenology in wet-dry tropical savanna near Darwin, Northern Australia. Australian Journal of Botany 45: 225–240. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [DOI] [PubMed] [Google Scholar]
- Nicotra BA, Chazdon RL, Schlichting CD. 1997. Patterns of genotypic variation and phenotypic plasticity of light response in two tropical Piper (Piperaceae) species. American Journal of Botany 84: 1542–1552. [PubMed] [Google Scholar]
- Nybom H, Bartish IV. 2000. Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspectives in Plant Ecology, Evolution and Systematics 3/2: 93–144. [Google Scholar]
- Oliveira PE. 1998. Fenologia e biologia reprodutiva das espécies de cerrado. In: Sano SM, de Almeida SP, eds. Cerrado: ambiente e flora. Planaltina: EMBRAPA. [Google Scholar]
- Rathcke B, Lacey EP. 1985. Phenological patterns of terrestrial plants. Annual Review of Ecology and Systematics 16: 179–214. [Google Scholar]
- Reich PB. 1995. Phenology of tropical forests: patterns, causes and consequences. Canadian Journal of Botany 73: 164–174 [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. 1992. Leaf life-span in relation to leaf, plant and stand characteristics among diverse ecosystems. Ecological Monographs 62: 365–392. [Google Scholar]
- Rivera G, Elliott S, Caldas LS, Nicolossi G, Coradin VTR. 2002. Increasing day-length induces spring flushing of tropical dry forest trees in the absence of rain. Trees 16: 445–456. [Google Scholar]
- Seghieri J, Simier S. 2002. Variations in phenology of a residual invasive shrub species in Sahelian fallow savannas, south-west Niger. Journal of Tropical Ecology 18: 897–912. [Google Scholar]
- Stallings JR, Fonseca GAB, Pinto LPS, Aguiar LMS, Sábato EL. 1991. Mamíferos do Parque Estadual do Rio Doce, Minas Gerais, Brasil. Revista Brasileira de Zoologia 7: 663–677. [Google Scholar]
- Stuessy TF. 1990.Plant taxonomy. New York: Columbia University Press. [Google Scholar]
- Warwick MC, Lewis GP. 2003. Revision of Plathymenia (Leguminosae—Mimosoideae). Edinburgh Journal of Botany 60: 111–119. [Google Scholar]