Abstract
• Background and Aims Seedling vigour is one of the major determinants for stable stand establishment in rice (Oryza sativa), especially in a direct seeding cropping system. The objectives of this study were to identify superior alleles with consistent effects on seedling vigour across different temperature conditions and to investigate genotype × environmental temperature interactions for seedling vigour QTL.
• Methods A set of 282 F13 recombinant inbred lines (RILs) derived from a rice cross were assessed for four seedling vigour traits at three temperatures (25 °C, 20 °C and 15 °C). Using a linkage map with 198 marker loci, the main-effect QTL for the traits were mapped by composite interval mapping.
• Key Results A total of 34 QTL for the four seedling vigour traits were identified. Of these QTL, the majority (82 %) were clustered within five genomic regions, designated as QTL qSV-3-1, qSV-3-2, qSV-5, qSV-8-1 and qSV-8-2. All of these five QTL had small individual effects on the traits, explaining 3·1–15·8 % of the phenotypic variation with a mean of 7·3 %. QTL qSV-3-1, qSV-3-2 and qSV-8-1 showed almost consistent effects on the traits across all three temperatures while qSV-5 and qSV-8-2 had effects mainly at the ‘normal’ temperatures of 20 °C and 25 °C. Among the five QTL identified, all and four showed additive effects on shoot length and germination rate, respectively. The contributions of these five QTL to shoot length and germination rate were also much larger than those to the other two traits.
• Conclusions A few of genomic regions (or QTL) were identified as showing effects on seedling vigour. For these QTL, significant genotype × environmental temperature interactions were found and these interactions appeared to be QTL-specific. Among the four seedling vigour traits measured, shoot length and germination rate could be used as relatively good indicators to evaluate the level of seedling vigour in rice.
Keywords: Rice (Oryza sativa L.), recombinant inbred line (RIL), seedling vigour, paper-roll tests, quantitative trait loci (QTL), genotype × environment interaction
INTRODUCTION
Seedling vigour is important for stable stand establishment and early vegetative growth in rice (Oryza sativa), especially in areas where sowing by directly planting germinated seeds in puddled soils is performed as an effective means to reduce production cost (Krishnasamy and Seshu, 1989; Dingkuhn et al., 1992; Yamauchi et al., 1993; Redoña and Mackill, 1996a). Previous studies have shown that poor seedling vigour frequently resulted in establishment failures. It has been difficult to improve the level of seedling vigour in rice via conventional breeding strategies due to its quantitative inherence (Li and Rutger, 1980; Redoña and Mackill, 1996b). Recently, much attention has been paid to the study of genetic control of seedling vigour, and a substantial amount of genetic variation for seedling vigour has been observed in rice (Yamauchi et al., 1993; Redoña and Mackill, 1996a), which could provide diverse gene resources for the improvement of the trait in breeding programs.
With the development of DNA markers and the construction of high-density linkage maps for many plant species, the QTL (quantitative trait loci) mapping technique has become a powerful tool for identifying genomic regions affecting quantitative traits by providing information on the map location, relative effect, gene action and dominance properties of each identified locus (Lander and Botstein, 1989; Tanksley, 1993). Recently, a few QTL for seedling vigour in rice have been identified by the QTL mapping approach, and some of these are expected to be useful for the improvement of seedling vigour by pyramiding the positive alleles via marker-assisted selection (MAS) (Redoña and Mackill, 1996b; Cui et al., 2002; Fujino et al., 2004; Zhang et al., 2004).
The significance of genotype × environment (GE) interactions in the genetic control of quantitative traits has long been recognized by quantitative geneticists (Moll et al., 1978). Because of the GE interaction, QTL that are important in one environment may not be as important as in another environment in determining the phenotype (Tanksley, 1993). For this reason, QTL with little GE interaction across a set of environments would be desirable in marker-assisted breeding programs. For seedling vigour in rice, environmental temperature is one of the most important factors affecting the performance of the trait (Chapman and Peterson, 1962; Redoña and Mackill, 1996a). Previous studies have confirmed that optimum temperatures for rice germination and early growth are 25–30 °C; however, water or soil temperatures during the sowing season vary greatly in different areas or in different cropping seasons in the same area. In temperate and high-elevation rice growing areas, the temperatures are even frequently below 15 °C (Chapman and Peterson, 1962). To identify superior and stable alleles, the seedling vigour of rice should therefore be tested in multiple temperature environments that take into consideration genotype × environmental temperature (GET) interactions. However, previous QTL mapping studies on seedling vigour of rice have usually been conducted in a single-temperature environment (Redoña and Mackill, 1996b; Cui et al., 2002; Fujino et al., 2004; Zhang et al., 2004), which have thus provided little information on the GET interactions for the trait.
‘Lemont’, a japonica cultivar used in southern California, USA, is insensitive to temperature in terms of seedling vigour, while ‘Teqing’, an indica cultivar used in Guangdong Province, China, shows high seedling vigour in the normal temperature range but is quite sensitive to low temperatures. In the current study, a recombinant inbred line (RIL) population derived from a cross between ‘Lemont’ and ‘Teqing’ was used to map QTL for seedling vigour under three different temperature conditions. The main objective was to investigate the GET interactions for the trait and identify QTL with consistent effects across multiple temperature environments.
MATERIALS AND METHODS
Rice RIL population and measurements of the traits
The RIL population used in this study consisted of 282 F13 RILs derived by single-seed descent from a cross between Oryza sativa L. vars. ‘Lemont’ (japonica) and ‘Teqing’ (indica). The plants of all RILs and the parents were planted in a field in the summer of 2002 in Wuhan, China. The mature seeds were harvested and stored at 10 °C until use. Phenotyping experiments were conducted from July to September in 2003. Prior to this, the germinability of the two parents was tested and germination rates of 100 % were observed for both parents after incubation for 5 d at 32 °C, indicating these seeds were viable and suitable for the analysis of seedling vigour.
The seedling vigour tests were performed in a randomized complete-block design with two replications (the parental varieties were replicated ten times). For each RIL in each replication, 40 manually selected grains were used. Four seedling vigour-related traits—germination rate, seedling root length, shoot length and dry weight—were measured by paper-roll tests according to Zhang et al. (2004) at 25 °C, 20 °C and 15 °C. Seeds with both coleoptile and radicle protrusion greater than or equal to the length of the seeds themselves were considered germinated. Numbers of germinated seeds for each RIL were recorded at 2, 3 and 10 d after incubation at 25 °C, 20 °C and 15 °C, respectively, and the germination rates were calculated as the percentages of seeds germinated. After 3 d (25 °C), 4 d (20 °C) and 15 d (15 °C) incubation, ten seedlings for each RIL were randomly sampled to measure their root and shoot lengths (mm). After the attached residual seed grains had been removed, the seedlings were dried in an oven for 2 d at 70 °C. Dry weight of seedlings for each RIL was measured, and was expressed as mg per seedling.
Marker genotyping and construction of the linkage map
Genomic DNA extraction and RFLP analysis were performed according to the protocol of Li et al. (1995). Microsatellite marker genotyping was conducted by the procedure of Wu and Tanksley (1993). A morphological marker (gl-1 for glabrous leaf) was evaluated for each RIL, and glabrous leaves were determined by touch. A complete linkage map was constructed as previously described by Zhang et al. (2004). This map consists of 198 marker loci including 91 RFLP, 106 microsatellite loci and a single gene locus (gl-1), covering all 12 rice chromosomes and spanning 1980·1 cM (Kosambi function) with an average interval of 10·7 cM between markers.
Data analyses and QTL mapping
The trait measurements averaged over two replications were used for the following data analyses and QTL mapping. Basic statistics and Pearson phenotypic correlations between the traits were calculated by SAS PROC CORR (SAS Institute, Inc., 1996). By controlling both main and epistatic effects of important markers, composite interval mapping was carried out to locate main-effect QTL using the computer program QTLMapper 1·0 (Wang et al., 1999) based on mixed linear models. A LOD score of 2·0 was used as the threshold to declare the presence of QTL. Genetic parameters (effects and test statistics) associated with significant QTL were estimated and the proportion of the phenotypic variance explained by each QTL was also calculated.
RESULTS
Phenotype analysis
The difference in seedling vigour between the two parental cultivars (‘Lemont’ and ‘Teqing’) was largely associated with temperature (Table 1). At 25 °C and 20 °C, the two parents differed significantly for germination rate, shoot length and dry weight (P < 0·01) and also for root length at 25 °C (P < 0·05). However, the degree of difference decreased or even disappeared when these traits were measured at 15 °C, when the two parents differed significantly only for shoot length and dry weight (P < 0·05). These results indicate that ‘Teqing’ is a high seedling-vigour parent at the normal temperatures of 20 °C and 25 °C, but it is quite sensitive to a low temperature of 15 °C, thus suggesting the presence of significant GET interaction for seedling vigour in rice.
Table 1.
Phenotypic analysis of seedling vigour related traits in the RIL population of rice
| RIL population |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature |
Traits† |
‘Lemont’ |
‘Teqing’ |
Mean ± s.d. |
Range |
Skewness |
Kurtosis |
|||
| 25 °C | Germination rate (GR) | 24·2 | 79·2** | 43·3 ± 24·11 | 2·5–100·0 | 0·08 | −1·01 | |||
| Root length (RL) | 39·2 | 44·6* | 37·2 ± 6·33 | 22·5–55·35 | 0·20 | 0·10 | ||||
| Shoot length (SL) | 13·4 | 21·4** | 15·8 ± 3·45 | 9·35–27·95 | 0·54 | 0·05 | ||||
| Dry weight (DW) | 1·45 | 1·78** | 1·50 ± 0·29 | 0·80–2·80 | 0·60 | 0·42 | ||||
| 20 °C | Germination rate (GR) | 14·6 | 62·9** | 45·9 ± 21·03 | 1·25–91·25 | 0·02 | −0·71 | |||
| Root length (RL) | 45·6 | 44·4 | 44·0 ± 8·55 | 20·3–81·6 | 0·73 | 0·80 | ||||
| Shoot length (SL) | 19·4 | 27·6** | 22·2 ± 5·67 | 9·5–40·45 | 0·32 | −0·20 | ||||
| Dry weight (DW) | 1·63 | 2·12** | 1·98 ± 0·41 | 1·00–3·40 | 0·35 | −0·06 | ||||
| 15 °C | Germination rate (GR) | 58·7 | 57·1 | 33·7 ± 18·36 | 0–81·4 | 0·40 | −0·68 | |||
| Root length (RL) | 25·5 | 26·0 | 22·8 ± 7·37 | 8·43–44·1 | 0·56 | −0·06 | ||||
| Shoot length (SL) | 26·1 | 30·6* | 29·1 ± 7·67 | 12·33–50·0 | 0·24 | −0·25 | ||||
| Dry weight (DW) | 2·73 | 2·97* | 3·08 ± 0·704 | 1·50–5·50 | 0·47 | 0·10 | ||||
Trait values under different temperature conditions can not be directly compared because of different incubation duration at different temperatures.
* and ** indicate significant differences between the two parental lines at P < 0·05 and P < 0·01, respectively.
In the RIL population, all of the four seedling vigour traits investigated showed continuous variation. Obvious transgressive segregation was observed for the four traits in the two directions, thereby implying that different positive alleles for the traits exist in both of the parental cultivars and the recombination of these positive alleles would produce genotypes with enhanced seedling vigour.
The skewness and kurtosis for the distributions of the four traits in the RIL population were all less than 1·0 in absolute values except germination rate at 25 °C, which had an absolute value of kurtosis slightly more than 1·0, suggesting that all of the traits approximately fit normal distributions and the data for the traits is suitable for QTL mapping.
QTL mapping
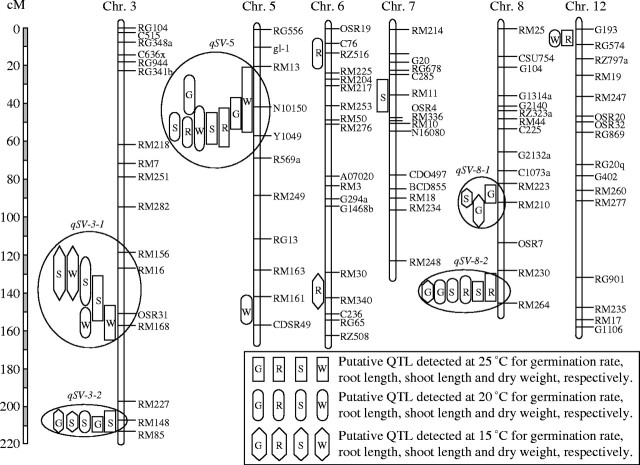
A scan of the whole genome was conducted using the software QTLMapper 1·0, and a total of 34 QTL for the four traits were detected at the three temperatures. Of these QTL, the majority (82 %) were clustered within five genomic regions on chromosomes 3, 5 and 8 (Table 2 and Fig. 1). Since the multiple traits affected by each of these five regions were those all associated with seedling vigour, each of these genomic regions could be assumed to be one putative QTL controlling seedling vigour and therefore they were designated as QTL qSV-3-1, qSV-3-2, qSV-5, qSV-8-1 and qSV-8-2 (Table 2 and Fig. 1). It should be noted, however, that each of these five QTL might be a single locus with pleiotropic effects on the multiple traits, or a group of tightly linked loci.
Table 2.
Comparative analysis of QTL for seedling vigour in rice under three temperature conditions
| 25 °C |
20 °C |
15 °C |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QTL |
Chromosome |
Marker intervals |
Traits† |
LOD |
A‡ |
R2§ |
LOD |
A |
R2 |
LOD |
A |
R2 |
||||||
| qSV-3-1 | 3 | RM16-OSR31-RM168 | SL | 3·8 | 0·82 | 6·3 | 5·7 | 1·85 | 10·3 | 3·6 | 1·92 | 6·6 | ||||||
| DW | 4·4 | 0·08 | 6·3 | 2·6 | 0·07 | 10·3 | 5·2 | 0·17 | 6·6 | |||||||||
| qSV-3-2 | 3 | RM148-RM85 | GR | 5·4 | −8·36 | 14·7 | 2·2 | −3·68 | 5·2 | |||||||||
| SL | 2·2 | −0·74 | 5·1 | 3·7 | −1·72 | 8·9 | 3·1 | −2·33 | 9·6 | |||||||||
| QSV-5 | 5 | RM13-N10150-Y1049 | GR | 3·2 | −0·82 | 6·3 | 3·5 | −5·10 | 6·9 | |||||||||
| RL | 5·5 | −2·05 | 10·9 | 2·7 | −1·93 | 5·4 | ||||||||||||
| SL | 4·2 | −0·83 | 6·3 | 3·0 | −1·36 | 6·3 | ||||||||||||
| DW | 3·5 | −0·07 | 6·5 | 10·3 | −0·14 | 15·8 | ||||||||||||
| qSV-8-1 | 8 | RM223-RM210-OSR7 | GR | 2·5 | 4·14 | 3·6 | 4·6 | 5·31 | 10·8 | |||||||||
| SL | 2·1 | 1·44 | 3·7 | |||||||||||||||
| qSV-8-2 | 8 | RM230-RM264 | GR | 2·6 | −4·34 | 5·0 | 3·1 | −3·55 | 4·8 | |||||||||
| RL | 5·3 | −1·97 | 10·1 | 3·8 | −2·02 | 5·9 | ||||||||||||
| SL | 2·2 | −0·58 | 3·1 | 2·2 | −1·07 | 3·4 | ||||||||||||
| 5 | RM161-CDSR49 | DW | 7·2 | −0·12 | 12·1 | |||||||||||||
| 6 | RM30-RM340 | RL | 4·9 | 2·43 | 13·2 | |||||||||||||
| QTL not in cluster | 6 | C76-RZ516 | RL | 4·4 | −2·22 | 7·2 | ||||||||||||
| 7 | C285-RM11 | SL | 5·9 | 1·07 | 10·7 | |||||||||||||
| 12 | G193-RG574 | RL | 2·2 | 1·52 | 6·0 | |||||||||||||
| DW | 2·0 | 0·07 | 3·4 | |||||||||||||||
GR, RL SL and DW represent germination rate, root length, shoot length and dry weight, respectively (see Table 1).
A = additive effect: the effect associated with substitution of a ‘Teqing’ allele by the corresponding ‘Lemont’ allele.
Variance explained by individual QTL.
Fig. 1.
Molecular linkage map of rice showing locations of putative QTL for rice seedling vigour detected under different temperature conditions. Short arms of chromosomes are at the top. Five chromosomal regions containing multiple QTL for seedling vigour traits on chromosomes 3, 5 and 8 are circled and designated as QTL qSV-3-1, qSV-3-2, qSV-5, qSV-8-1, and qSV-8-2.
Further analyses revealed several interesting properties about the five QTL. Firstly, all of the five QTL produced small individual effects on the seedling vigour-related traits at the three temperatures, explaining the trait phenotypic variation within a range of 3·1–15·8 %, with a mean of 7·3 %. This result was in good agreement with the observation that all the traits displayed continuous variation in the RIL population and that the frequency distributions approximately fit normal distributions, implying that seedling vigour in rice is controlled by many loci, each with small effects.
Secondly, qSV-3-1, qSV-3-2 and qSV-8-1 showed almost consistent effects across the three temperatures while qSV-5 and qSV-8-2 produced effects mainly at the normal temperatures of 20 °C and 25 °C, with no, or minor, effects at the low temperature of 15 °C. In other words, GET interactions appeared to be QTL-specific for seedling vigour. Significant GET interactions were found for qSV-5 and qSV-8-2 but not for qSV-3-1, qSV-3-2 and qSV-8-1.
Thirdly, each of the five regions simultaneously affected the multiple seedling vigour-related traits, with the additive effects in the same direction. For qSV-3-1 and qSV-8-1, the positive genotypes came from ‘Lemont’, while for the other three QTL (qSV-3-2, qSV-5 and qSV-8-2), the positive genotypes came from ‘Teqing’. Of the three QTL, two (qSV-5 and qSV-8-2) showed little effects at 15 °C, which seemed to provide a plausible explanation for the observation that the seedling vigour of ‘Teqing’ decreased greatly at 15 °C (Table 1).
Fourthly, among the five QTL, all and four showed additive effects on shoot length and germination rate, respectively, while only two affected root length and dry weight. Additionally, these five QTL in total explained 21 %, 29 %, and 20 % of shoot length variation at 25 °C, 20 °C and 15 °C, respectively. They also accounted for 25 %, 12 %, and 21 % of germination rate variation at the three temperatures, respectively. Overall, the contributions of the five QTL to shoot length and germination rate were much larger than those to the other two traits. Hence, these results led to the conclusion that shoot length and germination rate could be used as relatively good indicators to evaluate the level of seedling vigour in rice.
In addition, the traits affected by one QTL were not completely as the same as those affected by another one. For example, qSV-5 affected all the traits investigated while qSV-3-1 only affected shoot length and dry weight, and qSV-3-2 only affected shoot length and germination rate.
Trait correlations and the genetic explanations
As shown in Table 3, the phenotypic correlations between the four seedling vigour-related traits in the RIL population were all positive and highly significant (P < 0·01). This was consistent with the QTL mapping result that the majority of QTL for the traits were clustered within certain genomic regions, each with additive effects in the same direction (Table 2 and Fig. 1).
Table 3.
Correlation analysis of the seedling vigour-related traits under three temperature conditions
| 25 °C |
20 °C |
15 °C |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Traits |
1† |
2 |
3 |
1 |
2 |
3 |
1 |
2 |
3 |
||||||
| 1. Germination rate | 1 | 1 | 1 | ||||||||||||
| 2. Root length | 0·22** | 1 | 0·41** | 1 | 0·54** | 1 | |||||||||
| 3. Shoot length | 0·62** | 0·34** | 1 | 0·59** | 0·42** | 1 | 0·26** | 0·37** | 1 | ||||||
| 4. Dry weight | 0·50** | 0·49** | 0·63** | 0·54** | 0·58** | 0·62** | 0·19** | 0·33** | 0·60** | ||||||
1–3 denote germination rate, root length and shoot length, respectively.
Significant at P < 0·01.
Moreover, it was found that the strength of most correlations decreased markedly at the lower temperature of 15 °C as compared with those at 20 °C and 25 °C. For example, the correlation coefficients of germination rate with shoot length were 0·62, 0·59 and 0·26 at 25 °C, 20 °C and 15 °C, respectively, and those between germination rate and dry weight were 0·50, 0·54, and 0·19, respectively, at these temperatures. This observation seemed, to some extent, to be explained by the QTL mapping result that two (qSV-5 and qSV-8-2) of the five genomic regions associated with the four traits showed little effect at 15 °C.
DISCUSSION
In agreement with earlier investigations (Li and Rutger, 1980; Redoña and Mackill, 1996b), this study confirmed that the seedling vigour-related traits were normally distributed quantitative traits and that GE interactions could exert a large impact on the traits. Temperature is one of the most important environmental factors affecting seedling vigour in rice (Chapman and Peterson, 1962; Krishnasamy and Seshu, 1989; Redoña and Mackill, 1996a). Previous studies have demonstrated that different rice cultivars varied in their temperature effects. For example, indicas and tropical japonicas are quite sensitive to low temperatures as compared with temperate japonicas (Krishnasamy and Seshu, 1989). In the current study, another intriguing finding was that GET interactions for seedling vigour were QTL-specific. Among the five QTL identified here, three (qSV-3-1, qSV-3-2 and qSV-8-1) showed almost consistent effects on the traits across three temperatures, while the other two (qSV-5 and qSV-8-2) exerted effects mainly at 20 and 25 °C, with few effects at the lower temperature of 15 °C. Similarly, Li et al. (2003) reported that, for rice heading date and plant height QTL, the GE interaction could also be shown to be QTL-specific. Such information may be useful for the improvement of seedling vigour because it could help breeders determine which QTL could be effectively manipulated by MAS for cultivar development targeted for a given environment.
To date, several QTL mapping studies have reported the identification of a few QTL for rice seedling vigour-related traits. By comparing the QTL identified here to those previously identified at 18 °C based on the same population (Zhang et al., 2004), it was found that two QTL, qSV-3-2 and qSV-8-2, coincided well with those previously reported, in both the locations and directions of additive effects. QTL qSV-5 was within the interval RM13-N10150-Y1049 of chromosome 5. Next to this QTL, the earlier study detected a QTL with epistatic effects on seedling vigour-related traits. These results seem to indicate that there could be multiple loci within this genomic region of chromosome 5 showing main-effect and/or epistatic effects on seedling vigour. Even so, some discrepancies were observed between this study and the earlier one. The extreme example is that the two QTL, qSV-3-1 and qSV-8-1, were undetected in the earlier study and the QTL qSV-7 was undetected in this study. The earlier study was done at 18 °C, which is just between the normal temperatures (>20 °C) and the chilling temperatures (≤15 °C), while the current study was done at two normal temperatures (20 °C and 25 °C) and a chilling temperature of 15 °C. In a different study (Zhang et al., 2005), it was found that some of seedling vigour QTL expressed differentially under different cold environments. This is essentially in agreement with the argument that gene expression can be modified by interaction with other genes and by environmental effects (Atchley and Zhu, 1997). Therefore, the observed discrepancies described above could be mostly attributable to the temperature effects. These analyses also suggest that multiple environments should be considered in QTL mapping, especially for a trait like seedling vigour with significant GE interactions. In addition, the QTL qSV-8-1 identified here was found to share the same map location with a major locus for osmotic adjustment and dehydration tolerance in rice, reported by Lilley et al. (1996). The QTL qSV-3-1 was also reported by both Redoña and Mackill (1996b) and Cui et al. (2002). The other four QTL, i.e. a dry weight QTL on the distal end of the long arm of chromosome 5, two root length QTL within intervals C76-RZ516 and RM30-RM340 on chromosome 6, and a shoot length QTL marked by RM11 on chromosome 7 (Fig. 1), were overlapping with the corresponding seedling vigour QTL mapped to the same locations by Prasad et al. (2002). These coincidences of rice seedling vigour QTL indicated that a few QTL for the same or closely related traits could be detected by different studies even with quite different populations.
Plant growth under stress is a function of plant vigour and stress tolerance. By investigating the genetic control of salt tolerance in wheat Hordeum vulgare and H. chilense disomic addition lines, Forster et al. (1990) proposed that genes contributing to vigour might be different from genes conferring tolerance. This proposal was also supported by Foolad and Lin (2001) through genetic analysis of cold tolerance during vegetative growth in tomato (Lycopersicon esculentum). Based on previous information on temperature effects for seedling vigour in rice (Bertin et al., 1996), the three screening temperatures used in the present study can be classified into two categories: (a) non-chilling temperatures such as 20 °C and 25 °C, and (b) chilling temperature such as 15 °C. Accordingly, as shown in Table 2, the QTL identified in this study appeared to be grouped into three types. (1) QTL producing effects only at non-chilling temperatures, such as qSV-5. Since this kind of QTL was not detected at the low temperature, it was considered to contribute only to vigour. (2) QTL showing consistent effects on seedling vigour across the non-chilling and chilling temperatures, such as qSV-3-1 and qSV-3-2. This type of QTL contributed to both vigour and tolerance to the low temperature. (3) QTL showing effects only at the chilling temperature. Only one QTL, which was mapped within the interval RM30-RM340 on chromosome 6 (not being in a cluster with other QTL, Table 2) and accounted for 13·2 % of the phenotypic variation for root length, belonged to this kind, which should also be considered to contribute to both vigour and tolerance. In summary, all of the three kinds of QTL described contributed to vigour, but only the second and third kinds also contributed to tolerance to temperature stress. These results seemed to suggest that genes (or QTL) associated with vigour might not be clarified into two simple types: one contributing only to vigour and the other contributing only to tolerance. The low temperature, or other stress, may influence the expression of vigour genes and these genes may vary in response to the stress, which has been described as GE interaction (Moll et al., 1978; Tanksley, 1993). Since GE interactions for seedling vigour were gene-specific, as discussed above, some of these genes would show effects on vigour only under non-stress conditions, being considered to contribute only to vigour, while the others would be active across multiple conditions or only under stress conditions, contributing to both vigour and tolerance.
Tremendous genetic variation for seedling vigour has been identified. Previous studies have suggested that genetic donors for seedling vigour genes appear to be present mainly in indica and temperate japonica groups of rice (Redoña and Mackill, 1996a). In the present study, however, the identification of qSV-3-1, a QTL with positive alleles came from ‘Lemont’ (tropical japonica), indicating that tropical japonica also possesses favourable genes for seedling vigour that may merit further exploitation.
Seedling vigour itself is an abstract concept that needs to be assessed by other concrete criteria. Shoot length was reported to be the best and least variable determinant of seedling vigour under California conditions (Peterson et al., 1978). In an investigation by Redoña and Mackill (1996a), it was found that shoot weight, shoot length and coleoptile length were the best determinants to predict greenhouse and field seedling vigour of rice. In the current study, shoot length was found to be the only one associated with all of the five seedling vigour QTL identified here, thus providing strong support for the suggestion that shoot length is the best predictor of seedling vigour in rice. Despite of this, however, it is recommended that multiple related traits should be considered in experiments aimed at mapping of QTL for traits of quantitative inherence, such as seedling vigour. In this way, the power of QTL mapping can be considerably enhanced and the results for different related traits can be compared with each other, which may reveal genetic relationships among such complex traits.
Acknowledgments
We thank Dr B.Y. Fu and Dr Z.K. Li of the International Rice Research Institute (IRRI) for providing the ‘Lemont’/‘Teqing’ RIL population and the marker data. We also thank Professor S.B. Yu of Huazhong Agricultural University for his help in the construction of the genetic map. We are grateful to the undergraduate students A.L. Niu, X.L. Lu, H.B. Cui and Q.Y. Bi of the College of Life Sciences for helping to collect the phenotypic data. This work was supported by the State ‘973’ Project (2001CB108806) of China.
LITERATURE CITED
- Atchley WR, Zhu J. 1997. Developmental quantitative genetics, conditional epigenetic variability and growth in mice. Genetics 147: 765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin P, Kinet JM, Bouharmont J. 1996. Evaluation of chilling sensitivity in different rice varieties. Relationship between screening procedures applied during germination and vegetative growth. Euphytica 89: 201–210. [Google Scholar]
- Chapman AL, Peterson ML. 1962. The seedling establishment of rice under water in relating to temperature and dissolved oxygen. Crop Science 2: 391–395. [Google Scholar]
- Cui KH, Peng SB, Xing YZ, Xu CG, Yu SB, Zhang Q. 2002. Molecular dissection of seedling-vigour and associated physiological traits in rice. Theoretical and Applied Genetics 105: 745–753. [DOI] [PubMed] [Google Scholar]
- Dingkuhn M, De Datta SK, Pamplona R, Javellana C, Schnier HF. 1992. Effect of late-season N-fertilization on photosynthesis and the yield of transplanted and direct-seeded tropical flooded rice. 2. A canopy stratification study. Field Crops Research 28: 235–249. [Google Scholar]
- Foolad MR, Lin GY. 2001. Genetic analysis of cold tolerance during vegetative growth in tomato, Lycopersicon esculentum Mill. Euphytica 122: 105–111. [Google Scholar]
- Forster BP, Phillips MS, Miller TE, Baird E, Powell W. 1990. Chromosome location of genes controlling tolerance to salt (NaCl) and vigour in Hordeum vulgare and H. chilense Heredity 65: 99–107. [Google Scholar]
- Fujino K, Sekiguchi H, Sato T, Kiuchi H, Nonoue Y, Takeuchi Y, Ando T, Lin SY, Yano M. 2004. Mapping of quantitative trait loci controlling low-temperature germinability in rice (Oryza sativa L.). Theoretical and Applied Genetics 108: 794–799. [DOI] [PubMed] [Google Scholar]
- Krishnasamy V, Seshu DV. 1989. Seed germination rate and associated characters in rice. Crop Science 29: 904–908. [Google Scholar]
- Lander ES, Botstein D. 1989. Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121: 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CC, Rutger JN. 1980. Inheritance of cool-temperature seedling vigour in rice and its relationship with other agronomic characters. Crop Science 20: 295–298. [Google Scholar]
- Li ZK, Pinson SRM, Stansel JW, Park WD. 1995. Identification of quantitative trait loci (QTLs) for heading date and plant height in cultivated rice (Oryza sativa L.). Theoretical and Applied Genetics 91: 374–381. [DOI] [PubMed] [Google Scholar]
- Li ZK, Yu SB, Lafitte HR, Huang N, Courtois B, Hittalmani S, Vijayakumar CHM, Liu GF, Wang GC, Shashidhar HE, et al. 2003. QTL × environment interactions in rice. I. Heading date and plant height. Theoretical and Applied Genetics 108: 141–153. [DOI] [PubMed] [Google Scholar]
- Lilley JM, Ludlow MM, McCouch SR, O'Toole JC. 1996. Locating QTL for osmotic adjustment and dehydration tolerance in rice. Journal of Experimental Botany 47: 1427–1436. [Google Scholar]
- Moll RH, Cockerham CC, Stuber CW, Williams WP. 1978. Selections, responses, genetic-environmental interactions, and heterosis with recurrent parent selection for yield in maize. Crop Science 18: 641–645. [Google Scholar]
- Peterson ML, Jones DB, Rutger JN. 1978. Cool temperature screening of rice lines for seedling vigour. Riso 27: 269–274. [Google Scholar]
- Prasad SR, Prashanth GB, Hittalmani S, Shashidhar HE. 2002. Molecular mapping of quantitative trait loci associated with seedling tolerance to salt stress in rice(Oryza sativa L.). Current Science 78: 162–164. [Google Scholar]
- Redoña ED, Mackill DJ. 1996. Genetic variation for seedling-vigour traits in rice. Crop Science 36: 285–290. [Google Scholar]
- Redoña ED, Mackill DJ. 1996. Mapping quantitative trait loci for seedling-vigour in rice using RFLPs. Theoretical and Applied Genetics 92: 395–402. [DOI] [PubMed] [Google Scholar]
- SAS institute, Inc. 1996.SAS user's guide: statistics. Cary, NC: SAS Institute. [Google Scholar]
- Tanksley SD. 1993. Mapping polygenes. Annual Review of Genetics 27: 205–233. [DOI] [PubMed] [Google Scholar]
- Wang DL, Zhu J, Li ZK, Paterson AH. 1999. Mapping QTLs with epistatic effects and QTL × environment interactions by mixed linear model approaches. Theoretical and Applied Genetics 99: 1255–1264. [Google Scholar]
- Wu KS, Tanksley SD. 1993. Abundance, polymorphism and genetic mapping of microsatellites in rice. Molecular and General Genetics 241: 225–235. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Aguilar AM, Vaughan DA, Seshu DV. 1993. Rice germplasm suitable for direct sowing under flooded soil surface. Euphytica 67: 177–184. [Google Scholar]
- Zhang ZH, Yu SB, Yu T, Huang Z, Zhu YG. 2004. Mapping quantitative trait loci (QTLs) for seedling-vigour using recombinant inbred lines of rice (Oryza sativa L.). Field Crops Research (in press). [Google Scholar]
- Zhang ZH, Su L, Li W, Chen W, Zhu YG. 2005. A major QTL conferring cold tolerance at the early seedling stage using recombinant inbred lines of rice (Oryza sativa L.). Plant Science 168: 527–534. [Google Scholar]