Abstract
• Background and Aims Flowers are relatively invariant organs within species, but quantitative variation often exists among conspecifics. These variations represent the raw material that natural selection can magnify, eventually resulting in morphological divergence and diversification. This paper investigates floral variability in Rosmarinus officinalis, a Mediterranean shrub.
• Methods Nine populations were selected in three major southern Spanish habitats (coast, lowland and mountains) along an elevation gradient. Flower samples from randomly chosen plants were collected from each population, and a total of 641 flowers from 237 shrubs were weighed while still fresh to the nearest 0·1 mg. Leaves from the same plants were also measured. Variations among habitats, sites and plants were explored with general linear model ANOVA. Leaf–flower covariation was also investigated.
• Key Results Most (58 %) mass in flowers was accounted for by the corolla, whose linear dimensions correlated directly with flower mass. Averaged over plants, the mass of a flower varied between 12 mg and 38 mg. Habitat, site (within habitat) and shrub identity had significant effects on mass variance. Flowers from the coast were the smallest (17 mg) and those from the mountains the largest (25 mg on average). A pattern of continuously increasing flower size with elevation emerged which was largely uncoupled from the geographical pattern of leaf size variation.
• Conclusions As regards flower size, a great potential to local differentiation exists in Rosmarinus. Observed divergences accord with a regime of large-bodied pollinator selection in the mountains, but also with resource–cost hypotheses on floral evolution that postulate that reduced corollas are advantageous under prevailingly stressful conditions.
Keywords: Bees, coast, drought, flower size, Lamiaceae, Mediterranean, mountain, pollination, Rosmarinus officinalis, shrub, Spain
INTRODUCTION
Theory predicts that flowers are morphologically constant within species because of stabilizing selection (Berg, 1960; Stebbins, 1970; Feinsinger, 1983). Intraspecific differences as regards the flowers may easily turn into an obstacle for sexual reproduction and, as a result, a single integrated floral phenotype would usually be expected (Conner and Via, 1993; Cresswell, 2000; J. Herrera, 2001; but see Wilson, 1995; C. M. Herrera, 2001, 2002; J. Herrera, 2004). As a corollary to the invariant (relative to other organs) nature of flowers within species, the identification of Angiosperm taxa is customarily done on floral traits.
Nevertheless, flowers do present quantitative variation among conspecifics (reviewed in Cresswell, 1998; Galen, 1999). Even if small, these variations are worth studying because they represent the raw material which natural selection can magnify and, eventually, result in plant diversification. The need to match pollinators has traditionally been considered the major selective force at play during floral evolution, although this process is likely to be a manifold one responding also to plant enemies and even aspects of the abiotic environment (Conner and Rush, 1996; Mitchell et al., 1998; Cresswell et al., 2001; Galen and Cuba, 2001; Fenner et al., 2002).
Floral variation may occur at several hierarchical levels (reviewed in Williams and Conner, 2001) including among flowers within plants, among individuals that live in the same site, or among plants in different populations. The relative contribution of individuals and populations to overall variability has evolutionary implications and can give an insight on how inclined to local differentiation is that particular plant species. This paper reports natural quantitative floral variation in Rosmarinus officinalis, a sclerophyllous perennial shrub. The species is very common in the scrub vegetation of the Mediterranean region and, since it grows under a diversity of ecological conditions, may be a good subject for exploring incipient reproductive trait divergence.
STUDY SITES AND METHODS
The study was carried out in wild populations of Rosmarinus officinalis (Rosmarinus, hereafter) distributed across Andalucía, the southern-most region of the Iberian Peninsula. The region has a typically Mediterranean climate and supports a variety of sclerophyllous vegetation types, from Pinus pinea woodlands growing on stable sand dunes near the Atlantic coast, to evergreen-oak (Quercus rotundifolia) forests on the mountains. Understorey scrub formations may include (in addition to Rosmarinus and for example) Cistaceae, Ericaceae and Fabaceae (mostly Genisteae), as well as other Lamiaceae. A comprehensive description of vegetation types can be found in Rivas-Martínez (1987). A report of ecologically relevant characteristics relating to climate, soils and vegetation can be accessed on the Internet (http://www.juntadeandalucia.es/medioambiente/menu02.html). Local floras do not recognize subspecies or varieties of Rosmarinus in the geographical area of the present study (Valdés et al., 1987). Male sterility is often reported in the Lamiaceae, but only one female shrub was detected (and excluded from the sample) when collecting plants for this study. The remaining (237) shrubs sampled had perfect flowers.
Rosmarinus is a drought-tolerant, sun-loving shrub species without evident soil preferences. Nine populations were randomly chosen to represent three major habitats: coastal sandy areas (coast); low Guadalquivir River valley (lowland); and Sierra Morena–Subbetic Ranges (mountain). The two more distant populations were separated by 370 km. Table 1 presents names and a summary of the environmental characteristics of the study sites. As expected, coastal populations lived under considerably warmer and drier conditions than those in the mountains, and those in the lowlands in conditions intermediate between the other two.
Table 1.
Major environmental characteristics for nine southern Spanish populations of Rosmarinus
| Habitat |
Site* |
Rainfall† (mm) |
January temperature‡ (°C) |
Frosty days§ |
Altitude (m a.s.l.) |
|---|---|---|---|---|---|
| Coast | 1. Punta Umbría | 729 | 12·3 | <1 | 30 |
| 2. Mazagón | 562 | 12·3 | <1 | 15 | |
| 3. Barbate | 497 | 13·6 | <1 | 60 | |
| Lowland | 4. Hinojos | 576 | 11·1 | 1–20 | 100 |
| 5. Aznalcázar | 667 | 11·3 | 1–20 | 80 | |
| 6. Gerena | 647 | 11·2 | 1–20 | 150 | |
| Mountain | 7. Aracena | 874 | 10·7 | >20 | 600 |
| 8. Carcabuey | 794 | 9·6 | >20 | 700 | |
| 9. Cazorla | 1079 | 8·7 | >20 | 1100 |
The numbers identify the populations in Fig. 4.
Total precipitation during 2002.
Averaged lowest and highest temperatures during January 2002.
Yearly averages for the period 1961–1990.
Flowering branches from 19–41 randomly chosen plants were collected in each population and kept in separate, sealed plastic bags. These were taken to the laboratory and correlates of size noted for two or three flowers per plant within the following 24 h. Overall, 641 flowers from 237 shrubs were weighed while still fresh to the nearest 0·1 mg on an electronic balance. Furthermore, two or three fully developed, 1-year-old leaves from the previous growth season were randomly picked from each shrub, pressed between two glass slides and measured to the nearest 0·1 mm with digital callipers.
Mass allocation to floral parts was assessed in 25 shrubs from populations 1 and 6. From each plant one randomly chosen, fresh flower was dissected under a binocular microscope to separate the corolla, androecium and gynoecium. The detached parts were then weighed to the nearest 0·01 mg. Ovary and calyx cannot be readily separated in Rosmarinus so their joint mass is reported under ‘calyx + gynoecium’.
The area of the corolla is central to plant advertisement and pollinator attraction but, because of the elaborate and markedly tridimensional morphology of the flowers in Rosmarinus (Fig. 1), direct measurements of linear size or area are problematic. To investigate if the mass of a flower correlated well with its linear dimensions a simple estimate of size was devised by gently pressing a flower between two glass slides, then measuring the distance from the base of the calyx to the tip of the corolla lateral lobe. Measurements were performed on one flower from each of 106 shrubs spanning the whole range of floral variation. The same flowers were also weighed to the nearest 0·01 mg.
Fig. 1.
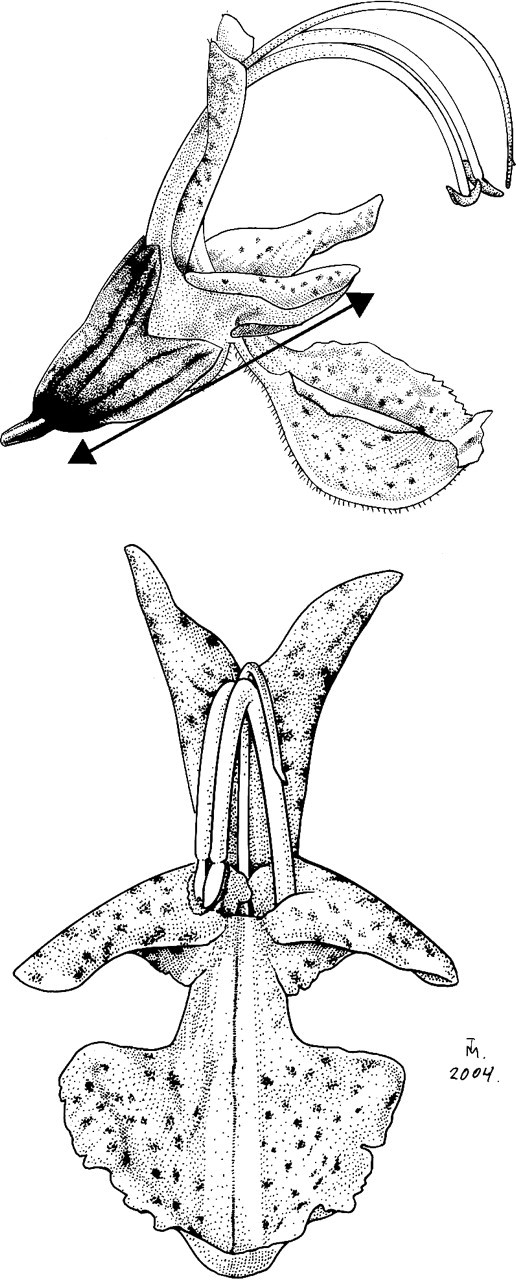
Front and side views of a Rosmarinus flower. The line indicates the estimate of linear size used in the present study.
Data analyses
A general linear model ANOVA (GLM module; StatSoft, 2001) was run on individual flower mass (log10-transformed), which had habitat, site (nested under habitat) and plant (nested under site and habitat) as categorical predictors. Means for the three levels of habitat (coast, lowland and mountain) were compared by a Fisher's least significant differences test. To compute the variance components for flower mass, ‘habitat’ was treated as a fixed factor, whereas the levels of site and plant were considered random.
If the floral variations that occurred among populations were to some extent environmentally induced, flowers and leaves should probably co-vary in size. This was worth studying but the nested data set used above (with flowers representing cases) did not have enough degrees of freedom left for leaf size to be added to the model. The data set was therefore rearranged so that individual plants became cases, and the flowers collected from each plant (most often three) viewed as trials for mass. The resulting, plant-based data set was analysed as a repeated measures design ANCOVA (GLM module; StatSoft, 2001) which included average leaf length (log10 transformed) as a continuous covariate of flower mass, along with habitat and site (nested within habitat) as categorical predictors. Since there were no significant within-subject (i.e. within-plant) effects for any factor, only between-subject effects are reported below.
RESULTS
Floral metrics
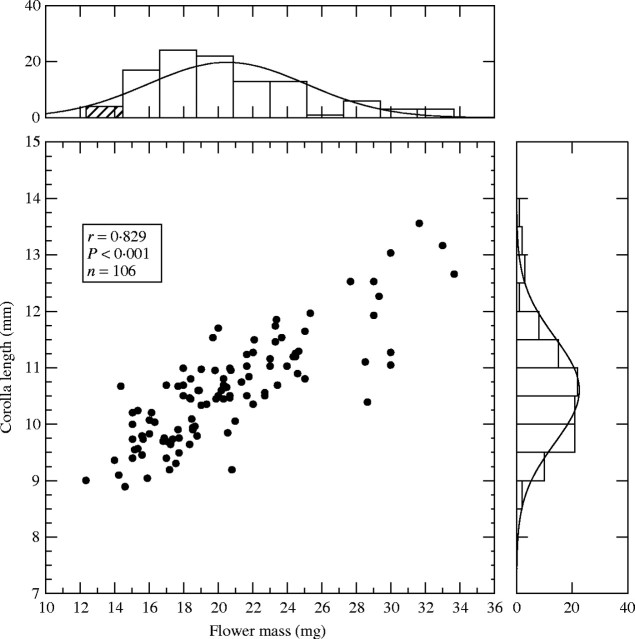
A significant positive correlation was found between linear size and mass (Fig. 2) indicating that heavier Rosmarinus flowers were in general showier. This refers to a sample that mixed flowers from several populations, but the relationship held also within populations (e.g. for site 1, r = 0·772, n = 20; site 4, r = 0·804, n = 19; site 9, r = 0·771, n = 20). The tight relationship between linear size and mass accords with the observation that more than half of the mass in a flower (58 % on average) is accounted for by the corolla (Table 2).
Fig. 2.
Scatterplot of flower mass vs. linear size in Rosmarinus officinalis. Histograms depicting variable distributions and normal smooth lines are also shown. Dots represent individual flowers, one from each of 106 plants.
Table 2.
Mass allocation to flower parts in Rosmarinus
| Mass (mg) |
|||||||
|---|---|---|---|---|---|---|---|
| Whole flower |
Corolla |
Androecium |
Gynoecium + calyx |
||||
| Mean | 18·7 | 10·8 | 3·7 | 4·3 | |||
| Standard error | 0·6 | 0·3 | 0·2 | 0·2 | |||
| Min–max | 15–25 | 9–13 | 2–6 | 3–7 | |||
| % of total mass | 100 | 58 | 20 | 22 | |||
| n | 25 | 25 | 25 | 25 | |||
Flower mass variations
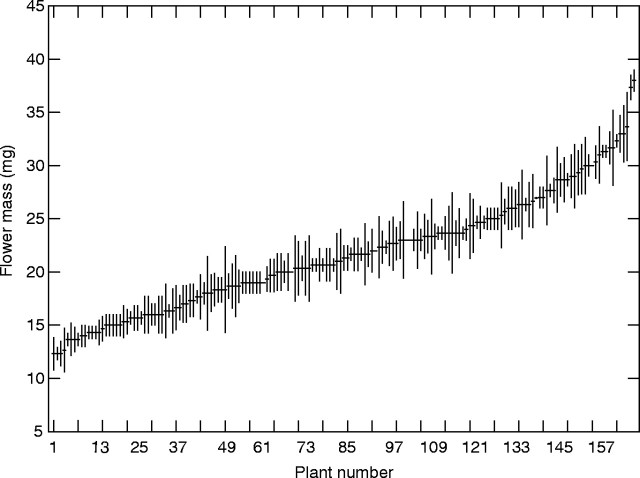
A fresh Rosmarinus flower weighs on average 21·13 ± 0·21 mg (mean ± standard error; median 20·6 mg; n = 641) but with extensive variation which ranges from 11 to 39 mg. Averaged over plants, flower mass varied from approx. 12 mg to approx. 38 mg (Fig. 3). The variation was also remarkably continuous. On the other hand, and as indicated by standard deviations that were small relative to overall variability, mass was relatively constant within plants. The largest range (i.e. largest minus smallest value) found within any shrub was 8 mg, although this was exceptional. More often, intra-plant oscillations were around 2 mg (mean 2·4 mg; median 2 mg; n = 237 plants), a range that contrasts with the overall range of 28 mg that existed across plants.
Fig. 3.
Average flower mass in 167 individual Rosmarinus shrubs, sorted from smallest to largest. Vertical lines span ± 1 s.d. around the mean, with n = 3 flowers per plant.
Results of the general linear model that tested habitat, site and plant identity as predictors of flower mass are presented in Table 3. All three factors proved statistically significant, although the effect of habitat was particularly strong. On average, flowers from coastal populations were the smallest, those from the mountains were the largest, and lowland ones were intermediate. Differences among means were all statistically significant. Variance components for (random) factors plant and site were 56 % and 26 %, respectively.
Table 3.
Flower mass in Rosmarinus as affected by habitat, site (within habitat), and individual plant
| ANOVA table |
||||||||
|---|---|---|---|---|---|---|---|---|
| Source |
d.f. |
MS |
F |
P |
||||
| Habitat | 2 | 1·503 | 1207·45 | <0·001 | ||||
| Site(Habitat) | 6 | 0·140 | 112·12 | <0·001 | ||||
| Plant(Site*Habitat) | 228 | 0·012 | 9·69 | <0·001 | ||||
| Error | 404 | 0·001 | ||||||
| Univariate statistics |
||||||
|---|---|---|---|---|---|---|
| Habitat |
Mean* |
s.e.m. |
n |
|||
| Coast | 17·202 a | 0·275 | 195 | |||
| Lowland | 20·752 b | 0·261 | 197 | |||
| Mountain | 25·047 c | 0·241 | 249 | |||
The nested design had fresh individual flower mass (in mg, log10 transformed) as the response variable.
For the whole model, r2 = 0·931.
Univariate statistics for the three habitats are also given.
The letters identify means significantly different at P < 0·001 (Fisher's least significant differences test).
Flower–leaf covariation
The shrub-based analysis confirmed the dependence of flower mass on habitat (MS = 0·750, F = 66·804, d.f. = 2, P < 0·001) and site (MS = 0·158, F = 14·065, d.f. = 4, P < 0·001), at the same time that revealed a weak, although statistically significant (MS = 0·061, F = 5·112, d.f. = 1, P = 0·02) trend of flowers to covary with leaves. This marginally significant covariation can be understood better by looking at Fig. 4, which depicts the contrasting patterns of geographical change for flowers and leaves. Although there were two instances (sites 1 and 2) in which both flowers and leaves were small, in general the sizes did not match. As regards site averages, flower and leaf size were not significantly correlated (r = 0·496, n = 9, P = 0·174).
Fig. 4.
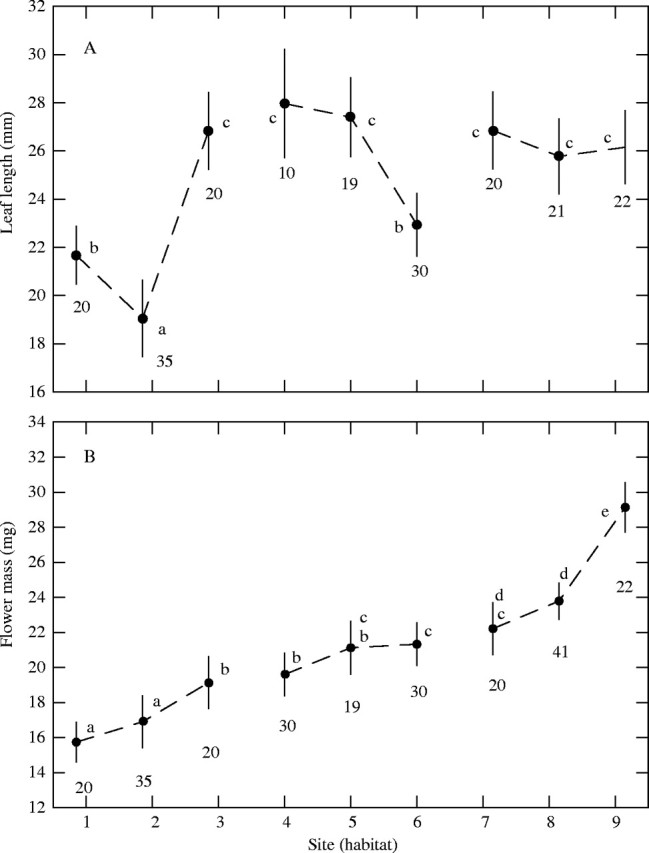
An interaction plot for (A) leaf and (B) flower size variation across habitats and sites in Rosmarinus. Dots mark site means and vertical lines represent 95 % confidence intervals. Numerals are sample sizes (i.e. shrubs). Within each graph, different lower-case letters denote groups of means significantly different at P = 0·05 (Fisher's least significant differences test). Sites 1–3 were at the coast, 4–6 in lowland, and 7–9 in mountain habitats (see Table 1 for names).
DISCUSSION
Corolla size is an important attribute in the reproductive biology of plants. Rosmarinus flowers varied in mass more than three-fold (12–38 mg) in the study area, and these variations translated directly into varying corolla dimensions. As a result, attractiveness will necessarily differ to some extent among individual shrubs, even if linear variations are dissimulated by the markedly tridimensional morphology of the corolla. More importantly, and because in general the size of the corolla correlates positively with nectar production (Plowright, 1981; Galen and Plowright, 1985; Harder et al., 1985; Stanton and Preston, 1988), Rosmarinus plants with larger flowers are also likely to be more rewarding on a per flower basis. By itself this has a great potential to modify pollination success (Cresswell and Galen, 1991) and suggests that the observed variations can bring tangible consequences for reproduction in this Mediterranean shrub.
Flowers decreased in mass as the habitat became drier and hotter from mountain to coast. A similar pattern of intraspecific variability is also exhibited by Polemonium viscosum (Polemoniaceae), a perennial whose flowers go smaller along a gradient of increasing aridity in the Rocky Mountains of North America (Galen et al., 1987). In annuals, on the other hand, small flower size associated to aridity can give way to differences in mating system (Jonas and Geber, 1999; Runions and Geber, 2000).
Divergence in flower size or shape among geographically isolated plant populations is often explained on the basis of pollinator-mediated selection (Miller, 1981; Robertson and Wyatt, 1990; Steiner et al., 1994; Johnson and Steiner, 1997). In southern Spain, a diverse bee guild, including small Halictidae, medium-sized Andrenidae, Anthophoridae, honeybees and Bombus species, pollinates Rosmarinus (J. Herrera, 1988; J. Herrera, unpubl. res.). Large thermoregulating bees such as Bombus become more abundant and diverse in the Iberian Peninsula as elevation increases (C. M. Herrera, 1988; Obeso, 1992), making it likely that the proportion of large bees will increase from coastal to mountain areas. At least in part, the direct relationship between flower size and altitude reported here might be accounted for by selection by differently sized bees although, to date, no study has specifically compared the identity of Rosmarinus's bees across habitats.
Explanations for the observed pattern of flower size change in Rosmarinus may include factors other than pollinator-mediated selection. Recent resource–cost hypotheses have postulated that reduced corollas can be advantageous for plants that live under prevailingly stressful conditions. In Polemonium viscosum, for example, large corollas incur physiological costs because of their greater water uptake (Galen, 1999, 2000). Plant water status and corolla size are also directly related in Epilobium angustifolium (Carroll et al., 2001). From this perspective, small-flowered Rosmarinus would be advantageous in relatively dry coastal areas, whereas the relatively moist and rich soils of the mountains would allow plants to produce larger flowers. Note that this resource–cost explanation does not exclude selection by larger pollinators as hypothesized above, it would just reinforce the pattern of increasing flower size with altitude.
Since plasticity has sometimes been demonstrated for flowers (Mazer and Schick, 1991; Holtsford and Ellstrand, 1992; Stratton, 1992; J. Herrera, 2004), one could reasonably wonder if (among-population) floral variability in Rosmarinus may result from a plastic response to the changing environment. If this were the case, the geographical pattern of floral variation should at least be similar to the one exhibited by leaves, but the patterns were unalike. Except for the two coastal populations which had small flowers and leaves, leaf size was largely uncoupled to flower mass for most of the geographical range of this study. Results do not support the notion that local conditions dictate flower mass in Rosmarinus, although only a common or garden approach could unquestionably solve the point.
Acknowledgments
C. M. Herrera, P. L. Ortiz and R. C. Soriguer assisted with the Rosmarinus flower collection. I am indebted to S. Andersson and an anonymous reviewer for constructive insights and criticism. This work was funded by Plan Andaluz de Investigación (Junta de Andalucía) and by grant BOS2000-0328 from the Spanish Dirección General de Enseñanza Superior e Investigación Científica.
LITERATURE CITED
- Berg RL. 1960. The ecological significance of correlation pleiades. Evolution 14: 171–180. [Google Scholar]
- Carroll AB, Pallardy SG, Galen C. 2001. Drought stress, plant water status, and floral trait expression in fireweed, Epilobium angustifolium (Onagraceae). American Journal of Botany 88: 438–446. [PubMed] [Google Scholar]
- Conner JK, Rush RS. 1996. Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum Oecologia 105: 509–516. [DOI] [PubMed] [Google Scholar]
- Conner J, Via S. 1993. Patterns of phenotypic and genetic correlations among morphological and life-history traits in wild radish, Raphanus raphanistrum Evolution 47: 704–711. [DOI] [PubMed] [Google Scholar]
- Cresswell JE. 1998. Stabilizing selection and the structural variability of flowers within species. Annals of Botany 81: 463–473. [Google Scholar]
- Cresswell JE. 2000. Manipulation of female architecture in flowers reveals a narrow optimum for pollen deposition. Ecology 81: 3244–3249. [Google Scholar]
- Cresswell JE, Galen C. 1991. Frequency-dependent selection and adaptive surfaces for floral character combinations—the pollination of Polemonium viscosum American Naturalist 138: 1342–1353. [Google Scholar]
- Cresswell JE, Hagen C, Woolnough JM. 2001. Attributes of individual flowers of Brassica napus L. are affected by defoliation but not by intraspecific competition. Annals of Botany 88: 111–117. [Google Scholar]
- Feinsinger P. 1983. Coevolution and pollination. In: Futuyma DJ, Slatkin M, eds. Coevolution. Sunderland, MA: Sinauer, 207–231. [Google Scholar]
- Fenner M, Cresswell JE, Hurley RA, Baldwin T. 2002. Relationship between capitulum size and pre-dispersal seed predation by insect larvae in common Asteraceae. Oecologia 130: 72–77. [DOI] [PubMed] [Google Scholar]
- Galen C. 1999. Why do flowers vary? The functional ecology of variation in flower size and form within natural plant populations. Bioscience 49: 631–640. [Google Scholar]
- Galen C. 2000. High and dry: drought stress, sex-allocation trade-offs, and selection on flower size in the alpine wildflower Polemonium viscosum (Polemoniaceae). American Naturalist 156: 72–83. [DOI] [PubMed] [Google Scholar]
- Galen C, Cuba J. 2001. Down the tube: pollinators, predators, and the evolution of flower shape in the alpine skypilot, Polemonium viscosum Evolution 55: 1963–1971. [DOI] [PubMed] [Google Scholar]
- Galen C, Plowright RC. 1985. The effects of nectar level and flower development on pollen carry-over in inflorescences of fireweed (Epilobium angustifolium) (Onagraceae). Canadian Journal of Botany 63: 488–491. [Google Scholar]
- Galen C, Zimmer KA, Newport ME. 1987. Pollination in floral scent morphs of Polemonium viscosum: a mechanism for disruptive selection on flower size. Evolution 41: 599–606. [DOI] [PubMed] [Google Scholar]
- Harder LD, Thomson JD, Cruzan MB, Unnasch RS. 1985. Sexual reproduction and variation in floral morphology in an ephemeral lily, Erythronium americanum Oecologia 67: 286–291. [DOI] [PubMed] [Google Scholar]
- Herrera CM. 1988. Variation in mutualisms: the spatio-temporal mosaic of a pollinator assemblage. Biological Journal of the Linnean Society 35: 95–125. [Google Scholar]
- Herrera CM. 2001. Deconstructing a floral phenotype: do pollinators select for corolla integration in Lavandula latifolia? Journal of Evolutionary Biology 14: 574–584. [Google Scholar]
- Herrera CM. 2002. Correlated evolution of fruit and leaf size in bird-dispersed plants: species-level variance in fruit traits explained a bit further? Oikos 97: 426–432. [Google Scholar]
- Herrera J. 1988. Pollination relationships in southern Spanish Mediterranean shrublands. Journal of Ecology 76: 274–287. [Google Scholar]
- Herrera J. 2001. The variability of organs differentially involved in pollination, and correlations of traits in Genisteae (Leguminosae: Papilionoideae). Annals of Botany 88: 1027–1037. [Google Scholar]
- Herrera J. 2004. Lifetime fecundity and floral variation in Tuberaria guttata (Cistaceae), a Mediterranean annual. Plant Ecology 172: 219–225. [Google Scholar]
- Holtsford TP, Ellstrand NC. 1992. Genetic and environmental variation in floral traits affecting outcrossing rate in Clarkia tembloriensis (Onagraceae). Evolution 46: 216–225. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Steiner KE. 1997. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae). Evolution 51: 45–53. [DOI] [PubMed] [Google Scholar]
- Jonas CS, Geber MA. 1999. Variation among populations of Clarkia unguiculata (Onagraceae) along altitudinal and latitudinal gradients. American Journal of Botany 86: 333–343. [PubMed] [Google Scholar]
- Mazer SJ, Schick CT. 1991. Constancy of population parameters for life-history and floral traits in Raphanus sativus L. 2. Effects of planting density on phenotype and heritability estimates. Evolution 45: 1888–1907. [DOI] [PubMed] [Google Scholar]
- Miller RB. 1981. Hawkmoths and the geographic patterns of floral variation in Aquilegia caerulea Evolution 35: 763–774. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Shaw RG, Waser NM. 1998. Pollinator selection, quantitative genetics, and predicted evolutionary responses of floral traits in Penstemon centranthifolius (Scrophulariaceae). International Journal of Plant Sciences 159: 331–33. [Google Scholar]
- Obeso JR. 1992. Geographic distribution and community structure of bumblebees in the northern Iberian Peninsula. Oecologia 89: 244–252. [DOI] [PubMed] [Google Scholar]
- Plowright RC. 1981. Nectar production in the boreal forest lily Clintonia borealis Canadian Journal of Botany 59: 156–160. [Google Scholar]
- Rivas-Martínez S. 1987.Memorias del mapa de series de vegetación de España. Madrid: Icona. [Google Scholar]
- Robertson JL, Wyatt R. 1990. Evidence for pollination ecotypes in the yellow-fringed orchid, Platanthera ciliaris Evolution 44: 121–133. [DOI] [PubMed] [Google Scholar]
- Runions CJ, Geber MA. 2000. Evolution of the self-pollinating flower in Clarkia xantiana (Onagraceae). I. Size and development of floral organs. American Journal of Botany 87: 1439–1451. [PubMed] [Google Scholar]
- Stanton ML, Preston RE. 1988. Ecological consequences and phenotypic correlates of petal size variation in wild radish, Raphanus sativus (Brassicaceae). American Journal of Botany 75: 528–539. [Google Scholar]
- StatSoft 2001.STATISTICA (data analysis software system), version 6. Tulsa, OK: StatSoft Inc. [Google Scholar]
- Stebbins GL. 1970. Adaptive radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Annual Review of Ecology and Systematics 1: 307–326. [Google Scholar]
- Steiner KE, Whitehead VB, Johnson SD. 1994. Floral and pollinator divergence in two sexually deceptive South African orchids. American Journal of Botany 81: 185–194. [Google Scholar]
- Stratton AD. 1992. Life-cycle components of selection in Erigeron annuus II. Genetic variation. Evolution 46: 107–120. [DOI] [PubMed] [Google Scholar]
- Valdés B, Talavera S, Galiano EF. 1987.Flora Vascular de Andalucía Occidental. Barcelona: Ketres. [Google Scholar]
- Williams JL, Conner JK. 2001. Sources of phenotypic variation in floral traits in wild radish, Raphanus raphanistrum (Brassicaceae). American Journal of Botany 88: 1577–1581. [PubMed] [Google Scholar]
- Wilson P. 1995. Selection for pollination success and the mechanical fit of Impatiens flowers around bumblebee bodies. Biological Journal of the Linnean Society 55: 355–383. [Google Scholar]