Abstract
• Background and Aims The paper by Monsi and Saeki in 1953 (Japanese Journal of Botany 14: 22–52) was pioneering not only in mathematical modelling of canopy photosynthesis but also in eco-developmental studies of seasonal changes in leaf canopies.
• Scope Construction and maintenance mechanisms of efficient photosynthetic systems at three different scaling levels—single leaves, herbaceous plants and trees—are reviewed mainly based on the nitrogen optimization theory. First, the nitrogen optimization theory with respect to the canopy and the single leaf is briefly introduced. Secondly, significance of leaf thickness in CO2 diffusion in the leaf and in leaf photosynthesis is discussed. Thirdly, mechanisms of adjustment of photosynthetic properties of the leaf within the herbaceous plant individual throughout its life are discussed. In particular, roles of sugar sensing, redox control and of cytokinin are highlighted. Finally, the development of a tree is considered.
• Conclusions Various mechanisms contribute to construction and maintenance of efficient photosynthetic systems. Molecular backgrounds of these ecologically important mechanisms should be clarified. The construction mechanisms of the tree cannot be explained solely by the nitrogen optimization theory. It is proposed that the pipe model theory in its differential form could be a potential tool in future studies in this research area.
Keywords: Monsi–Saeki theory, nitrogen optimization theory, pipe model theory, sugar-sensing, excitation pressure, cytokinin
INTRODUCTION
The paper by Monsi and Saeki (1953) provided plant ecologists with several key problems. Since the publication of this paper, optimization of the photosynthetic production system has been a major subject. For example, the leaf area index (LAI) that gives the maximum photosynthetic production (the optimum LAI) has been studied intensively. In strong light, the photosynthetic production per unit ground area increases with LAI (Hikosaka, 2005; Hirose, 2005). With the increase in LAI, leaves become more upright. Kuroiwa (1971) theoretically proposed a mode of change in leaf inclination from upright to horizontal with depth of the leaf canopy that homogenizes light absorption by the leaves. The predicted effects of leaf inclination were shown in an experiment using rice varieties differing in leaf angle (Tanaka et al., 1969). After Field (1983), Hirose and Werger (1987) pointed out the importance of nitrogen distribution within the leaf canopy, and nitrogen distribution has been analysed in many plants including trees based upon the nitrogen optimization theory.
The Monsi and Saeki method was originally developed to analyse herbaceous plant canopies. However, their approach has been used at other levels. For example, the method was applied to estimate primary production of forests (Monsi et al., 1973). It was also applied to the analysis of the photosynthetic system of a single leaf (Terashima, 1989; Terashima and Hikosaka, 1995).
Another important point of this seminal paper (Monsi and Saeki, 1953) is that it included analysis of seasonal changes in the productive structure of herbaceous plant communities. The importance of eco-developmental aspects had been recognized as long ago as 50 years.
The purpose of this review is to deal with ‘eco-developmental’ studies with respect to construction and maintenance of photosynthetic systems. In the first section, the optimum photosynthetic system is briefly described with respect to the optimization theory for nitrogen and light use. In the second section, the meaning of leaf thickness is discussed. In the Monsi and Saeki model, the leaf canopy is described by a few parameters, including those for leaf photosynthetic properties, and thereby the canopy size per se is not expressed in the model. In the actual leaf canopy, however, diffusion of the photosynthetic substrate, CO2, is affected by canopy size and CO2 diffusion often limit photosynthetic production (Monsi et al., 1973; Jones, 1992). Similarly, the thickness of the leaf is a very important determinant of the rate of leaf photosynthesis. Because there are many textbooks and reviews on gas diffusion in the plant canopy, the focus here is on the meaning of leaf thickness. The third section covers the physiological mechanisms of construction and maintenance of photosynthetic systems at various scaling levels, from a single leaf to the herbaceous plant individual. Construction of a tree photosynthetic system is separately treated in the last section. It is proposed that the pipe model theory in its differential form is a useful tool in analysing tree growth.
THE NITROGEN OPTIMIZATION THEORY: THE PHOTOSYNTHETIC SYSTEM THAT MOST EFFICIENTLY USES NITROGEN AND LIGHT
The plant individual
Because the nitrogen optimization theory is the main subject of Hikosaka (2005) and Hirose (2005) it will only be briefly outlined here. Let us consider the photosynthetic production of an individual plant in a leaf canopy. Nitrogen allocation among the leaves is optimized with respect to photosynthetic production when the following equation is satisfied for all the leaves located at various microsites within the plant:
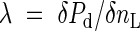 |
where Pd is daily net photosynthetic rate of the leaf, nL is nitrogen content per leaf area and λ is an unspecified Lagrange multiplier (Field, 1983, 1991; Field and Mooney, 1986; Hirose and Werger, 1987; Farquhar, 1989). λ changes with nitrogen availability: with the increase in nitrogen availability λ decreases.
Let us examine the solution of the above equation for a simple case. The instantaneous rate of net photosynthesis of a single leaf (Pn) can be expressed as a function of absorbed irradiance (A), maximum rate of gross photosynthesis (Pg,max), quantum yield on absorbed quantum basis (ϕa), and the respiration rate (r). Let us assume Pn is a first-order homogeneous function of A, Pg,max and r,
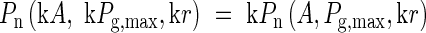 |
where k is an arbitrary constant. Pg,max, Pn,max, r, A and nL of the ith leaf of the plant under consideration are expressed as Pg,maxi, Pn,maxi, ri, Ai and nLi. If it is assumed that (1) ϕa is identical for all the leaves in the plant, (2) Pg,max, r, and thus Pn,max are linearly related to nL, (3) the ratio of light absorption among the leaves, A1 : A2 : A3 : …, does not change, and (4) the cost of nitrogen mobilization and re-mobilization is negligible, then eqn (1) is satisfied when Pn,max1:Pn,max2 : Pn,max3 : … = A1 : A2 : A3 : … (Farquhar, 1989; Field, 1991). This condition also realizes the maximum light use efficiency. In physiological terms, all the leaves in the plant should be in the same photosynthetic phase such as the initial-slope, transient or light-saturated phases such that light saturation of photosynthesis occurs simultaneously in all the leaves (Fig. 1).
Fig. 1.
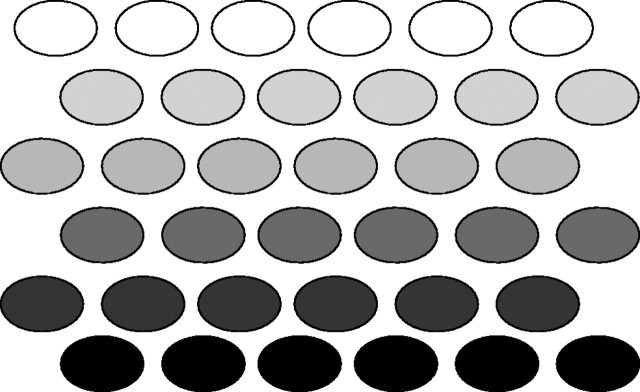
Photosynthetic subsystems in a photosynthetic system. For the photosynthetic system of a plant individual, subsystems can be regarded as the individual leaves. For the system of a leaf, subsystems are chloroplasts. Given that the photosynthetic properties of the subsystems are identical and light comes from above, the situation can be imagined in which upper subsystems are light-saturated while lower subsystems are light-limited. Under such conditions, light absorbed by the upper subsystems is wasted (or even harmful), and light is limited in the lower subsystems. In terms of nitrogen economy, this situation is paraphrased as follows. Nitrogen in the photosynthetic enzymes in the upper subsystems is used at maximum velocity, while nitrogen is wasted in the lower subsystems. To maximize the light and nitrogen use efficiencies, the system should be constructed such that all the subsystems reach light saturation at the same time. There are two ways to realize such conditions. One is to homogenize the light environment. The other is to adjust photosynthetic properties of the subsystems to their respective light absorption.
The above assumptions, however, may not be very realistic. For example, the relationship between Pn,max and nL is not necessarily linear but often saturating (Terashima and Evans, 1988; Evans, 1989; Makino et al., 1994, 1997; Eicheleman and Laisk, 1999). When the relationship is saturating and availability of nitrogen is high, the difference in nL giving the same λ between the uppermost leaves and lower leaves would become smaller than in the case where the relationship is linear. This is because the increase in nL in the leaves in the highest light conditions would cause only a slight increase in daily photosynthetic production due to the saturating nature of Pmax against nL. This leads to the situation in which light-saturation in the uppermost leaves occurs at lower irradiance (measured at the canopy surface) than that in the lower leaves. Obviously, this situation lowers light use efficiency. Another point is that the ratio of light absorption among leaves may not be constant; it may change depending on various factors such as time of day, solar elevation and cloud cover. Perhaps, the most misleading simplification among the above assumptions is that the cost of nitrogen mobilization/remobilization is negligible. In large plant individuals such as trees, the cost of nitrogen mobilization/remobilization would be particularly large, and this would explain why the gradient in nitrogen content is always smaller than that predicted by the theory, particularly in trees (Field, 1983).
Despite these problems, it is clear from the above theoretical consideration that homogenization of the light gradient by changing leaf angle and the formation of the gradient of nL within the canopy contribute to the efficient use of nitrogen and light (Fig. 1).
The leaf
The above theory for a plant individual can be applied to the leaf (Oja and Laisk, 1973; Terashima and Saeki, 1985; Farquhar, 1989; see Fig. 1). Let us assume that the leaf is composed of thin, serial layers. Given that the quantum yield of photosynthesis on absorbed light basis is identical among these layers, the sharpest light-response curve of the whole leaf is obtained when the ratio of the maximum net photosynthetic rate (pn,max) of the layer to light absorption is identical in all these layers. If pn,max of the thin layer is linearly related to the nitrogen content of the layer (nl), the light response with the sharpest transition also results in the most efficient nitrogen use.
The relationship between pn,max and nl may be saturating. If this is the case and the leaf realizes optimum nitrogen use efficiency, the chloroplasts in the uppermost layers will be light-saturated at lower irradiance than the chloroplasts in the lower layers as discussed for the plant individual. This situation leads to a blunter light-response curve, and lowers light use efficiency. The ratio of light absorption among the mesophyll layers may change to some extent due to external light conditions and internal factors such as chloroplast orientational movement. However, when the dorsiventral leaf is illuminated from the adaxial side with white light, as in nature, the ratio of light absorption among layers and that of pg,max agree well (Evans and Vogelmann, 2003).
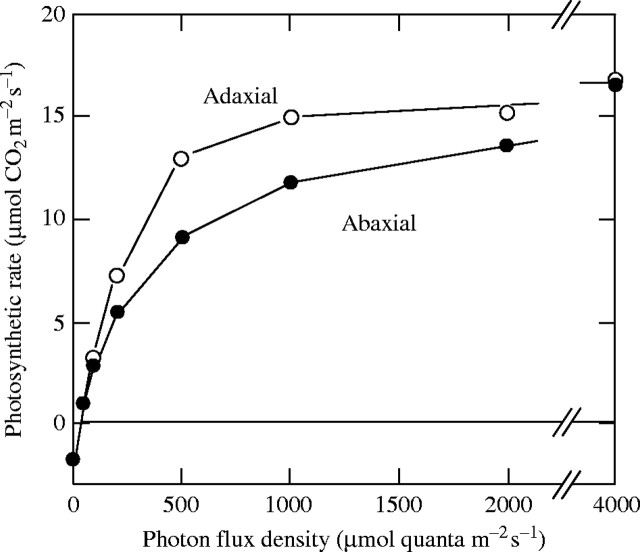
When the photosynthetic light-response curve of the dorsiventral leaf obtained by the adaxial side irradiation is compared with that obtained by abaxial (lower) side irradiation, effects of the disagreement of light absorption among layers and that of pg,max are evident (Fig. 2). pg,max in the lower side is smaller than that in the upper side, and the spongy tissue absorbs light more efficiently than the palisade tissue (Terashima, 1989; Terashima and Hikosaka, 1995). When the leaf is irradiated from the abaxial side, therefore, chloroplasts in the lower side are light-saturated at a very low irradiance, while those in the upper side are light-saturated only by very strong light. Thus, the transition from the initial slope phase to the light-saturated phase becomes much blunter (Oja and Laisk, 1976; Terashima and Saeki, 1985; Terashima, 1986).
Fig. 2.
Photosynthetic light-response curves measured in the same Glycine max leaf but by irradiation from the different sides. The curve obtained with irradiation from the adaxial side showed a transition from the light-limited to the light-saturated phases much sharper than that obtained with irradiation from the abaxial side. Redrawn from Terashima (1986).
MEANING OF LEAF THICKNESS
CO2 diffusion from the air to the chloroplast stroma
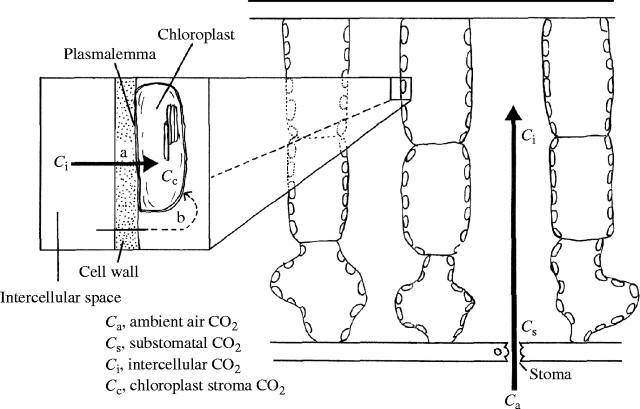
Because the problem of leaf thickness is closely related to CO2 diffusion, CO2 diffusion within a leaf will be considered first (Fig. 3). In photosynthesizing C3 leaves, CO2 concentration in the substomatal cavity, Cs, is lower than that in the ambient air, Ca, and CO2 diffuses into the leaf along the gradient of CO2 concentration. Cs can be estimated by the gas exchange technique. In a vigorously photosynthesizing leaf, the bulk CO2 concentration in the intercellular spaces, Ci, is lower than Cs due to the resistance to CO2 diffusion in the intercellular spaces (Parkhurst, 1994). CO2 concentration in the chloroplast stroma, Cc, in C3 plants is even lower than Ci. Recent technological innovations, including pulse-modulated fluorometry and concurrent measurement of carbon isotope discrimination and gas exchange, have enabled us to estimate Cc. For some species, Cc as low as half of Ca was reported (for a review, see Evans and Loreto, 2000). This indicates that resistance to CO2 diffusion from the ambient air to the chloroplast stroma is substantial.
Fig. 3.
Diffusion of CO2 from the atmosphere to chloroplast stroma. The inset shows two pathways. The diffusion of CO2 along pathway b is negligible compared with that along pathway a.
Conductance for CO2 diffusion through stomata (gs) has been well studied and the drawdown of CO2 concentration, Ca − Ci, is about 60–120 µmol mol−1 when Ca is 360 µmol mol−1 (Evans and Loreto, 2000). Stomatal conductance can be approximated as:
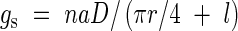 |
where n is stomatal density (m−2), a is stomatal pore area in m2, D is binary diffusion coefficient of CO2 in the air. In this approximation, a stoma is assumed to be a tube having radius r (m) and length l (m), respectively (Meidner and Mansfield, 1968). When stomata are open, na would be 0·005–0·02. Let us assume r is 5 µm and l is 10 µm. At 25°C, D is 1·55 × 10−5 m2 s−1. Then, gs ranges from 5·6 to 23 mm s−1 or 0·22 to 0·86 mol CO2 m−2 s−1. These are comparable to the reported stomatal conductance.
Similarly, the conductance in the intercellular spaces is approximated as gias = Dp/τd, where p is porosity, τ is tortuosity of the mesophyll intercellular spaces, and d is distance for CO2 diffusion. p usually ranges from 0·2 to 0·5. Let us assume τ = 1·5, for instance. Given that d is 100 µm (the average value for the leaf having a 200-µm-thick mesophyll), gias would range from 10 to 50 mm s−1 or, from 0·8 to 2·0 mol CO2 m−2 s−1. For the amphistomatous leaves, gias further increases by three- to four-fold (Parkhurst et al., 1988; Terashima et al., 2001). These values are much larger than the maximum stomatal conductance. Thus, it is unlikely that gias is a major limiting factor of leaf photosynthesis, in particular, in amphistomatous leaves.
The internal conductance (gi), the conductance for CO2 diffusion from the surface of mesophyll cell walls to the chloroplast stroma, via plasma membrane, cytoplasm and chloroplast envelope, is not very large, although it had been frequently assumed to be infinite. Evans and Loreto (2000) summarized the data on gi which ranges from 0·03 to 0·6 mol CO2 m−2 s−1 bar−1. At the atmospheric pressure of 1 bar (101 kPa), these gi values correspond to 0·03–0·6 mol CO2 m−2 s−1 of mature leaves and appear to differ depending on plant functional types (Fig. 4). gi values for annual herbs such as crop species are greatest and range from 0·2 to 0·6 mol CO2 s−1 bar−1 (Evans and Loreto, 2000). On the other hand, the values of evergreen broad-leaved trees are much lower, ranging from 0·03 to 0·2 mol CO2 m−2 s−1 bar−1 (Hanba et al., 1999; Evans and Loreto, 2000). In mesic deciduous trees, gi values are intermediate between those of annual herbs and evergreen trees. Among them, gi values for the tree species that develop leaves successively appear to be greater than those for flush type species (Hanba et al., 2001). Castanea sativa, a Mediterranean deciduous chestnut, had gi of 0·1 mol CO2 m−2 s−1 bar−1 (Lauteri et al., 1997). This is the lowest record for deciduous trees and is comparable to those of evergreen trees.
Fig. 4.
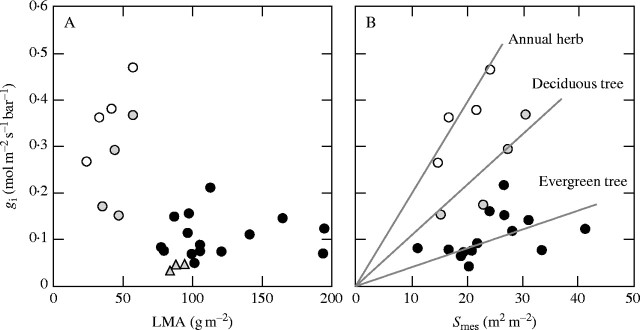
Internal conductance (gi) of various leaves plotted against (A) leaf dry mass per area (LMA) and (B) mesophyll surface area. Data from various sources. Open circles, annual herb (tobacco); grey circles, deciduous tree species; grey triangles, Castanea sativa (Mediterranean chestnut); solid circles, evergreen tree species.
gi in a pioneer clonal plant Polygonum cuspidatum (= Reynoutria japonica; Kogami et al., 2001; Sakata et al., 2002) decreased with increasing altitude: gi for the plants at the altitude of 10 m was 0·2 mol CO2 m−2 s−1 bar−1, while that for the plants at 2500 m was 0·076 mol CO2 m−2 s−1 bar−1 (Kogami et al., 2001). If this effect of altitude on gi is general, the trend of changes in carbon isotope composition with altitude (Körner et al., 1988) can be explained.
It has been shown that internal conductance increases with the increase in cumulated surface area of chloroplasts that face the intercellular spaces (Sc; Evans and Loreto, 2000). This indicates that increasing the effective area for CO2 dissolution increases internal conductance. In this respect, it is noteworthy that thick sun leaves have larger Sc than thin shade leaves. Although the surface area of mesophyll facing the intercellular spaces (Smes) is not as good a parameter as Sc, gi for the species of the same functional type appears to be roughly proportional to Smes. The decrease in distance from the mesophyll surface to the plasma membrane by having thin cell walls should be effective in increasing internal conductance because diffusion of CO2 in water is lower than that in air by 10−4. On the other hand, low gi values in evergreen tree leaves (Hanba et al., 1999; Miyazawa and Terashima, 2001) and alpine plants can be attributed to thick mesophyll cell walls.
Interestingly, gi can change drastically without marked changes in Sc and/or cell wall thickness. For example, the decrease in gi was reported for the plants under water stress (Flexas et al., 2002) or salt stress (Delfine et al., 1998, 1999) conditions. These studies indicate that CO2 permeability of membranes would change. Aquaporins, the most abundant proteins in plant plasma membranes, mainly transfer water molecules according to the gradient of the water potential. However, it was shown that animal aquaporin 1 transports CO2 as well as water (Cooper and Boron, 1998; Nakhoul et al., 1998; Yang et al., 2000). In these studies, Xenopus oocytes and/or liposomes were used and the CO2 permeability was monitored as changes in pH.
gi were estimated based on concurrent measurements of gas exchange and fluorescence in the leaves of Vicia faba and Phaseolus vulgaris, in the presence or absence of HgCl2, a potential inhibitor of most of the aquaporins. Because gi and hydraulic conductivity of the mesophyll cells decreased at the same concentration range of HgCl2, it is proposed that aquaporins are involved in diffusion of CO2 across the plasma membrane (Terashima and Ono, 2002). Temperature dependence of gi also indicated involvement of protein(s) in CO2 diffusion from the intercellular spaces to chloroplast stroma (Bernacchi et al., 2002). Very recently, it was shown that the aquaporin 1 from Nicotiana tabacum transfers CO2 using Xenopus oocytes (Uehlein et al., 2003). The study of Hanba et al. (2004) on transgenic rice plants, in which barley aquaporin was overexpressed, also confirmed their proposal; with the increase in aquaporin abundance, gi clearly increased. Besides abundance of aquaporin, conductance for CO2 through aquaporin could also be regulated by pH (Tourmaire-Roux, 2003), and by phosphorylation (Maurel et al., 1997; Kjellbom et al., 1999). In summary, gi is determined by Sc, wall thickness, and by abundance and the state or conductivity of aquaporins.
Why are sun leaves thicker than shade leaves?
When expressed on leaf area basis, the light-saturated rate of leaf photosynthesis in C3 plants strongly depends not only on nL, contents of the photosynthetic components including Rubisco, cytochrome f, H+-ATPase and reaction centres but also on structural parameters such as leaf thickness, leaf mass per area, mesophyll surface area (Smes) and chloroplast surface area (Sc). The correlation between Pmax and Sc is generally stronger than that between Pmax and Smes. Because diffusion of CO2 in water is 10−4 of that in the air, the flux via the pathway like b in Fig. 3 should be negligible compared with that of pathway a. Then, it is useless to have mesophyll cell surfaces without chloroplasts (Miyazawa et al., 2003). In fact, when grown with sufficient nutrients, most of the mesophyll cell surfaces facing the intercellular spaces are occupied by chloroplasts, although some unoccupied spaces are indispensable for the plants to re-acclimate to a brighter light environment (Oguchi et al., 2003).
Thickness of chloroplasts is also important. The drawdown of CO2 concentration from the intercellular spaces to the stroma, Ci − Cc, is proportional to the flux of CO2 across the liquid phase (including cell wall, plasma membrane, cytosol and chloroplast envelope) per unit chloroplast surface area and to the resistance to CO2 diffusion from the intercellular spaces to the stroma per unit chloroplast surface area. With the increase in the amount of Rubisco per unit chloroplast surface area, photosynthetic rate per unit chloroplast surface area increases. However, the photosynthetic rate per Rubisco decreases because Cc decreases. From the viewpoint of efficiency of Rubisco use (or nitrogen use), thicker leaves with greater Sc would be advantageous because the amount of Rubisco per unit chloroplast surface area becomes smaller, thereby Rubisco can operate at higher Cc. On the other hand, gias increases with leaf thickness, which causes a decrease in the bulk Ci. Moreoever, the construction and maintenance costs of thick leaves are expensive. In these aspects, thick leaves are not advantageous.
The effects of various aspects of mesophyll structure, in particular mesophyll thickness on photosynthesis, were evaluated, using a one-dimensional model of CO2 diffusion in the leaf (Terashima et al., 2001). First the thickness that gives the maximum photosynthetic rate for leaves with various Rubisco contents per leaf area was calculated. Interestingly, the maximum rate of photosynthesis occurred at an identical mesophyll thickness irrespective of Rubisco content per leaf area. Obviously, this does not explain why sun leaves are thicker than shade leaves. The construction/maintenance cost of the leaf was then taken into account. In this calculation, the mesophyll thickness was regarded as the cost. The mesophyll thickness that realized a given photosynthetic rate per unit mesophyll thickness was compared in model leaves with various Rubisco contents per leaf area. It was found that, with an increase in the Rubisco content per leaf area, the mesophyll thickness that realized a given photosynthetic rate per unit mesophyll thickness increased. This kind of constraint probably explains the strong relationship between the maximum rate of photosynthesis and leaf morphological parameters such as mesophyll thickness. In other words, leaf thickness is determined as a compromise between the increase in chloroplast surface area for CO2 dissolution and the decrease in the cost of the leaf.
In these simulations, the increase in mesophyll thickness simultaneously means a decrease in gias, an increase in Sc and an increase in construction and maintenance carbon costs of the leaf. Alternatively, the leaf can increase Sc and gias by decreasing cell size. The leaf with smaller cells is also mechanically tougher. Although xeric or alpine plants show such tendency, leaves do not have very small cells. This could be because leaves exhibiting considerable rates of leaf area expansion, adequate heat capacitance, high efficiency of resource use, etc. have been favoured by natural selection (Terashima et al., 2001).
MECHANISMS RESPONSIBLE FOR OPTIMUM NITROGEN ALLOCATION WITHIN HERBACEOUS PLANTS
Phenomenology
Formation of the gradient in nL within a plant would be dependent on either light environment or age. Hirose et al. (1988) observed that the gradient in nL in the individual plant of Lyschimacia vulgaris was less steep when the plants were grown in a thin leaf stand with much light penetration than in a dense stand, although the age of plants in these stands was identical. For a blade of Carex acutiformis (a sedge) in the stand, nL was greatest at the tip and decreased towards the blade base (Hirose et al., 1989). Because the blade of grass-type monocots elongate due to activity of the intercalary meristem at the blade base, the tip of the blade is more aged than the base. These results indicate that light is more important than age in determining nL. Analysing the light environments of leaves of various ages in a vine plant, Ackerly (1992) reached the same conclusion.
Hikosaka et al. (1994) devised an experimental system to examine separately the effects of light environment and age. They grew vines of Ipomoea tricolor horizontally to minimize the effects of mutual shading of the leaves. Photon irradiances of the respective leaves were controlled with small shade boxes. When nutrients were sufficient, nL was influenced by irradiance levels and nL was greater in the leaves receiving high light. In contrast, when the nutrient was limiting, nL decreased with leaf age even when all the leaves were exposed to the same irradiance (Hikosaka et al., 1994). Thus, age also appears to be important.
In the plant in the dense stand, there are vertical gradients in chloroplast properties such as Chl a/b and Rubisco/Chl as well as that in nL (Evans and Seemann, 1989; Terashima and Hikosaka, 1995). Such differences in chloroplast properties are well characterized by comparative studies in which plants were grown under high and low light conditions (Boardman, 1977; Anderson, 1986). When the leaves of I. tricolor that had been exposed were shaded, not only their nL but the ratios of Chl a/b and Rubisco/Chl decreased. In contrast, when nL decreased with age under constant photon irradiance, changes in Chl a/b and Rubisco/Chl were small (Hikosaka, 1996). These results suggest that re-acclimation of sun leaves to the shade is accompanied by both a decrease in nL and qualitative changes in chloroplast properties. Selective breakdown of the components related to energy transduction, such as Rubisco, and maintenance or synthesis of the components for the light harvesting system occur in re-acclimation to a shadier environment. On the other hand, when the leaves senesce in constant light, all photosynthetic components generally decreased (Hikosaka, 1996; Hikosaka, 2005).
Acclimation to sun and shade: formation of sun and shade leaves
Because construction and maintenance are complex processes, the processes should be dissected into several basic processes. The first deals with acclimation to the light environment in developing leaves and then senescing leaves.
Sun and shade leaves differentiate according to the light environments in which they are grown (Björkman, 1981). As already discussed, sun leaves are generally thicker than shade leaves. Thickening of sun leaves is mainly due to elongation of palisade tissue cells or to periclinal divisions in the palisade tissue cells, or both. By such thickening, mesophyll surface area per leaf area becomes greater in sun leaves than in shade leaves. Properties of chloroplasts are also different between the sun and shade leaves (Boardman, 1977; Anderson, 1986).
Anatomical differences between sun and shade leaves. Differentiation processes of sun and shade leaves were analysed using Chenopodium album, an annual herb. The plants were shaded in various ways and the effects of these shading treatments on the properties of the developing leaves were examined. Palisade tissue, two cell layers thick, was formed when mature leaves were exposed to high light, irrespective of the light environments of the developing leaves. On the other hand, when mature leaves were shaded, one-cell-layered palisade tissue was formed. These results clearly showed that anatomy of new leaves is determined by light environment of mature leaves. There must be a signal transduction system that conveys the signal(s) from the mature leaves to developing leaves (Yano and Terashima, 2001).
Further, a comparative developmental study of sun and shade leaves for C. album was conducted. Whether the plants were grown under typical sun or shade conditions, the number of cells in the palisade tissue per leaf was almost identical. Moreover, in sun leaves, anticlinal and periclinal divisions occur almost synchronously. Thus, the signal(s) from the mature leaves regulates the direction of cell division. In the future sun leaves, the signal probably induces periclinal division in addition to anticlinal division, while the signal from the shaded mature leaves only allows the cells to divide anticlinally (Yano and Terashima, 2004).
It is hypothesized that the signal is the abundance of photosynthate. When photosynthates from mature leaves are abundant, leaves would develop into sun leaves. Stomatal frequency could be also regulated by similar mechanisms (Lake et al., 2001).
In some deciduous trees, all leaves that will unfold in flush in the next year are prepared in the bud during the winter. In some species of this group, the anatomy of the next-year leaves is determined when they are in winter buds. For example, in Fagus crenata from the Pacific side of Japan (there are some difference among ecotypes; T. Koike, pers. comm.), the number of cell layers of the leaf is already determined in the winter bud (Uemura et al., 2000). The developing leaves may sense the light environment of the bud. However, it is more likely that differentiation between sun and shade leaves occur in the developing bud in response to matter production during the year. On the other hand, chloroplast properties are largely determined by the light environment of the developing leaves (Yano and Terashima, 2001; see below).
Roles of photoreceptors. In the plant stand, the decrease in PPFD with depth is always accompanied by a decrease in the red : far-red ratio (Anten et al., 2000; Smith, 2000). Thus, the role of phytochrome in the differentiation of sun and shade leaves has been studied.
Smith et al. (1993) demonstrated that shading of aurea mutants (deficient in phytochrome A, and also possibly other phytochromes) of tomato plants caused a similar decrease in Pmax as in wild-type plants. A series of studies using Arabidopsis thaliana with mutant photoreceptors clearly showed that phytochrome mutants are able to form sun and shade leaves in response to the light environment in which they are growing. These leaves differed in Pmax and chloroplast properties including Chl a/b and Rubisco/Chl. These results indicate that phytochromes do not play a central role in the differentiation of sun and shade leaves (Walters et al., 1999). Weston et al. (2000) examined the anatomy of leaves of photoreceptor mutants and confirmed their differentiation into sun and shade leaves.
Walters and Horton (1995) showed that acclimation of leaves was not dependent on the intensity of blue light. However, in the complete absence of blue light, acclimation to irradiance did not occur (Walters et al., 1999). In ecophysiological experiments, shading is usually done with a black screen, which does not change the spectrum of the light. In such experiments as well, differentiation between sun and shade leaves has been observed (for a review, see Björkman, 1981). Thus, sun and shade leaves develop even when the light spectrum is unchanged.
However, the above studies indicate that plants appear to sense whether they are in the light or in the dark using these photoreceptors. The decrease or increase in sugar content induces or suppresses expression of different genes (Koch, 1996) and the effects differ completely depending on whether leaves are in complete darkness or in the light (Fujiki et al., 2001).
In sucrose-uncoupling mutants, in which the expression of plastocyanin is not repressed by sucrose, various phytochrome-mediated phenomena become insensitive to sucrose (Dijkwel et al., 1997). It is also noteworthy that a mutant of A. thaliana that lacks the chloroplast inner envelope triose phosphate/phosphate translocator is defective in photosynthetic acclimation to the light environment (Walters et al., 2003). These results infer a close relationship between the sugar-sensing system or sugar metabolism and the photosensing system.
Redox control. The redox state of electron transport components between photosystems I and II reflects a balance between input and processing of light energy (Fujita, 1997). The balance can be assessed fluorometrically as the redox state of plastoquinone pool and is referred to as excitation pressure (Huner, 1998). In high light or other conditions that saturate or suppress the velocity of the Calvin–Benson cycle, the electron transport system is reduced. On the other hand, when the photosynthetic capacity is much greater than the incoming light, the electron transport system tends to be oxidized. When the leaves of Triticum aestivum and Secale cereale were developed under moderate light at a low temperature or under high light at an ordinary temperature, the maximum photosynthetic rates on a leaf area basis measured under the optimal condition increased (Gray et al., 1997). Chloroplasts in the plants developed under high excitation pressure are of the sun type.
The molecular identity of excitation pressure has been studied intensively. The redox state of plastoquinone itself appears to suppress Lhcb gene expression via phosphorelay in algae; while in higher plants, it is suppressed by reduced thioredoxin or glutathione. In the regulation of other genes, the phosphorelay, thioredoxin, glutathione and reactive oxygen species are involved (Hihara and Sonoike, 2001; Rodermel, 2001; Surpin et al., 2002; Muramatsu and Hihara, 2003). Walters et al. (1999) observed that A. thaliana defect in the y gene is not capable of light acclimation and proposed a central role of the COP/DET/FUS regulatory cluster, a focus for multiple signal transduction pathways including the redox signal.
Nitrogen abundance and cytokinin. Nitrogen availability affects the number or the total volume of chloroplasts in the leaf rather than their quality (Evans and Terashima, 1988; Terashima and Evans, 1988). Therefore, when the amounts of the photosynthetic components are expressed on a Chl basis, these values are not affected by nitrogen availability very much. However, when nitrogen is extremely limited, the quality of the chloroplast is also affected. For example, as predicted by the optimization model for nitrogen allocation (Hikosaka and Terashima, 1995), the Chl a/b ratio increases when the availability of nitrogen is extremely low (Kitajima and Hogan, 2003). On the other hand, when the nitrogen is abundant, the leaves tend to accumulate more Rubisco and thereby Rubisco/Chl increases (Terashima and Evans, 1988; Makino et al., 1994, 1997; Eichelmann and Laisk, 1999).
The abundance of nitrogen in the environment may be sensed as the concentration of nitrate or ammonium near the root surface. Nitrate also acts as a signal to induce the expression of enzymes involved in nitrogen assimilation (Crawford, 1995).
Cytokinin also plays a role as a signal. When maize seedlings are grown without exogenous nutrients, the leaves are metabolically more C3-like and their main carboxylating enzyme is Rubisco (Sugiharto et al., 1990). Addition of nitrate or ammonium to the intact nitrogen-deficient maize plants leads to rapid and marked increases in the Ppc transcript and in phosophoenolpyruvate carboxylase (PEPCase) activity. When nitrogen-deficient maize shoots without roots were fed with nitrate, PEPCase synthesis was not enhanced. Thus, the induction of PEPCase is not due to nitrate signal. Recent studies showed that cytokinin synthesized in roots in response to nitrate or ammonium is responsible for enhanced PEPCase synthesis (Takei et al., 2002). The concentration of cytokinin increases on application of nitrate or ammonium to the root. Perhaps, such regulation would occur in C3 plants as well. Light-harvesting complex II that are not essential to photosynthetic energy transduction may not be synthesized under the very limited nitrogen availability, which may result in a high Chl a/b ratio.
Appreciating importance of cytokinin from the root, Pons and Bergkotte (1996) hypothesized that the gradient in PPFD within a plant can be sensed by the different rates of transpiration in the leaves. Leaves in sunny microhabitats transpire more than those in shade microhabitats. To prove this hypothesis, they conducted an interesting experiment with growing and mature primary leaves of Phaseolus vulgaris. They enclosed one of the pair leaves and regulated the vapor pressure deficit (VPD) in the chamber. When they decreased the VPD in the chamber to suppress transpiration during leaf expansion, the leaves showed more shade-type characteristics than the leaves exposed to greater VPD. They argued that the cytokinin synthesized in the root system is preferentially transported by the transpiration stream to the leaves under high VPD and affects the nitrogen metabolism of these leaves. However, leaf expansion tends to be suppressed under dry conditions. Then, it is probable that the photosynthetic rate per leaf area increases with air dryness.
In nature, leaves exposed to the sun would be sun leaves irrespective of humidity. When the hypothesis of Pons and Bergotte (1996) for the differentiation of sun and shade leaves is followed, the cytokinin concentration in the xylem sap should increase in humid air. This possibility should be tested.
Acclimation of chloroplast properties within a leaf. In the leaf, the gradient of chloroplast properties across the leaf is formed due to acclimation of chloroplasts to the local light environment (Terashima and Inoue, 1985a, b; Yano and Terashima, 2001). The acclimation itself is not usually affected by nutrient status. The effects of local light environment were shown in the experiments in which the leaves were illuminated from the abaxial side. The inversion of a spinach leaf after full expansion induces complete inversion of the Chl a/b ratio and the partial inversion of the rate of electron transport activity (Terashima and Inoue, 1985b). The illumination of expanding leaves from the abaxial side leads to complete inversion of thylakoid morphology (Terashima et al., 1986). Analyses of the photosynthetic system in pendulous leaves such as those of Eucalyptus were also made. The results clearly showed that the Chl a/b ratio was highest on both sides and lowest in the middle (James et al., 1999). It is highly likely that the redox control is responsible for the gradient formation.
Factors regulating leaf senescence
In upright herbaceous plants, new leaves gradually shade old leaves. Senescence of the leaves is thus accompanied by shading by the upper leaves. Senescence processes may be superimposed by re-acclimation of the former sun leaves to the shade. When the whole plant is shaded, all the leaves retain their photosynthetic activity longer (Hidema et al., 1991). On the other hand, when particular leaves of the plants are shaded, such parts and leaves senesce faster (Weaver and Amasino, 2001). When all the leaves of the plant are under the same light conditions (this situation is possible in vine plants), the oldest leaves senesce fastest. Therefore, senescence patterns vary considerably. Thus, there is a need to dissect these complex experimental/natural conditions into several cases.
Phytochrome. In upright plants, young leaves shade old leaves, thereby the ratio of red light to far-red light (R/FR) received by the old leaves decreases. Phytochrome appears to be involved in changes in chloroplast properties in senescing leaves. Okada et al. (1992) and Okada and Katoh (1998) found that chlorophyll degraded rapidly in the dark in detached and attached Oryza sativa leaves, respectively. Chlorophyll degradation was reversibly suppressed by short exposure of the leaves to the weak red light. The effect of red light was suppressed by far-red light. An experiment with Helianthus annuus plants demonstrated that artificial far-red enrichment of the light penetrating through the canopy enhanced senescence of the lower leaves (Rousseaux et al., 1996). The results of these studies clearly indicate that the difference in the R/FR ratio received by leaves is involved in regulating leaf senescence (for review, see Anten et al., 2000; Smith, 2000).
Plant nitrogen status and cytokinin. When nitrogen nutrition is deficient, senescence is accelerated. The leaf may sense a decrease in nitrate and/or cytokinin levels. Effects of a decreased cytokinin level in the senescing process have been studied using transgenic tobacco plants expressing the isopentenyl transferase gene, a key enzyme of cytokinin synthesis, under the control of a highly senescence-specific promoter (SAG 12). Jordi et al. (2000) showed that the chlorophyll content was maintained in old senescing leaves in transgenic plants. However, an enhanced cytokinin level was less effective in maintaining Rubisco and Pmax. Using the same transgenic tobacco plants, Wingler et al. (1998) showed that cytokinin production decelerated the decrease in Rubisco content to some extent and that accumulation of sugars blocked the effects of cytokinin. These results indicate that enhanced production of cytokinin in the senescent leaves causes some deceleration of that senescence. Various factors regulate cytokinin production in the leaf (Jordi et al., 2000). Obviously, the cytokinin level decreases in the presence of sugar.
Cytokinin in the transpiration stream is involved in gene expression for nitrogen metabolism (Sakakibara et al., 2000; Takei et al., 2002) and the decrease in cytokinin input with the decrease in transpiration rate by shading could partly explain leaf senescence as Pons and Bergkotte (1996) and Pons et al. (2001) argued.
Sugar repression. High sugar concentration represses expression of genes for photosynthetic components (Sheen, 1990; Krapp and Stitt, 1995; Koch, 1996; Koch et al., 2000; Pego et al., 2000), which leads to the acceleration of senescence and re-translocation of nitrogenous compounds (Dai et al., 1999). Inversely, the low sugar concentration may be required for maintaining the high photosynthetic rate of the source leaf or for retardation of leaf senescence (Ono and Watanabe, 1997; Ono et al., 2001). There are some studies indicating interaction between photosensory and sugar-sensing pathways as mentioned above. Also, retardation of leaf senescence by cytokinin was cancelled by high sugar concentrations (Wingler et al., 1998). However, suppression of gene expression for photosynthetic components by sugar is strictly dependent on the developmental stage of the leaf. When leaves are actively expanding, sugar does not suppress an increase in photosynthetic activity (T. Araya, K. Noguchi and I. Terashima, unpubl. res.).
If a leaf can sense its light environment or photosynthetic status relative to those of other leaves within the plant and regulate the photosynthetic capacity accordingly, the nitrogen distribution within a plant would always change towards the optimal conditions (Ono et al., 2001). It is probable that a leaf senses its photosynthetic status by monitoring its sugar concentration. When the demand for photosynthates produced by the leaf under consideration is large, sugar concentration of this leaf would be low because of vigorous translocation to sink organs. On the other hand, when the demand for photosynthates produced by the leaf is small, its sugar concentration will increase.
Nitrogen deficiency suppresses plant growth, and thereby the sink activity of developing leaves is lowered. Then, translocation of carbon from the source leaves is also suppressed, this would lead to accumulation of carbohydrate under nitrogen deficiency (Radin and Eidenback, 1986; Paul and Stitt, 1993; Ono et al., 1996). Thus, by monitoring the abundance of non-structural carbon, nitrogen deficiency could be monitored indirectly. Perhaps, in addition to more direct nitrogen sensing systems, the leaves also sense the nitrogen status of a plant indirectly by monitoring its sugar concentration (Paul and Driscoll, 1997).
Redox control. When a leaf is shaded, the excitation pressure is lowered. This can be a signal for re-acclimation to shaded conditions (Huner et al., 1998). However, this possibility has not been tested.
Effects of partial and general shading. When the whole plants were generally shaded, senescence of all leaves was decelerated (Hidema et al., 1991). This could be explained as follows (Hikosaka, 1996, 2005). The amount of nitrogen needed for the construction of young organs will decrease due to suppression of growth. Moreover, photosynthetic production of the leaves decreases. Thus, nitrogen demand would not be large and sugar would not accumulate in all the leaves.
On the other hand, when particular leaves are shaded, they senesce faster (Weaver and Amasino, 2001). Because shading with a black cloth accelerated leaf senescence, the roles of phytochrome are not essential. Effects of such selective shading have not been assessed in detail. It is not known whether such shading increases sugar concentration. Obviously, excitation pressure decreases on shading, in particular, shade enriched with far-red light. Then, this can cue the senescence or adjustment of photosynthetic capacity to the low light conditions. Transpiration is also suppressed in shaded leaves. Thus, delivery of cytokinin also decreases.
Senescence in horizontally grown vine plants. When an Ipomoea tricolor plant was grown horizontally, all the leaves received the same irradiance. When the nitrogen nutrition was limited, nL of the oldest leaves started to decrease (Hikosaka et al., 1994). This apparently indicates the importance of actual age. In this study, however, all the lateral buds were removed to simplify the system, and only the terminal bud of the main stem was allowed to produce new leaves. Because it is known that sinks attract photosynthates from nearby leaves and young sink leaves are nearer to the apex of the main shoot, the photosynthates produced by these young leaves would be preferentially used for constructing new organs, which might keep their sugar concentration low. Then, the distance, or cost of re-translocation of carbon and nitrogen could be important. Plant hormone(s) could be involved in such regulation.
ANALYSES OF THE TREE PHOTOSYNTHETIC SYSTEM
The nitrogen optimization theory has been mainly applied to herbaceous plants. Analyses of nitrogen content and light level have been often confined to the leaves in the same shoot. Obviously, tree systems are more complicated and their properties should be examined, not only of leaves but also those of shoots, in relation to light environment.
Deciduous trees
With Fagus species, the effects of the current-year light environment on shoot and leaf properties were separated from those of the light environment of the previous year(s). Several leaf tiers located at different heights in a deciduous tree of Fagus japonica (about 15 m in height) were examined (Kimura et al., 1998). Among the properties of gross morphology of the shoot, the number of leaves per shoot and total leaf area of the shoot were largely determined by the light environment of the previous year. Although the light environment of the previous year largely determined the length of short shoots, current year light also contributed to the elongation of the long shoots. Leaf photosynthetic properties such as nL and Chl/nL, leaf thickness (leaf mass per area) depended strongly on the current-year light environment.
Fagus crenata in the same forest (Uemura et al., 2000) showed somewhat different results. Leaf properties such as leaf thickness and leaf mass per area were dependent both on the previous- and current-year light environment. The difference between these two Fagus species was partly due to the fact that palisade tissue of F. japonica leaves was one cell layer thick, irrespective of the current- and previous-year light environments, while, in F. crenata, palisade tissue of the sun leaves was two cell layers thick. The future palisade tissue had already two cell layers when the leaf primordia were in the winter bud. In F. crenata, artificial shading of the shoots that had expanding leaves with two-cell-layered palisade tissue, resulted in intermediate leaf thickness between full sun and shade leaves. On the other hand, when the leaf primordia that had developed in the shade box were exposed to full sun in spring, the thickness of these leaves was also intermediate. Pmax was more strongly dependent on the current-year light environment but was also intermediate between those of full-sun and full-shade leaves (Uemura et al., 2000). Thus, some photosynthetic characteristics are dependent not only on the current-year conditions but also on previous-year light.
Leaves developed in the winter buds in the sun-exposed shoots usually develop in sunny environments. However, the systems that determine photosynthetic capacity depend both on previous- and current-year light environments, which would potentially obscure the relationship between nL and the current-year light environment.
Evergreen trees
In evergreen trees, the situation is more complicated. In Quercus glauca, an evergreen tree, nL of the current-year leaves from various shoots depended on their relative photon flux density (RPFD). However, the correlation was very weak. For the 1-year-old leaves that were inevitably shaded by the current-year leaves, the correlation between nL and RPFD was not significant (Miyazawa et al., 2004). Similar results were reported for evergreen coniferous trees (Shoettle and Smith, 1998). Nitrogen in the current-year leaves on the shoot (cumulated leaf area of current-year leaves × nL) was also strongly dependent on RPFD. On the other hand, the amount of nitrogen retained in the 1-year-old leaves for the shoot was negatively correlated with the RPFD of the shoot, because of leaf shedding. The remobilized nitrogen from 1-year-old leaves and the leaves that were eventually to fall was estimated. If it is assumed that nitrogen from old leaves and those about to fall are translocated to new leaves on the same shoot, the share of the remobilized nitrogen in the new leaves can be estimated. Such values were large for the shoots that were in low RPFD, while most of leaf nitrogen in the shoots in high RPFD was from other sources. Obviously, vigorous shoots in high light attracted more nitrogen from other sources. So the leaves of different ages, even on the same shoot, should be dealt with separately.
Nitrogen optimization theory and the tree architectural models
Systems governing matter flow in trees are complicated and the translocation and re-translocation costs of nitrogen appear to be expensive. Further studies are needed in this field. Reassessment of the pipe model theory could be a breakthrough.
The pipe model theory, which was originally proposed by Shinozaki et al. (1964a, b), has been extensively used for examining the architecture of non-photosynthetic organs of a tree. This theory claims that, for a tree or a forest canopy, the cumulated dry mass of the leaves above the height z is proportional to the sum of cross-sectional areas of the branches at the height z. Although the statement is very simple, many studies have supported this theory.
However, physiological bases of the pipe model theory are not necessarily clear. In some studies, the pipe is considered literally as the water conducting system, and structure and allometry of trees were studied based on analyses of the water-conducting system (Tyree and Ewers, 1991; West et al., 1999; Magnani et al., 2000). However, the light factor, which exerts crucial effects on photosynthesis and transpiration, has not been analysed.
The pipe model theory was used to understand the construction mechanisms of trees of Acer mono var. marmoratum f. dissetum and A. rufinerve Sieb. et Zucc. (Sone et al., 2005). In particular, the role of the light factor in tree architecture was examined. In these Acer species, the growth of the stem or trunk occurs well after the full expansion of the current year leaves (Komiyama et al., 1987). Thus, the growth of non-photosynthetic organs would be mostly attributed to the current-year photosynthetic production. Then, it was possible to deal separately with the effect of current-year photosynthetic production on wood growth.
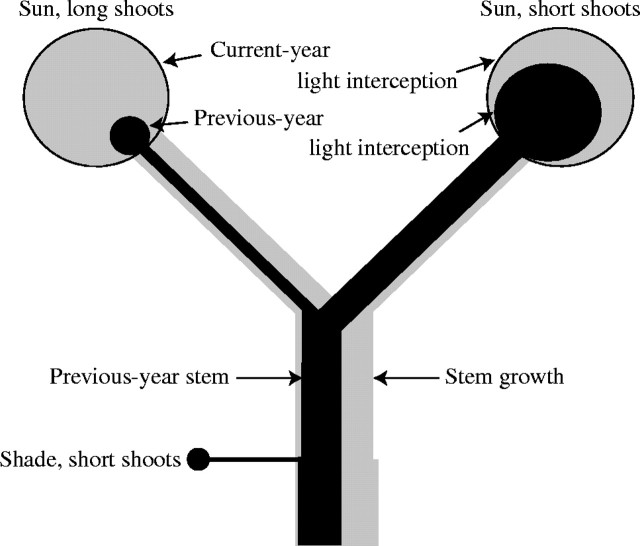
There is a strong relationship between cumulated leaf mass and cross-sectional area of the shoot for the shoots from various positions of a tree. This clearly indicates that the pipe model theory is relevant in analysing architecture of non-photosynthetic organs including very young shoots. Interestingly, there were no clear relationships between the cumulated light interception of the shoot and its current-year cross-sectional growth, ΔAb, taken at various positions within a tree. However, when the same was made for a particular branch, there was a strong relationship between the shoot light interception and ΔAb. The branches that showed larger ΔAb for the same shoot light interception were found to have many long shoots, while those that showed smaller ΔAb had mostly short shoots (data not shown). These results indicate that branches having many long shoots in the sun use their photosynthates preferentially, while branches having more short shoots allocate more photosynthates to lower organs (Fig. 5; see also Terashima et al., 2002; Sone et al., 2005). Long/short shoot differentiation was thus crucial and this could be related to the branch order (Suzuki, 2002, 2003).
Fig. 5.
A model for branch growth and carbon allocation. Areas of circles indicate cumulated light interceptions by the branches. The shaded and solid circles indicate cumulated light interceptions by the branches in the current year and previous year, respectively. Shaded parts of the stems indicate the wood produced in the current year. Solid parts of the stems denote the wood existed in the previous year. An original drawing by K. Sone.
It has been shown that light interception and branch priority are most important factors governing tree architecture. Nitrogen translocation problems should be studied in relation to these factors.
CONCLUDING REMARKS
The paper by Monsi and Saeki (1953) is important not only for practical use, but also as the source of various challenging problems. In one sense, therefore, their study was provocative. The same can be said for the nitrogen optimization theory. Through numerous studies based on the nitrogen optimization theory for nearly 20 years, various key physiological problems have been identified. However, in understanding tree architecture, various difficulties are encountered that will not be solved solely by modification of the nitrogen optimization theory. Perhaps, other provocative models should be constructed to overcome these difficulties. It is proposed that the branch autonomy/priority model based on the differential version of the pipe model theory is a useful framework that accommodates the nitrogen optimization theory as its sub-model.
Acknowledgments
Critical reading of earlier versions of this review by Drs Ko Noguchi, Sachiko Funayama-Noguchi and Arata Antonio Suzuki is gratefully acknowledged. We dedicate this review to Professor Emeritus Toshiro Saeki who passed away on 15 April 2004, aged 76. The studies in our laboratory have been supported by grants from the Japanese Society for the Promotion of Science.
Footnotes
Present address: Centre for Forest Biology, Department of Biology, University of Victoria, PO Box 3020, Victoria, BC, Canada V8W 3N5.
Present address: Department of Bio-environmental Science, National Institute for Basic Biology, Myodaiji, Okazaki 444-8585, Japan.
LITERATURE CITED
- Ackerly DD. 1992. Light, leaf age and nitrogen concentration in a tropical vine. Oecologia 89: 596–600. [DOI] [PubMed] [Google Scholar]
- Anderson JM. 1986. Photoregulation of the composition, function, and structure of thylakoid membranes. Annual Review of Plant Physiology 37: 93–136. [Google Scholar]
- Anten NPR, Hikosaka K, Hirose T. 2000. Nitrogen utilisation and the photosynthetic system. In: Marshall B, Roberts JA, eds. Leaf development and canopy growth. Sheffield: Sheffield Academic Press, 171–203. [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP. 2002. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo Plant Physiology 130: 1992–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman O. 1981. Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, eds. Physiological plant ecology. I. Responses to the physical environment. Berlin: Springer-Verlag, 57–107. [Google Scholar]
- Boardman NK. 1977. Comparative photosynthesis of sun and shade plants. Annual Review of Plant Physiology 28: 355–377. [Google Scholar]
- Cooper GJ, Boron WF. 1998. Effects of PCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its 189S mutant. American Journal of Physiology 275: C1481–1486. [DOI] [PubMed] [Google Scholar]
- Crawford NM. 1995. Nitrate: nutrient and signal for plant growth. Plant Cell 7: 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai N, Schaffer A, Petreikov M, Shahak Y, Giller Y, Ratner K, Levine A, Granot D. 1999. Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell 11: 1253–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfine S, Alvino A, Villani MC, Loreto F. 1999. Restrictions to CO2 conductance and photosynthesis in spinach leaves recovering from salt stress. Plant Physiology 119: 1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfine S, Alvino A, Zacchini M, Loreto F. 1998. Consequences of salt stress on conductance to CO2 diffusion, Rubisco characteristics and anatomy of spinach leaves. Australian Journal of Plant Physiology 25: 395–402. [Google Scholar]
- Dijkwel PP, Huijser C, Weisbeek PJ, Chua N-H, Smeekens SCM. 1997. Sucrose control of phytochrome A signaling in Arabidopsis Plant Cell 9: 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelmann H, Laisk A. 1999. Ribulose-1,5-bisphosphate carboxylase/oxygenase content, assimilatory charge, and mesophyll conductance in leaves. Plant Physiology 119: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19. [DOI] [PubMed] [Google Scholar]
- Evans JR, Loreto F. 2000. Acquisition and diffusion of CO2 in higher plant leaves. In: Leegood RC, Sharkey TD, von Caemmerer S. eds, Photosynthesis: physiology and metabolism. Dordrecht: Kluwer Academic Publishers, 321–351. [Google Scholar]
- Evans JR, Seemann JR. 1989. The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences and control. In: Briggs WR, ed. Photosynthesis. New York: Alan R. Liss, 183–205. [Google Scholar]
- Evans JR, Terashima I. 1988. Photosynthetic characteristics of spinach leaves grown with different nitrogen treatments. Plant and Cell Physiology 29: 157–165. [Google Scholar]
- Evans JR, Vogelmann TC. 2003. Profiles of 14C fixation through spinach leaves in relation to light absorption and photosynthetic capacity. Plant, Cell & Environment 26: 547–560. [Google Scholar]
- Farquhar GD. 1989. Models of integrated photosynthesis of cells and leaves. Philosophical Transactions of the Royal Society of London, Series B. Biological Sciences 323: 357–367. [Google Scholar]
- Field C. 1983. Allocating leaf nitrogen for the maximization of carbon gain: leaf age as a control on the allocation program. Oecologia 56: 341–347. [DOI] [PubMed] [Google Scholar]
- Field C. 1991. Ecological scaling of carbon gain to stress and resource availability. In: Mooney HA, Winner WE and Pell EJ, eds. Response of plants to multiple stresses. Cambridge: Cambridge University Press, 35–65. [Google Scholar]
- Field C, Mooney HA. 1986. The photosynthesis–nitrogen relationship in wild plants. In: Givnish TJ, ed. On the economy of form and function. Cambridge: Cambridge University Press, 25–55. [Google Scholar]
- Fujita Y. 1997. A study on the dynamic features of photosystem stoichiometry: accomplishments and problems for future studies. Photosynthesis Research 53: 83–93. [Google Scholar]
- Flexas J, Bota J, Escalona JM, Sampol B, Medrano H. 2002. Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Functional Plant Biology 29: 461–471. [DOI] [PubMed] [Google Scholar]
- Fujiki Y, Ito M, Nishida I, Watanabe A. 2001. Multiple signaling pathways in gene expression during sugar starvation. Pharmacological analysis of din Gene expression in suspension-cultured cells of Arabidopsis Plant Physiology 124: 1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GR, Savitch LV, Ivanov AG, Huner NPA. 1997. Cold acclimation and freezing tolerance—a complex interaction of light and temperature. Plant Physiology 114: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanba YT, Miyazawa S-I, Kogami H, Terashima I. 2001. Effects of leaf age on internal CO2 transfer conductance and photosynthesis in tree species having different types of shoot phenology. Australian Journal of Plant Physiology 28: 1075–1084. [Google Scholar]
- Hanba YT, Miyazawa S-I, Terashima I. 1999. Influences of leaf thickness on internal resistance to CO2 diffusion and δ13C in leaf dry matter. Functional Ecology 13: 632–639. [Google Scholar]
- Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M. 2004. Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant and Cell Physiology 45: 521–529. [DOI] [PubMed] [Google Scholar]
- Hidema J, Makino A, Mae T, Ojima K. 1991. Photosynthetic characteristics of rice leaves under different irradiances from full expansion through senescence. Plant Physiology 97: 1287–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hihara Y, Sonoike K. 2001. Regulation, inhibition and protection of photosystem I. In: Aro E-M, Andersson B. eds. Regulation of photosynthesis. Dordrecht: Kluwer, 507–531. [Google Scholar]
- Hikosaka K. 1996. Effects of leaf age, nitrogen nutrition and photon flux density on the organization of the photosynthetic apparatus in leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading. Planta 198: 144–150. [DOI] [PubMed] [Google Scholar]
- Hikosaka K. 2005. Leaf canopy as a dynamic system: ecophysiology and optimality in leaf turnover. Annals of Botany 95: 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K, Terashima I. 1995. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant, Cell & Environment 18: 605–618. [Google Scholar]
- Hikosaka K, Terashima I, Katoh S. 1994. Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading. Oecologia 97: 451–457. [DOI] [PubMed] [Google Scholar]
- Hirose T. 2005. Development of the Monsi–Saeki theory on canopy structure and function. Annals of Botany 95: 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Werger MJA. 1987. Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 72: 520–526. [DOI] [PubMed] [Google Scholar]
- Hirose T, Werger MJA, Pons TL, Rheenen JWA. 1988. Canopy structure and leaf nitrogen distribution in a stand of Lysimachia vulgaris L. as influenced by stand density. Oecologia 77: 145–150. [DOI] [PubMed] [Google Scholar]
- Hirose T, Werger MJA, Rheenen JWA. 1989. Canopy development and leaf-nitrogen distribution in a stand of Carex acutiformis Ecology 70: 1610–1618. [Google Scholar]
- Hollinger DY. 1996. Optimality and nitrogen allocation in a tree canopy. Tree Physiology 16: 627–634. [DOI] [PubMed] [Google Scholar]
- Huner NPA, Öquist G, Sarfan F. 1998. Energy balance and acclimation to light and cold. Trends in Plant Science 3: 224–230. [Google Scholar]
- James SA, Smith WK, Vogelmann TC. 1999. Ontogenetic differences in mesophyll structure and chlorophyll distribution in Eucalyptus globulus ssp. globulus (Myrtaceae). American Journal of Botany 86: 198–207. [PubMed] [Google Scholar]
- Jones HG. 1992.Plant and microclimate. Cambridge: Cambridge University Press. [Google Scholar]
- Jordi W, Schapendonk A, Davelaar E, Stoopen GM, Pot CS, De Visser R, Van Rhijn JA, Gan S, Amasino RM. 2000. Increased cytokinin levels in transgenic PSAG12-IPT tobacco plants have large direct and indirect effects on leaf senescence, photosynthesis and N partitioning. Plant, Cell & Environment 23: 279–289. [Google Scholar]
- Kimura K, Ishida A, Uemura A, Matsumoto Y, Terashima I. 1998. Effects of current-year and pevious-year PPFDs on shoot gross morphology and leaf properties in Fagus crenata Tree Physiology 18: 459–466. [DOI] [PubMed] [Google Scholar]
- King D. 1990. Allometry of saplings and understorey trees of a Panamanian forest. Functional Ecology 4: 27–32. [Google Scholar]
- Kitajima K, Hogan KP. 2003. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant, Cell & Environment 26: 857–865. [DOI] [PubMed] [Google Scholar]
- Kjellbom P, Karlsson C, Johansson I, KarlssonM, Johanson U. 1999. Aquaporins and water homeostasis in plants. Trends in Plant Science 4: 308–314. [DOI] [PubMed] [Google Scholar]
- Koch KE. 1996. Carbohydrate-modulated gene expression in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 509–540. [DOI] [PubMed] [Google Scholar]
- Koch KE, Ying Z, Wu Y, Avigne WT. 2000. Multiple paths ofsugar-sensing and a sugar/oxygen overlap for genes of sucrose and ethanol metabolisms. Journal of Experimental Botany 51: 417–427. [DOI] [PubMed] [Google Scholar]
- Kogami H, Hanba YT, Kibe T, Terashima I, Masuzawa T. 2001. CO2 transfer conductance, leaf structure and carbon isotope composition of Polygonum cuspidatum leaves from low and high altitudes. Plant, Cell & Environment 24: 529–538. [Google Scholar]
- Komiyama A, Inoue S, Ishikawa T. 1987. Characteristics of the seasonal diameter of twenty-five species of deciduous broad-leaved trees. Japanese Journal of Forest Science 69: 379–385. [Google Scholar]
- Körner C, Farquhar GD, Roksandic Z. 1988. A global survey of carbon isotope discrimination in plants from high altitude. Oecologia 88: 30–40. [DOI] [PubMed] [Google Scholar]
- Krapp A, Stitt M. 1995. An evaluation of direct and indirect mechanisms for the ‘sink-regulation’ of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195: 313–323. [Google Scholar]
- Kuroiwa S. 1971. Total photosynthesis of a foliage in relation to inclination of leaves. In: Setlik I, ed. Prediction and measurement of photosynthetic productivity. Wageningen: Pudoc, 79–89. [Google Scholar]
- Lake JA, Quick WP, Beerling DJ, Woodward FI. 2001. Signal from mature to new leaves. Nature 411: 154. [DOI] [PubMed] [Google Scholar]
- Lauteri M, Scartazza A, Guido MC, Brugnoli E. 1997. Genetic variation in photosynthetic capacity, carbon isotope discrimination and mesophyll conductance in provenances of Castanea sativa adapted to different environments. Functional Ecology 11: 675–683. [Google Scholar]
- Magnani F, Mencuccini M, Grace J. 2000. Age-related differences in stand productivity: the role of structural acclimation under hydraulic constraints. Plant, Cell & Environment 23: 251–263. [Google Scholar]
- Makino A, Nakano H, Mae T. 1994. Responses of ribulose-1,5-bisphosphate carboxylase, cytochrome f, and sucrose synthesis enzymes in rice leaves to leaf nitrogen and their relationships to photosynthesis. Plant Physiology 105: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Shimada T, Takumi S, Kaneko K, Matsuoka M, Shimamoto K, Nakano H, Miyao-Tokutomi M, Mae T, Yamamoto N. 1997. Does decrease in ribulose-1,5-bisphosphate carboxylase by antisense RbcS lead to a higher N-use efficiency of photosynthesis under conditions of saturating CO2 and light in rice plants? Plant Physiology 114: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C. 1997. Aquaporins and water permeability of plant membranes. Annual Review of Plant Physiology and Plant Molecular Biology 48: 399–429. [DOI] [PubMed] [Google Scholar]
- Meidner H, Mansfield TA. 1968.Physiology of stomata. London: McGraw-Hill. [Google Scholar]
- Miyazawa S-I, Terashima I. 2001. Slow chloroplast development in the evergreen broad-leaved tree species: relationship between leaf anatomical characteristics and photosynthetic rate during leaf development. Plant, Cell & Environment 24: 279–291. [Google Scholar]
- Miyazawa S-I, Makino A, Terashima I. 2003. Changes in mesophyll anatomy and sink-source relationships during leaf development in Quercus glauca, an evergreen tree showing delayed leaf greening. Plant, Cell & Environment 26: 745–755. [Google Scholar]
- Miyazawa S-I, Suzuki AA, Sone K, Terashima I. 2004. Relationship between light, leaf nitrogen and nitrogen remobilization in the crowns of mature evergreen trees, Quercus glauca Tree Physiology 24: 1157–1164. [DOI] [PubMed] [Google Scholar]
- Monsi M, Saeki T. 1953. Über den Lichtfaktor in den Pflanzengesellschaften und seine Bedeutung für die Stoffproduktion. Japanese Journal of Botany 14: 22–52. [Google Scholar]
- Monsi M, Uchijima Z, Oikawa T. 1973. Structure of foliage canopies and photosynthesis. Annual Review of Ecology and Systematics 4: 301–327. [Google Scholar]
- Muramatsu M, Hihara Y. 2003. Transcriptional regulation of genes encoding subunits of photosystem I during acclimation to high-light conditions in Synechocystis sp. PCC 6803. Planta 216: 446–453. [DOI] [PubMed] [Google Scholar]
- Nakhoul NL, Davis BA, Romero MF, Boron WF. 1998. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. American Journal of Physiology 274C: 543–548. [DOI] [PubMed] [Google Scholar]
- Oguchi R, Hikosaka K, Hirose T. 2003. Does the photosynthetic light-acclimation need change in leaf anatomy? Plant, Cell & Environment 26: 505–512. [Google Scholar]
- Okada K, Katoh S. 1998. Two long-term effects of light that control the stability of proteins related to photosynthesis during senescence of rice leaves. Plant and Cell Physiology 39: 394–404. [Google Scholar]
- Okada K, Inoue Y, Satoh K, Katoh S. 1992. Effects of light on degradation of chlorophyll and proteins during senescence of detached rice leaves. Plant and Cell Physiology 33: 1183–1191. [Google Scholar]
- Ono K, Watanabe A. 1997. Levels of endogenous sugars, transcripts of rbcS and rbcL, and RuBisCO protein in sunflower leaves. Plant and Cell Physiology 38: 1032–1038. [Google Scholar]
- Ono K, Nishi Y, Watanabe A, Terashima I. 2001. Possible mechanisms of adaptive leaf senescence. Plant Biology 3: 234–243. [Google Scholar]
- Ono K, Terashima I, Watanabe A. 1996. Interaction between nitrogen deficit of a plant and nitrogen content in the old leaves. Plant and Cell Physiology 37: 1083–1089. [Google Scholar]
- Oja V, Laisk A. 1976. Adaptation of the photosynthetic apparatus to the profile of light in the leaf. Fiziologoa Rastenii 23: 445–451 [Soviet Plant Physiology 23: 381–386]. [Google Scholar]
- Parkhurst DF. 1994. Diffusion of CO2 and other gases inside leaves. New Phytologist 126: 449–479. [DOI] [PubMed] [Google Scholar]
- Parkhurst DF, Wong S-C, Farquhar GD, Cowan IR. 1988. Gradients of intercellular CO2 levels across the leaf mesophyll. Plant Physiology 86: 1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Driscoll SP. 1997. Sugar repression of photosynthesis: the role of carbohydrates in signaling nitrogen deficiency through source:sink imbalance. Plant, Cell & Environment 20: 110–116. [Google Scholar]
- Paul MJ, Stitt M. 1993. Effects of nitrogen and phosphorus deficiencies on levels of carbohydrates, respiratory enzymes and metabolites in seedlings of tobacco and their response to exogenous sucrose. Plant, Cell & Environment 16: 1047–1057. [Google Scholar]
- Pego JV, Kortstee AJ, Huijser C, Smeekens SCM. 2000. Photosynthesis, sugars and the regulation of gene expression. Journal of Experimental Botany 51: 407–416. [DOI] [PubMed] [Google Scholar]
- Pons TL, Bergkotte M. 1996. Nitrogen allocation in response to partial shading of a plant: possible mechanisms. Physiologia Plantarum 98: 571–577. [Google Scholar]
- Pons TL, Jordi W, Kuiper D. 2001. Acclimation of plants to light gradients in leaf canopies: evidence for a possible role for cytokinins transported in the transpiration stream. Journal of Experimental Botany 52: 1563–1574. [DOI] [PubMed] [Google Scholar]
- Radin JW, Eidenback MP. 1986. Carbon accumulation during photosynthesis in leaves of nitrogen and phosphorus-stressed cotton. Plant Physiology 82: 869–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodermel S. 2001. Pathway of plastid-to-nucleus signaling. Trends in Plant Science 6: 471–478. [DOI] [PubMed] [Google Scholar]
- Rousseaux MC, Hall AJ, Sánchex RA. 1996. Far-red enrichment and photosynthetically active radiation level influence leaf senescence in field-grown sunflower. Physiologia Plantarum 96: 217–224. [Google Scholar]
- Sakakibara H, Taniguchi M, Sugiyama T. 2000. His-Asp phosphorelay singaling: a communication avenue between plants and their environment. Plant Molecular Biology 42: 273–278. [DOI] [PubMed] [Google Scholar]
- Sakata T, Yokai Y. 2002. Analysis of the O2 dependency in leaf level photosynthesis of two Raynoutria japonica populations growing at different altitudes. Plant, Cell & Environment 25: 65–74. [Google Scholar]
- Sheen J. 1990. Metabolic repression of transcription in higher plants. Plant Cell 2: 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yoda K, Hozumi K, Kira T. 1964. A quantitative analysis of plant form—the pipe model theory. I. Basic analysis. Japanese Journal of Ecology 14: 133–139. [Google Scholar]
- Shinozaki K, Yoda K, Hozumi K, Kira T. 1964. A quantitative analysis of plant form—the pipe model theory. II. Further evidence of the theory and its application in forest ecology. Japanese Journal of Ecology 14: 133–139. [Google Scholar]
- Shoettle AS, Smith WK. 1998. Interrelationships among light, photosynthesis and nitrogen in the crown of mature Pinus contorta ssp. latifolia Tree Physiology 19: 13–22. [DOI] [PubMed] [Google Scholar]
- Smith H. 2000. Plant architecture and light signals. In: Marshall B, Roberts JA. eds. Leaf development and canopy growth. Sheffield: Sheffield Academic Press, 118–144. [Google Scholar]
- Smith H, Samson G, Fork DC. 1993. Photosynthetic acclimation to shade: probing the role of phytochromes using photomorphogenetic mutants of tomato. Plant, Cell & Environment 16: 929–937. [Google Scholar]
- Sone K, Noguchi K, Terashima, I. 2005. Dependency of branch diameter growth in young Acer trees on light intensity and shoot growth activity. Tree Physiology (in press). [DOI] [PubMed] [Google Scholar]
- Sugiharto B, Miyake K, Nakamoto H, Sasakawa H, Sugiyama T. 1990. Regulation of expression of carbon assimilating enzymes by nitrogen in maize leaf. Plant Physiology 92: 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surpin M, Larkin RM, Chory J. 2002. Signal transduction between the chloroplast and the nucleus. Plant Cell (Suppl.): S327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A. 2002. Influence of shoot architectural position on shoot growth and branching patterns in Cleyera japonica Tree Physiology 22: 885–890. [DOI] [PubMed] [Google Scholar]
- Suzuki AA. 2003. Shoot growth patterns in saplings of Cleyera japonica in relation to light and architectural position. Tree Physiology 23: 67–71 [DOI] [PubMed] [Google Scholar]
- Takei K, Takahashi T, Sugiyama T, Yamaya T, Sakakibara H. 2002. Multiple routes communicating nitrogen availability from roots to shoots: a signal transduction pathway mediated by cytokinin. Journal of Experimental Botany 53: 971–977. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Matsushima S, Kojyo S, Nitta H. 1969. Analysis of yield-determining process and its application to yield-prediction and culture improvement of lowland rice. XV. On the relation between the plant type of rice plant community and the light curve of carbon assimilation. Proceedings of Crop Science Society of Japan 38: 287–293. [Google Scholar]
- Terashima I. 1986. Dorsiventrality of photosynthetic light response curve of a leaf. Journal of Experimental Botany 37: 399–405. [Google Scholar]
- Terashima I. 1989. Productive structure of a leaf. In: Briggs W, ed. Photosynthesis. New York: Alan R. Liss, 207–226. [Google Scholar]
- Terashima I, Evans JR. 1988. Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant and Cell Physiology 29: 143–155. [DOI] [PubMed] [Google Scholar]
- Terashima I, Hikosaka K. 1995. Comparative ecophysiology/anatomy of leaf and canopy photosynthesis. Plant, Cell & Environment 18: 1111–1128. [Google Scholar]
- Terashima I, Inoue Y. 1985. Palisade tissue chloroplasts and spongy tissue chloroplasts in spinach: biochemical and ultrastructural differences. Plant and Cell Physiology 26: 63–75. [Google Scholar]
- Terashima I, Inoue Y. 1985. Vertical gradient in photosynthetic properties of spinach chloroplasts dependent on intra-leaf light environment. Plant and Cell Physiology 26: 781–785. [Google Scholar]
- Terashima I, Kimura K, Sone K, Noguchi K, Ishida A, Uemura A, Matsumoto Y. 2002. Differential analyses of the effects of the light environment on development of deciduous trees: basic studies for tree growth modeling. In: Nakashizuka T, Matsumoto Y. eds. Diversity and interaction in a temperate forest community: Ogawa Forest Reserve of Japan. Tokyo: Springer, 187–200. [Google Scholar]
- Terashima I, Miyazawa S-I, Hanba YT. 2001. Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. Journal of Plant Research 114: 93–105. [Google Scholar]
- Terashima I, Ono K. 2002. Effects of HgCl2 on CO2 dependence of leaf photosynthesis: evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane. Plant and Cell Physiology 43: 70–78. [DOI] [PubMed] [Google Scholar]
- Terashima I, Saeki T. 1985. A new model for leaf photosynthesis incorporating the gradients of light environment and of photosynthetic properties of chloroplasts within a leaf. Annals of Botany 56: 489–499. [Google Scholar]
- Terashima I, Sakaguchi S, Hara N. 1986. Intra-leaf and intracellular gradients in chloroplast ultrastructure of dorsiventral leaves illuminated form the adaxial or abaxial side during their development. Plant and Cell Physiology 27: 1023–1031. [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu D-T, Bligny R, Maurel C. 2003. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425: 393–397. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Ewers FW. 1991. The hydraulic architecture of trees and other woody plants. New Phytologist 119: 345–360. [Google Scholar]
- Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R. 2003. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425: 734–737. [DOI] [PubMed] [Google Scholar]
- Uemura A, Ishida A, Nakano T, Terashima I, Tanabe H, Matsumoto Y. 2000. Acclimation of leaf characteristics of Fagus species to previous-year and current-year solar irradiances. Tree Physiology 20: 945–951. [DOI] [PubMed] [Google Scholar]
- Walters RG, Horton P. 1995. Acclimation of Arabidopsis thaliana to the light environment: regulation of chloroplast composition. Planta 197: 475–481. [DOI] [PubMed] [Google Scholar]
- Walters RG, Rogers JJM, Shephard F, Horton P. 1999. Acclimation of Arabidopsis thaliana to the light environment: the role of photoreceptors. Planta 209: 517–527. [DOI] [PubMed] [Google Scholar]
- Walters RG, Shephard F, Rogers JJM, Rolfe SA, Horton P. 2003. Identification of mutants of Arabidopsis defective in acclimation of photosynthesis to the light environment. Plant Physiology 131: 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LM, Amasino RM. 2001. Senescence is induced in individually darkened Arabidopsis leaves, but inhibited in whole darkened plants. Plant Physiology 127: 876–886. [PMC free article] [PubMed] [Google Scholar]
- West GB, Brown JH, Enquist BJ. 1999. A general model for the structure and allometry of plant vascular systems. Nature 400: 664–667. [Google Scholar]
- Weston E, Thorogood K, Vinti G, López-Juez E. 2000. Light quantity controls leaf-cell and chloroplast development in Arabidopsis thaliana wild type and blue-light-perception mutants. Planta 211: 807–815. [DOI] [PubMed] [Google Scholar]
- Wingler A, von Schaewen A, Leegood RC, Lea PJ, Quick WP. 1998. Regulation of leaf senescence by cytokinin, sugars and light. Effects on NADH-dependent hydroxypyruvate reductase. Plant Physiology 116: 329–335. [Google Scholar]
- Yang B, Fukuda N, Van Hoek A, Matthay MA, Ma T, Verkman AS. 2000. Carbon dioxide permeability of aquaporin-1 measured in erythrocytes and lung of aquaporin-1 nul mice and in reconstituted proteoliposomes. Journal of Biological Chemistry 275: 2686–2692. [DOI] [PubMed] [Google Scholar]
- Yano S, Terashima I. 2001. Separate localization of light signal perception for sun or shade type chloroplast and palisade tissue differentiation in Chenopodium album Plant and Cell Physiology 42: 1303–1310. [DOI] [PubMed] [Google Scholar]
- Yano S, Terashima I. 2004. Developmental process of sun and shade leaves in Chenopidium album L. Plant, Cell & Environment 27: 781–793. [Google Scholar]