Abstract
• Background and Aims In a leaf canopy, there is a turnover of leaves; i.e. they are produced, senesce and fall. These processes determine the amount of leaf area in the canopy, which in turn determines canopy photosynthesis. The turnover rate of leaves is affected by environmental factors and is different among species. This mini-review discusses factors responsible for leaf dynamics in plant canopies, focusing on the role of nitrogen.
• Scope Leaf production is supported by canopy photosynthesis that is determined by distribution of light and leaf nitrogen. Leaf nitrogen determines photosynthetic capacity. Nitrogen taken up from roots is allocated to new leaves. When leaves age or their light availability is lowered, part of the leaf nitrogen is resorbed. Resorbed nitrogen is re-utilized in new organs and the rest is lost with dead leaves. The sink–source balance is important in the regulation of leaf senescence. Several models have been proposed to predict response to environmental changes. A mathematical model that incorporated nitrogen use for photosynthesis explained well the variations in leaf lifespan within and between species.
• Conclusion When leaf turnover is at a steady state, the ratio of biomass production to nitrogen uptake is equal to the ratio of litter fall to nitrogen loss, which is an inverse of the nitrogen concentration in dead leaves. Thus nitrogen concentration in dead leaves (nitrogen resorption proficiency) and nitrogen availability in the soil determine the rate of photosynthesis in the canopy. Dynamics of leaves are regulated so as to maximize carbon gain and resource-use efficiency of the plant.
Keywords: Canopy photosynthesis, canopy structure, cost–benefit analysis, evolutionarily stable strategy, leaf area index, leaf lifespan, leaf senescence, leaf turnover, nitrogen resorption, nitrogen turnover, nitrogen use efficiency, optimality model
INTRODUCTION
At the ecosystem level, leaf canopy is the unit of photosynthesis. Photosynthetic rates of the canopy vary depending on climate and on structure and physiology of individuals in the canopy. Understanding factors that affect canopy photosynthesis would contribute to agriculture, ecology, meteorology, and global science.
Leaf area distribution is an important determinant of rates of photosynthesis in the canopy (Monsi and Saeki, 1953). When the leaf area index (LAI; leaf area per unit ground area) increases, photon flux density (PFD) captured by the canopy increases, leading to higher photosynthetic production in the canopy. However, when leaves at the bottom receive PFD that is lower than the compensation point of photosynthesis, further increase in LAI decreases canopy photosynthesis. There is an optimal LAI that maximizes rates of photosynthesis (Monsi and Saeki, 1953; Saeki, 1960). Leaf nitrogen is another important factor for canopy photosynthesis. Since about half of leaf nitrogen is invested in photosynthetic proteins, there is a strong correlation between the photosynthetic capacity (the light-saturated rate of photosynthesis at an ambient condition) and leaf nitrogen content per unit area (Field and Mooney, 1986; Evans, 1989a). Thus, to have high rates of photosynthesis, canopies should accumulate a large amount of nitrogen in their leaves (Hirose and Werger, 1987b).
Neither LAI nor nitrogen content of the canopy is a static parameter, but changes dynamically with canopy growth. A leaf canopy can be regarded as a population of leaves (Harper, 1989). Leaves are produced and senesce with a certain lifespan. When the canopy is young, the number of produced leaves is larger than that of senescing leaves. When the canopy is mature the turnover of leaves may be close to steady state. Similarly, nitrogen is taken up from soil and is lost with dead leaves. Standing LAI and nitrogen content are a result of the turnover of leaves and nitrogen.
Here is a review of the dynamics of leaf area and nitrogen in leaf canopies. In the first part, two components of leaf turnover—leaf production and senescence—are discussed in relation to canopy photosynthesis. The discussion is focused, particularly, on the role of nitrogen. It has been argued that plants regulate leaf longevity so as to maximize their fitness (Chabot and Hicks, 1982; Kikuzawa, 1991). Then in the second part, leaf turnover is discussed with regard to its optimization.
ECOPHYSIOLOGY OF LEAF TURNOVER
Leaf production
Pattern of leaf production varies among species. Most annual herbs and many tropical pioneer trees produce their leaves continuously during vegetative growth (Ackerly, 1996; Kikuzawa, 2003), whereas many deciduous trees and shrubs produce leaves simultaneously at the beginning of the growing season without later leaf production (Kikuzawa, 1983, 2003). Kikuzawa (1983, 1984) categorized the pattern of leaf production into flush, succeeding and intermediate types. When species with similar growth forms are compared, the flush type is usually found in species with a longer leaf lifespan (Koike, 1988; Reich, 1993).
The rate of leaf area production, Gcan (m2 m−2 d−1) may be defined as follows:
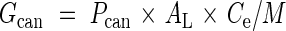 |
where Pcan is the rate of canopy photosynthesis per unit ground area (mol C m−2 d−1), AL is the fraction of assimilates allocated for leaf construction, Ce is the conversion efficiency from assimilates to biomass (g mol−1 C) and M is leaf mass per area (LMA) of new leaves (g m−2) (Ackerly, 1996; Hikosaka, 2003). Equation (1) assumes the succeeding type of leaf production, but it is also applicable to the flush type if the average production rate is calculated for a longer period than the interval of flushes. Note that this equation does not necessarily indicate the production rate of individual leaves. When plants produce leaves with a smaller size under less productive conditions, Gcan becomes lower even when the rate of individual leaf production is constant.
According to the canopy photosynthetic model, the rate of photosynthesis in the canopy (Pcan) is determined by three factors: vertical profile of PFD in the canopy, LAI and photosynthetic capacity of leaves in the canopy (Monsi and Saeki, 1953; see Hirose, 2005). AL is sensitive to changes in the environment of the plant. For example, nitrogen deficiency decreases AL with increased allocation of biomass to roots (Brouwer, 1962; Hirose, 1987; Wilson, 1988). This is regarded as an adaptive response to compensate for low rates of nitrogen uptake. As the plant height increases, the ratio of leaf to stem mass may decrease to maintain mechanical stability (McMahon, 1973; Givnish, 1982).
LMA is different among species. Higher LMA is found in species with an inherently slow growth rate (Poorter and Remkes, 1990; Poorter and Evans, 1998) and with a longer leaf lifespan (Reich et al., 1991, 1992, 1997, 1999), and may be a factor leading to lower productivity (see eqn 1). However, an increase in LMA has been shown to contribute to leaf toughness, suggesting that higher LMA is necessary to maintain leaves for a longer period (Reich et al., 1991; Wright and Cannon, 2001; Wright and Westoby, 2002). Within a species, LMA is sensitive to environmental conditions, especially to light availability. Sun leaves generally have a higher LMA than shade leaves to accomodate more chloroplasts per unit leaf area (Björkman, 1981; Terashima et al., 2001; Oguchi et al., 2003).
The inverse of Ce is the construction cost of a leaf. Construction cost has been estimated with several methods. One is based on the relationship between respiration and growth (Merino et al., 1982). When the specific respiration rate is plotted against the relative growth rate, the relationship is a linear function with a positive Y-intercept (Thornley, 1970; Kimura et al., 1978). The intercept gives the maintenance respiration rate and the slope the conversion efficiency from glucose (respiratory substrate) to biomass. The other method uses construction costs of leaves (glucose equivalent) calculated from the chemical composition (Penning de Vries et al., 1974; McDermitt and Loomis, 1982; Chapin, 1989) or determined from the heat of combustion (Williams et al., 1987). Many authors studied the interspecific variation in Ce to test the hypothesis that leaves with a longer lifespan may have a high Ce because of costly compounds for chemical defense or physical toughness (Coley et al., 1985). However, they failed to find a clear difference in Ce among leaves with different lifespans, or between evergreen and deciduous leaves. None of the following are correlated with leaf lifespan: the slope of the relationship between respiration and growth (Merino et al., 1982); the construction cost calculated from its chemical composition (Chapin, 1989); or the heat of combustion (Williams et al., 1989). Compiling data of the heat of combustion from 203 woody species, however, Poorter and Villar (1997) found a small but significant difference between evergreen and deciduous leaves. Similar results were obtained in a literature survey by Villar and Merino (2001). Navas et al. (2003) also found a weak but significantly positive correlation between lifespan and construction cost of leaves among Mediterranean species. Similar values of construction cost in leaves with a different leaf lifespan may have come from a trade-off between costly chemicals. Leaves with a longer lifespan tend to accumulate carbon-based secondary compounds such as phenolics, while those with a shorter lifespan contain a large amount of proteins (Chapin, 1989; Poorter and Villar, 1997).
Nitrogen allocation to leaves
Nitrogen is one of the elements most limiting plant growth in many ecosystems (Aerts and Chapin, 2000). This is partly because plants need a large amount of nitrogen for photosynthesis. About half of leaf nitrogen is invested in the photosynthetic apparatus (Evans and Seemann, 1989; Hikosaka and Terashima, 1996). Within a species, there is a strong correlation between the photosynthetic capacity and nitrogen content of a leaf (Makino et al., 1983; Hirose and Werger, 1987a; Evans, 1989a) and the rate of photosynthesis in a canopy increases as its nitrogen content increases (Hirose and Werger, 1987b).
Within a canopy, plants allocate nitrogen among leaves such that leaves exposed to higher PFDs have higher nitrogen contents (Field, 1983; Hirose and Werger, 1987b; Hirose et al., 1988; Hollinger, 1989; Ellsworth and Reich, 1993; Niinemets, 1997; Osada et al., 2003). At a high PFD, a greater investment of nitrogen increases photosynthetic production while, at a low PFD, rates of photosynthesis respond little to nitrogen content (Hirose and Werger, 1987a). Thus as PFD increases the optimal nitrogen content that maximizes photosynthetic productivity per unit nitrogen increases (Hirose and Werger, 1987a; Hikosaka and Terashima, 1995; Terashima and Hikosaka, 1995). Theoretical models have shown that a non-uniform allocation of leaf nitrogen leads to efficient use of nitrogen in canopy photosynthesis (Pons et al., 1989; Schieving et al., 1992; Evans, 1993; Anten et al., 1995a).
The slope of the photosynthesis–nitrogen relationship is different among species (Field and Mooney, 1986; Evans, 1989a). Since leaf nitrogen content per unit area is not very different among species (Reich et al., 1991, 1992, 1999), the slope, which is intimately related to the photosynthetic nitrogen use efficiency (PNUE, photosynthetic capacity per unit leaf nitrogen), is the most important factor for the interspecific difference in photosynthetic capacity. PNUE has been studied as an inherent trait of species (Pons et al., 1994). It has been shown that less steep slopes of the photosynthesis–nitrogen relationship tend to be found in tree species rather than in herbs (Evans, 1989a; Hikosaka et al., 1998), in C3 than in C4 species (Sage and Pearcy, 1987; Anten et al., 1995a), in slow-growing species (Poorter et al., 1990; Pons et al., 1994), in species with a long leaf lifespan (Reich et al., 1992), in species inhabiting high altitudes (Westbeek et al., 1999; Hikosaka et al., 2002), and in species with a high LMA (Poorter and Evans, 1998). Variation in the slope is caused by several factors such as nitrogen allocation to the photosynthetic apparatus, the specific activity of photosynthetic enzymes, and conductance for CO2 diffusion from air to chloroplasts (Lloyd et al., 1992; Hikosaka et al., 1998; Poorter and Evans, 1998; Westbeek et al., 1999).
Why do some species have a low PNUE? A negative relationship between PNUE and LMA implies that investments in physical toughness sacrifices PNUE (Reich et al., 1991; Pons et al., 1994; Hikosaka et al., 1998). Recently, Takashima et al. (2004) determined nitrogen allocation in leaves of evergreen and deciduous Quercus species. They found that evergreen species allocate more nitrogen to cell wall proteins at the expense of nitrogen allocation to the photosynthetic apparatus. Onoda et al. (2004) found that Polygonum cuspidatum, a perennial herb, alters nitrogen allocation in leaves depending on the germination time such that early germinators invest more nitrogen in cell wall proteins and less to photosynthetic proteins. In both studies, the fraction of leaf nitrogen allocated to cell walls was positively correlated with leaf mass per area. There may be a trade-off in nitrogen partitioning between the photosynthetic apparatus and cell wall proteins.
Leaf senescence and shedding
Leopold (1961) categorized leaf senescence into four types: (1) ‘over-all senescence’ in monocarpic species, in which the entire individual dies in an abrupt manner; (2) ‘top senescence’ in perennial herbs, in which the whole above-ground part dies; (3) ‘deciduous senescence’ in deciduous woody species, in which only leaves dies; and (4) ‘progressive senescence’ in the growing season when younger leaves are still active. The first three types are strategies to avoid stress in a non-favourable season. In the following the progressive senescence is discussed.
The progressive senescence has been studied intensively for herbaceous species. In many herbaceous plants photosynthetic capacity and nitrogen content of a leaf are highest at its full expansion and thereafter decline linearly with time (Thomas and Stoddart, 1980; Smart, 1994). Parallel changes in photosynthetic capacity and nitrogen content are caused by the photosynthetic proteins that are degraded through senescence with a constant specific activity (Makino et al., 1983). Degraded nitrogenous compounds, mainly amino acids, are translocated to other organs, leading to reduction in the leaf nitrogen content (Makino et al., 1983; Hidema et al., 1991; Hikosaka, 1996). Thus it is widely accepted that leaf senescence is accompanied by recycling of nitrogen within a plant (Thomas and Stoddart, 1980; Smart, 1994).
Plants alter the rate of leaf senescence depending on growth environment. Light is one of factors responsible for regulation of leaf senescence. According to the theory of nitrogen allocation among leaves, retranslocation of nitrogen from shaded to sunlit leaves will increase photosynthetic gain of the whole plant. Several studies have tested the hypothesis that shading a leaf accelerates retranslocation of nitrogen from the leaf. Hirose et al. (1988) established stands of Lysimachia vulgaris, a perennial herb, with different plant densities and found that the vertical gradient of leaf nitrogen content was steeper in the dense stand, suggesting that mutual shading accelerated retranslocation of nitrogen. Several experiments in which parts of leaves were artificially shaded showed that nitrogen is retranslocated from shaded to unshaded leaves (Evans, 1989b; Hikosaka et al., 1994; Pons and Pearcy, 1994; Ackerly and Bazzaz, 1995).
The effect of shading is not simple because shading of the whole plant delays leaf senescence (Hidema et al., 1991; Ono et al., 1996; Terashima et al., 2005), which seems to contradict the studies above. Hikosaka (1996) suggested that leaf senescence due to shading is affected by the manner of shading, i.e. whether part or all of leaves are shaded. Different nitrogen demand in sink organs influences the pattern of leaf senescence. Retranslocation of nitrogen from old leaves is affected not only by physiological status of the leaf but also by development of sink organs; when plants are growing fast, much nitrogen is required for construction of new tissues, which accelerates senescence in old leaves (Nambias and Fife, 1987; Ono et al., 1996). When all leaves of the plant are shaded, plant growth is limited by light. Retranslocation of nitrogen from older leaves may be slow owing to low sink activities. When part of leaves of a plant is shaded with the other leaves receiving strong light, higher plant growth rates are maintained by photosynthesis of the latter. In this case, nitrogen retranslocation is accelerated with great demand of nitrogen in developing organs (Fig. 1).
Fig. 1.
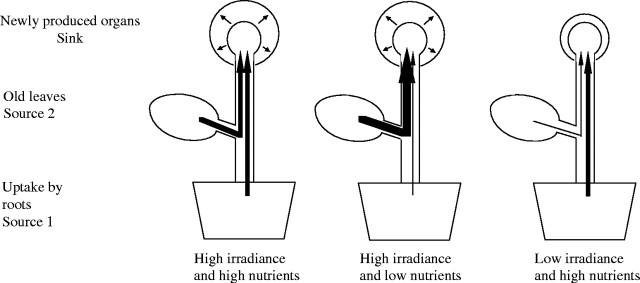
Sink and source relationship for nitrogen in plants. Developing organs require nitrogen, which is supplied either from nitrogen uptake in roots (source 1) or retranslocation from old leaves (source 2). When the plant is growing at high irradiance and high nutrient availability, nitrogen is supplied from the two sources. Retranslocation from old leaves is accelerated when nutrient supply is limited, and lowered when irradiance is low.
This idea may be supported by several branch-level studies. Stoll and Schmid (1998) determined branch growth of trees at the edge and at the centre of a tree patch. Branch growth was greatest in sun branches and, interestingly, shade branches of the trees at the edge had lower growth rates than shade branches of the trees at the centre, though the former received higher PFDs. Thus branch growth was not a simple function of PFD. Takenaka (2000) studied the relationship between the mortality rate and PFD in branches of plants growing at different PFDs. When compared among branches that receive similar PFDs, the mortality rate of the branch was higher in plants growing at higher PFDs. These results have been interpreted as an optimal foraging strategy; plants may allocate more resources to branches in favourable conditions at the expense of growth and survivorship of branches in unfavourable conditions.
Nitrogen deficiency is known to accelerate leaf senescence (Thomas and Stoddart, 1980; Makino et al., 1983, 1984; Guitman et al., 1991; Smart, 1994). This is also related to nitrogen demand in sink organs. For production of new tissues, plants have two sources of nitrogen. One is uptake by roots and the other is retranslocation from old organs (Fig. 1). If rates of nitrogen uptake in roots are low, sink organs may accelerate senescence in old leaves to satisfy their nitrogen demand. Ono et al. (1996) showed that nitrogen deficiency in new organs varies with sink development and nitrogen uptake rates, and that the rate of leaf senescence is well correlated with nitrogen deficiency.
Several ecological studies have shown that the leaf lifespan is longer in plants growing under lower nutrient availability (see Aerts and Chapin, 2000), which seems to contradict the results from physiological studies. This may be explained by different regimes of nitrogen deficiency (Hikosaka, 2003). Ecologists usually use field plants where nitrogen turnover is nearly at a steady state, while physiological experiments often subject plants to a sudden change in nitrogen supply. Plants growing under chronic nitrogen deficiencies have a lower productivity, which leads to a smaller demand for nitrogen in sink organs. On the other hand, when plants that have been under high nitrogen availability are subjected to a sudden reduction in nitrogen supply, nitrogen becomes deficient in the growing organs. This will accelerate nitrogen translocation from old organs. Mutual shading may also be responsible for the different results between ecological and physiological studies in relation to response to nutrient deficiency. In plants under chronic nitrogen deficiency LAI is small (Aerts and de Caluwe, 1994a; Anten et al., 1995b), which lowers mutual shading and may thus retard leaf senescence.
Leaf ageing has a significant effect on photosynthesis even when environmental factors do not change. Hikosaka et al. (1994) studied leaf senescence in the vine Ipomoea tricolor grown horizontally to avoid mutual shading. When all leaves were exposed to full sunlight, allocation of leaf nitrogen was affected by nutrient availability. At a lower nitrogen availability nitrogen was retranslocated from old to new leaves, leading to a steeper gradient of leaf nitrogen content, while at a high nitrogen availability the oldest leaves kept a high nitrogen content, which was comparable to that of new leaves. High nitrogen content in old leaves did not mean a high photosynthetic capacity in this case. Hikosaka (1996) determined nitrogen allocation within leaves of I. tricolor. Old leaves in plants with high nitrogen availability without any shading maintained a high nitrogen content but had smaller amounts of photosynthetic proteins such as ribulose-1,5-bisphosphate carboxylase, cytochrome f and photosystems, leading to low photosynthetic capacities. This result suggests that photosynthetic capacity is not necessarily coupled with nitrogen content.
Patterns of leaf senescence in evergreen woody species are not the same as those in herbaceous species. First, in the temperate region, changes in leaf nitrogen contents are not simply age-dependent but affected by the seasonal environment. For example, in the understorey of deciduous forests, light availability is high and air temperature is low in winter. These environmental factors induce an increase in the leaf nitrogen content. Thus the leaf nitrogen content and photosynthetic capacity of evergreen understorey species show large fluctuations within a year (Kume and Ino, 1993; Skillman et al., 1996). Secondly, in evergreen trees with a longer leaf lifespan, decrease in photosynthetic capacity is not necessarily coupled with that in leaf nitrogen content. A parallel decline in photosynthetic capacity and leaf nitrogen content has been observed in some species (Field and Mooney, 1983; Kitajima et al., 1997), while there are also reports that photosynthetic capacity gradually decreases while nitrogen content remains relatively constant until the end of the life of a leaf (Kitajima et al., 2002, 2005; Escudero and Mediavilla, 2003). Causes of the decrease in photosynthetic capacity in such species have not been clarified.
There may be two factors regulating photosynthetic capacity; one is the age-dependent decrease in photosynthetic capacity and the other is the protein degradation controlled by the sink–source relationship (Fig. 1). In fast-growing plants, leaf senescence is almost always coupled with nitrogen re-allocation because the high growth rate accelerates retranslocation of nitrogen from an old leaf, which may occur faster than the age-dependent reduction in photosynthetic capacity of the leaf. Whether it is also the case for species with a long leaf lifespan is questionable, but Kikuzawa (2003) reported that photosynthetic capacity declined with time in a shoot of Fagus crenata, a flush-type deciduous tree, although the light environment did not change much throughout year.
Nitrogen resorption
When leaves senesce, some nitrogen in the leaf is resorbed while the other remaining in the leaf is lost from plants with shedding. This is also the case in species that maintain a high leaf nitrogen content until the end of the life of a leaf (Escudero and Mediavilla, 2003). Since nitrogen is a limiting element in most plants, recycling of nitrogen is beneficial. May and Killingbeck (1992) showed that plant growth is suppressed when nitrogen resorption is prevented. The nitrogen resorption efficiency is defined as the fraction of nitrogen resorbed from living leaves (Aerts and Chapin, 2000). The larger the nitrogen resorption efficiency is, the more nitrogen is reutilized by the plant. There has been much discussion on the factors that determine the nitrogen resorption efficiency. Some suggested that plants in lower nutrient availabilities have a higher nitrogen resorption efficiency (Boerner, 1984; Shaver and Melillo, 1984; Aerts and de Caluwe, 1994b), while many others did not find such relationships (Schlesinger et al., 1989; Chapin and Moilanen, 1991; Escudero et al., 1992a). From a literature survey, Aerts (1996) concluded that there is no consistent trend in nitrogen resorption efficiency against nitrogen availabilities.
Killingbeck (1996) argued that nitrogen resorption is determined by its end-point rather than the fraction of resorption. He defined the nitrogen resorption proficiency, which is the nitrogen concentration on a mass- or an area-basis of dead leaves. From the survey of various studies, he suggested that 0·7 % (mass-basis) or 0·5 g m−2 (area-basis) is the end-point and that plants with the nitrogen content in dead leaves higher than these values may indicate incomplete nitrogen resorption.
Several studies have argued that there is a certain amount of nitrogen that cannot be retranslocated (non-labile nitrogen), which is derived from the X-intercept in the linear regression of photosynthetic capacity and nitrogen content (Charles-Edwards et al., 1987; Anten et al., 1995a). This idea is supported by the fact that the same photosynthesis–nitrogen relationship holds across growth conditions and leaf ageing within a species. However, it is still uncertain whether the X-intercept actually represents non-labile nitrogen. Recently, Yasumura et al. (2004) showed, in three deciduous tree species, that the nitrogen content in dead leaves was higher than the X-intercept values.
LEAF TURNOVER AS A STRATEGY
Carbon balance, nitrogen use and leaf lifespan
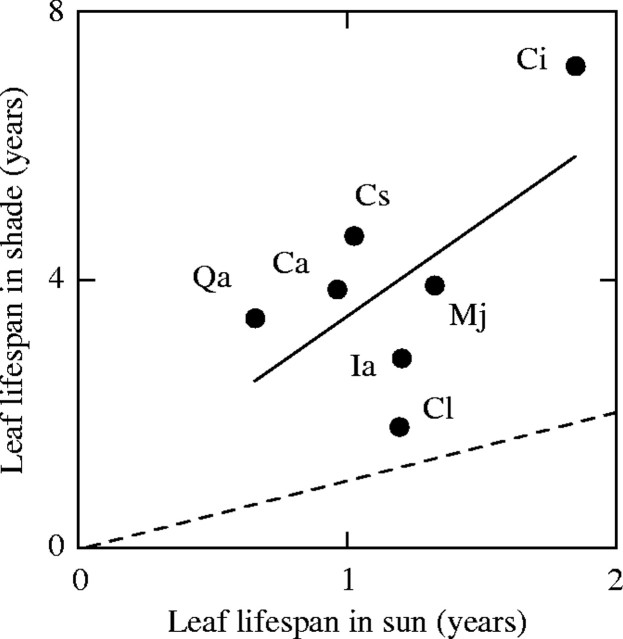
Leaf lifespan, an inverse of the turnover rate of leaves, varies among species by two orders of magnitude (Reich et al., 1992). A longer leaf lifespan tends to be found more in woody than in herbaceous species (Reich, 1993), in species living in habitats with poor nutrient availability (Aerts and Chapin, 2000), and in slow-growing species (Reich et al., 1992). Now it is widely accepted that there is a convergence of leaf traits; species with a longer leaf lifespan have a lower photosynthetic capacity per unit mass, a lower photosynthetic capacity per unit area, a lower nitrogen concentration per unit mass, and a higher LMA irrespective of life form, phylogeny and biomes (Reich et al., 1997, 1999; Wright et al., 2004), though the relationship slightly shifts depending on habitats (Reich et al., 1999; Wright et al., 2001, 2004). Within a species, leaf lifespan varies with growth environment. Many studies have shown that low growth irradiance extends leaf lifespan (Chabot and Hicks, 1982; Kikuzawa, 1988; Williams et al., 1989; Osada et al., 2001, 2003; Fig. 2).
Fig. 2.
Leaf lifespan of evergreen species at a forest edge (sun) and understorey (shade) Cs, Castanopsis sieboldii; Qa, Quercus acuta; Ca, Camellia japonica; Cl, Cleyera japonica; Ci, Cinnamomum japonicum; Ia, Illicium anisatum; Mj, Maesa japonica. Continuous and dotted line denote regression and 1 : 1 line, respectively (K. Hikosaka, unpubl. res.). For description of the site, see Hikosaka and Hirose (2000).
In 1980s, several authors incorporated a cost–benefit theory to analyse the variation in leaf lifespan (Chabot and Hicks, 1982; Mooney and Gulmon, 1982). A leaf needs to acquire a certain amount of carbon, at least, to construct one new leaf to maintain the number of leaves in the canopy. Thus leaves with lower rates of photosynthesis should have a longer lifespan to pay back the construction cost. Williams et al. (1989) determined photosynthesis, leaf construction cost, and leaf lifespan of seven Piper species in different light environments in a tropical rain forest. They found that the ratio of the construction cost to daily photosynthesis, which they called ‘payback time’, was positively correlated with actual leaf lifespan. Similar correlations have been observed in other studies (Sobrado, 1991; Emaus and Prichard, 1998; Navas et al., 2003; Oikawa et al., 2004). These results support the cost–benefit hypothesis.
Several authors noticed that the rate of photosynthesis per unit mass scales the −2/3 power of leaf longevity in the broad interspecific comparison conducted by Reich et al. (1999) (Westoby et al., 2000; Givnish, 2002). This implies that, if no reduction in leaf photosynthesis is assumed, a leaf with a longer lifespan has a greater life-time carbon gain that is the product of the rate and duration of photosynthesis. Givnish (2002) suggested that species with a longer leaf lifespan may need greater carbon gain than expected when one focuses on leaf performance only, because they invest more biomass in roots to survive in low-productive, highly infertile or dry habitats. Westoby et al. (2000) considered the effect of time discounting on the value of captured light or photosynthesis. Persistence of leaves for a longer period is disadvantageous due to overshading by surrounding vegetation or due to risk of damage such as herbivory. Furthermore, earlier carbon gain in a leaf may be more valuable because it can be reinvested in new leaves, resulting in a greater compound-interest (the opportunity–cost effect; for detail see also Harper, 1989). However, field studies have suggested that life-time carbon gain is not very different between leaves with different longevity (Chabot and Hicks, 1982; Diemer and Körner, 1996; Mediavilla and Escudero, 2003). Comparing Mediteranean evergreen species, Mediavilla and Escudero (2003) showed that the effect of the reduction in the photosynthetic capacity on life-time carbon gain was greater in species with a longer lifespan. Thus the time-discounting effect may mainly be ascribed to leaf senescence.
Leaf lifespan has been discussed in relation to nitrogen use in leaves. Small (1972) showed that deciduous species have a higher rate of photosynthesis, while evergreen species utilize nitrogen for a longer period. This idea was extended to a plant-level nitrogen use efficiency (NUE), defined as the biomass production per unit nitrogen taken up. NUE has been expressed as the product of nitrogen productivity (NP; biomass production per unit nitrogen in the plant body) and mean residence time of nitrogen (MRT) (Berendse and Aerts, 1987). MRT is a positive function of leaf lifespan (Escudero et al., 1992b; Aerts and Chapin, 2000). It was shown that deciduous species have a higher NP, whereas evergreen species have a longer MRT (Berendse and Aerts, 1987; Aerts, 1990; Eckstein and Karlsson, 1997), leading to a similar NUE between species.
Why are there no species that have both high productivity (photosynthetic capacity or NP) and long persistence (leaf lifespan or MRT)? One of important reasons may be structural and physiological constraints (Reich et al., 1991). As mentioned above, leaves with a high photosythetic capacity have a lower leaf toughness (Reich et al., 1991; Wright and Westoby, 2002). Having higher photosynthetic capacity or PNUE may require a considerable investment of nitrogen in the photosynthetic apparatus at the expense of the investment in cell wall proteins (Onoda et al., 2004; Takashima et al., 2004). A leaf may not satisfy both at the same time. On the other hand, species with a low productivity and short persistence may have been eliminated by natural selection (Reich et al., 1991).
Such physiological constraints, however, do not necessarily explain all the variations in leaf lifespans. For example, as mentioned above, sun leaves have a shorter lifespan than shade leaves, although sun leaves may have a greater leaf toughness due to higher LMA. If sun leaves had a leaf lifespan that was comparable to that of shade leaves, they would realize the highest carbon gain. Why do sun leaves fall earlier than shade leaves? Optimality models are useful for comprehensive understanding of leaf lifespan.
Optimality models of leaf turnover
Kikuzawa (1991) extended the cost–benefit theory to a simple optimality model of leaf lifespan. He assumed (a) that construction cost of a leaf (C) is incurred at leaf birth, (b) that rate of photosynthesis of a leaf decreases linearly with time, (c) that the number of leaves in the canopy is constant, and (d) leaf lifespan is regulated to maximize carbon gain at the canopy level, not at the leaf level. Then he derived the optimal leaf lifespan (Lopt):
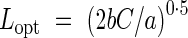 |
where a is the maximum rate of photosynthesis of a leaf at leaf birth, b the time when the rate of photosynthesis becomes zero (potential leaf longevity) and C the construction cost of a leaf. a/b indicates the rate of decline in the rate of photosynthesis. The model predicts that a leaf is shed when the net gain by a leaf per unit time over the entire lifespan becomes maximal even when the leaf could continue to photosynthesize. Although production of a new leaf requires an investment of biomass, the replacement improves the carbon gain of the canopy instead of maintaining old, less productive leaves. This model explains variation in leaf lifespan; it predicts that plants will have a shorter leaf lifespan if leaves have higher rates of photosynthesis, a higher rate of decline in rate of photosynthesis or smaller construction cost. Kikuzawa and Ackerly (1999) have shown that the calculated Lopt for actual plants was strongly correlated with the observed leaf lifespan.
The model of Kikuzawa (1991) is not sufficient to explain the behaviour of the leaf canopy because it does not predict the total number of leaves in the canopy. As discussed above, photosynthetic traits of a leaf are strongly influenced by shading in the canopy, suggesting that leaf senescence is dependent on position rather than age (Ackerly, 1999). Ackerly (1996) showed that if a steady state is assumed for turnover of leaves, leaf lifespan (Ln) is expressed by:
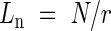 |
where N is the number of leaves and r is the leaf birth rate (the number of leaves produced per unit time). If we use LAI and the rate of leaf production instead of N and r, respectively, lifespan at a canopy level (La) in terms of leaf area can be expressed as
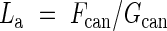 |
where Fcan is the leaf area index (LAI) and Gcan is the rate of leaf area production (see eqn 1). Ln is equal to La if the area of a leaf is constant. Ackerly (1999) proposed an alternative optimality model in which the leaf birth rate is maximized. In a competing system, height growth is important to outcompete neighbours (Weiner, 1990; Nagashima and Terashima, 1995). The height growth of plants depends on the addition and elongation of internodes, and each internode is produced in association with a new leaf (Ackerly, 1999). Ackerly (1999) assumed that the rate of photosynthesis is position-dependent, in which the number of younger leaves determines the rate of photosynthesis of older leaves, and derived the optimal solution to maximize the leaf birth rate (Lr):
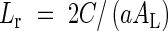 |
where AL is biomass allocation to leaves (see eqn 1). In this optimal solution, the oldest leaf drops when its photosynthesis becomes zero.
Kikuzawa's model assumes that assimilated carbon minus carbon invested for leaf construction (i.e. revenue minus cost) per unit time is maximized, while Ackerly's solution maximizes the leaf production rate, which is proportional to the amount of carbon invested for leaf construction. Thus the two solutions are different from each other. Ackerly (1999) compared the leaf turnover predicted by his model and Kikuzawa's with observation of pioneer species in a tropical forest. Observed values were correlated with prediction by both models, but the difference between observed and predicted values was smaller in the model of Ackerly (1999).
Recently, Escudero and Mediavilla (2003) presented a new hypothesis to explain leaf lifespan. In Mediterranean evergreen species, the photosynthetic capacity of leaves decreased with leaf ageing. Since the leaf nitrogen content does not decrease until the end of the life of a leaf, the decrease in photosynthetic capacity was ascribed to a reduction in PNUE. Photosynthetic gain in a plant is maximized by shedding older leaves only when photosynthesis by retranslocated nitrogen in new leaves exceeds the photosynthesis of the leaves lost (Franklin and Ågren, 2002). This condition is satisfied when the ratio of PNUE in the old leaves to PNUE in the young leaves becomes lower than the nitrogen resorption efficiency. Escudero and Mediavilla (2003) showed that, in the nine species that they studied, the ratio of PNUE in the oldest leaves to PNUE in the youngest leaves was similar or lower than the nitrogen resorption efficiency, which is consistent with their hypothesis.
To extend the hypothesis of Escudero and Mediavilla (2003) to an optimality model, however, turnover of nitrogen needs to be incorporated. Since plants lose nitrogen with dead leaves, plants should drop leaves after they take up nitrogen in an amount large enough to compensate for the lost nitrogen. Otherwise the amount of nitrogen in the canopy would decrease. Thus the optimal leaf lifespan may be a function of two factors: the rate of nitrogen uptake and of PNUE reduction.
Leaf turnover and canopy photosynthesis
The canopy photosynthesis model has been used to analyse canopy structure and function (Monsi and Saeki, 1953; see Hirose, 2005). In previous studies the canopy has been treated as a static system and dynamic aspects have been paid less attention. However, the canopy photosynthesis model may also be useful for the analysis of canopy dynamics such as leaf lifespan. Leaf production is determined by the rate of photosynthesis of the canopy (eqn 1) and leaf shedding is a removal of excessive leaves that do not contribute to canopy photosynthesis. Hikosaka (2003) incorporated dynamics of leaves and nitrogen into a canopy photosynthesis model. Figure 3 outlines the structure of the model. Leaf area in the canopy increases with the production of new leaves, which is proportional to the rate of photosynthesis in the canopy (eqn 1). Uptake of nitrogen from the soil increases the amount of nitrogen in the canopy. The optimal LAI that maximizes canopy photosynthesis is calculated. If leaf area is in excess, old leaves are eliminated, and part of nitrogen is lost with dead leaves. Consequently a new canopy having an optimal LAI with an optimal amount of nitrogen is obtained. Repeating this process gives growth of the leaf canopy.
Fig. 3.
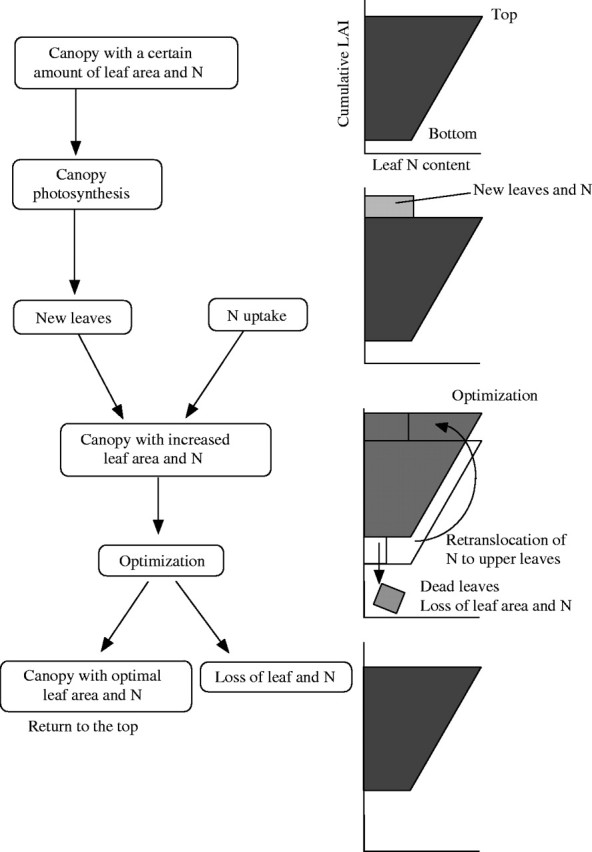
A schematic diagram of the leaf turnover model of Hikosaka (2003). See text for details.
One of the important assumptions in the model of Hikosaka (2003) is the optimization of LAI. Earlier models for canopy photosynthesis have predicted that the canopy should increase LAI until PFD at the bottom of canopy equals the compensation point of daily photosynthesis (e.g. Saeki, 1960; Verhagen et al., 1963). However, LAI is determined not only by light, but also by nutrient availability (Aerts and de Caluwe, 1994a; Ackerly and Bazzaz, 1995; Anten et al., 1995b). When the amount of nitrogen in the canopy is limited, mean nitrogen content per unit leaf area decreases with increasing LAI, resulting in low canopy photosynthesis. Anten et al. (1995b) calculated the effect of LAI on the rate of photosynthesis in the canopy with the amount of nitrogen in the canopy being kept constant, where nitrogen distributes optimally among leaves. They found an optimal LAI that maximizes canopy photosynthesis for a given canopy nitrogen, which showed a strong correlation with observed LAI. Their theory has been incorporated in the model of Hikosaka (2003).
The amount of nitrogen lost with dead leaves has two important effects on leaf turnover. First, it affects optimal LAI (Franklin and Ågren, 2002). Consider a canopy having a LAI that is higher than the optimal LAI at a given canopy nitrogen. Some leaves may be in excess, but leaf shedding decreases canopy nitrogen content as dead leaves contain some nitrogen. The canopy should shed leaves only when shedding increases canopy photosynthesis. Franklin and Ågren (2002) showed that the canopy that has smaller nitrogen resorption efficiency should have a higher LAI.
The second, more important effect is that the nitrogen concentration in dead leaves determines the rate of leaf turnover (Hikosaka, 2003). A leaf canopy absorbs carbon and nitrogen to produce leaves and loses them with dead leaves. When leaf turnover is at a steady state, by definition, the rate of leaf mass production is equal to the rate of leaf mass loss, and the rate of nitrogen uptake in leaves is equal to the rate of nitrogen loss. Nitrogen concentration of dead leaves is, also by definition, equal to the ratio of the loss rate of nitrogen to the loss rate of leaf mass (Vitousek, 1982; Yasumura et al., 2002). Thus the rate of leaf mass production is given as the ratio of the nitrogen uptake rate to the nitrogen concentration of dead leaves. Even if the canopy has a potential of higher canopy photosynthesis, the rate of photosynthesis in the canopy is regulated so as to meet the nitrogen uptake rate. Lower nitrogen concentrations in dead leaves may be advantageous at low nutrient availability because it allows a high canopy photosynthetic capacity at a given nitrogen uptake rate.
When a factor other than nitrogen (such as light) limits canopy photosynthesis, low nitrogen concentrations in dead leaves may be disadvantageous. If the ratio of the nitrogen uptake rate to leaf mass production is higher than the nitrogen concentration of dead leaves, nitrogen continues to accumulate in the leaf canopy, which would lead to excessively high nitrogen contents in leaves. Excessive nitrogen, increasing respiration, would not increase carbon gain and may only attract herbivores. When nutrient availability is relatively high, plants need to decrease nitrogen uptake rates or to increase nitrogen concentration of dead leaves to avoid excessive accumulation of nitrogen. A canopy with low nitrogen concentrations in dead leaves would not be favoured at high nitrogen availabilities. Supraoptimal nitrogen contents of living leaves are often observed in sun species that are growing in extremely low light conditions (Anten and Werger, 1996; Hikosaka and Terashima, 1996; Anten et al., 1998; Hikosaka et al., 1999). Such plants sometimes shed green leaves that contain a considerable amount of nitrogen (Y. Yasumura, pers. comm.). Plants balance the flux of nitrogen and carbon in the canopy.
These results suggest that nitrogen loss from the canopy plays an important role in leaf dynamics. It should be noted that the leaf turnover rate depends more strongly on nitrogen concentration of dead leaves than on nitrogen concentration of living leaves or on nitrogen resorption. Thus the nitrogen resorption proficiency (Killingbeck, 1996) can be the most important parameter to determine leaf dynamics.
The model of Hikosaka (2003) incorporated several physiological processes to evaluate environmental responses of canopy structure and leaf turnover.
Nitrogen availability. Higher nitrogen availability leads to the greater LAI. This is because higher nitrogen uptake rates allow higher rates of photosynthesis in the canopy. Leaf lifespan is predicted to be slightly longer at lower nitrogen availabilities (Fig. 4A), which is due partly to lower rates of canopy photosynthesis (see eqn 3). This result is consistent with previous studies that showed shorter leaf lifespan at higher nutrient availability (see Aerts and Chapin, 2000).
Light availability. Leaf lifespan is longer at low irradiance (Fig. 4B), which also results from the low rate of canopy photosynthesis. This is consistent with previous studies (Chabot and Hicks, 1982; Kikuzawa, 1988; Osada et al., 2001).
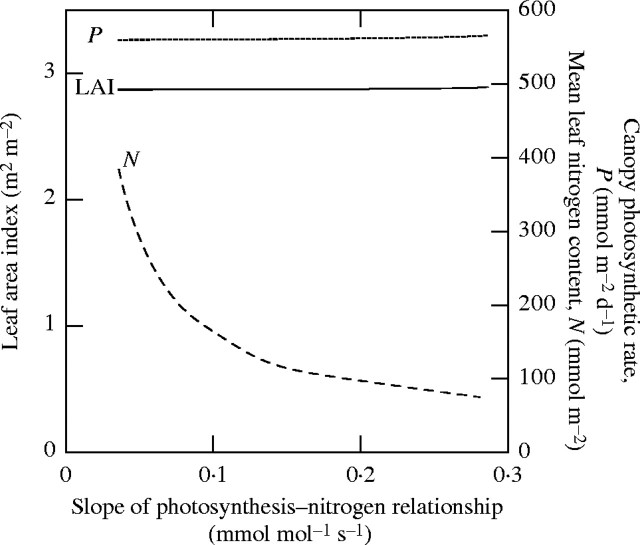
PNUE or elevated CO2. Effects of the slope of the photosynthesis–nitrogen relationship are shown in Fig. 5. Interestingly, increase in the slope increases neither the rate of photosynthesis in the canopy nor the LAI at a steady-state leaf turnover. This is because the rate of photosynthesis in the canopy is always proportional to the ratio of nitrogen uptake rate to nitrogen concentration in dead leaves irrespective of other factors. As the slope of the photosynthesis–nitrogen relationship increases, the mean nitrogen content per unit leaf area in the canopy decreases in a counteracting manner (Fig. 5). This may explain the long-term response to elevated CO2 (CO2 acclimation); elevated CO2 increases the slope of the photosynthesis–nitrogen relationship but long-term growth at elevated CO2 often reduces the leaf nitrogen content such that the in situ rate of photosynthesis in the leaf is similar to that in leaves growing at normal CO2 (Sage, 1994; Makino and Mae, 1999).
LMA. The model predicts that an increase in LMA extends leaf lifespan because it decreases the rate of leaf area production (see eqn 1). This is consistent with field observations (e.g. Reich et al., 1992).
Fig. 4.

(A) Predicted leaf lifespan as a function of nitrogen uptake rate (a measure of nitrogen availability) and (B) noon irradiance (a measure of light availability). Noon irradiance is assumed to be 2000 μmol m−2 s−1 in (A) and nitrogen uptake rate is assumed to be maximal in (B) (the maximal nitrogen uptake rate is the upper limit where the leaf turnover is maintained at a steady state and increases with noon irradiance). Redrawn from Hikosaka (2003).
Fig. 5.
Influence of the slope of the photosynthesis–nitrogen relationship on canopy characteristics. Noon irradiance and nitrogen uptake rate are 2000 μmol m−2 s−1 and 4 mmol m−2 d−1, respectively. Redrawn from Hikosaka (2003).
Leaf turnover and evolutionary game
The optimal strategy that maximizes photosynthesis of the whole canopy would not be evolutionarily stable when the revenue of an individual is affected by neighbours' strategy (Givnish, 1982; Maynard-Smith, 1982). In plant canopies, especially in herbaceous canopies, individual plants compete for light with their neighbours and light interception of an individual is strongly affected by neighbours (Hikosaka et al., 2001). Hikosaka and Hirose (1997) incorporated the game theory into a canopy photosynthesis model and examined evolutionarily stable leaf angle. The photosynthetic capacity of canopies with vertical leaves exceeds those with horizontal leaves, because more light passes through to reach deeper layers, resulting in more uniform distribution of light within the canopy as mentioned above. However, horizontal leaves intercept light more than vertical leaves under the same light intensity. If a mutant with horizontal leaves appears in a stand of individuals with vertical leaves, photosynthesis of the mutant would exceed that of neighbours because of its greater light interception. Therefore, the evolutionarily stable leaf angle is expected to be more horizontal than the ‘optimal’ leaf angle that maximizes whole canopy photosynthesis. Similar logic has been applied to the evolutionarily stable LAI. The evolutionarily stable LAI is shown to be greater than the optimal LAI (Schieving and Poorter, 1999; Anten, 2002; see Anten, 2005).
No one seems to have discussed the evolutionarily stable leaf turnover rate. The pattern of leaf turnover that maximizes the leaf birth rate (Ackerly, 1999) may be closer to the evolutionarily stable strategy because, as mentioned above, height growth is important to compete with neighbours. If the evolutionarily stable canopy has a greater LAI than the optimal canopy, the former has a lower rate of photosynthesis than the optimal canopy (Hikosaka and Hirose, 1997; Anten, 2002). Then it will extend the leaf lifespan of the plant (see eqn 3).
CONCLUSIONS
Leaf dynamics are affected by various environmental and internal factors, where nitrogen plays an important role. The effects of nitrogen can be summarized as follows. (a) Nitrogen is a determinant of the rate of photosynthesis. Its amount in the canopy and its allocation among leaves have large effects on canopy photosynthesis. (b) Nitrogen is utilized efficiently by retranslocation from old leaves. Old leaves are an alternative source of nitrogen for sink organs when nitrogen uptake of roots does not meet sink demand. If sink organs require more nitrogen, senescence of old leaves is accelerated. (c) When leaf turnover is at a steady state, the ratio of biomass production to nitrogen uptake (i.e. NUE) is equal to the ratio of litter fall to nitrogen loss, which is an inverse of the nitrogen concentration in dead leaves. Thus nitrogen concentration in dead leaves (nitrogen resorption proficiency) and nitrogen availability in the soil determine the rate of photosynthesis in the canopy. Leaf turnover is an important process leading to maximization of carbon gain and resource-use efficiency. It may also affect success in competition with neighbours. The optimality and the game theory may be useful tools to analyse and to predict environmental response of canopy characteristics.
Acknowledgments
I thank T. Hirose, S. Oikawa and Y. Yasumura for comments. This study was supported in part by grants from the Japanese Ministry of Education, Science, Sports, and Culture.
LITERATURE CITED
- Ackerly DD. 1996. Canopy structure and dynamics: integration of growth processes in tropical pioneer trees. In: Mulkey SS, Chazdon RL,Smith AP, eds. Tropical forest plant ecophysiology. New York:Chapman and Hall, 619–658. [Google Scholar]
- Ackerly DD. 1999. Self-shading, carbon gain and leaf dynamics: a test of alternative optimality models. Oecologia 119: 300–310. [DOI] [PubMed] [Google Scholar]
- Ackerly DD, Bazzaz FA. 1995. Leaf dynamics, self shading and carbon gain in seedlings of a tropical pioneer tree. Oecologia 101: 289–298. [DOI] [PubMed] [Google Scholar]
- Aerts R. 1990. Nitrogen use efficiency in evergreen and deciduous species from heathlands. Oecologia 84: 391–397. [DOI] [PubMed] [Google Scholar]
- Aerts R. 1996. Nutrient resorption from senescing leaves of perennials: are there general patterns? Journal of Ecology 84: 597–608. [Google Scholar]
- Aerts R, de Caluwe H. 1994. Effects of nitrogen supply on canopy structure and leaf nitrogen distribution in Carex species. Ecology 75: 1482–1490. [Google Scholar]
- Aerts R, de Caluwe H. 1994. Nitrogen use efficiency of Carex species in relation to nitrogen supply. Ecology 75: 2362–2372. [Google Scholar]
- Aerts R, Chapin FS III. 2000. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Advances in Ecological Research 30: 1–67. [Google Scholar]
- Anten NPR. 2002. Evolutionarily stable leaf area production in plant populations. Journal of Theoretical Biology 217: 15–32. [DOI] [PubMed] [Google Scholar]
- Anten NPR. 2005. Canopy structure and species coexistence analysed with individual plant-based canopy models. Annals of Botany 95: 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anten NPR, Miyazawa K, Hikosaka K, Nagashima H, Hirose T. 1998. Leaf nitrogen distribution in relation to leaf age and photon flux density in dominant and subordinate plants in dense stands of a dicotyledonous herb. Oecologia 113: 247–259. [DOI] [PubMed] [Google Scholar]
- Anten NPR, Schieving F, Medina E, Werger MJA, Schuffelen P. 1995. Optimal leaf area indices in C3 and C4 mono- and dicotyledonous species at low and high nitrogen availability. Physiologia Plantarum 95: 541–550. [Google Scholar]
- Anten NPR, Schieving F, Werger MJA. 1995. Patterns of light and nitrogen distribution in relation to whole canopy gain in C3 and C4 mono- and dicotyledonous species. Oecologia 101: 504–513. [DOI] [PubMed] [Google Scholar]
- Anten NPR, Werger MJA. 1996. Canopy structure and nitrogen distribution in dominant and subordinate plants in a dense stand of Amaranthus dubius (L.) with a size hierarchy of individuals. Oecologia 105: 30–37. [DOI] [PubMed] [Google Scholar]
- Berendse E, Aerts R. 1987. Nitrogen use efficiency: a biologically meaningful definition? Functional Ecology 1: 293–296. [Google Scholar]
- Björkman O. 1981. Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, eds. Encyclopedia of plant physiology. I. New series. Vol. 12A. Berlin: Springer, 57–107. [Google Scholar]
- Boerner REJ. 1984. Foliar nutrient dynamics and nutrient use efficiency of 4 deciduous tree species in relation to site fertility. Journal of Applied Ecology 21: 1029–1041. [Google Scholar]
- Brouwer R. 1962. Nutritive influences on the distribution of dry matter in the plant. Netherlands Journal of Agricultural Science 10: 399–408. [Google Scholar]
- Chabot BF, Hicks DJ. 1982. The ecology of leaf life spans. Annual Review of Ecology and Systematics 13: 229–259. [Google Scholar]
- Chapin FS III. 1989. The cost of tundra plant structures: evaluation of concepts and currencies. American Naturalist 133: 1–19. [Google Scholar]
- Chapin FS III, Moilanen L. 1991. Nutritional controls over nitrogen and phosphorus resorption from Alaskan birch leaves. Ecology 72: 709–715. [Google Scholar]
- Charles-Edwards DA, Stutzel H, Ferraris R, Beech BF. 1987. An analysis of spatial variation in the nitrogen content of leaves from different horizons within a canopy. Annals of Botany 60: 421–426. [Google Scholar]
- Coley PD, Bryant JP, Chapin FS III. 1985. Resource availability and plant anti-herbivore defense. Science 230: 895–899. [DOI] [PubMed] [Google Scholar]
- Diemer M, Körner Ch. 1996. Lifetime leaf carbon balances of herbaceous perennial plants from low and high altitudes in the central Alps. Functional Ecology 10: 33–43. [Google Scholar]
- Eckstein RL, Karlsson PS. 1997. Aboveground growth and nutrient use by plants in a subarctic environment: effects of habitat, life-form and species. Oikos 79: 311–324. [Google Scholar]
- Ellsworth DS, Reich PB. 1993. Canopy structure and vertical patterns of photosynthesis and related leaf traits in a deciduous forest. Oecologia 96: 169–178. [DOI] [PubMed] [Google Scholar]
- Emaus D, Prichard H. 1998. A cost–benefit analysis of leaves of four Australian savanna species. Tree Physiology 18: 537–545. [DOI] [PubMed] [Google Scholar]
- Escudero A, Del Arco JM, Garrido MV. 1992. The efficiency of nitrogen retranslocation from leaf biomass in Quercus ilex ecosystems. Vegetatio 99–100: 225–237. [Google Scholar]
- Escudero A, Del Arco JM, Sanz IC, Ayala J. 1992. Effects of leaf longevity and retranslocation efficiency on the retention time of nutrients in the leaf biomass of different woody species. Oecologia 90: 80–87. [DOI] [PubMed] [Google Scholar]
- Escudero A, Mediavilla S. 2003. Decline in photosynthetic nitrogen use efficiency with leaf age and nitrogen resorption as determinants of leaf life span. Journal of Ecology 91: 880–889. [Google Scholar]
- Evans JR. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19. [DOI] [PubMed] [Google Scholar]
- Evans JR. 1989. Partitioning of nitrogen between and within leaves grown under different irradiance. Australian Journal of Plant Physiology 16: 533–548. [Google Scholar]
- Evans JR. 1993. Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. II. stability through time and comparison with a theoretical optimum. Australian Journal of Plant Physiology 20: 69–82. [Google Scholar]
- Evans JR, Seemann JR. 1989. The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences and control. In:Briggs WR, ed. Photosynthesis. New York: Alan R. Liss, 183–205. [Google Scholar]
- Field C. 1983. Allocating leaf nitrogen for the maximisation of carbon gain: leaf age as a control on the allocation program. Oecologia 56: 341–347. [DOI] [PubMed] [Google Scholar]
- Field C, Mooney HA. 1983. Leaf age and seasonal effects on light, water, and nitrogen use efficiency in a California shrub. Oecologia 56: 348–355. [DOI] [PubMed] [Google Scholar]
- Field C, Mooney HA. 1986. The photosynthesis–nitrogen relationship in wild plants. In: Givnish TJ, ed. On the economy of form and function. Cambridge: Cambridge University Press, 25–55. [Google Scholar]
- Franklin O, Ågren GI. 2002. Leaf senescence and resorption as mechanisms of maximizing photosynthetic production during canopy development at N limitation. Functional Ecology 16: 727–733. [Google Scholar]
- Givnish TJ. 1982. On the adaptive significance of leaf height in forest herbs. American Naturalist 120: 353–381. [Google Scholar]
- Givnish TJ. 2002. Adaptive significance of evergreen vs. deciduous leaves: solving the triple paradox. Silva Fennica 36: 703–743. [Google Scholar]
- Guitman MR, Arnozis PA, Barneix AJ. 1991. Effect of source-sink relations and nitrogen nutrition on senescence and N remobilization in the flag leaf of wheat. Physiologia Plantarum 82: 278–284. [Google Scholar]
- Harper JL. 1989. The value of a leaf. Oecologia 80: 53–58. [DOI] [PubMed] [Google Scholar]
- Hidema J, Makino A, Mae T, Ojima K. 1991. Photosynthetic characteristics of rice leaves aged under different irradiances from full expansion through senescence. Plant Physiology 97: 1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K. 1996. Effects of leaf age, nitrogen nutrition and photon flux density on the photosynthetic apparatus of leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Planta 198: 144–150. [DOI] [PubMed] [Google Scholar]
- Hikosaka K. 2003. A model of dynamics of leaves and nitrogen in a canopy: an integration of canopy photosynthesis, leaf life-span, and nitrogen-use efficiency. American Naturalist 162: 149–164. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Hirose T. 1997. Leaf angle as a strategy for light competition: optimal and evolutionarily stable light-extinction coefficient within a canopy. Écoscience 4: 501–507. [Google Scholar]
- Hikosaka K, Hirose T. 2000. Photosynthetic nitrogen-use efficiency in species coexisting in a warm-temperate evergreen forest. Tree Physiology 20: 1249–1254. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Terashima I. 1995. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant, Cell & Environment 18: 605–618. [Google Scholar]
- Hikosaka K, Terashima I. 1996. Nitrogen partitioning among photosynthetic components and its consequence in sun and shade plants. Functional Ecology 10: 335–343. [Google Scholar]
- Hikosaka K, Hanba YT, Hirose T, Terashima I. 1998. Photosynthetic nitrogen-use efficiency in woody and herbaceous plants. Functional Ecology 12: 896–905. [Google Scholar]
- Hikosaka K, Nagamatsu D, Ishii HS, Hirose T. 2002. Photosynthesis–nitrogen relationships in species at different altitudes on Mount Kinabalu, Malaysia. Ecological Research 17: 305–313. [Google Scholar]
- Hikosaka K, Nagashima H, Harada Y, Hirose T. 2001. A simple formulation of interaction between individuals competing for light in a monospecific stand. Functional Ecology 15: 642–646. [Google Scholar]
- Hikosaka K, Sudoh S, Hirose T. 1999. Light acquisition and use of individuals competing in a dense stand of an annual herb, Xanthium canadense Oecologia 118: 388–396. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Terashima I, Katoh S. 1994. Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Oecologia 97: 451–457. [DOI] [PubMed] [Google Scholar]
- Hirose T. 1987. A vegetative plant growth model: adaptive significance of phenotypic plasticity in matter partitioning. Functional Ecology 1: 195–202. [Google Scholar]
- Hirose T. 2005. Development of the Monsi-Saeki theory on canopy structure and function. Annals of Botany 95: 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Werger MJA. 1987. Nitrogen use efficiency in instantaneous and daily photosynthesis of leaves in the canopy of Solidago altissima stand. Physiologia Plantarum 70: 215–222. [Google Scholar]
- Hirose T, Werger MJA. 1987. Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 72: 520–526. [DOI] [PubMed] [Google Scholar]
- Hirose T, Werger MJA, Pons TL, van Rheenen JWA. 1988. Canopy structure and leaf nitrogen distribution in a stand of Lysimachia vulgaris L. as influenced by stand density. Oecologia 77: 145–150. [DOI] [PubMed] [Google Scholar]
- Hollinger DY. 1989. Canopy organization and foliage photosynthetic capacity in a broad-leaved evergreen montane forest. Functional Ecology 3: 53–62. [Google Scholar]
- Kikuzawa K. 1983. Leaf survival of woody plants in deciduous broad-leaved forests. 1. Tall trees. Canadian Journal of Botany 62: 2551–2556. [Google Scholar]
- Kikuzawa K. 1984. Leaf survival of woody plants in deciduous broad-leaved forests. 2. Small trees and shrubs. Canadian Journal of Botany 61: 2133–2139. [Google Scholar]
- Kikuzawa K. 1988. Leaf survivals of tree species in deciduous broad-leaved forests. Plant Species Biology 3: 67–76. [Google Scholar]
- Kikuzawa K. 1991. A cost–benefit analysis of leaf habit and leaf longevity of trees and their geographical pattern. American Naturalist 138: 1250–1263. [Google Scholar]
- Kikuzawa K. 2003. Phenological and morphological adaptations to the light environment in two woody and two herbaceous plant species. Functional Ecology 17: 29–38. [Google Scholar]
- Kikuzawa K, Ackerly DD. 1999. Significance of leaf longevity in plants. Plant Species Biology 14: 39–45. [Google Scholar]
- Killingbeck KT. 1996. Nutrients in senescent leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77: 1716–1727. [Google Scholar]
- Kimura M, Yokoi Y, Hogetsu K. 1978. Quantitative relationships between growth and respiration. II. Evaluation of constructive and maintenance respiration in growing Helianthus tuberosus leaves. Botanical Magazine, Tokyo 91: 43–56. [Google Scholar]
- Kitajima K, Mulkey SS, Samaniego M, Wright SJ. 2002. Decline of photosynthetic capacity with leaf age and position in two tropical pioneer tree species. American Journal of Botany 89: 1925–1932. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Mulkey SS, Wright SJ. 1997. Decline of photosynthetic capacity with leaf age in relation to leaf longevities for five tropical canopy tree species. American Journal of Botany 84: 702–708. [PubMed] [Google Scholar]
- Kitajima K, Mulkey SS, Wright SJ. 2005. Variation in crown light utilization characteristics among tropical canopy trees. Annals of Botany 95: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume A, Ino Y. 1993. Comparison of ecophysiological responses to heavy snow in two varieties of Aucuba japonica with different areas of distribution. Ecological Research 8: 111–121. [Google Scholar]
- Koike T. 1988. Leaf structure and photosynthetic performance as related to the forest succession of deciduous broad-leaved trees. Plant Species Biology 3: 77–87. [Google Scholar]
- Leopold AC. 1961. Senescence in plant development. Science 134: 1727–1732. [DOI] [PubMed] [Google Scholar]
- Lloyd J, Syvertsen JP, Kriedemann PE, Farquhar GD. 1992. Low conductances for CO2 diffusion from stomata to the sites of carboxylation in leaves of woody species. Plant, Cell & Environment 15: 873–899. [Google Scholar]
- McDermitt DK, Loomis RS. 1982. Elemental composition of biomass and its relation to energy content, growth efficiency, and growth yield. Annals of Botany 48: 275–290. [Google Scholar]
- McMahon T. 1973. Size and shape in biology. Science 179: 1201–1204. [DOI] [PubMed] [Google Scholar]
- Makino A, Mae T. 1999. Photosynthesis and plant growth at elevated levels of CO2 Plant and Cell Physiology 40: 999–1006. [Google Scholar]
- Makino A, Mae T, Ohara K. 1983. Photosynthesis and ribulose 1,5-bisphosphate carboxylase in rice leaves. Plant Physiology 73: 1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Mae T, Ohara K. 1984. Relation between nitrogen and ribulose-1,5-bisphosphate carboxylase in rice leaves from emergence through senescence. Plant and Cell Physiology 25: 429–437. [Google Scholar]
- May JD, Killingbeck KT. 1992. Effects of preventing nutrient resorption on plant fitness and foliar nutrient dynamics. Ecology 73: 1868–1878. [Google Scholar]
- Maynard-Smith J. 1982.Evolution and the theory of games. Cambridge: Cambridge University Press. [Google Scholar]
- Mediavilla S, Escudero A. 2003. Photosynthetic capacity, integrated over the lifetime of a leaf, is predicted to be independent of leaf longevity in some tree species. New Phytologist 159: 203–211. [DOI] [PubMed] [Google Scholar]
- Merino J, Field C, Mooney HA. 1982. Construction and maintenance costs of Mediterranean climate evergreen and deciduous leaves. I. Growth and CO2 exchange analysis. Oecologia 53: 208–213. [DOI] [PubMed] [Google Scholar]
- Monsi M, Saeki T. 1953. Über den Lichtfaktor in den Pflanzengesellschaften und seine Bedeutung für die Stoffproduktion. Japanese Journal of Botany 14: 22–52. [Google Scholar]
- Mooney HA, Gulmon SL. 1982. Constraints on leaf structure and function in reference to herbivory. BioScience 32: 198–206. [Google Scholar]
- Nagashima H, Terashima I. 1995. Relationships between height, diameter and weight distributions of Chenopodium album plants in stands: effects of dimension and allometry. Annals of Botany 75: 181–188. [Google Scholar]
- Nambias EKS, Fife DN. 1987. Growth and nutrient retranslocation in needles of radiata pine in relation to nitrogen supply. Annals of Botany 60: 147–156. [Google Scholar]
- Navas M-L, Ducout B, Roumet C, Richarte J, Garnier J, Garnier E. 2003. Leaf life span, dynamics and construction cost of species from Mditerranean old-fields differing in successional status. New Phytologist 159: 213–228. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. 1997. Role of foliar nitrogen in light harvesting and shade tolerance of four temperate deciduous woody species. Functional Ecology 11: 518–531. [Google Scholar]
- Oguchi R, Hikosaka K, Hirose T. 2003. Does the photosynthetic light-acclimation need change in leaf anatomy? Plant, Cell & Environment 26: 505–512. [Google Scholar]
- Oikawa S, Hikosaka K, Hirose T, Hori Y, Shiyomi M, Takahashi S. 2004. Cost–benefit relationships in leaves emerging at different times in a deciduous fern Pteridium aquilinum Canadian Journal of Botany 82: 521–527. [Google Scholar]
- Ono K, Terashima I, Watanabe A. 1996. Interaction between nitrogen deficit of a plant and nitrogen content in the old leaves. Plant and Cell Physiology 37: 1083–1089. [Google Scholar]
- Onoda Y, Hikosaka K, Hirose T. 2004. Allocation of nitrogen to cell walls decreases photosynthetic nitrogen use efficiency. Functional Ecology 18: 419–425. [Google Scholar]
- Osada N, Takeda H, Furukawa A, Awang M. 2001. Leaf dynamics and maintenance of tree crowns in a Malaysian rain forest stand. Journal of Ecology 89: 774–782. [Google Scholar]
- Osada N, Takeda H, Kitajima K, Pearcy RW. 2003. Functional correlates of leaf demographic response to gap release in saplings of a shade-tolerant tree, Elateriospermum tapos Oecologia 137: 181–187. [DOI] [PubMed] [Google Scholar]
- Penning de Vries FWT, Brunsting AHM, van Laar HH. 1974. Products, requirements and efficiency of biosynthesis: a quantitative approach. Journal of Theoretical Biology 45: 339–377. [DOI] [PubMed] [Google Scholar]
- Pons TL, Pearcy RW. 1994. Nitrogen reallocation and photosynthetic acclimation in response to partial shading in soybean plants. Physiologia Plantarum 92: 636–644. [Google Scholar]
- Pons TL, Schieving F, Hirose T, Werger MJA. 1989. Optimization of leaf nitrogen allocation for canopy photosynthesis in Lysimachia vulgaris In: Lambers H, ed. Causes and consequences of variation in growth rate and productivity of higher plants. The Hague: SPB Academic Publishing, 175–186. [Google Scholar]
- Pons TL, van der Werf A, Lambers H. 1994. Photosynthetic nitrogen use efficiency of inherently low- and fast-growing species: possible explanations for observed differences. In: Roy J, Garnier E, eds. A whole plant perspective on carbon–nitrogen interactions. The Hague: SPB Academic Publishing, 61–77. [Google Scholar]
- Poorter H, Evans JR. 1998. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific area. Oecologia 116: 26–37. [DOI] [PubMed] [Google Scholar]
- Poorter H, Remkes C. 1990. Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83: 553–559. [DOI] [PubMed] [Google Scholar]
- Poorter H, Remkes C, Lambers H. 1990. Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiology 94: 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, Villar R. 1997. The fate of acquired carbon in plants: chemical composition and construction costs. In: Bazzaz FA, Grace J, eds. Plant resource allocation. San Diego: Academic Press, 39–72. [Google Scholar]
- Reich PB. 1993. Reconciling apparent discrepancies among studies relating life span, structure and function of leaves in contrasting plant life forms and climates: ‘the blind men and the elephant retold’. Functional Ecology 10: 768–776. [Google Scholar]
- Reich PB, Ellsworth DS, Walters MB, Vose JM, Gresham C, Volin JC, Bowman WD. 1999. Generality of leaf trait relationships: a test across six biomes. Ecology 80: 1955–1969. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. 1991. Leaf lifespan as a determination of leaf structure and function among 23 Amazonian tree species. Oecologia 86: 16–24. [DOI] [PubMed] [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. 1992. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecological Monographs 62: 365–392. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. 1997. From tropics to tundra: global convergence in plant functioning. Proceedings of the National Academy of Science of the USA 94: 13730–13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki T. 1960. Interrelationships between leaf amount, light distribution and total photosynthesis in a plant community. Bonanical Magazine, Tokyo 73: 55–63. [Google Scholar]
- Sage RF. 1994. Acclimation of photosynthesis to increasing atmospheric CO2: the gas exchange perspective. Photosynthesis Research 39: 351–368. [DOI] [PubMed] [Google Scholar]
- Sage RF, Pearcy RW. 1987. The nitrogen use efficiency of C3 and C4 plants. II. Leaf nitrogen effects on the gas exchange characteristics of Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiology 84: 959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieving F, Pons TL, Werger MJA, Hirose T. 1992. Vertical distribution of nitrogen in photosynthetic activity at different plant densities in Carex acutiformis Plant and Soil 142: 9–17. [Google Scholar]
- Schieving F, Poorter H. 1999. Carbon gain in a multispecies canopy: the role of specific leaf area and photosynthetic nitrogen-use efficiency in the tragedy of the commons. New Phytologist 143: 201–211. [Google Scholar]
- Schlesinger WH, DeLucia EH, Billings WD. 1989. Nutrient-use efficiency of woody plants on contrasting soils in the Western Great Basin, Nevada. Ecology 70: 105–113. [Google Scholar]
- Shaver GR, Melillo JM. 1984. Nutrient budgets of marsh plants: efficiency concepts and relation to availability. Ecology 65: 1491–1510. [Google Scholar]
- Skillman JB, Strain BR, Osmond CB. 1996. Contrasting patterns of photosynthetic acclimation and photoinhibition in two evergreen herbs from a winter deciduous forest. Oecologia 107: 446–455. [DOI] [PubMed] [Google Scholar]
- Small E. 1972. Photosynthetic rates in relation to nitrogen recycling as an adaptation to nutrient deficiency in peat bog plants. Canadian Journal of Botany 50: 2227–2233. [Google Scholar]
- Smart CM. 1994. Gene expression during leaf senescence. New Phytologist 126: 419–448. [DOI] [PubMed] [Google Scholar]
- Sobrado MA. 1991. Cost–benefit relationships in deciduous and evergreen leaves of tropical dry forest species. Functional Ecology 5: 608–616. [Google Scholar]
- Stoll P, Schmid B. 1998. Plant foraging and dynamic competition between branches of Pinus sylvestris in contrasting light environments. Journal of Ecology 86: 934–945. [Google Scholar]
- Takashima T, Hikosaka K, Hirose T. 2004. Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant, Cell & Environment 27: 1047–1054. [Google Scholar]
- Takenaka A. 2000. Shoot growth responses to light microenvironment and correlative inhibition in tree seedlings under a forest canopy. Tree Physiology 20: 987–991. [DOI] [PubMed] [Google Scholar]
- Terashima I, Araya T, Miyazawa SI, Sone K, Yano S. 2005. Construction and maintenance of the optimal photosynthetic systems of the leaf, hervaceous plant and tree: an eco-developmental treatise. Annals of Botany 95: 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima I, Hikosaka K. 1995. Comparative ecophysiology of leaf and canopy photosynthesis. Plant, Cell & Environment 18: 1111–1128. [Google Scholar]
- Terashima I, Miyazawa SI, Hanba YT. 2001. Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. Journal of Plant Research 114: 93–105. [Google Scholar]
- Thomas H, Stoddart JL. 1980. Leaf senescence. Annual Review of Plant Physiology 31: 83–111. [Google Scholar]
- Thornley JHM. 1970. Respiration, growth and maintenance in plants. Nature 227: 304–305. [DOI] [PubMed] [Google Scholar]
- Verhagen AMW, Wilson JH, Britten EJ. 1963. Plant production in relation to foliage illumination. Annals of Botany 27: 627–640. [Google Scholar]
- Villar R, Merino J. 2001. Comparison of leaf construction costs in woody species with differing leaf life-spans in contrasting ecosystems. New Phytologist 151: 213–226. [DOI] [PubMed] [Google Scholar]
- Vitousek PM. 1982. Nutrient cycling and nitrogen use efficiency. American Naturalist 119: 553–572. [Google Scholar]
- Weiner J. 1990. Asymmetric competition in plant populations. Trends in Ecology and Evolution 5: 360–364. [DOI] [PubMed] [Google Scholar]
- Westbeek HMH, Pons TL, Cambridge ML, Atkin OK. 1999. Analysis of differences in photosynthetic nitrogen use efficiency of alpine and lowland Poa species. Oecologia 120: 19–26. [DOI] [PubMed] [Google Scholar]
- Westoby M, Warton D, Reich PB. 2000. The time value of leaf area. American Naturalist 155: 649–656. [DOI] [PubMed] [Google Scholar]
- Williams KB, Field CB, Mooney HA. 1989. Relationships among leaf construction cost, leaf longevity, and light environment in rain forest plants of the genus Piper American Naturalist 133: 198–211. [Google Scholar]
- Williams K, Percival F, Merino J, Mooney HA. 1987. Estimation of tissue construction cost from heat of combution and organic nitrogen content. Plant, Cell & Environment 10: 725–734. [Google Scholar]
- Wilson JB. 1988. A review of evidence on the control of shoot : root ratio, in relation to models. Annals of Botany 61: 433–449. [Google Scholar]
- Wright IJ, Cannon K. 2001. Relationships between leaf lifespan and structural defences in a low-nutrient, sclerophyll flora. Functional Ecology 15: 351–359. [Google Scholar]
- Wright IJ, Reich PB, Westoby M. 2001. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Functional Ecology 15: 423–434. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavendar-Bares J, Chapin T, Cornelissen JHC, Diemer M, et al. 2004. The leaf economics spectrum worldwide. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Westoby M. 2002. Leaves at low versus high rainfall: coordination of structure, lifespan and physiology. New Phytologist 155: 403–416. [DOI] [PubMed] [Google Scholar]
- Yasumura Y, Hikosaka K, Matsui K, Hirose T. 2002. Leaf-level nitrogen-use efficiency of canopy and understorey species in a beech forest. Functional Ecology 16: 826–834. [Google Scholar]
- Yasumura Y, Onoda Y, Hikosaka K, Hirose T. 2004. Nitrogen resorption from leaves with different growth irradiance in three deciduous woody species. Plant Ecology (in press). [Google Scholar]