Abstract
• Background and Aims Light extinction through crowns of canopy trees determines light availability at lower levels within forests. The goal of this paper is the exploration of foliage distribution and light extinction in crowns of five canopy tree species in relation to their shoot architecture, leaf traits (mean leaf angle, life span, photosynthetic characteristics) and successional status (from pioneers to persistent).
• Methods Light extinction was examined at three hierarchical levels of foliage organization, the whole crown, the outermost canopy and the individual shoots, in a tropical moist forest with direct canopy access with a tower crane. Photon flux density and cumulative leaf area index (LAI) were measured at intervals of 0·25–1 m along multiple vertical transects through three to five mature tree crowns of each species to estimate light extinction coefficients (K).
• Results Cecropia longipes, a pioneer species with the shortest leaf life span, had crown LAI <0·5. Among the remaining four species, crown LAI ranged from 2 to 8, and species with orthotropic terminal shoots exhibited lower light extinction coefficients (0·35) than those with plagiotropic shoots (0·53–0·80). Within each type, later successional species exhibited greater maximum LAI and total light extinction. A dense layer of leaves at the outermost crown of a late successional species resulted in an average light extinction of 61 % within 0·5 m from the surface. In late successional species, leaf position within individual shoots does not predict the light availability at the individual leaf surface, which may explain their slow decline of photosynthetic capacity with leaf age and weak differentiation of sun and shade leaves.
• Conclusion Later-successional tree crowns, especially those with orthotropic branches, exhibit lower light extinction coefficients, but greater total LAI and total light extinction, which contribute to their efficient use of light and competitive dominance.
Keywords: Anacardium excelsum, Antirrhoea trichantha, architecture, Castilla elastica, Cecropia longipes, crown LAI, forest canopy, leaf angle, light extinction coefficient, Luehea seemannii, photosynthesis, tropical trees
INTRODUCTION
Light available to individual leaves decreases according to the Beer's law of light extinction within a plant canopy (Monsi and Saeki, 1953). Since no leaf should exist below the light compensation point, the leaf area index (LAI), the total leaf area per square meter of ground, is ultimately constrained by the pattern of light extinction in the canopy. Typical LAI is in the range of 5–7 in temperate broad-leaved forests, and 6–8 in lowland tropical forests (Leigh, 1999). Although herbaceous communities may have LAI as high as in forests, light utilization by forest plants differs from those in herbaceous communities in many aspects. Vertical distribution of leaves takes place over a much greater distance in forests than in herbaceous communities, and this allows forest plants to exploit available light with a wide range of morphologies (e.g. trees, treelets, shrubs, vines, epiphytes, etc.). The great height of canopy trees in particular poses biomechanical challenges to optimize foliage distribution and to supply adequate amounts of water (Borchert and Tomlinson, 1984; Niklas, 1994). Consequently, leaf and light distributions in the forest canopy are expected to be spatially and temporally complex, especially in tropical forests where taxonomic and morphological diversities are high (Corner, 1964; Hallé et al., 1978; Leigh, 1990). Due to this complexity and the difficulty of reaching the canopy, analysis of light extinction through forest tree crowns as a function of cumulative LAI poses a much greater challenge than similar analyses for herbaceous communities. Monsi and Saeki (1953) predicted that plants in full sun should have inclined leaves to achieve lower light extinction coefficients and high LAI to maximize canopy photosynthesis (see Hirose, 2005). Greater inclination of not only leaves, but also terminal shoots, should result in more uniform distribution and efficient use of light in canopy tree crowns. In this paper is reported how light utilization characteristics of crowns of tall mature trees in a tropical forest vary among species in relation to differences in their architecture and leaf arrangement patterns.
Although irradiance generally decreases as one descends from the canopy of a tropical forest (Yoda, 1974; Yoda et al., 1983), there is a great variation from point to point (Koike and Syahbuddin, 1993; Baldocchi and Collineau, 1994; Parker and Brown, 2000; Montgomery and Chazdon, 2001). Koike and Syahbuddin (1993) concluded from their study of a West Sumatran forest that there is no continuous stratified upper canopy layer, but that the upper canopy consists of individual crowns that protrude upwards. Theories predict that architecture of tree crowns and patterns of leaf arrangement should affect the light extinction characteristics of the canopy (Anderson, 1966; Horn, 1971; Kuuluvainen and Pukkala, 1989). Because solar tracks run near the zenith throughout the year at tropical latitudes, light exploitation characteristics of individual tree crowns should exert a greater influence on the light environment below them in tropical than in temperate forests (Horn, 1971; Terborgh, 1985). Although significant effects of overall tree architecture (e.g. the ratio of projected crown area to height) on understorey light environments have been demonstrated (e.g. Canham et al., 1994; Kabakoff and Chazdon, 1996; Montgomery and Chazdon, 2001), effects of species differences in shoot architecture and leaf arrangements on light extinction characteristics within individual tree crowns have rarely been examined.
Leaf distribution within a crown can be viewed as an outcome of evolution for optimal spatial distribution of carbon and nutrients to photosynthetic and support tissues (Mooney et al., 1981; Field, 1983; Hirose and Werger, 1987a; Sands, 1995). Architecture and leaf phenology interact to create a gradient of leaf age and self-shading within a tree crown (Kikuzawa, 1995; Kikuzawa et al., 1996). For example, many pioneer trees exhibit orthotropic (vertically oriented) shoots that successively produce leaves in spiral or decussate phyllotaxy (Ashton, 1978; Shukla and Ramakrishnan, 1986). Consequently, such plants exhibit a clear gradient of self-shading with leaf position, as well as accompanying gradients of leaf nitrogen and photosynthetic capacity as predicted by theories of optimal nitrogen allocation (Field, 1983; Hirose and Werger, 1987a; Traw and Ackerly, 1995; Hikosaka, 1996; Kitajima et al., 2002). Other species, including many understorey and later successional trees have plagiotropic (horizontally inclined) shoots that produce leaves in distinctive seasonal or annual flushes (Kohyama, 1991; Kikuzawa et al., 1996). In species with plagiotropic shoots, light incident on the individual leaf surface is affected more by among-branch shading than by within-branch shading. However, the orthotropic–plagiotropic dichotomy is an oversimplification as a correlate of successional status. Ackerly (1996) concludes that no single architectural model is ‘typical or optimal’ for pioneer species. For example, some tropical pioneer trees produce a combination of vertical and horizontal shoots, with plagiotropic lateral branches that are successively produced along orthotropic leader shoots or from the main trunk. In such cases, self-shading gradients are expected to occur at two levels of organization: shading of lower horizontal branches by younger horizontal branches that are successively produced above them, as well as gradients within each horizontal branch from younger leaves produced at more distal positions to old leaves located near central leader shoots that are heavily shaded by higher branches. Whereas many studies have addressed the effects of crown architecture on light utilization for understorey saplings and treelets (Kohyama, 1987; Kohyama and Hotta, 1990; King et al., 1997; Valladares et al., 2002), little information is available about the link between shoot architecture and light absorption characteristics of dominant canopy trees.
For crowns of dominant canopy trees, it may be useful to examine the link between architecture and light-utilization patterns at three hierarchical levels: crown as a whole, canopy crust and individual shoots. The canopy crust is defined as the dense layer of leaves borne on terminal shoots at the outermost position of an individual crown (e.g. within 1–2 m from the external surface of the crown). Light extinction can be as much as 94 % over short distances within the canopy crust (Johnson and Atwood, 1970). Many canopy trees have protruding crowns (Koike and Syahbuddin, 1993), and light availability at the surface of the canopy crust should also differ depending on the position relative to the apex of the crown. Orthotropic shoot orientation and steeper leaf angle should result in lower light extinction coefficients but greater total LAI and total light extinction through the crown than plagiotropic shoots. Also predicted is that leaves at lower shaded branches should be more horizontally oriented than leaves on well-exposed branches at the crown surface.
Here are presented the results of a study that explored the species differences in vertical distribution of light and foliage in the tree crowns of a tropical seasonal forest, using a tower crane for access to the canopy. Five common canopy tree species that differ in their successional status, architecture and typical leaf angles were chosen. Also documented is light utilization at the surface and inside the canopy crust of an emergent crown of a dominant, late successional species. Whether the light environment at the surface of a leaf is predictable by its position within a terminal shoot in the upper canopy was examined in three species with contrasting shoot architecture. It was found that tree crowns as a whole exhibited predictable patterns of light extinction in relation to leaf and shoot angles, but that leaf position within a shoot was not correlated with light incident on individual leaves. Also, the photosynthetic traits of leaves at sunny and shaded locations of trees were documented to explore possible relationships of leaf photosynthetic traits with architecture and crown light utilization patterns.
MATERIALS AND METHODS
Study site, canopy access and species
The study was conducted in a seasonal tropical forest the Parque Natural Metropolitano near Panama City, Republic of Panama. It is a 75–150-year-old stand that consists of pioneers as well as early successional tree species that persist in later successional stands, with tree heights ranging up to 38 m (Table 1). Annual rainfall averages 1740 mm, the majority of which occurs during the rainy season of mid-May through to mid-December. Light availability is 48 % greater in February and March than in the cloudy wet season (Kitajima et al., 1997b). The upper canopy of this forest was approached from above with a 42-m-tall construction crane maintained by the Smithsonian Tropical Research Institute. A metal cage (gondola) suspended from the 51-m-long arm of the crane could carry up to three researchers to any height within the radius of the crane's horizontal arm.
Table 1.
Relevant characteristics of five tree species studied for vertical leaf and light distribution
| Species |
Successional status |
Branch orientation |
N |
Height (m) |
Crown depth (m) |
Crown radius (m) |
Leaf contacts |
Leaf angle (°) |
Leaf absorptance |
|---|---|---|---|---|---|---|---|---|---|
| Anacardium excelsum | Early–late | Orthotropic | 4 (71) | 22·6–28·9 | 7·0 (4·6) | 8·61 (2·92) | 5·0ab | 50·6a | 0·89bc |
| Luehea seemannii | Early–late | Plagiotropic | 4 (41) | 18·4–26·2 | 6·3 (4·3) | 3·96 (1·75) | 3·9b | 31·5b | 0·91a |
| Antirrhoea trichantha | Early | Orthotropic | 3 (33) | 12·0–18·4 | 4·8 (2·3) | 2·64 (1·04) | 5·9a | 22·8c | 0·87d |
| Castilla elastica | Early | Plagiotropic | 5 (45) | 13·8–19·1 | 1·8 (1·1) | 3·40 (1·65) | 1·8c | 33·6b | 0·88cd |
| Cecropia longipes | Pioneer | Orthotropic | 4 (56) | 16·3–23·3 | 1·1 (0·7) | 3·24 (2·36) | 0·3d | 27·7bc | 0·90ab |
N is the number of trees sampled followed by the total number of vertical transects in parentheses. Height is the range of height among study trees. All others are means followed by standard deviation in parentheses or letters indicating significant difference between species (Tukey–HSD, P < 0·05).
Leaf contacts, the total number of leaf contact per vertical transects; leaf angle, the angle from the horizontal plane; leaf absorptance, PFD absorptance for individual leaf lamina.
At least three individuals for each of five common tree species growing within the reach of the crane were sampled (Table 1). For each tree, crown radius was determined as the mean distance from the trunk to the projected edge in eight compass directions, while crown depth was measured as the distance between the crown apex and the lowest foliated branch. These species contrast in overall architecture and successional status (Fig. 1). Anacardium excelsum (Anacardiaceae) and Luehea seemannii (Tiliaceae) have maximum height approx. 30 m; they are common in early successional stands, but persist in late successional and mature forests. Antirrhoea trichantha (Rubiaceae) and Castilla elastica (Moraceae) have maximum height <20 m; they are restricted to early successional stands <100 years old. Cecropia longipes (Moraceae) is a pioneer typical of early successional stands, but is also found as a rare element in late successional stands as a gap specialist. Species nomenclature is based on D'Arcy (1987) and successional status is taken from Croat (1978) and personal observations. Hereafter the species are referred to by their genera, as species within the same genus usually exhibit similar branch architecture.
Fig. 1.
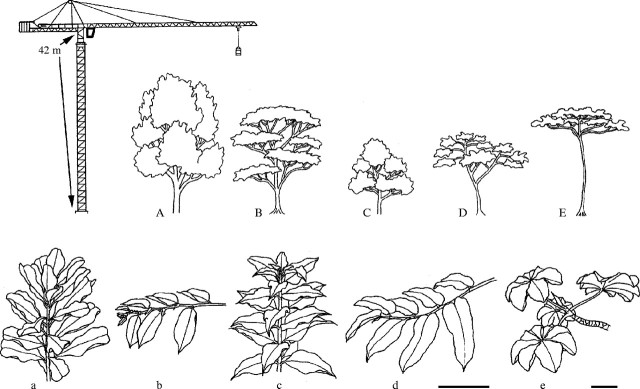
Line drawings of typical mature trees (A–E) and terminal shoots (a–e) of the study species in the reversed order of successional status: Anacardium excelsum (late successional species with the greatest leaf longevity among the study species (A and a); Luehea seemannii (B and b); Antirrhoea trichantha (C and c); Castilla elastica (D and d); Cecropia longipes (shortest-lived pioneer; E and e). The whole trees are drawn to the scale of the tower crane (42 m tall) to show their relative sizes and crown shapes. Scales for branch drawings are shown as bars at the bottom (0·2 m, shared for a–d, separate for e).
Leaf production and survival were recorded monthly for marked leaves on three exposed branches per individual for all species, and also on three shaded lower branches per tree for the two late successional species, Anacardium and Luehea, for 1–3 years (duration varied among species). From these measurements, seasonal changes in standing leaf crop were estimated. Details of phenology, leaf longevities and photosynthetic characteristics of these species are reported in previous publications from the same site (Mulkey et al., 1995; Kitajima et al., 1997a, b, 2002).
The five study species represent three contrasting architecture models according to the classification schemes of Hallé et al. (1978) and Leigh (1990). Anacardium and Antirrhoea have relay branches developing orthotropically that are bunched around monopodial trunks (Scarrone's model; Fig. 1A and C). Cecropia also has relay branches attached to a monopodial main trunk; however, branching is sparse and each branch grows relatively horizontally before tipping upward (Fig. 1E). Leaves of Anacardium and Antirrhoea are arranged in dense spirals around branches, which creates strong self-shading within each shoot (Fig. 1a and c), while leaves of Cecropia are arranged sparsely around branch tips and have long petioles which minimize overlap and self-shading (Fig. 1e). Luehea exhibits sympodial trunk development, in which relay branches sprout upwards but curve evenly to end as horizontal terminal shoots that usually develop second-order horizontal branches (Troll's model; Fig. 1B). Leaves of this species are distichously arrayed on horizontal branches, and often hang down by 10–40° from the horizontal plane (Fig. 1b). Castilla also has plagiotropic branches that are arranged continuously along monopodial trunks and bear distichous leaves (Cook's model; Fig. 1D and d); these branches do not develop second-order branches and are shed as units. However, branches of Castilla are often in layers or whorls, in which case the architecture follows Massart's model.
Vertical light gradients through whole crowns
Photon flux density (PFD) was sampled along vertical transects spaced every 1 m over the surface of each tree crown in January–March, 1993 (see Table 1 for sample size). Sampling was between 1000 and 1430 h local time on predominantly clear days. Based on standing leaf crop estimated from monthly leaf censuses, the study species exhibited the maximum standing leaf number between August and November, but still had 60–90 % of the maxima at the time of measurements (beginning of dry season). With the gondola positioned above each crown and from its sunny side, a quantum sensor (LI-190SA, Li-Cor, Lincoln, NE, USA) mounted on a self-levelling (gimbaled) platform (10 cm × 15 cm) along each vertical transect, was lowered to set depths below the crown surface. These depths were each 0·25 m for the first 2 m from the crown surface, and then at 1-m intervals until there was no more foliage of the study tree present directly below. The exception was Luehea, for which the first 3 m of the crown was sampled at 0·25, 0·75, 1·25 and 3 m from the crown surface. Instantaneous PFD was recorded with a data logger (LI-1000, LI-COR) connected with a long cable to the sensor at these depths. The data logger also simultaneously recorded instantaneous PFD read by a reference (unshaded) sensor placed in direct beam irradiance 1 m from the sunny side of the gondola. From these measurements, % PFD transmission (%T) was calculated for each measurement point along the vertical transects. All tree crowns were at the uppermost layer of the canopy, and well exposed to direct sunlight during the measurements. The gondola and the cables hanging the PFD platform never shaded the sensor during the measurements, and their effects on diffuse radiation should be negligible.
The cumulative leaf area to each depth into the crown was estimated from the number of leaves intersecting the vertical transect. A metal rod, 1 m in length and 6 mm in diameter, was attached beneath the PFD sensor platform. As the platform and rod descended, observers in the gondola and on the ground recorded the number of leaves contacted by the rod during each vertical interval, with the aid of binoculars. The cumulative LAI was then estimated by dividing the cumulative leaf number by the cosine of the median leaf angle for each species (Table 1). Leaf angle was measured for a total of 60–155 leaves of each species with a similar sample size from upper and lower crown positions. Leaf angle was measured with a protractor attached to a level as the tilt of the leaf blade (approximated as a flat plate) from the horizontal plane (0–90°, read as positive values whether leaves are tipped up or hanging down from the petiole). With the exception of Luehea, in most species, there was no significant difference in leaf angle between upper and lower crown. Thus, the median leaf angle was applied for the entire crown for the cosine correction for estimating LAI from leaf contact number. For Luehea, leaves on shade branches were more horizontal (20°) than those on upper canopy branches (35°) (P = 0·0002 with Wilcoxon Rank test). However, since the majority of leaves were found in the upper canopy branches, the median angle for the upper canopy branches was used for Luehea.
Canopy crust measurements
For a single 34-m Anacardium tree, two transects were laid with a metre tape along the crown surface from the crown summit in two directions to the edge of the crown: south-east (125° from north, 8 m long horizontally, 34–19 m above the ground) and north-west (300° from north, 13 m long horizontally, 34–15 m above the ground). The transect orientation was chosen to sample east- and west-facing sides of the crown, while minimizing the effect of the crane's tower and gondola position on the measurements. At every 1 m along each transect, the height from the ground was determined to describe the vertical profile of the transects, and light and foliage distribution at the crown surface and 0·5 m into the canopy crust were optically measured with a LAI-2000 canopy analyser (Li-Cor) and a quantum sensor mounted 8 cm behind the LAI-2000's optical sensor. All measurements were taken under overcast conditions in the mid-wet season (August 1994) when Anacardium trees exhibited their maximum standing leaf number. Simultaneous measurements were taken every 15 s with another unit of the canopy analyser placed at the top of the canopy crane's central tower. The LAI-2000's wand was mounted on a 2-m-long beam to place the sensor horizontally at 1·5 m away from the gondola's edge. The quarter of the optical sensor in the direction of the operator was masked to remove the effect of the operator and gondola, and the same sky sector was masked in the reference sensor. LAI was estimated with LI-COR C2000 Software (Welles and Norman, 1991). This optically estimated LAI reflects not only the leaves directly above the point of measurements but also other foliage and objects within the 148° view of the sensor. Furthermore, because of non-random distribution of shoots and leaves, optically estimated LAI should be considered as a correlate rather than as a measure of true LAI. From the PFD recorded by the quantum sensors mounted on the canopy analysers, % PFD transmission (%T) was calculated as the ratio of PFD at measurement locations to open sky PFD measured above canopy.
Leaf position effects within shoots
How light availability at the surface of individual leaves was affected by their positions (measured by the number of leaves distal to the focal leaf within the same terminal shoot) was examined for three species with contrasting architecture and successional status (Anacardium, Luehea and Cecropia; Table 1). Two or three terminal shoots were selected in well-lit positions (sun branches) and an equal number from lower shaded positions (shade branches) of each species, and selected leaves at five randomly selected positions within each shoot for measurements. For Cecropia, only sun branches existed. A single GaAsP photodiode (G1118, Hamamatsu, Japan) calibrated against a LI-190SA quantum sensor was attached to the adaxial surface near the centre of each sampled leaf. Total daily PFD was calculated from 10-min averages for PFD sampled every 5 s recorded with LI-1000 data loggers. Care was taken to maintain the natural angle and orientation of measured leaves during the measurements. For typical leaf and solar angles, cosine-error correction for these sensors would have little effect on estimated total daily PFD (Pearcy et al., 1990). Each leaf was measured continuously for 3–5 full days, and % total daily PFD was calculated for each leaf for each day as a percentage of total daily PFD at the horizontal plane above the forest canopy. From this, mean % total daily PFD was calculated for each leaf over the measurement duration that encompassed variable weather conditions. These measurements for Anacardium and Luehea were taken in January–February 1995, when these two evergreen species had 60–90 % of the maximum standing leaf number, while measurements for Cecropia were taken in June–July 1996 when Cecropia canopy had 80–90 % of its maximum leaf number.
Leaf photosynthesis
Photosynthetic rate at light saturation (Amax) and stomatal conductance to water vapour (gs) were measured in situ for mature, non-senescent leaves of known age (1–3 months old) in branches at well-lit and shaded locations within crowns of study trees (except in Cecropia which had leaves only in well-exposed locations). For shade branches of Anacardium and Luehea, % total daily PFD was quantified for a subsample of the leaves as described in the previous section. Shade branches of Antirrhoea and Castilla were chosen from those below the middle of the crown that were shaded by larger branch systems above. Based on ambient PFD during the gas exchange measurements, these shade leaves received 3–6 % of above canopy PFD around midday under clear-to-lightly overcast conditions (above canopy PFD >700 µmol m−2 s−1). Field-portable infrared gas analysers for CO2 and water were used that provided supplemental light at 1100–1300 µmol photons m−2 s−1 and supplied CO2 at 38 Pa to the leaf chamber (CIRAS-1; PP Systems, Hertfordshire, UK for measurements in June–August, 1994 for Anacardium, Luehea and Antirrhoea, and in April 1995 for Anacardium; LI-6400, Li-Cor, for measurements in June–July, 1996 for Antirrhoea, Castilla and Cecropia). Leaf disks (10 cm2) were sampled from the leaves measured for gas exchange and other leaves of similar age, to determine leaf absorptance of PFD with a LI-1800 spectroradiometer with an external integrating sphere (Li-Cor), and leaf mass per area (LMA) after drying at 60°C.
Data analysis
Within a species, light and leaf distributions along vertical transects were similar among individuals, and the data pooled for each species was analysed. From these two measurements, we calculated the light extinction coefficients for each species (K) as the Model II regression slope for the natural logarithm of mean %T vs. mean cumulative LAI, following Monsi and Saeki (1953). Model II regression was used because random error was associated with both axes. K was also calculated from the relationship between %T and optically estimated LAI for each canopy crust transect on an Anacardium tree, using only the measurements at 0·5 m below the canopy surface. All analyses were performed with JMP software (Version 3·1, SAS Institute, USA).
RESULTS
Foliage and light distribution through the tree crown
The study species differed in canopy depth and mean number of leaf contacts per vertical transect (Table 1). Many of the vertical transects through the periphery of the crowns were very short. There was also a large within-species variance in cumulative leaf contact number at a given depth, with greater variance within the first few metres [coefficient of variance (CV) >100 %] than at greater depth (50–90 % depending on species). Percentage PFD transmission also varied, with CV ranging from 60–150 % below 1 m depth without clear inter- or intraspecific trends. The later successional species with greater maximum height (Anacardium and Luehea) had larger crown radius and crown depth. The mean number of leaf contacts per transect was a function of crown depth, branch architecture and crown allometry (Table 1). The mean number of leaf contacts per transect was similarly high for Anacardium and Antirrhoea with densely arranged leaves on orthotropic branches. Between these two, there were slightly more leaf contacts per transect in Antirrhoea with less tilted leaves and a more compact crown (low ratio of crown radius to crown depth) than in Anacardium, despite lower crown depth in the former. The mean number of leaf contacts per transect was intermediate to low for plagiotropic species (Luehea and Castilla), and lowest for Cecropia, which presents leaves in a manner to avoid overlap at the tip of terminal branches (Fig. 1). Absorptance of individual leaves did not differ significantly between well-lit sun branches and shade branches, except for Anacardium (0·87 for shade leaves vs. 0·90 for sun leaves, P = 0·02). Thus, species medians for pooled data were compared among species (Table 1). Leaf angle and absorptance differed significantly among species but lacked any apparent relationship with branch orientation, overall architecture or successional status. Anacardium had more steeply angled leaves than all other species.
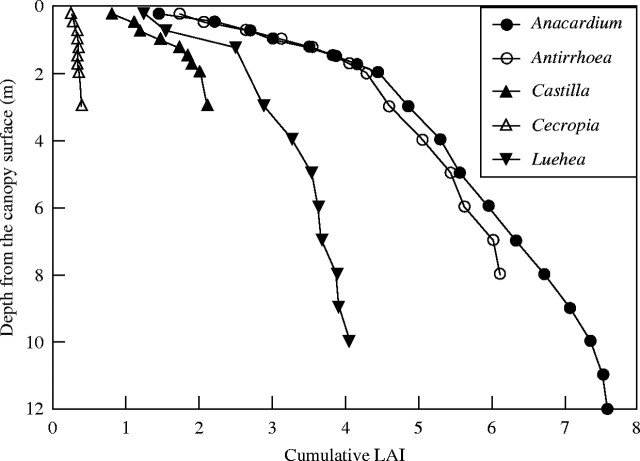
The five species differed greatly in the average leaf area accumulation rates with depth, as well as the maximum crown LAI (Fig. 2). Branch orientation appeared to be more important as a determinant of the patterns of LAI increase with crown depth than successional status. Two species with orthotropic branches, Ancardium and Antirrhoea, accumulated LAI very rapidly within the top 2 m from the crown surface (Fig. 2). They continuously accumulated LAI at slower rates at deeper crown depths to achieve high total crown LAI, that was greater for Anacardium with a greater crown depth than for Antirrhoea. Two species with plagiotropic branches, Luehea and Castilla, accumulated LAI rapidly within the first 1 m of the outermost crown surface, and then more slowly to result in much lower total LAI. Their LAI accumulation was slower with a lower maximum than that of Anacardium and Antirrhoea. Cecropia, a pioneer species with non-overlapping leaves at branch tips (Fig. 1E and J) had low crown LAI (maximum <0·5).
Fig. 2.
Mean cumulative leaf area index (horizontal axis) as a function of depth from the crown surface (vertical axis) for five canopy trees. Leaf contacts were counted for every 0·25–1 m down to the lowest leaf along each vertical transect through each crown. The cumulative LAI was estimated by dividing cumulative leaf contact with cosine of the median leaf angle of each species.
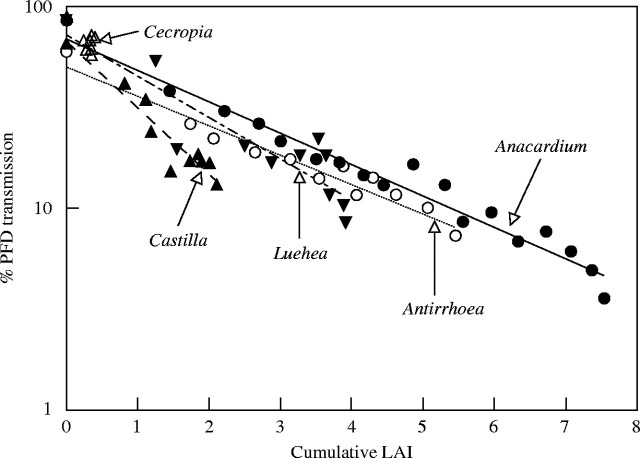
Light transmitted through tree crowns decreased as an exponential function of cumulative LAI in all species but Cecropia as predicted by the Beer's law of light extinction (Fig. 3). Unlike the other four species, Cecropia did not show any predictable light extinction, as LAI did not accumulate through the crowns of this species (open triangles clustered at the upper left corner in Fig. 3). The remaining four species differed in light extinction coefficients in relation to their terminal shoot inclination (Fig. 3). Anacardium and Antirrhoea with orthotropic branches showed similarly low extinction coefficients (K = 0·347 and 0·345, respectively) even though individual leaves are more inclined in Anacardium than in Antirrhoea (Table 2). Anacardium had higher %T for a given value of cumulative LAI (Fig. 3) because Anacardium trees were taller and received, on average, greater light at the crown surface than Antirrhoea. However, the minimal PFD was lower for Anacardium due to its greater total crown LAI and canopy depth than Antirrhoea. Two species with plagiotropic terminal shoots had significantly greater extinction coefficients than those with orthotropic terminal shoots. Luehea with curving relay branches that end with plagiotropic terminal shoots had an extinction coefficient of 0·526, whereas Castilla whose horizontal branches cluster around a monopodial trunk had an even higher coefficient of 0·795. Thus, Luehea with more inclined branches on average had a slower rate of light extinction than Castilla, even though their median leaf angles were similar (Table 1). However, it was darker under later successional Luehea than under Castilla as a result of the greater total LAI of the former (Fig. 3).
Fig. 3.
Light extinction as a function of cumulative leaf area index (LAI) through crowns of five neotropical tree species (see Table 1 for full names). Mean % PFD transmission (vertical axis) at a given depth from the canopy surface is plotted against mean LAI (horizontal axis) above that depth.
Table 2.
Means (s.d.) for gas exchange characteristics and leaf mass per area (LMA) for leaves grown in exposed (sun) and shaded (shade) regions of the crowns of five canopy tree species
| Species |
Branch light |
N |
Median leaf life span (d) |
N |
Amax (µmol m−2 s−1) |
gs (mol m−2 s−1) |
N |
LMA (g m−2) |
|---|---|---|---|---|---|---|---|---|
| Anacardium excelsum | Sun | 5873 | 250 | 82 | 7·3 (2·8)** | 0·213 (0·098)* | 20 | 111·9 (23·4)*** |
| Shade | 1739 | 186 | 28 | 5·3 (3·6) | 0·164 (0·128) | 20 | 90·7 (13·0) | |
| Luehea seemannii | Sun | 5736 | 158*** | 16 | 10·5 (3·1)* | 0·505 (0·283)** | 25 | 138·1 (28·8)*** |
| Shade | 1448 | 189 | 10 | 8·0 (2·5) | 0·217 (0·069) | 19 | 78·6 (13·1) | |
| Antirrhoea trichantha | Sun | 2764 | 155 | 26 | 10·8 (3·0)*** | 0·412 (0·182)*** | 6 | 65·3 (8·8)*** |
| Shade | – | – | 12 | 5·5 (2·8) | 0·264 (0·192) | 6 | 32·7 (2·9) | |
| Castilla elastica | Sun | 515 | 180 | 13 | 13·7 (2·2)*** | 0·276 (0·083)*** | 13 | 87·2 (6·5)*** |
| Shade | – | – | 16 | 4·5 (1·5) | 0·139 (0·084) | 16 | 32·7 (8·7) | |
| Cecropia longipes | Sun | 699 | 84 | 10 | 25·5 (3·6) | 0·800 (0·345) | 15 | 88·7 (13·2) |
Samples size (N) for gs is the same as that for Amax.
Note that there were no shade leaves for Cecropia.
The median leaf life span (data for shade branches available only for the first two species) is indicated.
Asterisks indicate significant difference between sun and shade branches (*P < 0·05; **P < 0·005; ***P < 0·0005).
Light extinction in the canopy crust of Anacardium
The crown surface of an emergent Anacardium tree showed asymmetric topography in both horizontal and vertical directions (Fig. 4A and B). The crown spread was much more extensive to north-west than to south-east; along the north-west transect, crown surface sloped more gently, and foliated branches were found as far as 19 m below the crown apex, and 13 m away in the horizontal direction. Optically estimated LAI at the surface of and 0·5 m inside the canopy crust increased with descent from the apex along each transect (Fig. 4C and D). The optically estimated LAI reflects leaf area distribution within the 148° view angle above the point of measurement. Consequently, optically estimated LAI at the crown surface was greater than zero at lower heights (Fig. 4C and D, open circles) because of neighbour trees as well as high portions of the same tree crown. The optical LAI at 0·5 m inside the canopy crust reflected these neighbour effects plus those of the foliage immediately over the measurement positions (Fig. 4C and D, lines through closed circles). The LAI difference between the surface and inside of the canopy crust, which could be considered as an estimate of the LAI of the 0·5 m deep crown crust itself, was generally <3·0 at a given measurement position but somewhat greater at lower heights. The maximum values of optical LAI was similar between the two transects, even though the north-west transect extended to a lower height from the ground. The ratio of PFD inside to that at the surface of the crown crust was 0·04–1·00 with a mean of 0·39 (i.e. a 0·5-m-deep layer of crown crust caused 61 % reduction of PFD). The PFD extinction as a function of optical LAI was steeper inside than at the surface of the canopy crust in the south-east transect, but it was similar in the north-west transect (Fig. 4E and F). Light extinction coefficients estimated as regression slopes for 0·5 m inside the crown crust were 0·621 and 0·760 for north-west and south-east transects, respectively (Fig. 4E and F, closed symbols). These values based on optical LAI were higher than the extinction coefficient estimated from vertical transects for the whole crown for the same species (0·347; Fig. 3).
Fig. 4.
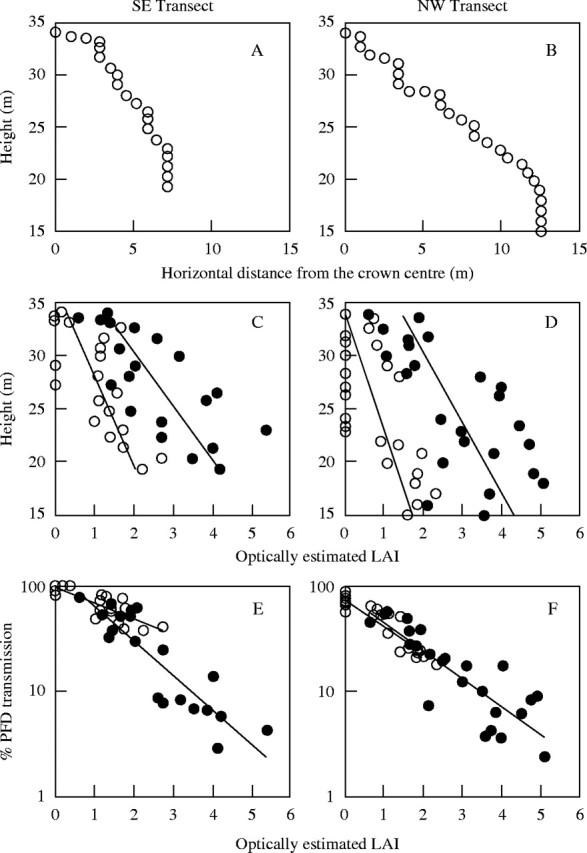
Foliage and light distribution at the surface (open circles) and 0·5 m inside (closed circles) of the crown crust of an emergent Anacardium excelsum tree. (A, B) Canopy surface topography along two transects laid from the apex of the crown to south-east (125°) and north-west (300°); height from the ground and horizontal distance from the apex was measured at every 1-m interval along the transects. (C, D) LAI estimated optically with a canopy analyser (LAI-2000, Li-Cor) as a function of the height from the ground. (E, F) Percentage PFD transmission as a function of optically estimated LAI within the canopy crust.
Leaf position effects within branches
Relative total daily PFD at the individual leaf surface, expressed as the percentage of the above-canopy daily PFD, showed a significant negative correlation with leaf position for shade branches of Anacardium (Fig. 5A; r2 = 0·59, P = 0·0014) and sun branches of Cecropia (Fig. 5C; r2 = 0·59, P = 0·0008). Although older leaves at more proximal positions (i.e. farther away from the tip) should experience greater degrees of self-shading, the light environment at the surface of a leaf was independent of its position within sun branches of Anacardium (Fig. 5A) and both types of branches of Luehea (Fig. 5B). Examination of data for individual shoots did not reveal better relationships. In all cases, leaves at similar positions experienced widely different relative daily PFD, probably due to variation in leaf orientation. Even leaves at the most distal positions near the tips of well-exposed sun branches usually experienced PFD much less than 100 % PFD at the horizontal plane, probably due to leaf angles, leaf orientation and effects of neighbouring branches. Not surprisingly, leaves on the shade branches received less light than those on the sun branches (closed vs. open circles, Fig. 5A and B). No leaves existed below 10 % of full sun for Cecropia, which lacked shade branches.
Fig. 5.
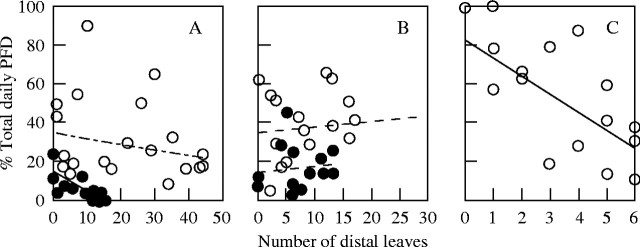
The relationship of % total daily PFD at individual leaf surface (100 × total daily PFD per unit leaf area divided by above-canopy PFD on the horizontal plane, vertical axis) to leaf position within the terminal shoots (indicated by the number of distal leaves relative to the focal leaf, horizontal axis) for three canopy tree species with contrasting branch architecture. (A) Anacardium with steeply angled leaves around orthotropic shoots, (B) Luehea with variously angled leaves along plagiotropic shoots, and (C) Cecropia with minimally overlapping leaves at the tips of orthotropic shoots. Open circles, upper canopy branches; closed circles, shade branches in the lower part of the crown. No shade branch existed for Cecropia. Solid and broken lines indicate significant (P < 0·05) and non-significant regressions, respectively, for sun and shade branches.
Leaf traits in sun vs. shade branches
Leaves on sun branches had higher photosynthetic rates (Amax) and stomatal conductance (gs) under saturating light than those on shade branches (Table 2). These differences were accompanied by differences in leaf mass per area (LMA), but little difference in photosynthetic rates per unit mass (Amax/LMA). Two later successional species, Anacardium and Luehea, showed smaller differences between sun and shade branches than did two early successional species, Antirrhoea and Castilla. Leaf life-span data for shade branches were available only for Anacardium and Luehea. Leaves on sun branches had shorter life span than those on shaded branches in Luehea, but there was no significant difference in Anacardium (Table 2).
DISCUSSION
The inclination of individual leaves determines the light extinction coefficient in herbaceous canopies (Monsi and Saeki, 1953; Saeki, 1960). In contrast, the inclination of terminal shoots, rather than individual leaves, appears to be more important for light extinction through tree canopies. Light measured on a horizontal plane declined more slowly along the vertical transects through crowns of the species with orthotropic shoots (Anacardium and Antirrhoea) than those with plagiotropic shoots (Luehea and Castilla). The milder gradient of PFD in crowns consisting of orthotropic shoots was accompanied by a greater total LAI and darker environment underneath. Within each group, later successional species exhibited a greater total LAI and light extinction than did early successional species (Anacardium > Antirrhoea; Luehea > Castilla). Further, light availability at the individual leaf surface was not a simple function of leaf position within the terminal shoot in canopy trees. Rather, light availability at individual leaves varied widely at similar positions within the terminal shoot due to variation in leaf orientation and angle. The causes and implications of these findings are discussed in relation to species differences in architecture and leaf arrangement.
Light extinction through the whole crown
The light extinction characteristics of tree crowns differed among species in relation to several traits, including successional status, tree and branch architecture, and leaf angles. Estimated light extinction coefficients (0·37–0·80) generally corresponded to those for steeply inclined leaves in herbaceous communities (<1·0). Measurements were taken mostly under clear sky conditions, which should result in a greater light extinction coefficient for a given leaf angle than under overcast conditions (Saeki, 1963). Under overcast conditions, extinction coefficients should be even lower. Both LAI and light intensity showed large variation at a given depth among trees within each species; but on average, they exhibited patterns that could be linked to species-specific architecture. However, the light incident on individual leaves was unrelated to the position of leaves within shoots in the upper crown for both orthotropic Anacardium and plagiotropic Luehea (Fig. 5). Thus, simulations of tree architecture and light utilization, which treat foliated branches as a unit, are appropriate for most tree species (e.g. Takenaka, 1994). Crowns of later successional species exhibited greater canopy depth and total crown LAI. This explains the pattern found by Brown and Parker (1994) at the community level for a temperate broad-leaved forest; early successional stands have higher extinction coefficients and lower total LAI than late successional stands. The greater total LAI and total light extinction exhibited by individual crowns of later successional species may be an adaptive strategy to extract light resources and cast shade upon their subdominant neighbours (Horn, 1971; Canham et al., 1994). Although canopy species composition can significantly affect light transmittance at the stand level in the absence of gaps (Messier and Bellefleur, 1988; Canham et al., 1994; Kabakoff and Chazdon, 1996), the understorey light environment is affected also by subcanopy trees and understorey plants (Nicotra et al., 1999; Denslow and Guzman, 2000; Montgomery and Chazdon, 2001). At our site, canopy openness and total daily PFD at 1·8 m above the ground directly below crowns of Anacardium, Luehea and Castilla did not differ significantly among species despite strong interspecific differences in total crown LAI and percentage transmittance, except in the late dry season when Castilla crowns were completely deciduous (K. Kitajima, S. S. Mulkey and S. J. Wright, unpubl. res.).
Canopy crust topography and light utilization
The arrangement of the photosynthetic surface in curved or tilted layers results in more uniform light distribution among leaves and enhances the photosynthetic productivity of the canopy as a whole, such that stand-level productivity per unit ground area continuously increases with increasing PFD (Boysen Jensen, 1932; Monsi and Saeki, 1953). The rough surface topography of forest tree crowns achieves this on a large spatial scale. The height of the forest canopy surface at the present study site was very heterogeneous, partly because this stand included both tall species that persist into late successional stands (e.g. Anacardium and Luehea) and shorter species that are restricted to early successional stands (e.g. Antirrhoea and Castilla). Community-level measurements of canopy topography at this study site (G. Parker, pers. comm.) also support this view (also see Parker and Brown, 2000). Thus, the heterogeneity of canopy structure at the present study site (a 75–100-year-old successional stand) resembles mature stands more than the successional stands (15–20 years old) in Costa Rica studied by Montgomery and Chazdon (2001).
Both height and orientation are important aspects of crown surface topography. Tree crown development is often asymmetric because crowns tend to grow away from near neighbours and into adjacent tree-fall gaps (Jones and Harper, 1987; Young and Hubbell, 1991; Young and Perkocha, 1994; Umeki, 1995; Olsen, 2001). In contrast, variation in crown topography relative to the diurnal course of the sun has not been well studied. Zotz et al. (1995) found that east-facing leaves that received direct sunlight during the early morning achieved greater photosynthetic production and higher photosynthetic water use efficiency than west-facing leaves for a canopy tree, Ficus insipida, at the present study site. The more moderate sloping of crown surface to west than to east (Fig. 4A) thus increased interception of more valuable morning light for the crown as a whole than would a completely symmetrical crown. The effects of orientation on crown topology and leaf physiology may be worth examining in the future. Overall, light extinction at 0·5 m below the crown surface appeared to follow Beer's law of light extinction, but this analysis is somewhat circular because LAI was optically estimated. The heterogeneous distribution of foliage was likely to cause an underestimation of LAI by optical method (Welles and Norman, 1991), and consequently a greater estimated light extinction coefficient for the crown crust (Fig. 4) than for the whole crown of the same species (Fig. 3). Nevertheless, the optical method may be useful in assessing the effects of neighbours at different positions in the upper canopy.
Lack of light gradients within terminal shoots
Unlike pioneer trees with orthotropic terminal shoots (Fig. 5C; also Ackerly, 1999; Kitajima et al., 2002), two mature canopy tree species exhibited no relationship between leaf position and leaf light environment within sun branches (Fig. 5A and B). Luehea with plagiotropic branches showed a complete lack of relationship both for sun branches and shade branches, because self-shading was caused by upper branches rather than by distal leaves within a horizontal branch. Anacardium with orthotropic branches showed a non-significant trend in sun branches and a significant gradient in shade branches. This species displays steeply inclined leaves in dense spirals (Fig. 1a), which probably resulted in unpredictable shading patterns within terminal sun branches. But, in shade branches that received most light as diffused radiation, there was a more predictable self-shading gradient even though shade branches are inclined more horizontally. Light availability at individual leaf surfaces may be predicted only through analysis with a three-dimensional architecture program that takes into account the precise orientation and angle of leaves, as well as shading by neighbouring leaves and branches (Pearcy and Yang, 1996).
Whether light availability predictably declines with leaf position within shoots should affect the rate at which leaf nitrogen content and photosynthetic capacity change with leaf age. Grassland plants and pioneer trees develop leaves successively at regular intervals, and leaf nitrogen content and photosynthetic capacity decline linearly with leaf age and position within shoots. The relatively short leaf life spans of such species are predicted to equal the leaf age at which the daily net photosynthetic rate declines to zero by an optimality model in which the lifetime carbon gain for each leaf is maximized (Saeki, 1960; Mooney et al., 1981; Hikosaka et al., 1994; Hikosaka, 1996; Ackerly, 1999; Kitajima et al., 2002). In contrast, later successional trees extend shoots and produce new leaves in pulses, resulting in non-steady development of self-shading with leaf ageing, especially in species with plagiotropic terminal shoots (Kikuzawa et al., 1996). This decoupling of leaf age and leaf position, in combination with the lack of predictable self-shading in relation to a leaf position (Fig. 5A and B), provide an explanation for slower declines of photosynthetic rates with leaf age in late successional species with long leaf life spans, such as Anacardium and Luehea (Kitajima et al., 1997a). Indeed, for these species, the observed leaf life span is predicted only by an alternative optimality model in which daily carbon gain rate averaged across leaf lifetime is maximized (Kikuzawa, 1991; Kitajima 1997a; Kikuzawa and Ackerly, 1999; Hikosaka, 2005). It is concluded that late successional trees exhibit patterns of leaf display that result in more equal sharing of available light among leaves within shoots, which leads to lower overall light extinction coefficients at the crown level and slower declines of leaf nitrogen content and photosynthetic rate in relation to leaf age.
Sun vs. shade branches
Unlike most herbaceous plants and pioneer trees that produce leaves only near their upper part, trees produce leaves in both well-lit and shaded positions of the crown. This allows tree crowns to exploit light more completely (Terashima et al., 2005). Three of our study species exhibited the classical phenotypic differentiation of sun vs. shade leaves (Table 2); higher leaf mass, photosynthetic capacity and stomatal conductance per unit leaf area for leaves on sun branches than those on shaded branches. In addition, the shorter leaf life span of Luehea for sun branches than shade branches was also expected because greater productivity and growth in the upper canopy layer should result in shorter mean leaf longevity and greater leaf turn-over rates (Koike, 1986; Osada et al., 2001).
Anacardium exhibited only weak differentiation to sun vs. shade phenotypes and showed no difference in leaf life span between sun and shade branches (Table 2). Phenotypic plasticity of leaf photosynthetic traits is often weaker for late successional species (Strauss-Debenedetti and Bazzaz, 1991; Valladares et al., 2000). Lack of sun-shade acclimation of leaves is uncommon, but it is found among various life forms of tropical forest plants (Mulkey, 1986; Hogan, 1988; Mulkey et al., 1991; Kitajima, 1994). The steeply inclined leaves of Anacardium, which are presented in dense whorls, resulted in more uniform sharing of light among leaves and smaller differences in leaf phenotype between upper and lower branches within the crown. During the period of active leaf production (February–March), Anacardium leaves on sun branches become shaded by newer leaves that are successively produced above them even before they mature physiologically. Only half of the Anacardium leaves on sun branches received >20 % total daily PFD (Fig. 5A). Lack of strongly sun-acclimated leaves in this species is part of a strategy to distribute light more uniformly within its crown through strongly inclined leaves surrounding orthotropic branches.
Diversity of leaf display strategies in time and space
Only a small fraction of the diversity of leaf presentation strategies observed among tropical trees is reported here. For understorey plants, Valladares et al. (2002) concluded that various architecture and leaf display patterns result in surprisingly similar light use efficiency estimated by a three dimensional simulation. In contrast, the present analysis of canopy trees suggests a large variation in light use efficiency among species. Functional diversity in light utilization characteristics by tree crowns should be examined not only in space, but also in time (Mulkey et al., 1996; Wright, 1996). Water becomes increasingly limiting during the dry season, whereas the heavy cloud cover during the wet season limits light availability and canopy productivity (Wright and Van Schaik, 1994; Graham et al., 2003). In most species in the present study site, canopy leaf area builds up over the course of 5–8 months during the wet season, except for Anacardium which produces leaves during the dry season (S. J. Wright, unpubl. res.). In four of the study species, leaves produced during the early wet season have lower LMA and photosynthetic capacity than those produced immediately before the dry season (Kitajima et al., 1997b). In a much shorter time scale, Luehea leaves increase their angles by >30°, on average, in response to water and light stress later in the day (K. Kitajima, S. S. Mulkey and S. J. Wright, unpubl. res.). Canopy trees acclimate to daily and seasonal heterogeneity in resource availability by adjusting when to display what types of leaves at what angles and degrees of self-shading.
Optimization of carbon gain with respect to availability of light and water is achieved through a diversity of leaf displays in time and space. Greater inclination of leaves and shoots are often, but not always associated with more uniform sharing of light within a crown, which in turn enables greater total LAI and more complete light exploitation. Crowns of later successional trees often, but not necessarily cast deeper shade underneath. Combinations of certain traits, such as orientation of terminal shoots and leaves, leaf longevity and leaf phenology, provide useful guidelines to understand functionally convergent patterns of leaf display among forest trees.
Acknowledgments
We received financial support from the National Science Foundation (IBN-9220759 to S.S.M. and S.J.W.; BIR-9419994 to S.S.M.), the Smithsonian Scholarly Studies Program (to S.S.M. and S.J.W.), and the Andrew W. Mellon Foundation (to S.J.W. and K.K.). Figure 1 was drawn by Donna Conlon. We thank M. Samaniego and M. Garcia for their valuable assistance in the field, and G. Parker, D. Ackerly, E. G. Leigh and R. Montgomery for constructive comments.
LITERATURE CITED
- Ackerly D. 1996. Canopy structure and dynamics: integration of growth processes in tropical pioneer trees. In: Mulkey SS, Chazdon RL, Smith AP, eds. Tropical forest plant ecophysiology. New York: Chapman and Hall, 619–658. [Google Scholar]
- Ackerly D. 1999. Self-shading, carbon gain and leaf dynamics: a test of alternative optimality models. Oecologia 119: 300–310. [DOI] [PubMed] [Google Scholar]
- Anderson MC. 1966. Stand structure and light penetration. II. A theoretical analysis. Journal of Applied Ecology 3: 41–54. [Google Scholar]
- Ashton PS. 1978. Crown charactersitics of tropical trees. In: Tomlinson PB, Zimmerman MH, eds. Tropical trees as living systems. Cambridge: Cambridge University Press, 591–615. [Google Scholar]
- Baldocchi D, Collineau S. 1994. The physical nature of solar radiation in heterogeneous canopies: spatial and temporal attributes. In: Caldwell MM, Pearcy RW, eds. Exploitation of environmental heterogeneity by plants. San Diego: Academic Press, 21–71. [Google Scholar]
- Borchert R, Tomlinson PB. 1984. Architecture and crown geometry in Tabebuia rosea (Bignoniaceae). American Journal of Botany 71: 958–969. [Google Scholar]
- Boysen Jensen P. 1932.Die stoffproduktion der pflanzen. Juna: Gustav Fischer. [Google Scholar]
- Brown MJ, Parker GG. 1994. Canopy light transmittance in a chronosequence of mixed-species deciduous forest. Canadian Journal of Forest Research 24: 1694–1703. [Google Scholar]
- Canham CD, Finzi AD, Pacala SW, Burbank DH. 1994. Causes and consequences of resource heterogeneity in forests: interspecific variation in light transmission by canopy trees. Canadian Journal of Forest Research 24: 337–340. [Google Scholar]
- Corner EJH. 1964.The life of plants. Cleveland, Ohio: World Publishing. [Google Scholar]
- Croat TB. 1978.The Flora of Barro Colorado Island. Stanford: Stanford University Press. [Google Scholar]
- D'Arcy WG. 1987.Flora of Panama. Monographs in systematic botany, Vol. 18. St Louis: Missouri Botanical Garden. [Google Scholar]
- Denslow JS, Guzman S. 2000. Variation in stand structure, light and seedling abundance across a tropical moist forest chronosequence, Panama. Journal of Vegetation Science 11: 201–212. [Google Scholar]
- Field CB. 1983. Allocation of leaf nitrogen for the maximization of carbon gain: leaf age as a control of the allocation program. Oecologia 56: 341–347. [DOI] [PubMed] [Google Scholar]
- Graham EA, Mulkey SS, Kitajima K, Phillips NG, Wright SJ. 2003. Cloud cover limits net CO2 updtake and growth of a rainforest tree during tropical rainy seasons. Proceedings of the National Academy of Science of the USA 100: 572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallé F, Oldeman RAA, Tomlinson PB. 1978.Tropical trees and forest. Springer: Berlin. [Google Scholar]
- Hikosaka K. 1996. Effects of leaf age, nitrogen nutrition and photon flux density on the organization of the photosynthetic apparatus in leaves of a vine (Ipomoea tricolor Cav) grown horizontally to avoid mutual shading of leaves. Planta 198: 144–150. [DOI] [PubMed] [Google Scholar]
- Hikosaka K. 2005. Leaf canopy as a dynamic system: ecophysiology and optimality in leaf turnover. Annals of Botany 95: 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K, Terashima I, Katoh S. 1994. Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Oecologia 97: 451–457. [DOI] [PubMed] [Google Scholar]
- Hirose T. 2005. Development of the Monsi-Saeki theory on canopy structure and function. Annals of Botany 95: 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Werger MJA. 1987. Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 72: 520–526. [DOI] [PubMed] [Google Scholar]
- Hirose T, Werger MJA. 1987. Nitrogen use efficiency in instantaneous and daily photosynthesis in the canopy of Solidago altissima stand. Physiologia Plantarum 70: 215–222. [Google Scholar]
- Hogan KP. 1988. Photosynthesis in two neotropical palm species. Functional Ecology 2: 371–377. [Google Scholar]
- Horn H. 1971.Adaptive geometry of trees. Princeton, NJ: Princeton University Press. [Google Scholar]
- Johnson PL, Atwood DM. 1970. Aerial sensing and photographic study of the El Verde rain forest. In: Odum HT, ed. A tropical rain forest. Washington, DC: Atomic Energy Commission. [Google Scholar]
- Jones M, Harper FRH. 1987. The influence of neighbours on the growth of trees. II. The fate of buds on long and short shoots in Betula pendula Proceedings of the Royal Society of London B 232: 19–33. [Google Scholar]
- Kabakoff RP, Chazdon RL. 1996. Effects of canopy species dominance on understory light availability in low-elevation secondary forest stand in Costa Rica. Journal of Tropical Ecology 12: 779–788. [Google Scholar]
- Kikuzawa K. 1991. A cost-benefit analysis of leaf habit and leaf longevity of trees and their geographical pattern. American Naturalist 138: 1250–1263. [Google Scholar]
- Kikuzawa K. 1995. Leaf phenology as an optimal strategy for carbon gain in plants. Canadian Journal of Botany 73: 158–163. [Google Scholar]
- Kikuzawa K, Ackerly D. 1999. Significance of leaf longevity in plants. Plant Species Biology 14: 39–45. [Google Scholar]
- Kikuzawa K, Koyama H, Umeki K, Lechowicz MJ. 1996. Some evidence for an adaptive linkage between leaf phenology and shoot architecture in sapling trees. Functional Ecology 10: 252–257. [Google Scholar]
- King DA, Leigh EG, Condit R, Foster RB, Hubbell SP. 1997. Relationships between branch spacing, growth rate and light in tropical forest saplings. Functional Ecology 11: 627–635. [Google Scholar]
- Kitajima K. 1994. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98: 419–428. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Mulkey SS, Samaniego M, Wright SJ. 2002. Decline of photosynthetic capacity with leaf age and position in two tropical pioneer tree species. American Journal of Botany 89: 1925–1932. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Mulkey SS, Wright SJ. 1997. Decline of photosynthetic capacity with leaf age in relation to leaf longevities for five tropical canopy tree species. American Journal of Botany 84: 702–708. [PubMed] [Google Scholar]
- Kitajima K, Mulkey SS, Wright SJ. 1997. Seasonal leaf phenotypes in the canopy of a tropical dry forest: photosynthetic characteristics and associated traits. Oecologia 109: 490–498. [DOI] [PubMed] [Google Scholar]
- Kohyama T. 1987. Significance of architecture and allometry in saplings. Functional Ecology 1: 399–404. [Google Scholar]
- Kohyama T. 1991. A functional model describing sapling growth under a tropcial forest canopy. Functional Ecology 5: 83–90. [Google Scholar]
- Kohyama T, Hotta M. 1990. Significance of allometry in tropical saplings. Functional Ecology 4: 515–521. [Google Scholar]
- Koike F. 1986. Canopy dynamics estimated from shoot morphology in an evergreen broad-leaved forest. Oecologia 70: 348–350. [DOI] [PubMed] [Google Scholar]
- Koike F, Syahbuddin. 1993. Canopy structure of a tropical rain forest and the nature of an unstratified upper layer. Functional Ecology 7: 230–235. [Google Scholar]
- Kuuluvainen T, Pukkala T. 1989. Effects of crown shape and tree distribution on the spatial distribution of shade. Agricultural and Forest Meteorology 40: 215–231. [Google Scholar]
- Leigh EG. 1990. Tree shape and leaf arrangement: a quantitative comparison of montane forests, with emphasis on Malaysia and South India. In: Daniel JC, Serrao JS, eds. Conservation in developing countries: problems and prospects. Bombay: Oxford University Press, 119–174. [Google Scholar]
- Leigh EG, Jr. 1999.Tropical forest ecology. a view from Barro Colorado Island. New York: Oxford University Press. [Google Scholar]
- Messier C, Bellefleur P. 1988. Light quantity and quality on the forest floor of pioneer and climax stages in a birch-beech-sugar maple stand. Canadian Journal of Forest Research 18: 615–622. [Google Scholar]
- Monsi M, Saeki T. 1953. Über den lichtfaktor in den pflanzengesellschaften und seine bedeutung füe die stoffproduktion. Japanese Journal of Botany 14: 22–52. [Google Scholar]
- Montgomery RA, Chazdon RL. 2001. Forest structure, canopy architecture, and light transmittance in tropical wet forests. Ecology 82: 2707–2718. [Google Scholar]
- Mooney HA, Field C, Gulmon SL, Bazzaz FA. 1981. Photosynthetic capacity in relation to leaf position in desert versus old-field annuals. Oecologia 50: 109–112. [DOI] [PubMed] [Google Scholar]
- Mulkey SS. 1986. Photosynthetic acclimation and water-use efficiency of three species of understory herbaceous bamboo (Gramineae) in Panama. Oecologia 70: 514–519. [DOI] [PubMed] [Google Scholar]
- Mulkey SS, Kitajima K, Wright SJ. 1995. Photosynthetic capacity and leaf longevity in the canopy of a dry tropical forest. Selbyana 16: 169–173. [Google Scholar]
- Mulkey SS, Kitajima K, Wright SJ. 1996. Plant physiological ecology of forest canopies. Trends in Ecology and Evolution 11: 408–412. [DOI] [PubMed] [Google Scholar]
- Mulkey SS, Smith AP, Wright SJ. 1991. Comparative life history and physiology of two understory Neotropical herbs. Oecologia 88: 263–273. [DOI] [PubMed] [Google Scholar]
- Nicotra AB, Chazdon RL, Iriarte SVB. 1999. Spatial heterogeneity of light and woody seedling regeneration in tropical wet forests. Ecology 80: 1908–1926. [Google Scholar]
- Niklas KJ. 1994.Plant allometry. The scaling of form and process. Chicago: University of Chicago. [Google Scholar]
- Olsen T. 2001. Architecture of a cool-temperate forest canopy. Ecology 82: 2719–2730. [Google Scholar]
- Osada N, Takeda H, Furukawa A, Awang M. 2001. Leaf dynamics and maintenance of tree crowns in a Malaysian rain forest stand. Journal of Ecology 89: 774–782. [Google Scholar]
- Parker GG, Brown MJ. 2000. Forest canopy stratification—is it useful? American Naturalist 155: 473–484. [DOI] [PubMed] [Google Scholar]
- Pearcy RW, Yang WM. 1996. A three-dimensional crown architecture model for assessment of light capture and carbon gain by understory plants. Oecologia 108: 1–12. [DOI] [PubMed] [Google Scholar]
- Pearcy, RW, Roden JS, Gamon JA. 1990. Sunfleck dynamics in relation to canopy structure in a soybean (Glycine max (L.) Merr.) canopy. Agricultural and Forest Meteorology 52: 359–372. [Google Scholar]
- Saeki T. 1960. Interrelationships between leaf amount, light distribution and total photosynthesis in a plant community. Botanical Magazine, Tokyo 73: 55–63. [Google Scholar]
- Saeki T. 1963. Light relations in plant communities. In: Evans LT, ed. Environmental control of plant growth. New York: Academic Press, 79–94. [Google Scholar]
- Sands PJ. 1995. Modelling canopy production. 1. Optimal distribution of photosynthetic resources. Australian Journal of Plant Physiology 22: 593–601. [Google Scholar]
- Shukla RP, Ramakrishnan PS. 1986. Architecture and growth strategies of tropical trees in relation to successional status. Journal of Ecology 74: 33–46. [Google Scholar]
- Strauss-Debenedetti S, Bazzaz FA. 1991. Plasticity and acclimation to light in tropical Moraceae of different successional positions. Oecologia 87: 377–387. [DOI] [PubMed] [Google Scholar]
- Takenaka A. 1994. A simulation-model of tree architecture development based on growth-response to local light environment. Journal of Plant Research 107: 321–330. [Google Scholar]
- Terashima I, Araya T, Miyazawa S, Sone K, Yano S. 2005. Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: an eco-developmental treatise. Annals of Botany 95: 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terborgh J. 1985. The vertical component of plant species diversity in temperate and tropical forests. American Naturalist 126: 760–776. [Google Scholar]
- Traw MB, Ackerly DD. 1995. Leaf position, light levels, and nitrogen allocation in five species of rain forest pioneer trees. American Journal of Botany 82: 1137–1143. [Google Scholar]
- Umeki K. 1995. A comparison of crown asymmetry between Picea abies and Betula maximowicziana Canadian Journal of Forest Research 25: 1876–1880. [Google Scholar]
- Valladares F, Skillman JB, Pearcy RW. 2002. Convergence in light capture efficiencies among tropical forest understory plants with contrasting crown architectures: a case of morphological compensation. American Journal of Botany 89: 1275–1284. [DOI] [PubMed] [Google Scholar]
- Valladares F, Wright SJ, Lasso E, Kitajima K, Pearcy RW. 2000. Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 81: 1925–1936. [Google Scholar]
- Welles J, Norman J. 1991. An instrument for indirect measurement of canopy architecture. Agronomy Journal 83: 818–825. [Google Scholar]
- Wright SJ. 1996. Phenological responses to seasonality in tropical forest plants. In: Mulkey SS, Chazdon RL and Smith AP, eds. Tropical forest plant ecophysiology. New York: Chapman and Hall, 440–460. [Google Scholar]
- Wright SJ, van Schaik CP. 1994. Light and the phenology of tropical trees. American Naturalist 143: 192–199. [Google Scholar]
- Yoda K. 1974. Three-dimensional distribution of light intensity in a tropical rain forest of west Malaysia. Japanese Journal of Ecology 24: 247–254. [Google Scholar]
- Yoda K, Nishioka M, Dhanmanonda P. 1983. Vertical and horizontal distribution of relative illuminance in the dry and wet seasons in a tropical dry-evergreen forest in Sakaerat, NE Thailand. Japanese Journal of Ecology 33: 97–100. [Google Scholar]
- Young TP, Hubbell SP. 1991. Crown asymmetry, treefalls, and repeat disturbance of broad-leaved forest gaps. Ecology 72: 1464–1471. [Google Scholar]
- Young TP, Perkocha V. 1994. Tree falls, crown asymmetry and buttresses. Journal of Ecology 82: 319–324. [Google Scholar]
- Zotz G, Harris G, Koniger M, Winter K. 1995. High rates of photosynthesis in the tropical pioneer tree Ficus insipida Willd. Flora 190: 265–272. [Google Scholar]