Abstract
• Background and Aims The regrowth dynamics after defoliation of the invasive grass Calamagrostis epigejos were studied. As nitrogen (N) reserves have been shown to play an important role during plant regrowth, the identity, location and relative importance for regrowth of N stores were determined in this rhizomatous grass.
• Methods Plant growth, nitrate uptake and root respiration were followed during recovery from defoliation. Water soluble carbohydrates, nitrate, free amino acids and soluble proteins were analysed in the remaining organs.
• Key Results Nitrate uptake and root respiration were severely reduced during the first days of regrowth. Roots were the main net source of mobilized N. The quantitatively dominant N storage compounds were free amino acids. Free amino acids and soluble proteins in the roots decreased by 55 and 50 %, respectively, and a substantial (∼38 %) decrease in stubble protein was also observed. Although the relative abundance of several soluble proteins in roots decreased during the initial recovery from defoliation, no evidence was found for vegetative storage protein (VSP). Furthermore, rhizomes did not act as a N storage compartment.
• Conclusions Production of new leaf area was entirely reliant, during the first week after defoliation, on N stores present in the plant. Mobilized N originated mainly from free amino acids and soluble proteins located in roots, and less so from proteins in stubble. Presence of VSP in the roots was not confirmed. The data suggest that rhizomes played an important role in N transport but not in N storage.
Keywords: Calamagrostis epigejos L. Roth, invasive species, nitrate uptake, N storage, N mobilization, N reserves, rhizome, root respiration, vegetative storage protein, VSP
INTRODUCTION
Plant adaptations to defoliation include a suite of morphological and physiological responses (Richards, 1993). The ability of plants to use internal stores of carbon (C) and nitrogen (N) to re-establish photosynthetically active leaf area rapidly and to supply maintenance demands of the remaining organs is one of the key factors affecting plant survival during the first days of regrowth (Volenec et al., 1996). Regrowing plants may be limited more by N than by C stores, since N uptake can be severely reduced or stopped for several days following defoliation (Clement et al., 1978; Jarvis and Macduff, 1989).
Organic nitrogen and nitrate represent the major stores of N in plants (Millard, 1988). In grasses, free amino acids have been shown to play an important role in N storage (Nordin and Näsholm, 1997; Gloser, 2002), but a decrease in the soluble protein content coupled with an increase in the proteinase activity after defoliation has suggested a significant role for soluble proteins in supplying N for regrowing leaves in the grass Lolium perenne (Ourry et al., 1989). Specialized vegetative storage proteins (VSP; Staswick, 1994) have been identified in several herbaceous and tree species (Hendershot and Volenec, 1993a, b; Stepien et al., 1994; Corre et al., 1996), but were not found in L. perenne (Louahlia et al., 1999). No other grass species has been studied in this respect.
Calamagrostis epigejos is a perennial rhizomatous grass that is expanding vigorously into many natural and semi-natural habitats in Eurasia, changing plant community composition. Attempts to reduce the abundance of C. epigejos by cultivation or burning are usually not successful since site disturbance can actually enhance its spread (Rebele and Lehmann, 2001). On the other hand, from forest management practices, it is known that selective mowing reduces the competitiveness of C. epigejos (Rebele and Lehmann, 2001). However, knowledge about recovery from defoliation for this species is limited to field observations (Jakrlová and Sedláková, 1998; Rebele and Lehmann, 2002). An analysis of physiological mechanisms underlying the response of C. epigejos to defoliation is lacking. Levels of N-compounds in C. epigejos have shown seasonal dynamics (Gloser, 2002) that are typical of N reserves in perennial species (e.g. Cyr et al., 1990). For instance, the content of free amino acids and soluble proteins in overwintering organs of C. epigejos halved during the first month after the onset of growth (Gloser, 2002), suggesting an extensive use during spring regrowth in field conditions. However, knowledge of the use of N stores in rhizomatous grasses after defoliation is limited.
In this study, the regrowth dynamics of C. epigejos plants after a single severe defoliation were analysed. The aim was to determine the identity, location and relative importance for regrowth of N stores in this rhizomatous grass.
MATERIALS AND METHODS
Plant material and growth conditions
Caryopses of Calamagrostis epigejos were collected in a forest near Brno, Czech Republic. They were sown in containers filled with washed quartz sand and the resulting seedlings were transferred into aerated hydroponic culture 3 weeks after sowing. The modified Hoagland's solution used contained 603 μm Ca(NO3)2, 795 μm KNO3, 190 μm KH2PO4, 270 μm MgSO4, 2 μm MnSO4, 0·85 μm ZnSO4, 0·15 μm CuSO4, 20 μm H3BO3, 0·25 μm Na2MoO4 and 40·5 μm FeNa-EDTA. Twelve plants were grown in each of 7 L plastic containers and the nutrient solution was renewed weekly. The pH of the nutrient solution was kept at 5·8 by regular adjustment with sulphuric acid. Growth chamber conditions were 338 ± 16 µmol m−2 s−1 photosynthetically active radiation and 20/15 °C (16 h light/8 h dark).
Defoliation and harvests
Plants were defoliated at 5 cm above the shoot/root junction 7 weeks after germination. Four plants were sampled immediately before defoliation (day 0) and then at 2, 4, 6, 11 and 21 days after defoliation. Harvests started 6 h into the light period. The length of the shoot was measured as the distance between the tip of the longest leaf and the shoot/root junction, and the leaf area of the whole plant was determined as the sum of areas of all leaf blades from digitalized images. Plants were separated into roots, rhizomes, stubble (basal 2 cm of the shoot, composed mainly of leaf sheaths), leaf sheaths and leaf blades. Samples were frozen in liquid nitrogen, freeze-dried, ground, and stored at −20 °C.
Specific nitrate uptake rate (NUR) and root respiration rate
Four individual plants, randomly selected for subsequent harvesting, were transferred into 300 mL cylinders filled with aerated nutrient solution 18 h before NUR measurements. An open-flow system supplied nutrient solution continuously so that the nitrate concentration was maintained constant. After plant acclimation, the flow of the nutrient solution was stopped and nitrate depletion in the solution was measured. Measurements started 4 h into the light period on days 0, 2, 4, 6 and 11 after defoliation, but were not done on day 21 because the roots were too big to fit available cylinders. Samples of the solution were taken at the beginning and at the end of the 2-h exposure and nitrate concentration was determined colorimetrically (Cataldo et al., 1975). Root respiration was measured on the same plants immediately after NUR measurements. An excised root sample was placed in an airtight cuvette equipped with a Clark-type oxygen electrode (Labio, Praha, Czech Republic), filled with stirred, air-saturated nutrient solution maintained at 20 °C. The rate of decrease of oxygen concentration in the solution was measured polarographically for 20–30 min (Lambers et al., 1993).
Chemical analyses
N-compounds were extracted from plant material with 50 mm phosphate buffer (pH 7·6); nitrate content was determined colorimetrically (Cataldo et al., 1975); soluble proteins were assayed according to Bradford (1976) using BSA as a standard; and the content of free amino acids was estimated using a reaction with ninhydrin, with leucin as a standard (Rosen, 1957). Before the analysis of amino-N, proteins were precipitated with 5 % sulphosalicylic acid to prevent interference with the assay. Total water-soluble carbohydrates (WSC) were determined according to Schnyder and de Visser (1999). N-compound contents were expressed per unit structural dry weight, estimated as the difference between total dry weight of the sample and its WSC content.
Electrophoresis of soluble proteins. Soluble proteins from 100–150 mg of freeze-dried samples were extracted twice with 3·5 mL of 50 mm Tris/Cl buffer (pH 7·5, 10 μm leupeptin, 100 mm dithiothreitol, 1 mm EDTA). After centrifugation (11 000 g, 10 min), the two supernatants were combined. Nucleic acids were precipitated with protamine sulphate (1 mg mL−1) and the pellet was discarded after centrifugation (30 000 g, 10 min). Proteins in the supernatant were precipitated by the sodium deoxycholate–trichloracetic acid method (Peterson, 1983). The protein pellet was washed with ice-cold acetone, dried and stored at −70 °C. All procedures were carried out at 4 °C. The pellet was resuspended in the sample buffer (5 min, 100 °C). Because of large changes in total protein content, and for comparison with published results, root extracts were selected for analysis of changes in protein composition. SDS–PAGE was run according to Coligan et al. (1995) on the Protean II xi Cell system (Bio Rad, USA). One well was used for molecular weight markers (Molecular Weight Standard Broad Range, Bio Rad, USA). Equal amounts of total soluble protein were loaded. Gels were silver-stained according to Damerval et al. (1987) and scanned (Personal Densitometer SI, Molecular Dynamics, USA). Quantitative image analyses were run in ImageQuant (Molecular Dynamics, USA).
Statistics
Data were analysed in STATISTICA 5·5 (Statsoft Inc., Tulsa, USA) in the module ‘General Linear Model’. The homogeneity of variances between groups was checked with Hartley's and Cochran's tests, and normality of residuals with K-S and Shapiro–Wilk's test. Heterogeneous data sets were transformed. Comparisons between control values of plants just before defoliation (day 0) and the data of defoliated plants during regrowth (day 2–day 21) were based on the Dunnett test.
RESULTS
Plant growth and morphology after defoliation
Defoliation removed all leaf area, and resulted in a low leaf mass ratio of 0·02 kg blade kg−1 total plant mass 2 d after defoliation. A 22 % decline in the total plant dry weight occurred between day 2 and day 6 of regrowth (P < 0·05; Fig. 1A), despite the fact that the mass of leaf blades was increasing. This initial decline in the total plant biomass was due to a slight decrease in the dry weight of all remaining organs (Table 1). Thereafter, plant biomass increased rapidly and, after 21 d of regrowth, the plants had 50 % more total dry weight than plants harvested just before defoliation (P < 0·05). Leaf area per plant after 21 d of regrowth was 64 % higher than in plants just before defoliation (P < 0·05; Fig. 1B), and the number of leaves per plant had doubled (an increase from 45 to 95 leaves per plant, data not shown). However, the shoot length after 21 d of regrowth was reduced by 23 %, compared with the value before defoliation (P < 0·05; Fig. 1C).
Fig. 1.
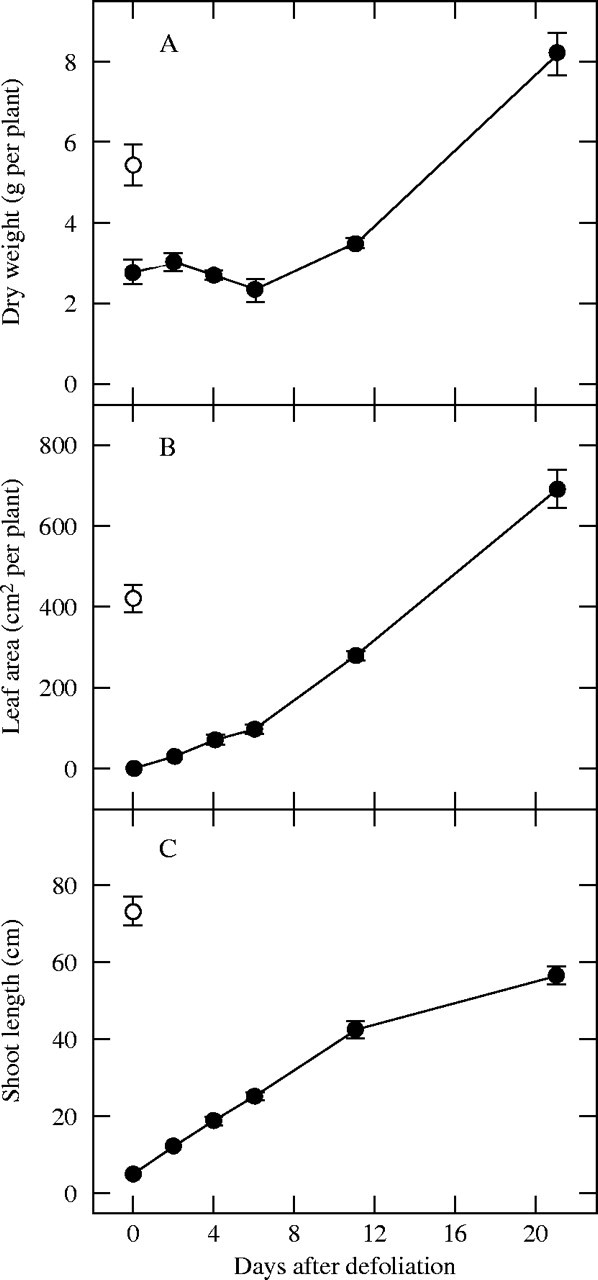
Effect of defoliation on (A) total plant dry weight, (B) leaf area and (C) shoot length of C. epigejos plants during 21 d of regrowth following defoliation (closed symbols). The open symbols represent values for plants just before defoliation. Values are mean ± s.e. of four replicates.
Table 1.
Changes in the plant dry weight of compartments of C. epigejos plants during regrowth after defoliation. Day 0 indicates values for plants just prior to defoliation
| Days after defoliation |
Leaf blade (g) |
Leaf sheath (g) |
Stubble (g) |
Rhizome (g) |
Root (g) |
|---|---|---|---|---|---|
| 0 | 2·27 ± 1·43 | 1·25 ± 0·14 | 0·52 ± 0·05 | 0·36 ± 0·05 | 1·03 ± 0·17 |
| 2 | 0·06 ± 0·01 | 0·59 ± 0·05 | 0·56 ± 0·03 | 0·39 ± 0·04 | 1·39 ± 0·13 |
| 4 | 0·20 ± 0·03 | 0·51 ± 0·04 | 0·46 ± 0·03 | 0·39 ± 0·04 | 1·54 ± 0·05 |
| 6 | 0·25 ± 0·04 | 0·45 ± 0·07 | 0·36 ± 0·05 | 0·32 ± 0·05 | 0·95 ± 0·13 |
| 11 | 0·92 ± 0·06 | 0·66 ± 0·04 | 0·50 ± 0·01 | 0·40 ± 0·06 | 1·01 ± 0·08 |
| 21 | 2·96 ± 0·21 | 1·51 ± 0·10 | 1·12 ± 0·08 | 0·65 ± 0·03 | 1·94 ± 0·23 |
| MSD* | 0·55 | 0·37 | 0·22 | 0·20 | 0·64 |
Minimum significant differences from the Dunnett test (MSD; P = 0·05) for comparison of the control value (day 0) with values during regrowth are included for each parameter. Values represent means ± s.e. of four replicates.
Specific nitrate uptake rate and root respiration rate
Defoliation reduced the NUR in the first 2 d after defoliation to less than 1 % of the NUR of plants just before defoliation (P < 0·05; Fig 2). NUR was negative 4 d after cutting and started to recover from day 6 after defoliation. Since root respiration rate was also reduced during the first 4 d of regrowth (P < 0·05; Fig. 2), root respiration rate and NUR were positively correlated (r = 0·90, P < 0·05; Fig. 2).
Fig. 2.
Specific nitrate uptake rate (NUR, open symbols) and root respiration rate (R, closed symbols) of C. epigejos plants during 11 d of regrowth after defoliation. The relationship between NUR and R is shown as an inset. Values are means ± s.e. of four replicates.
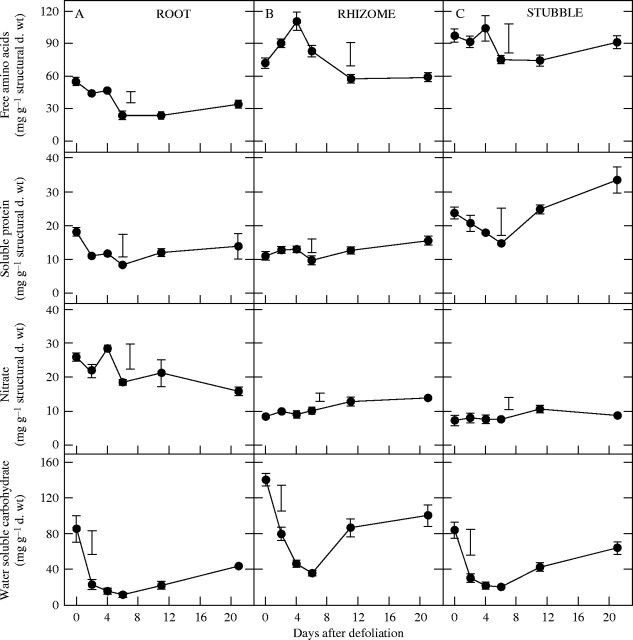
Content of N-compounds and water-soluble carbohydrates
Defoliation had immediate effects on the content of N-compounds, which were specific to both compartment and N-compound (Fig. 3). Quantitatively, the most important changes occurred in the content of free amino acids and soluble proteins in the roots, which decreased during the first 6 d of regrowth by 58 % and 54 %, respectively (P < 0·05; Fig. 3A). This corresponds to a decrease of 35 mg of free amino acids and 11 mg of soluble proteins per plant (Fig. 3 and Table 1). Although the content of amino acids in the root increased thereafter, it was still significantly lower at day 21 than before defoliation (P < 0·05). The nitrate content in roots did not vary significantly between day 0 and day 11 (P > 0·05).
Fig. 3.
Content of free amino acids, soluble protein, nitrate and total water soluble carbohydrate present in (A) roots, (B) rhizomes and (C) stubble bases per unit of biomass of C. epigejos plants during regrowth after defoliation. Bars indicate minimum significant differences (P = 0·05) from the Dunnett test for comparison between control (day 0) and values during the regrowth. Values are means ± s.e. of four replicates.
In the rhizomes, the content of free amino acids increased during the first 4 d of regrowth by 53 % (P < 0·05; Fig. 3B). From day 4 to day 21, free amino acids declined to values similar to those of plants just before defoliation (P > 0·05). Total soluble protein and nitrate contents in rhizomes were effectively constant during the first phase of regrowth.
Free amino acids and nitrate content in the stubble during the regrowth period did not differ from values of plants just before defoliation (P > 0·05; Fig. 3C), but soluble protein content decreased by 38 % from defoliation to day 6, which represents 7 mg of soluble protein per plant (Fig. 3C and Table 1). Afterwards, it increased steadily.
The content of water-soluble carbohydrates (WSC) was strongly reduced by defoliation in all measured compartments (Fig. 3). At day 6 after defoliation, WSC content was reduced to 14 %, 25 % and 24 % of the value of plants just before defoliation in roots, rhizomes and stubble, respectively (P < 0·05).
One-dimensional SDS–PAGE profile of soluble proteins
Major variation in protein content occurred in roots, where total soluble proteins decreased from 18·7 to 7·8 mg plant−1 during the first 6 d of regrowth. A representative gel showing the changes in root soluble proteins is shown in Fig. 4. Changes in the relative abundance of different molecular weight protein bands in the roots occurred. For example, the relative abundance of 63·5 kDa (initially 2 % of total protein), 55 kDa (4 %), 41 kDa (5 %) and 28 kDa (4 %) bands decreased in the first 2 d after cutting, recovering thereafter.
Fig. 4.
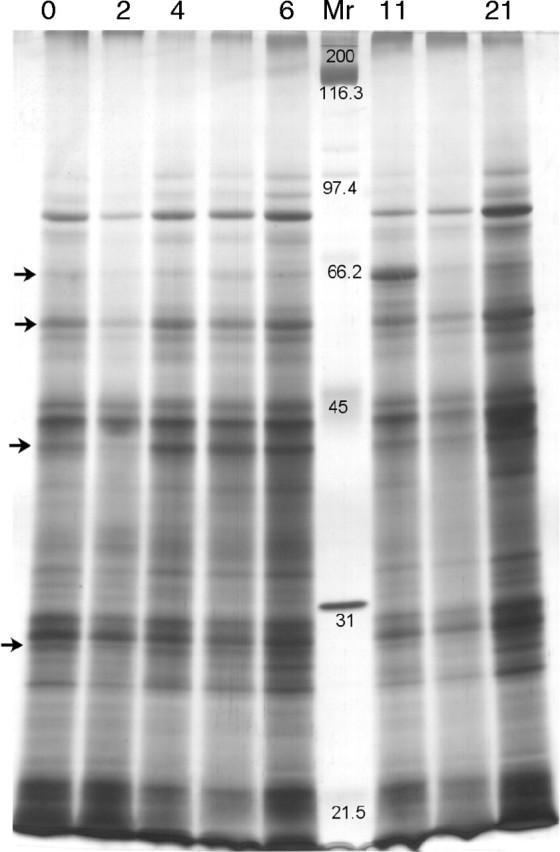
One-dimensional SDS–PAGE profile of soluble proteins from roots of C. epigejos plants during regrowth after defoliation. The numbers above the lanes refer to sampling day (days after defoliation). Lanes without numbers are not presented in the Results section. The positions of molecular weight markers (kDa) are depicted in the ‘Mr’ lane. The positions of bands affected by defoliation (63·5, 55, 41 and 28 kDa) are indicated by arrows. The same amount of total soluble protein was loaded into each well and proteins were visualized by silver staining.
DISCUSSION
Plant growth and morphology after defoliation
Two distinct phases could be distinguished in the time course of regrowth of C. epigejos plants: 0–6 d, and 6–21 d. During the initial phase the total plant biomass decreased, but it was rapidly restored to pre-defoliation levels by the next week. Plant height was still reduced at the end of the regrowth period, despite the fact that leaf dry weight and leaf area had already surpassed the values of plants just before defoliation. This is consistent with the observation that repeated mowing in field conditions reduced the height of the C. epigejos canopy (Jakrlová and Sedláková, 1998). Such regrowth dynamics, generally including reduction of individual tiller size, is a common morphological response of grasses to defoliation (Chapman and Lemaire, 1993; Louahlia et al., 2000). The results of the present experiment show that C. epigejos has a similar morphological response to defoliation to that of forage grass species.
The use of internal N stores during regrowth
In the initial phase of C. epigejos regrowth, NUR was reduced to nearly zero. This result is analogous to that found in Lolium perenne grown with a non-limiting external N supply both before and after defoliation (Clement et al., 1978; Louahlia et al., 1999). The negative value of NUR in the early phase of regrowth may be due to both a reduction in influx and an increase in efflux of nitrate, as for reported for defoliated ryegrass (Macduff and Jackson, 1992). The root respiration rate of C. epigejos plants was reduced during the first phase of regrowth, as also found in Dactylis glomerata (Davidson and Milthorpe, 1966). Although the WSC content of the roots decreased after defoliation, there was no correlation between root respiration rate and WSC (P > 0·10). The 60 % decrease in root respiration rate may be partly related to the reduction in root growth rate (Bingham et al., 1996), but may also be due to the decrease in NUR, since a substantial proportion of respiratory energy in roots is used for nutrient uptake (Poorter et al., 1991).
Due to the decrease in NUR, the initial re-establishment of leaf area relied entirely on N-compounds already present in the plant at the time of defoliation. During the initial phase of regrowth, roots were a net source of N, providing most of the mobilized N, as shown by the ∼50 % decrease in free amino acids and soluble protein content in roots of defoliated plants (Fig. 3). Similarly, in the grass Festuca rubra, defoliation caused a substantial mobilization of N from roots to growing leaves (Thornton et al., 2002). Decreases in free amino acids and soluble protein content may have resulted from an increase in mobilization, and/or reduction of synthesis and/or deposition rate and may be related to the observed slow-down in root growth rate. The sampling strategy used may have slightly underestimated the mobilization from the remaining leaf sheath bases, as the stubble was composed of source (sheaths) as well as sink tissue (active leaf meristems), but the predominant net mobilization of N from roots in C. epigejos plants is in agreement with the results obtained in Lolium perenne (Louahlia et al., 2000).
The role of soluble proteins as a store of N for regrowth
Although, quantitatively, the most important changes occurred in free amino acids, the content of total soluble proteins in the roots and stubble of C. epigejos was also substantially reduced in the first days after defoliation. Further, the relative abundance of several root soluble proteins (63·5, 55, 41 and 28 kDa) decreased (Fig. 4). A similar decrease in the abundance of specific proteins was also observed in stubble and roots of L. perenne during the first 4 d of regrowth (Louahlia et al., 1999). Several criteria have been proposed for defining vegetative storage proteins (Cyr and Bewley, 1990; Staswick, 1994): VSP should be (1) prominent in the protein spectrum, (2) mobilized readily, but (3) should not have an enzymatic function. In both C. epigejos and L. perenne, the detected proteins were not of major abundance in the soluble protein profile, and thus the criteria were not satisfied. The increase in the decomposition rate and/or decrease in the rate of synthesis of soluble proteins in roots following defoliation was selective. However, these proteins were probably involved in inhibited processes (e.g. N uptake and assimilation) rather than constituting true VSP. In contrast to dicotyledons (Hendershot and Volenec, 1993a, b), VSPs seem to have no relevant role in N storage in grasses.
The role of rhizomes in N storage
Rhizomes can serve as storage organs for C and N in perennial plants with clonal growth (Suzuki and Stuefer, 1999). However, in the present experiment, rhizomes were not a net source of N. The content of free amino acids in rhizomes increased immediately after defoliation, in contrast to the changes typically observed during reserve mobilization in storage organs (Hendershot and Volenec, 1993a, b). Moreover, the increase in free amino acids was not accompanied by a concomitant change in soluble protein content. In a previous study, the total amount of N per plant present in rhizomes during the annual cycle was lower than in roots or stubbles (Gloser, 2002), due to relatively low allocation of biomass to rhizomes. Thus, rhizomes may have an important function in transport of N stores from source organs of mature tillers to young tillers initiated at the distal limits of rhizomes, but they play only a minor role in N storage.
CONCLUSIONS
Regrowth dynamics of the rhizomatous grass C. epigejos following severe defoliation included morphological (reduction of plant height) and physiological responses. As in other grasses, internal N stores were extensively used to support leaf (re)growth following defoliation. Mobilized N was mainly free amino acids (from roots), but soluble proteins (from roots and stubble) also contributed. No evidence of VSP was found. Along with prior results, this suggests that VSPs may be of no relevance as N stores for grasses. Likewise, rhizomes were not a source of mobilized N, but they may play an important role in transport of stored N between tillers.
Supplementary Material
Acknowledgments
We thank Oldřich Jůza for assistance with chemical analyses. WSC were analysed at the Chair of Grassland Science, Technische Universität München, Germany. This research was supported by the Grant Agency of the Czech Republic, grant No. 206/98/P268.
Footnotes
Current address: Chair of Grassland Science, Technische Universität München, D-85350 Freising-Weihenstephan, Germany
LITERATURE CITED
- Bingham IJ, Panico A, Stevenson EA. 1996. Extension rate and respiratory activity in the growth zone of wheat roots: time-course for adjustments after defoliation. Physiologia Plantarum 98: 201–209. [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Cataldo DA, Haroon M, Schrader LE, Youngs VL. 1975. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Communications in Soil Science and Plant Analysis 6: 71–80. [Google Scholar]
- Chapman DF, Lemaire G. 1993. Morphogenetic and structural determinants of plant regrowth after defoliation. In: Baker MJ, ed. Proceedings of the XVII International Grassland Congress. Wellington: SIR Publishing, 95–104. [Google Scholar]
- Clement CR, Hopper MJ, Jones LHP, Leafe EL. 1978. The uptake of nitrate by Lolium perenne from flowing nutrient solution 2. Effect of light, defoliation, and relationship to CO2 flux. Journal of Experimental Botany 29: 1173–1183. [Google Scholar]
- Coligan JE, Dunn BM, Ploegh HL, Speicher DW, Wingfield PT. 1995. One-dimensional SDS gel electrophoresis of proteins. In: Chanda VB, ed. Current protocols in protein science. New York: John Wiley and Sons. Unit 10·1. [Google Scholar]
- Corre N, Bouchart V, Ourry A, Boucaud J. 1996. Mobilization of nitrogen reserves during regrowth of defoliated Trifolium repens L. and identification of potential vegetative storage proteins. Journal of Experimental Botany 47: 1111–1118. [Google Scholar]
- Cyr DR, Bewley JD. 1990. Proteins in the roots of the perennial weeds chicory (Cichorium intybus L.) and dandelion (Taraxacum officinale Weber) are associated with overwintering. Planta 182: 370–374. [DOI] [PubMed] [Google Scholar]
- Cyr DR, Bewley JD, Dumbroff EB. 1990. Seasonal dynamics of carbohydrate and nitrogenous components in the roots of perennial weeds. Plant, Cell & Environment 13: 359–365. [Google Scholar]
- Damerval C, Leguilloux M, Blaisonneau J, Devienne D. 1987. A simplification of Heukeshoven and Dernicks silver staining of proteins. Electrophoresis 8: 158–159. [Google Scholar]
- Davidson JL, Milthorpe FL. 1966. The effect of defoliation on the carbon balance in Dactylis glomerata Annals of Botany 30: 185–198. [Google Scholar]
- Gloser V. 2002. Seasonal changes of nitrogen storage compounds in a rhizomatous grass Calamagrostis epigeios Biologia Plantarum 45: 563–568. [Google Scholar]
- Hendershot KL, Volenec JJ. 1993. Nitrogen pools in taproots of Medicago sativa L. after defoliation. Journal of Plant Physiology 141: 129–135. [Google Scholar]
- Hendershot KL, Volenec JJ. 1993. Taproot nitrogen accumulation and use in overwintering alfalfa (Medicago sativa L). Journal of Plant Physiology 141: 68–74. [Google Scholar]
- Jakrlová J, Sedláková I. 1998. Vliv kosení na produkci a parametry růstu populace Calamagrostis epigejos. In: Eliáš P, ed. Populačná biológia rastlín, Bratislava-Nitra: SEKOS, 78–82. [Google Scholar]
- Jarvis SC, Macduff JH. 1989. Nitrate nutrition of grasses from steady-state supplies in flowing solution culture following nitrate deprivation and/or defoliation. 1. Recovery of uptake and growth and their interactions. Journal of Experimental Botany 218: 965–975. [Google Scholar]
- Lambers H, van der Werf A, Bergkotte M. 1993. Respiration: the alternative pathway. In: Hendry GAF, Grime JP, eds. Methods in comparative plant ecology. A laboratory manual. London: Chapman and Hall, 140–144. [Google Scholar]
- Louahlia S, Laine P, Thornton B, Ourry A, Boucaud J. 2000. The role of N-remobilisation and the uptake of NH4+ and NO3− by Lolium perenne L. in laminae growth following defoliation under field conditions. Plant and Soil 220: 175–187. [Google Scholar]
- Louahlia S, Macduff JH, Ourry A, Humphreys M, Boucaud J. 1999. Nitrogen reserve status affects the dynamics of nitrogen remobilization and mineral nitrogen uptake during recovery of contrasting cultivars of Lolium perenne from defoliation. New Phytologist 142: 451–462. [Google Scholar]
- Macduff JH, Jackson SB. 1992. Influx and efflux of nitrate and ammonium in italian ryegrass and white clover roots: comparisons between effects of darkness and defoliation. Journal of Experimental Botany 43: 525–535. [Google Scholar]
- Millard P. 1988. The accumulation and storage of nitrogen by herbaceous plants. Plant, Cell & Environment 11: 1–8. [Google Scholar]
- Nordin A, Näsholm T. 1997. Nitrogen storage forms in nine boreal understorey plant species. Oecologia 110: 487–492. [DOI] [PubMed] [Google Scholar]
- Ourry A, Bigot J, Boucaud J. 1989. Protein mobilization from stubble and roots, and proteolytic activities during post-clipping re-growth of perennial ryegrass. Journal of Plant Physiology 134: 298–303. [Google Scholar]
- Peterson GL. 1983. Determination of total protein. Methods in Enzymology 91: 95–119. [DOI] [PubMed] [Google Scholar]
- Poorter H, Van der Werf A, Atkin OK, Lambers H. 1991. Respiratory energy requirements of roots vary with the potential growth rate of plant species. Physiologia Plantarum 83: 469–475. [Google Scholar]
- Rebele F, Lehmann C. 2001. Biological flora of Central Europe: Calamagrostis epigejos (L.) Roth. Flora 196: 325–344. [Google Scholar]
- Rebele F, Lehmann C. 2002. Restoration of a landfill site in Berlin, Germany by spontaneous and directed succession. Restoration Ecology 10: 340–347. [Google Scholar]
- Richards JH. 1993. Physiology of plants recovering from defoliation. In: Baker MJ, ed. Proceedings of the XVII International Grassland Congress. Wellington: SIR Publishing, 85–94. [Google Scholar]
- Rosen H. 1957. A modified ninhydrin colorimetric analysis for amino acids. Archives of Biochemistry and Biophysics 67: 10–15. [DOI] [PubMed] [Google Scholar]
- Schnyder H, de Visser R. 1999. Fluxes of reserve-derived and currently assimilated carbon and nitrogen in perennial ryegrass recovering from defoliation. The regrowing tiller and its component functionally distinct zones. Plant Physiology 119: 1423–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE. 1994. Storage proteins of vegetative plant tissues. Annual Review of Plant Physiology and Plant Molecular Biology 45: 303–322. [Google Scholar]
- Stepien V, Sauter JJ, Martin F. 1994. Vegetative storage proteins in woody plants. Plant Physiology and Biochemistry 32: 185–192. [Google Scholar]
- Suzuki JI, Stuefer J. 1999. On the ecological and evolutionary significance of storage in clonal plants. Plant Species Biology 14: 11–17. [Google Scholar]
- Thornton B, Paterson E, Kingston-Smith AH, Bollard AL, Pratt SM, Sim A. 2002. Reduced atmospheric CO2 inhibits nitrogen mobilization in Festuca rubra Physiologia Plantarum 116: 62–72. [DOI] [PubMed] [Google Scholar]
- Volenec JJ, Ourry A, Joern BC. 1996. A role for nitrogen reserves in forage regrowth and stress tolerance. Physiologia Plantarum 97: 185–193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.