Abstract
• Background and Aims Under high photon flux, excitation energy may be in excess in aluminum (Al)-treated leaves, which use a smaller fraction of the absorbed light in electron transport due to decreased CO2 assimilation compared with normal leaves. The objectives of this study were to test the hypothesis that the antioxidant systems are up-regulated in Al-treated citrus leaves and correlate with protection from photoxidative damage, and to test whether xanthophyll cycle-dependent thermal energy dissipation is involved in dissipating excess excitation energy.
• Methods ‘Cleopatra’ tangerine seedlings were fertilized and irrigated daily for 8 weeks with quarter-strength Hoagland's nutrient solution containing Al at a concentration of 0 or 2 mM from Al2(SO4)3.18H2O. Thereafter, leaf absorptance, chlorophyll (Chl) fluorescence, Al, pigments, antioxidant enzymes and metabolites were measured on fully expanded leaves.
• Key Results Compared with control leaves, energy was in excess in Al-treated leaves, which had smaller thermal energy dissipation, indicated by non-photochemical quenching (NPQ). In contrast, conversion of violaxanthin (V) to antheraxanthin (A) and zeaxanthin (Z) at midday increased in both treatments, but especially in Al-treated leaves, although A + Z accounted for less 40 % of the total xanthophyll cycle pool in them. Activities of superoxide dismutase (SOD), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR) and catalase (CAT), and concentrations of ascorbate (AsA), dehydroascorbate (DASA), reduced glutathione (GSH) and oxidized glutathione (GSSG) were higher in Al-treated than in control leaves.
• Conclusions These results corroborate the hypothesis that, compared with control leaves, antioxidant systems are up-regulated in Al-treated citrus leaves and protect from photoxidative damage, whereas thermal energy dissipation was decreased. Thus, antioxidant systems are more important than thermal energy dissipation in dissipating excess excitation energy in Al-treated citrus leaves.
Key words: Aluminum, antioxidant systems, Citrus reshni, ‘Cleopatra’ tangerine, non-photochemical quenching, thermal energy dissipation, xanthophyll cycle
INTRODUCTION
Aluminum (Al) in mildly acidic or neutral soils occurs primarily as insoluble deposits and is essentially biologically inactive. However, in many acid soils Al toxicity is a major factor limiting crop productivity; it is particularly important throughout the tropics and subtropics.
Since CO2 assimilation decreases in leaves supplied with Al (Pereira et al., 2000; Chen et al., 2005), only a fraction of the absorbed light energy is used in electron transport. As a result, there is more excess excitation energy in leaves with large Al content than in normal leaves, particularly under high photon flux (PF). Excess absorbed light can be dissipated as heat through xanthophyll cycle-dependent thermal energy dissipation in the antenna pigment complexes of PSII (Demmig-Adams and Adams, 1996; Niyogi et al., 1998). However, Lu et al. (2003) reported that although excess excitation energy increased in the salt-acclimated halophyte Artimisia anethifolia, there was no change in thermal energy dissipation, indicated by non-photochemical quenching (NPQ). It was suggested that the Mehler reaction and/or photorespiration could be enhanced to dissipate excess excitation energy. The NPQ is highly correlated with the concentration of antheraxanthin (A) + zeaxanthin (Z) (Demmig-Adams and Adams, 1996; Chen and Cheng, 2003; Cheng, 2003). However, some studies have shown no correlation between NPQ and A + Z concentration (Förster et al., 2001; Cousins et al., 2002). Although the effects of many environmental stresses (water, temperature, nutrients, salt) on xanthophyll cycle-dependent thermal energy dissipation have been examined in some detail (Adams et al., 1994; Cousins et al., 2002; Chen and Cheng, 2003; Cheng, 2003; Lu et al., 2003), little is known about the response of xanthophyll cycle-dependent thermal energy dissipation of leaves to Al.
An alternative route for energy dissipation and consumption of photosynthetic electrons is directly in the water–water (Asada) cycle or indirectly in photorespiration (Asada, 1999). Many enzymes such as superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), monodehydroascorbate reductase (MDAR) and dehydroascorbate reductase (DHARR), and antioxidant metabolites such as ascorbate (AsA), and reduced glutathione (GSH), all involved in the water–water cycle, as well as catalase (CAT) involved in scavenging the bulk H2O2 generated by photorespiration may be enhanced in Al-treated leaves, serving to protect them from photo-oxidative damage under high light. In a study, Kuo and Kao (2003) investigated the effects of Al on activities of SOD, CAT, GR and APX in detached rice (Oryza sativa ‘Taichung Native 1’) leaves. Aluminum decreased SOD activity, but increased CAT and GR activities. APX activity was increased only after prolonged treatment. However, activities of MDAR and DHAR and the concentrations of AsA and GSH were not determined.
Potentially, Al might alter the synthesis and breakdown of particular components and so affect the mechanisms of energy dissipation in citrus leaves. Citrus belongs to evergreen subtropical fruit trees and is known to be sensitive to Al. Low pH and high Al concentration are the factors contributing to poor citrus growth and shortened lifespan of trees (Lin and Myhre, 1990). Although the effects of Al on mineral nutrient (Lin and Myhre, 1991b) and CO2 assimilation (Pereira et al., 2000; Chen et al., 2005) of citrus have been investigated by a few researchers, very little is known about the effects of Al on the photoprotectives system of citrus leaves. Therefore it is a useful plant to analyse the responses of different mechanisms of energy dissipation in photosynthetic metabolism to Al.
The aims of this study were to test the hypothesis that the antioxidant systems are up-regulated and function to protect from photo-oxidative damage, and to establish whether xanthophyll cycle-dependent thermal energy dissipation is involved in dissipating the increased excess excitation energy in Al-treated citrus leaves.
MATERIALS AND METHODS
Plant culture and Al treatments
Seeds of ‘Cleopatra’ tangerine (Citrus reshni Hort. ex Tanaka), an Al-resistant rootstock used in citrus cultivation (Lin and Myhre, 1991a), were germinated in plastic trays containing MetroMix 360 (The Scotts Co., Marysville, OH, USA), and irrigated when necessary with quarter-strength Hoagland's nutrient solution. Four weeks after germination, uniform seedlings with a single stem were selected, transplanted into 1·7 L plastic pots containing MetroMix 360, and grown in a greenhouse under a natural photoperiod. Each pot contained one seedling, and was supplied twice weekly with 150 mL of quarter-strength Hoagland's nutrient solution. Five weeks after transplanting, each seedling was supplied daily until dripping with quarter-strength Hoagland's nutrient solution at an Al concentration of 0 or 2 mm from Al2(SO4)3.18H2O. The pH of both control and treatment solutions was adjusted to 4·1 using 0·1 m HCl or 0·1 m NaOH. There were 30 trees per Al treatment in a completely randomized design. Seventeen weeks after germination, leaf absorptance, chlorophyll (Chl) fluorescence, activities of antioxidant enzymes, and contents of Al, pigments and antioxidant metabolites were measured.
Measurements of leaf absorptance, Chl fluorescence and CO2 assimilation
Leaf reflectance and transmittance were measured with a LI-1800 spectroradimeter and 1800-12S integrating sphere (Li-Cor Inc., Lincoln, Nebraska, USA) (Chen and Cheng, 2003). There were five replicates per treatment (one leaf per replicate, one leaf per plant).
Chlorophyll fluorescence was measured with a pulse-modulated fluorometer FMS2 (Hansatech Instruments Ltd., Norfolk, UK) both at midday under full sun [photon flux (PF) of 1020 ± 34 µmol m−2 s−1; henceforth ‘full sun’ refers to this value] and before dawn (Chen and Cheng, 2003). Non-photochemical quenching (NPQ) was calculated according to Bilger and Björkman (1990) and maximum PSII efficiency of dark-adapted leaves (Fv/Fm) was calculated as in van Kooten and Snel (1990). Photochemical quenching coefficient qP, efficiency of excitation transfer to open PSII centres (Fv′/Fm′), and PSII quantum efficiency were calculated as in Genty et al. (1989). The rate of electron transport was estimated from (Fm′ − Fs)/Fm′ × 0·5 × leaf absorptance × PF, with PSI photochemistry assumed to equal PSII (Genty et al., 1990). The rate of excess energy production was estimated according to Kato et al. (2003). There were eight replicates per treatment (one leaf per replicate, one leaf per plant).
Leaf CO2 assimilation was measured with a CIRAS-1 portable photosynthesis system (PP systems, Herts, UK) at ambient CO2 concentration (360 µmol mol−1) in natural PF of 1020 ± 34 µmol m−2 s−1 from 1100 h to 1200 h on a clear day. During measuring, leaf temperature and ambient vapour pressure were 28 ± 0·1 °C and 1·48 ± 0·01 kPa, respectively. There were eight replicates per treatment (one leaf per replicate, one leaf per plant).
Analysis of leaf pigments
Immediately before Chl fluorescence measurements, one disc (1 cm2) was punched from the leaf used for fluorescence and gas exchange measurements and frozen in liquid N2, then stored at −80 °C until analysis. Pigments were measured by HPLC (Cheng, 2003). There were seven replicates per treatment (one leaf per replicate, one leaf per plant).
Assay of leaf superoxide anion generation
Superoxide anion generation was determined by reduction of nitroblue tetrazolium (NBT; Doke, 1983). Five fresh leaf discs (total of 5 cm2) from a leaf taken under full sun at midday were immersed immediately in 3 mL of a mixture containing 10 mm Na2HPO4–KH2PO4 (pH 7·8), 0·05 % (w/v) NBT and 10 mm NaN3 for 1 h. Then the mixture was incubated for 15 min at 85 °C and cooled rapidly. Absorbance at 580 nm was recorded and the reduction of NBT was expressed as increased OD580 m−2 s−1. There were five replicates per treatment (one leaf per replicate, one leaf per plant).
Assay of leaf H2O2 production
H2O2 production was measured according to Orendi et al. (2001). Six leaf discs (total of 6 cm2) from a leaf taken under full sun at midday were immediately incubated in 2 mL of 50 mm phosphate buffer (pH 7·0), 0·05 % (w/v) guaiacol (440 μL L−1) and horseradish peroxidase (2·5 U mL−1) for 2 h at room temperature in the dark. Absorbance was measured immediately at 470 nm. There were five replicates per treatment (one leaf per replicate, one leaf per plant).
Leaf lipid peroxidation
Three leaf discs (total of 3 cm2) from a leaf were taken at midday under full sun, frozen in liquid N2, and stored at −80 °C until assay. Lipid peroxidation was determined by measuring the malondialdehyde (MDA) concentration (Hodges et al., 1999). There were five replicates per treatment (one leaf per replicate, one leaf per plant).
Extraction and assay of leaf antioxidant enzymes
Leaf discs (1 cm2 in size) from a leaf were taken at midday under full sun, frozen in liquid N2, and stored at −80 °C until being assayed. There were four replicates per treatment (one leaf per replicate, one leaf per plant). SOD (EC 1.15.1.1), APX (EC 1.11.1.1.11), MDAR (EC 1.6.5.4), DHAR (EC 1.8.5.1), GR (EC 1.6.4.2) and CAT (EC 1.11.1.16) were extracted according to Chen and Cheng (2003). SOD activity was assayed according to McCord and Fridovich (1969). APX, CAT, MDAR, DHAR and GR activities were measured according to Chen and Cheng (2003).
Extraction and analysis of leaf antioxidant metabolites
Leaf discs were taken from the same leaf used for antioxidant enzyme measurements under full sun at midday, frozen in liquid N2, and stored at −80 °C until assay. There were four replicates per treatment (one leaf per replicate, one leaf per plant). Two leaf discs (total of 2 cm2) were ground in 1 mL of ice-cold 7 % (w/v) 5-sulfosalicylic acid. GSH and GSSG were determined according to Griffith (1980). AsA and DAsA were measured according to Logan et al. (1998). Briefly, one leaf disc (1 cm2) was ground in 1 mL of ice-cold 6 % (v/v) HClO4, the extract was centrifuged at 10 000 g for 10 min at 2 °C and immediately used for measurements. 30 mL of 1·5 m Na2CO3 was added to 100 mL of the supernatant to raise the pH to approx. 1–2. AsA was assayed at 265 nm in 200 mm sodium acetate buffer (pH 5·6), before and after 15 min incubation with 1·5 units AsA oxidase (EC 1.10.3.3). For total ascorbate, 30 mL of 1·82 m Na2CO3 was added to 100 mL of extract to raise the pH to approx. 6–7 and incubated for 30 min at room temperature with an equal volume (130 mL) of 20 mM GSH in 100 mM Tricine-KOH (pH 8·5). Total ascorbate was assayed as above. DAsA was calculated as the difference between total ascorbate and AsA.
Assay of leaf Al
Leaf Al was assayed by ICP emission spectrometry (Lin and Myhre, 1991b). There were five replicates per treatment (two leaves per replicate, two leaves per plant).
Statistical analysis
Experiments were performed with 4–8 replicates (one plant per replicate). Results represented the mean ± s.e. for n = 4–8. Unpaired t-tests were applied for comparison between two means.
RESULTS
Leaf Al and absorptance
Leaf Al concentration in Al-treated and control leaves was 23·4 and 31·1 µg g−1 DW, respectively. Both Al-treated leaves and control leaves showed very similar absorption spectra in the PAR (photosynthetically active radiation, approx. 400–700 nm) region except for a slight decrease in the Al-treated leaves compared with control leaves (mean leaf absorptance: 91·8 vs. 92·8 %).
CO2 assimilation, Chl fluorescence variables, electron transport and excess energy
CO2 assimilation (Fig. 1A), non-photochemical quenching (NPQ; Fig. 1B), photochemical quenching coefficient (qP; Fig. 1C), PSII quantum efficiency (Fig. 1D), and pre-dawn maximum PSII quantum efficiency (Fv/Fm) (0·80 vs. 0·83 respectively) were decreased by Al, whereas Al-treated and control leaves were not significantly different in the efficiency of excitation transfer (Fv′/Fm′) (0·66 vs. 0·62 respectively).
Fig. 1.
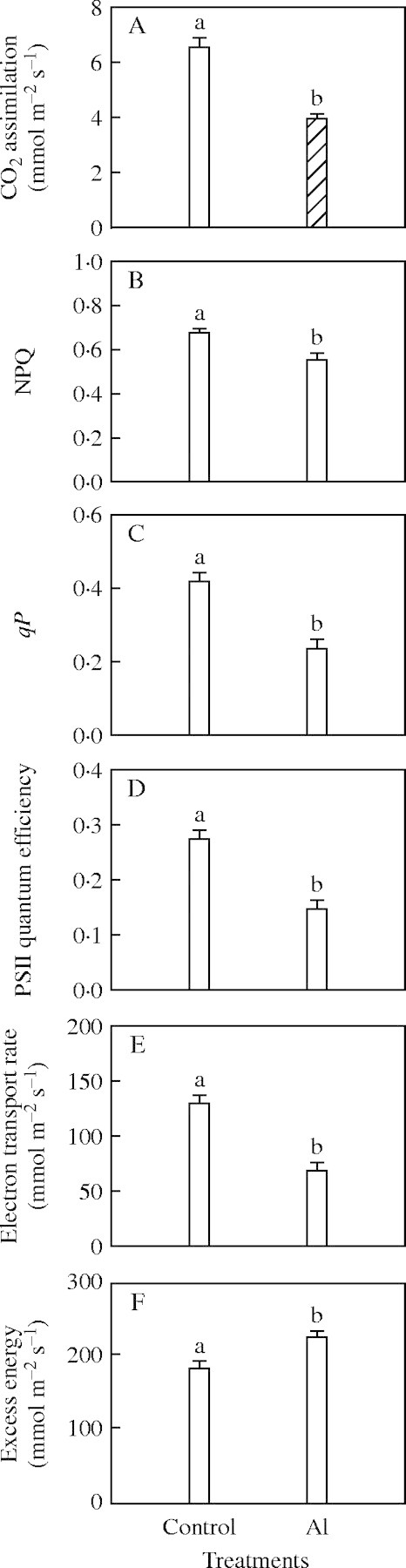
(A) CO2 assimilation; (B) non-photochemical quenching, NPQ; (C) photochemical quenching coefficient, qP; (D) PSII quantum efficiency; (E) electron transport rate; and (F) excess energy in Al-treated and control leaves. Bars represent mean ± s.e. (n = 8). Different letters above the bars indicate a significant difference at P < 0·05.
Since Al-treated leaves used a smaller fraction of the absorbed light in electron transport compared with control leaves (Fig. 1E), they had more excess excitation energy (Fig. 1F).
Leaf pigments
Chlorophyll concentration at both midday and pre-dawn was lower in Al-treated than in control leaves (Fig. 2A), whereas Chl a/b ratio at midday and before dawn was higher (Fig. 2B).
Fig. 2.
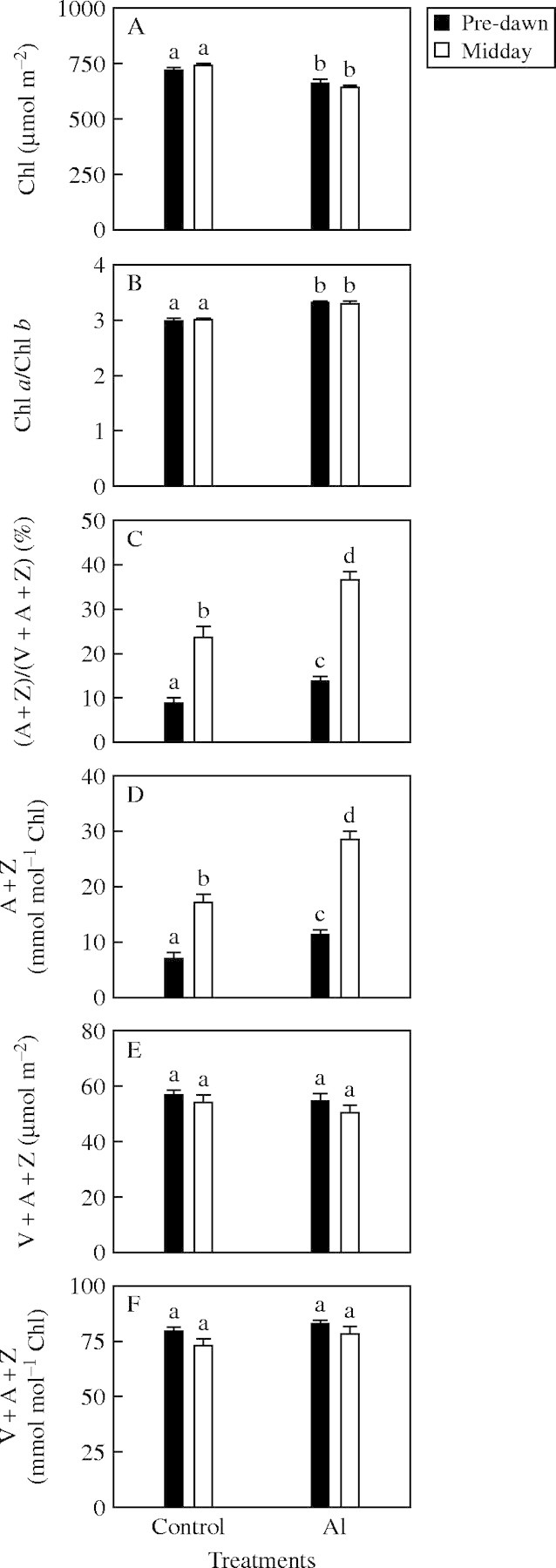
(A) Chlorophyll (Chl); (B) Chl a/b; (C, D) A + Z expressed on the basis of xanthophyll cycle pool or Chl; and (E, F) xanthophyll cycle pool expressed on the basis of area or Chl before dawn and midday in Al-treated and control leaves. Bars represent mean ± s.e. (n = 7). Significant differences were tested between pre-dawn and midday data for the same type of leaves, and Al-treated and control leaves taken at the same time. Different letters above the bars indicate a significant difference at P < 0·05.
A+Z expressed on a xanthophyll cycle pool basis (Fig. 2C) or a Chl basis (Fig. 2D) before dawn and at midday was higher in Al-treated than in control leaves, but no significant difference was observed in [violaxanthin (V) + A + Z] expressed on an area or a Chl basis on either occasion (Fig. 2E, F). At midday, A + Z expressed on a xanthophyll cycle pool basis accounted for less 40 % of the total xanthophyll cycle pool even in Al-treated leaves (Fig. 2C), with the balance in V.
NBT reducing activity, H2O2 production and lipid peroxidation
Aluminum increased NBT reducing activity in leaves compared with controls (0·84 vs. 0·59 OD580 m−1 s−1), an indication of superoxide generation, and H2O2 production (3·34 vs. 1·96 nmol m−1 s−1). However, no significant difference was found in MDA concentrations (an indicator of oxidative lipid metabolism) between Al-treated and control leaves (8·34 compared to 8·13 µmol m−2).
Antioxidant enzymes and metabolites
Activities of SOD, APX, MDAR, DHAR, GR and CAT (Fig. 3), and concentrations of AsA, DASA, GSH, and GSSG were higher in Al-treated than in control leaves (Fig. 4A–D), whereas the ratios of both AsA/DAsA and GSH/GSSG were lower (Fig. 4E, F).
Fig. 3.
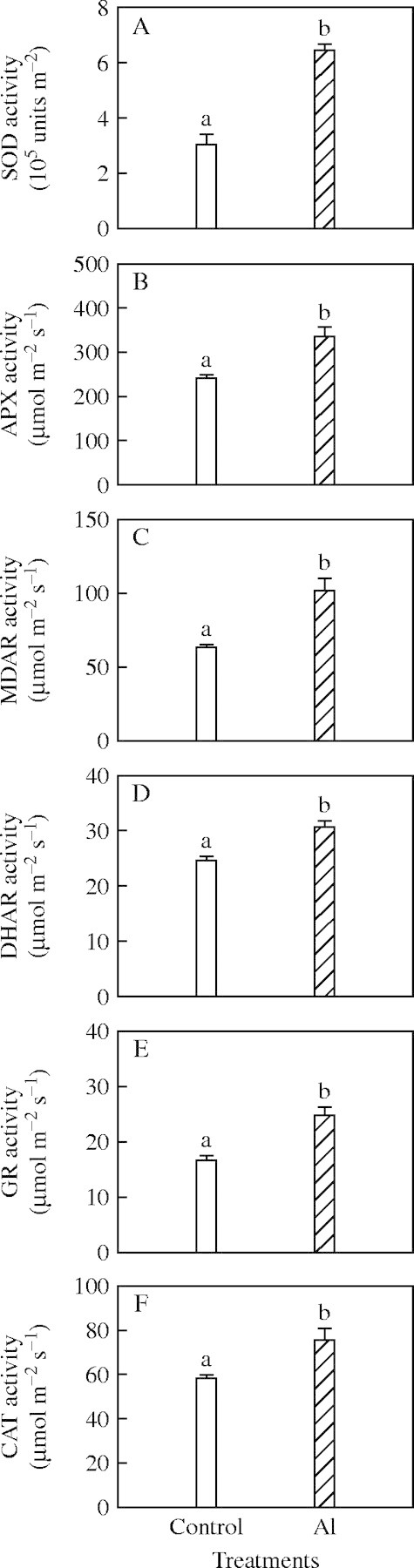
Activities of (A) superoxide dismutase (SOD); (B) ascorbate peroxidase (APX); (C) monodehydroascorbate reductase (MDAR); (D) dehydroascorbate reductase (DHAR); (E) glutathione reductase (GR); (F) and catalase (CAT) in Al-treated and control leaves. Bars represent mean ± s.e. (n = 4). Different letters above the bars indicate a significant difference at P < 0·05.
Fig. 4.
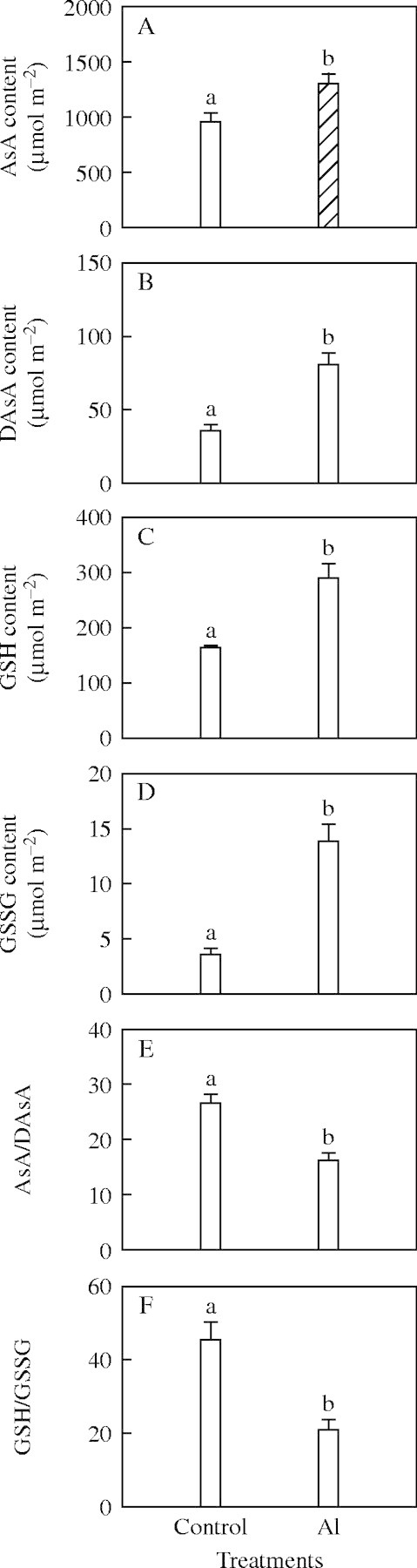
(A) Concentrations of ascorbate (AsA); (B) dehydroascorbate (DAsA); (C) reduced glutathione (GSH); and (D) oxidized glutathione (GSSG); and ratios of (E) AsA/DAsA and (F) GSH/GSSG in Al-treated and control leaves. Bars represent mean ± s.e. (n = 4). Different letters above the bars indicate a significant difference at P < 0·05.
DISCUSSION
The Al-treatment increased leaf Al content per unit dry weight by 33 % compared with controls, resulting in use of a smaller fraction of the absorbed light in electron transport (Fig. 1E) since CO2 assimilation decreased to a greater degree (40 %, Fig. 1A) than Chl content per unit area (8 % at pre-dawn and 13 % at midday, Fig. 2A) or light absorption (1 %). As a result, more excess excitation energy existed in Al-treated leaves (223·3 vs. 181·2 µmol m−2 s−1) compared with control leaves under high PF at midday (Fig. 1F). It has been suggested that excess light can be dissipated as heat in the antenna pigment complexes (Demmig-Adams and Adams, 1996; Niyogi et al., 1998). However, in this study, thermal energy dissipation, measured as NPQ, was lower in Al-treated than in control leaves (Fig. 1B). Similar results were observed in the halophyte Artimisia anethifolia when salt-acclimated (Lu et al., 2003). This suggests that metabolic pathways rather than thermal dissipation may dissipate the increased excess excitation energy in Al-treated leaves.
Our results showed that under Al treatment, the changes in NPQ (Fig. 1B) did not correspond to changes in A + Z expressed on a xanthophyll cycle pool basis (Fig. 2C). Similar results have been obtained in Chlamydomonas reinhardtii (Förster et al., 2001) and in Sorghum bicolor (Cousins et al., 2002). This suggests that under Al toxicity NPQ development may be impaired and/or A + Z has functions other than involvement in thermal energy dissipation. The increase in the conversion of V to A and Z may help to quench 1O2 as production of 1O2 increases in Al-treated leaves under high light due to increased closure of PSII (Fig. 1C), because both Z and A are better photoprotectors than V, with a higher efficiency for de-exciting 1O2 (Mathews-Roth et al., 1974).
Since thermal energy dissipation was lower in Al-treated leaves (Fig. 1B), both the water–water cycle and photorespiration in Al-treated leaves may be up-regulated to cope with the increased excess excitation energy (Fig. 1F). As expected, the activities of SOD, APX, MDAR, DHAR and GR (Fig. 3A–E) and the concentrations of AsA, DAsA, GSH and GSSG (Fig. 2A–D), all involved in the water–water cycle, as well as the activity of CAT (Fig. 3F) involved in scavenging the bulk H2O2 generated by photorespiration, increased in Al-treated leaves. Closure of PSII reaction centres results in formation of toxic activated oxygen species. The up-regulation of enzymatic (Fig. 3) and non-enzymatic (Fig. 4A–D) antioxidants also agreed with the increased requirement for scavenging reactive species in Al-treated leaves due to increased closure of PSII reaction centres (Fig. 1C).
AsA and GSH account for most of the total ascorbate pool and glutathione pool, respectively, in leaves under normal conditions (Polle, 1997). The ratios of AsA to DAsA and of GSH to GSSG decrease under oxidative stress (Law et al., 1983; Gossett et al., 1994). Although the ratios of both AsA/DAsA and GSH/GSSG were lower in Al-treated than in control leaves, AsA and GSH still accounted for more than 90 % of the total glutathione pool and ascorbate pool in the Al-treated leaves (Fig. 4). Furthermore, Al-treated leaves also had a pre-dawn maximum PSII efficiency (Fv/Fm) of approximately 0·80 and no difference in MDA concentration compared with control leaves, although superoxide generation and H2O2 production increased in Al-treated leaves. This indicates that up-regulation of the antioxidant system provided considerable protection to Al-treated leaves against photo-oxidative damage. Thus, the lower ratios of both AsA/DAsA and GSH/GSSG in Al-treated compared with control leaves do not necessarily imply that Al-treated leaves are damaged by photo-oxidation under high light. This is consistent with results observed in nitrogen-limited grape (Vitis labrusca ‘Concord’) leaves (Chen and Cheng, 2003).
In conclusion, our findings support the hypothesis that antioxidant systems are up-regulated and are more important than thermal energy dissipation by the xanthophyll cycle in Al-treated citrus leaves and serve to protect them from photo-oxidative damage.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30270930). We acknowledge the invaluable help of Dr L. Cheng (Cornell University, USA) during the study.
LITERATURE CITED
- Adams WW, Demmig-Adams B, Verhoeven AS, Barker DH. 1994. ‘Photoinhibition’ during winter stress: involvement of sustained xanthophyll cycle-dependent energy dissipation. Australian Journal of Plant Physiology 22: 261–276. [Google Scholar]
- Asada K. 1999. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 50: 601–639. [DOI] [PubMed] [Google Scholar]
- Bilger W, Björkman OB. 1990. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis Photosynthesis Research 25: 173–185. [DOI] [PubMed] [Google Scholar]
- Chen L-S, Cheng L. 2003. Both xanthophyll cycle-dependent thermal dissipation and the antioxidant system are up-regulated in grape (Vitis labrusca L. cv. Concord) leaves in responses to N limitation. Journal of Experimental Botany 54: 2165–2175. [DOI] [PubMed] [Google Scholar]
- Chen L-S, Qi Y-P, Smith BR, Liu X-H. 2005. Aluminum-induced decrease in CO2 in citrus seedlings is unaccompanied by decreased activities of key enzymes involved in CO2 assimilation. Tree Physiology 25: 317–324. [DOI] [PubMed] [Google Scholar]
- Cheng L. 2003. Xanthophyll cycle pool size and composition in relation to the nitrogen content of apple leaves. Journal of Experimental Botany 54: 385–393. [DOI] [PubMed] [Google Scholar]
- Cousins AB, Adam NR, Wall GW, Kimball BA, Pinter PJ Jr, Ottman MJ, Leavitt SW, Webber AN. 2002. Photosystem II energy use, non-photochemical quenching and the xanthophyll cycle in Sorghum bicolor grown under drought and free-air CO2 enrichment (FACE) conditions. Plant, Cell and Environment 25: 1551–1559. [Google Scholar]
- Demmig-Adams B, Adams III WW. 1996. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends in Plant Science 1: 21–26. [Google Scholar]
- Doke N. 1983. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiological Plant Pathology 23: 345–357. [Google Scholar]
- Förster B, Osmond CB, Boynton JE. 2001. Very high light resistant mutants of Chlamydomonas reinhardtii: responses of photosynthem II, nonphotochemical quenching and xanthophyll pigments to light and CO2 Photosynthesis Research 67: 5–15. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. 1989. The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92. [Google Scholar]
- Genty B, Harbinson J, Baker NR. 1990. Relative quantum efficiencies of the two photosystems of photorespiratory and non-respiratory conditions. Plant Physiology and Biochemistry 28: 1–10. [Google Scholar]
- Gossett DR, Millhollon EP, Lucas MC. 1994. Antioxidant responses to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Science 34: 706–714. [Google Scholar]
- Griffith OW. 1980. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Analytical Biochemistry 106: 207–212. [DOI] [PubMed] [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK. 1999. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207: 604–611. [DOI] [PubMed] [Google Scholar]
- Kato MC, Hikosaka K, Hirotsu N, Makino A, Hirose T. 2003. The excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms determines the rate of photoinactivation in photosystem II. Plant and Cell Physiology 44: 318–325. [DOI] [PubMed] [Google Scholar]
- van Kooten O, Snel JFH. 1990. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynthesis Research 25: 147–150. [DOI] [PubMed] [Google Scholar]
- Kuo MC, Kao CH. 2003. Aluminum effects on lipid peroxidation and antioxidative enzyme activities in rice leaves. Biologia Plantarum 46: 149–152. [Google Scholar]
- Law MY, Charles SA, Halliwell B. 1983. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts: the effect of hydrogen peroxide and of paraquat. Biochemical Journal 210: 899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Myhre DL. 1990. Citrus root growth as affected by soil aluminum level under field conditions. Soil Science Society of American Journal 54: 1340–1344. [Google Scholar]
- Lin Z, Myhre DL. 1991. Differential response of citrus rootstocks to aluminum levels in nutrient solutions: I. plant growth. Journal of Plant Nutrition 14: 1223–1238. [Google Scholar]
- Lin Z, Myhre DL. 1991. Differential response of citrus rootstocks to aluminum levels in nutrient solutions: II. Plant mineral concentratios. Journal of Plant Nutrition 14: 1239–1254. [Google Scholar]
- Logan BA, Grace SC, Adams III WW, Demmig-Adams B. 1998. Seasonal differences in xanthophyll cycle characteristics and antioxidants in Mahonia repens growing in different light environments. Oecologia 116: 9–17. [DOI] [PubMed] [Google Scholar]
- Lu C, Qiu N, Lu Q. 2003. Photoinhibition and the xanthophyll cycle are not enhanced in the salt-acclimated halophyte Artimisia anethifolia Physiologia Plantarum 118: 532–537. [Google Scholar]
- Mathews-Roth MM, Wilson T, Fujimori E, Krinsky NI. 1974. Carotenoid chromophore length and protection against photosensitization. Photochemistry and Photobiology 19: 217–222. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. 1969. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). Journal of Biological Chemistry 244: 6049–6055. [PubMed] [Google Scholar]
- Niyogi KK, Grossmn AR, Björkman O. 1998.Arabidopsis mutants define a central role of the xanthophyll cycle in the regulation of photosynthetic energy conversion. The Plant Cell 10: 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orendi G, Zimmermann P, Baar C, Zentgraf U. 2001. Loss of stress-induced expression of catalase3 during leaf senescence in Arabidopsis thaliana is restricted to oxidative stress. Plant Science 161: 301–314. [DOI] [PubMed] [Google Scholar]
- Pereira WE, de Siqueira DL, Martinez CA, Puiatti M. 2000. Gas exchange and chlorophyll fluorescence in four citrus rootstocks under aluminum stress. Journal of Plant Physiology 157: 513–520. [Google Scholar]
- Polle A. 1997. Defense against photoxidative damage in plants. In: Scandalios JG, ed. Oxidative stress and the molecular biology of antioxidant defenses. New York: Spring Harbor Laboratory Press, 623–666. [Google Scholar]


