Abstract
• Background and Aims High-temperature environments with >30 °C during flowering reduce boll retention and yield in cotton. Therefore, identification of cotton cultivars with high-temperature tolerance would be beneficial in both current and future climates.
• Methods Response to temperature (10–45 °C at 5 °C intervals) of pollen germination and pollen tube growth was quantified, and their relationship to cell membrane thermostability was studied in 12 cultivars. A principal component analysis was carried out to classify the genotypes for temperature tolerance.
• Key Results Pollen germination and pollen tube length of the cultivars ranged from 20 to 60 % and 411 to 903 µm, respectively. A modified bilinear model best described the response to temperature of pollen germination and pollen tube length. Cultivar variation existed for cardinal temperatures (Tmin, Topt and Tmax) of pollen germination percentage and pollen tube growth. Mean cardinal temperatures calculated from the bilinear model for the 12 cultivars were 15·0, 31·8 and 43·3 °C for pollen germination and 11·9, 28·6 and 42·9 °C for pollen tube length. No significant correlations were found between pollen parameters and leaf membrane thermostability. Cultivars were classified into four groups based on principal component analysis.
• Conclusions Based on principal component analysis, it is concluded that higher pollen germination percentages and longer pollen tubes under optimum conditions and with optimum temperatures above 32 °C for pollen germination would indicate tolerance to high temperature.
Key words: Cotton, Gossypium hirsutum, high temperature, cell membrane thermostability, principal component analysis, pollen germination, pollen tube, relative injury
INTRODUCTION
Global surface temperature has increased by approx. 0·6 °C since the late 19th century and is projected to increase by 1·4–5·8 °C by the end of the current century (Houghton et al., 2001). Further, extreme events such as warmer days with decrease in diurnal temperature range are projected to occur more frequently in the future climates (Dai et al., 2001). Temperature is the important factor controlling plant growth and development. Suitability of a crop to a given location depends not only on the threshold temperatures but also on the length of the growing season. Daily or seasonal temperatures above optimum and temperature extremes, should they coincide with critical stages of plant development, will become a major factor limiting crop production (Hall, 1992). Fruit-set in many agronomic crops is sensitive to high temperature (Reddy et al., 1991, 1992; Peet et al., 1998). Fruit set was reduced on exposure to daytime temperatures of >30 °C for about 13 h in Upland (Gossypium hirsutum) and Pima (G. barbadense) cottons (Reddy et al., 1992), 35°C for 4 h in Brassica napus (Young et al., 2004), >28 °C for 12 h during flowering in tomato (Lycopersicon esculentum) (Peet et al., 1998; Sato et al., 2002). Seed yield of wheat (Triticum aestivum) (Saini and Aspinall, 1982), corn (Zea mays) (Mitchell and Petolino, 1988) and rice (Oryza sativa) (Matsui et al., 1997) were reduced on exposure to daytime temperatures of 30 °C for 16 h, 38 °C for 16 h and >36 °C for 6 h, respectively. Similarly, pod-set was reduced at day temperatures >28 °C for 12 h in bean (Phaseolus vulgaris) (Prasad et al., 2002) and >28 °C for 12 h in groundnut (Arachis hypogaea) (Prasad et al., 1999a, 2003).
Conventional and transgenic cultivars of cotton are grown across the USA in about 5·3 Mha (Economic Research Service, 2003) and 31·6 Mha around the world under diverse temperature regimes (15–45 °C). Cotton plants aborted most of the squares and flowers when day/night temperatures were >30/20 °C for 13 h (Reddy et al., 1991, 1993). At extremely high day temperatures such as 40 °C for 13 h, all existing squares and flowers were aborted in several Upland cotton cultivars (Reddy et al., 1992, 1995), whereas Pima cotton was highly sensitive and failed to produce fruiting branches (Reddy et al., 1995).
Pollen grains once released from anthers act as independent functional units and are exposed to ambient environment. Therefore, episodes of high temperature during flowering would more severely affect pollen than the deeply seated ovules. In cotton, anther dehiscence occurs during the morning hours of 0700 to 1100 depending on the prevailing weather conditions while pollen germination occurs within 30 min upon contact of a receptive stigma (Pundir, 1972). Actual fertilization however, occurs anywhere between 12 and 24 h once pollen is released, due to slow growth of the pollen tube (Pundir, 1972). Therefore, high-temperature damage occurring during anthesis is likely to include failure of pollination and/or fertilization, resulting in lower boll-set. Weaver and Timm (1988) suggested that pollen is more sensitive to high temperatures than female reproductive organs, which could account for a lack of fertilization under high-temperature stress. Recent studies have shown that micro- and mega-sporogenesis are injured by high temperature, resulting in reduced fruit set (Cross et al., 2003; Young et al., 2004) but they also suggest that pollen plays a major role in fruit-set under high-temperature conditions. Tomato plants grown at 32/26 °C temperature for 0–15 d before anthesis failed to set fruit due to disruption of anther components (Sato et al., 2002). Young et al. (2004) demonstrated the importance of pollen in fruit-set through reciprocal crossing studies where fruit set was reduced by 88 % when pollen donor plants were treated with high temperature (35 °C for 4 h during day), while fruit set was reduced by 37 % when emasculated receptor plants were treated with high temperature.
Results from in vitro studies with peanuts showed that genotypes varied in response to temperature for cardinal temperatures (Tmin, Topt and Tmax), pollen germination percentage and maximum pollen tube length (Kakani et al., 2002). The differences in cardinal temperature were mainly responsible for tolerance/susceptibility of peanut genotypes to high temperature (Kakani et al., 2002; Craufurd et al., 2003). Upland cotton genotypes were bred for heat tolerance by selecting progenies developed from surviving pollen grains when exposed to 35 °C for 15 min (Rodriguez-Garay and Barrow, 1988), suggesting that pollen could be used to screen cotton cultivars for high-temperature tolerance. Recently Burke et al. (2004) reported an optimum temperature of 28 °C for in vitro pollen germination with greenhouse-grown cotton cultivar Gregg 65. However, variation in cardinal temperatures for pollen germination and pollen tube growth in cotton cultivars have not been studied. Therefore, identification of cardinal temperatures for pollen germination and pollen tube growth and developing response functions will be useful for understanding mechanisms of high-temperature tolerance.
A vegetative physiological parameter widely used to study plant tolerance to temperature is cell membrane thermostability. It was successfully used to screen cotton cultivars for high-temperature tolerance (Ashraf et al., 1994; ur Rahman et al., 2004). Cultivars showing high membrane thermostability gave higher seed cotton yield under high-temperature conditions during flowering and boll-filling period (Malik et al., 1999; ur Rahman et al., 2004). Recent studies in peanuts showed that cell membrane thermostability was not highly correlated with yield loss or pollen germination under high-temperature conditions (Kakani et al., 2002; Craufurd et al., 2003). Thus, it is essential to understand the relationships between responses of pollen to high temperature and leaf membrane thermostability in cotton. The objectives of this study were to (a) quantify the effect of temperature on pollen germination and pollen tube growth of different cotton cultivars, (b) determine cardinal temperatures for pollen germination and pollen tube growth, and (c) compare pollen (total germination and maximum pollen tube growth) response to temperature with leaf membrane thermostability.
MATERIALS AND METHODS
Plant growth
Twelve cotton cultivars representing traditional, improved and transgenics expressing variable tolerance to drought, high temperature and other biotic stresses were evaluated in the present study (Table 1). The plants were grown during the summer of 2002 in an experimental field at the R. R. Foil Plant Science Research Center, Mississippi State University (33°28′N, 88°47′W). Plants were grown under recommended cultural practices for commercial production. The growing temperatures were optimum during the squaring and flower collection period. The mean temperatures during the period of 3 weeks prior to flower collection were 27 ± 0·24 °C and during the flower collection period were 28 ± 0·31 °C.
Table 1.
Trade name, maturity group, leaf-type and specific traits of 12 cotton cultivars evaluated for tolerance to high temperature (Cotton Farming, 2002)
| Cultivar* |
Maturity group |
Leaf type |
Special traits |
|---|---|---|---|
| (1) Acala 1517–99 | Full | Hairy | Verticillium tolerant |
| (2) BXN 49 B† | Early–mid | Hairy | Contains BXN and BG genes |
| (3) DP 458 B/RR‡ | Mid–full | Smooth | Good heat tolerance |
| (4) DP 5415 RR | Mid–full | Smooth | Outstanding yield potential |
| (5) FM 832 | Med–full | Okra/smooth | Adaptable to drought |
| (6) FM 832 B | Med–full | Okra/smooth | Good water use efficiency |
| (7) NuCOTN 33 B | Mid–full | Smooth | Widely adapted |
| (8) NuCOTN 35 B | Mid–full | Smooth | Good fibre quality |
| (9) ST 457 | Early–mid | Hairy | Conventional cultivar |
| (10) ST 4793 R | Early–mid | Hairy | Contains RR genes |
| (11) ST 4892 BR | Early–mid | Hairy | Contains BG and RR genes |
| (12) STV 825 | Full | Smooth | Conventional cultivar |
(1), New Mexico State University, Las Cruces, NM; (2), (9), (10) and (11), Stoneville Pedigree Seed Company, Memphis TN; (3), (4), (7) and (8), Delta and Pine Land Company, Scott, MS; (5) and (6), Bayer CropScience US, Kansas City, MO; (12), conventional.
B, Bollgard®
R, Roundup Ready® (both trademarks of Monsanto Technology, LLC).
Pollen collection and growth medium
The flowers for this study were collected from the first fruiting position between 55 and 60 d after emergence. Plant to plant variation can also be a significant source of variation in pollen germination measurements (Sari-Gorla et al., 1994). To minimize the effect of this variation without having to perform individual determinations on many plants of single cultivars, pollen from flowers on different plants was taken as a sample in the present study. Fresh cotton flowers were collected at the time of anther dehiscence, between 0730 and 0830 h, from ten plants per each cultivar, and immediately placed in plastic bags and carried to the laboratory. The improved pollen growth medium of Taylor (1972), consisting of 2 g agar, 30 g C12H22O11, 5·3 mg KNO3, 51·7 mg MnSO4, 10·3 mg H3BO3, 10·3 mg MgSO4·7H2O made up to 100 mL with deionized water, was used in this study. The medium was placed in Petri dishes and temperature equilibrated before sprinkling the pollen on the medium. Pollen was sprinkled on the media by gently tapping a set of three flowers directly above the surface of the medium in each Petri dish. Approximately, 800–1000 pollen grains were sprinkled on each Petri dish. Three Petri dishes of each genotype at each temperature treatment were used as replications. The whole procedure was completed within 30 min to avoid pollen desiccation. Partial opening of the Petri dish lids allowed a relative humidity of about 50 % to be maintained and also prevented moisture accumulation on germinating pollen grains and avoided pollen rupture as cotton pollen is highly sensitive to moisture (Burke et al., 2003).
Temperature treatments
Petri dishes with media containing pollen were incubated in the dark at temperatures between 10 and 45 °C at 5 °C intervals in growth cabinets (Percival Scientific, Inc., Perry, IA, USA) and observed for germination. As pollen did not germinate at temperatures of 10 and 45 °C, additional incubation temperatures of 12·5 and 42·5 °C were included. On a given day, all 12 cultivars were tested at a given temperature. Growth cabinets were maintained at pre-determined temperature and temperature of cabinet and media were recorded at 1-h intervals using a Campbell CR10X data logger. No differences were observed between measured cabinet and media temperatures. The average temperature of the growth cabinet during pollen germination was used in the analysis.
Pollen germination and pollen tube measurements
Pollen germination (PG) was determined by direct microscopic observation (Nikon Scientific, Kanagawa, Japan). A pollen grain was considered germinated when pollen tube length (PTL) was at least equal to or greater than the grain diameter (Kakani et al., 2002). Germination percentage was determined by dividing the number of germinated pollen grains per field of view by the total number of pollen per field of view and expressed as percentage. Measurements of pollen tube length were recorded directly by an ocular micrometer fitted to the eyepiece of the microscope. Mean pollen tube length was calculated as the average length of 20 pollen tubes measured from each Petri dish after 24 h. The replicated values on maximum pollen germination and tube length were analysed using the one-way ANOVA procedure (SAS Institute, 1997).
Curve fitting and analysis
Maximum pollen germination percentage and pollen tube length recorded after 24 h of incubation, at each temperature, were analysed using linear and nonlinear regression techniques to quantify developmental responses to temperature. Quadratic (Yan and Wallace, 1998), cubic or higher order polynomial (Tollenaar et al., 1979) and modified broken-stick or bilinear (Omanga et al., 1995) equations were applied to data and examined to determine the best-fit model.
The modified bilinear equation (eqn 1) provided the greatest R2 value and smallest root mean squared deviation (r.m.s.d.) for both pollen germination and pollen tube length and was used to estimate cardinal temperatures, minimum (Tmin), optimum (Topt) and maximum (Tmax), for pollen germination and pollen tube length of all cultivars (Kakani et al., 2002). The PROC NLIN procedure in SAS (SAS Institute, 1997) was used to estimate parameters in the modified bilinear equation. A modified Newton–Gauss iterative method was used to determine Topt based on the lowest r.m.s.d. values between observed and predicted values. Values of Tmin and Tmax were estimated using parameters derived from the modified bilinear equations (eqns 2 and 3). Replicated values of cardinal temperatures were then analysed using the one-way ANOVA procedure in SAS (SAS Institute, 1997).
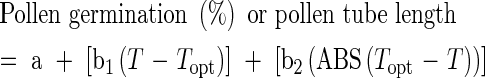 |
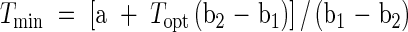 |
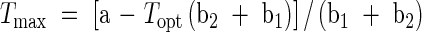 |
where a, b1 and b2 are equation constants, T the various temperatures at which germination and tube growth were studied, and Topt the optimum temperature for germination or pollen tube growth.
Cell membrane thermostability measurements
At the time of pollen sampling, cell membrane thermostability of leaves was measured using the procedure described by Martineau et al. (1979). Each sample assay consisted of two sets of five leaf discs cut with a 1·2-cm-diameter punch from five fully expanded leaves on the main stem. Samples were replicated three times each. Before each assay, the two paired sets of leaf discs were placed into two separate test tubes with 20 mL of deionized water, after washing them thoroughly with at least four changes of deionized water to remove electrolytes released from cut cells at the periphery of the discs. To avoid evaporation and leakage of contents, test tubes were sealed with aluminum foil. One set of test tubes was incubated for 20 min at 55 °C in a temperature-controlled water bath, whilst the other set was left at room temperature of approx. 25 °C. Test tubes were then immediately incubated at 10 °C for 12 h and inverted several times to mix the contents. After incubation, the initial measurement of conductance was measured by an electrical conductivity meter (Corning Checkmate II; Corning Inc., New York, USA), after which tubes were sealed with aluminum foil and autoclaved at 120 °C and 0·15 MPa for 20 min to kill leaf tissues. Autoclaved tubes were cooled to 25 °C, contents mixed thoroughly and final conductance was recorded. Relative injury (RI) to cell membranes resulting from the temperature treatments was calculated using eqn (4)
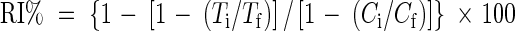 |
where T and C refer to the conductance of the treatment (55 °C) and control (25 °C) solution, respectively, and the subscripts i and f indicate initial and final conductance, respectively. The ratio of the initial to the final conductance (Ti/Tf) is a relative measure of electrolyte leakage caused by elevated temperature and consequently a measure of the extent of damage to cellular membranes. One-way ANOVA in SAS (SAS Institute, 1997) was carried out to identify cultivar differences.
Principal component analysis (PCA)
A PCA using PROC PRINCOMP of SAS (SAS Institute, 1997) was applied to pollen germination and pollen tube growth parameters to identify the parameters that best describe cultivar tolerance to temperature. Values of maximum pollen germination percentage (PG%max) and pollen tube length (PTLmax), cardinal temperatures (Tmin, Topt and Tmax) for pollen germination and pollen tube length and RI of 12 cultivars were included in the PCA. Eigenvectors generated by PCA were used to identify parameters that best differentiated cultivars for temperature tolerance. The first two PC scores, PC1 and PC2 that accounted for maximum variability of the parameters tested, were used to group the cultivars. The cultivars which had +PC1 and +PC2 scores were classified as tolerant, +PC1 and −PC2 scores as moderately tolerant, −PC1 and +PC2 as moderately susceptible and finally −PC1 and −PC2 as susceptible.
RESULTS
Pollen germination
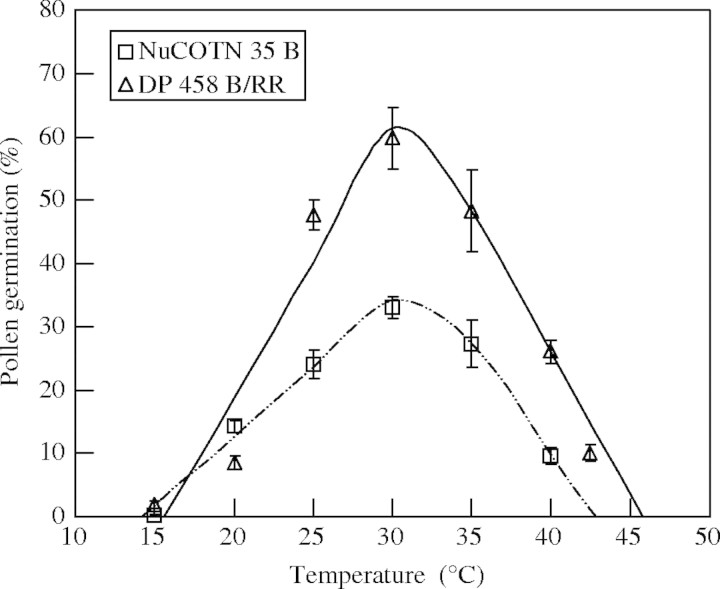
Pollen grains started germinating in about 10 min on contact with the in vitro medium. Figure 1 shows the variation for pollen germination in response to temperature of two cultivars for clarity. Cultivar differences for both germination percentage and cardinal temperatures were observed (Table 2). Maximum percentage of germination ranged from 33 (NuCOTN 35 B) to 60 % (DP 458 B/RR), with a mean of 44 %. The modified bilinear equation provided best-fit to predict the cultivars pollen germination response to temperature (Fig. 1). The average R2 value for all cultivars tested was 88 % (Table 2). Cardinal temperatures for pollen germination differed greatly among cultivars. Values of Tmin ranged from 11·1 °C (BXN 49B) to 20·2 °C (ST 457) with an average of 15·1 °C. Optimum temperature (Topt) ranged from 28·4 °C for ST 4793 R to 35·4 °C for ST 4892 BR with an average Topt of 31·4 °C. The Tmax values ranged from 40·8 °C for ST 4892 BR to 46·2 °C for STV 825 with an average Tmax of 43·3 °C (Table 2).
Fig. 1.
Pollen germination in response to temperature (symbols) and their fitted lines based on modified bilinear equation of two cotton cultivars (DP 458 B/RR and NuCOTN 35 B). Cultivars with variation for maximum pollen germination are presented for clarity. Error bars indicate ± s.e.
Table 2.
Maximum pollen germination percentage, modified bilinear equation constants, and cardinal temperatures for pollen germination of 12 cotton cultivars in response to temperature
| Equation constants |
Cardinal temperatures (°C) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar |
Maximum pollen germination (%) |
a |
b1 |
b2 |
R2 |
Tmin |
Topt |
Tmax |
|||||
| DP 458 B/RR | 59·8 | 66·0 | −0·11 | −4·36 | 83·5 | 15·5 | 31·1 | 45·8 | |||||
| STV 825 | 52·8 | 53·4 | 0·09 | −3·36 | 85·0 | 14·3 | 29·8 | 46·2 | |||||
| FM 832 B | 49·8 | 50·4 | −0·06 | −3·70 | 85·5 | 15·8 | 29·6 | 43·0 | |||||
| NuCOTN 33 B | 46·8 | 50·4 | 0·10 | −3·57 | 79·6 | 14·9 | 28·7 | 43·2 | |||||
| ST 4892 BR | 45·9 | 48·7 | −3·35 | −5·58 | 96·0 | 13·6 | 35·4 | 40·8 | |||||
| Acala 1517–99 | 45·5 | 51·0 | −1·87 | −4·61 | 89·6 | 15·5 | 34·2 | 42·0 | |||||
| DP 5415 RR | 44·3 | 56·6 | −1·40 | −4·52 | 89·9 | 14·7 | 32·9 | 42·4 | |||||
| FM 832 | 42·5 | 44·7 | 0·25 | −3·20 | 79·7 | 16·0 | 28·9 | 44·0 | |||||
| ST 4793 R | 36·4 | 38·3 | 0·25 | −2·58 | 94·2 | 14·8 | 28·4 | 44·8 | |||||
| ST 457 | 35·6 | 41·8 | −1·48 | −3·56 | 90·4 | 20·2 | 33·8 | 44·1 | |||||
| BXN 49B | 35·5 | 42·7 | −1·46 | −3·47 | 89·6 | 11·1 | 32·3 | 41·0 | |||||
| NuCOTN 35 B | 33·0 | 38·2 | −0·68 | −2·84 | 97·7 | 14·3 | 31·9 | 42·7 | |||||
| Mean | 43·99 | – | – | – | 88·4 | 15·1 | 31·4 | 43·3 | |||||
| s.e.d. | 4·03*** | – | – | – | – | 0·20*** | 0·38*** | 0·16*** | |||||
Significant at P = 0·001 level.
–, Data not analysed statistically.
Pollen tube growth
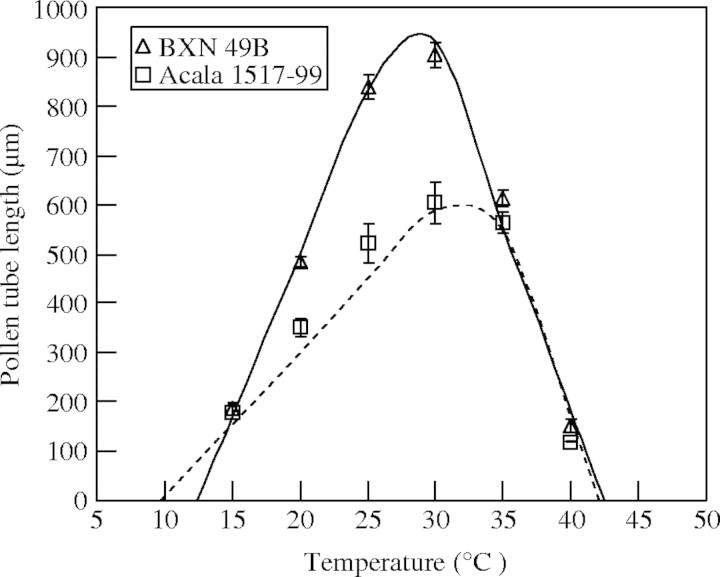
Cultivars differed significantly in pollen tube length at optimum temperatures (Fig. 2). Pollen tubes remained stable without rupturing for 24 h after germination on the in vitro medium. Pollen tube length ranged from 605 µm for Acala 1517–99 to 903 µm for BXN 49B, with an average of 778 µm (Table 3). Similar to pollen germination, the modified bilinear function described the response of pollen tube length to temperature. The modified bilinear model fit is shown for two cultivars that had high variation in pollen tube length and cardinal temperatures for pollen tube growth (Fig. 2). The Tmin ranged from 9·8 °C for Acala 1517–99 to 13·4 °C for NuCOTN 35 B with an average Tmin of 12·1 °C. The Topt ranged from 25·9 °C for STV 825 to 33·3 °C for Acala 1517–99 with an average Topt of 28·3 °C. Values of Tmax ranged from 42·1 °C for Acala 1517–99, FM 832 and NuCOTN 33 B to 44·3 °C for ST 457 with an average of 42·8 °C (Table 3).
Fig. 2.
Pollen tube length in response to temperature (symbols) and their fitted lines based on modified bilinear equation of two cotton cultivars (BXN 49B and Acala 1517-99). Cultivars with variation for maximum pollen tube length are presented for clarity. Error bars indicate ± s.e.
Table 3.
Relative injury, maximum pollen tube length, modified bilinear equation constants and cardinal temperatures for pollen tube length of 12 cotton cultivars in response to temperature
| Equation constants |
Cardinal temperatures (°C) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar |
Relative injury (%) |
Maximum pollen tube length (µm) |
a |
b1 |
b2 |
R2 |
Tmin |
Topt |
Tmax |
|||||
| DP 458 B/RR | 41·0 | 880 | 1086·2 | 0·24 | −7·12 | 92·8 | 11·8 | 26·5 | 42·3 | |||||
| ST 4892 BR | 49·2 | 766 | 898·1 | −0·36 | −5·80 | 96·0 | 12·3 | 28·8 | 43·4 | |||||
| FM 832 B | 51·4 | 673 | 874·4 | −0·72 | −6·01 | 97·1 | 12·6 | 29·2 | 42·2 | |||||
| ST 4793 R | 54·4 | 815 | 975·6 | 0·09 | −6·23 | 94·2 | 12·1 | 27·5 | 43·4 | |||||
| Acala 1517-99 | 61·9 | 605 | 685·0 | −2·41 | −5·33 | 78·4 | 9·8 | 33·3 | 42·1 | |||||
| ST 457 | 63·1 | 796 | 999·8 | 0·19 | −6·19 | 90·4 | 12·0 | 27·7 | 44·3 | |||||
| DP 5415 RR | 65·1 | 838 | 949·6 | 0·72 | −6·25 | 94·2 | 12·4 | 26·1 | 43·3 | |||||
| FM 832 | 67·2 | 675 | 866·7 | −1·63 | −6·21 | 95·4 | 12·1 | 31·0 | 42·1 | |||||
| NuCOTN 33 B | 71·2 | 723 | 908·7 | −0·18 | −6·08 | 92·5 | 12·2 | 27·6 | 42·1 | |||||
| STV 825 | 71·4 | 875 | 1065·0 | 0·73 | −6·72 | 85·0 | 11·6 | 25·9 | 43·6 | |||||
| BXN 49B | 72·6 | 903 | 1053·4 | −0·43 | −7·04 | 99·0 | 12·4 | 28·4 | 42·5 | |||||
| NuCOTN 35 B | 75·7 | 786 | 884·8 | 0·01 | −6·15 | 93·3 | 13·4 | 27·8 | 42·2 | |||||
| Mean | 62·1 | 778 | – | – | – | 93·0 | 12·1 | 28·3 | 42·8 | |||||
| s.e.d. | 11·39* | 7·6*** | – | – | – | – | 0·47*** | 0·15*** | 0·28*** | |||||
***, *, Significant at P = 0·001 and 0·05 levels, respectively.
–, Data not analysed statistically.
Cell membrane thermostability
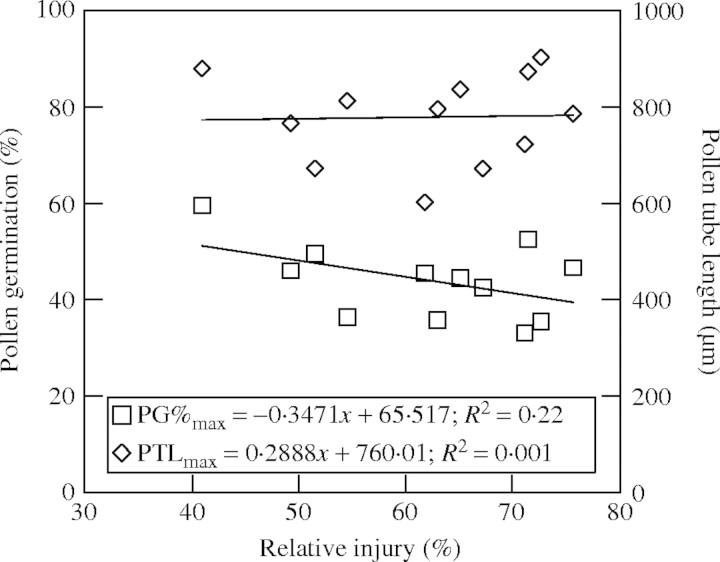
The leaf cell membrane thermostability expressed as percentage relative injury (RI%) differed significantly among cultivars and ranged from 41 % for DP 458 B/RR to 76 % for NuCOTN 35 B with an average of 62 % (Table 3). Relative injury had poor correlation with pollen germination and pollen tube length (Fig. 3).
Fig. 3.
Relationship between cell membrane thermostability expressed as relative injury (%) and maximum pollen germination percentage (PG%max) and pollen tube length (PTLmax) recorded at optimum temperature of 12 cotton cultivars.
Principal component analysis
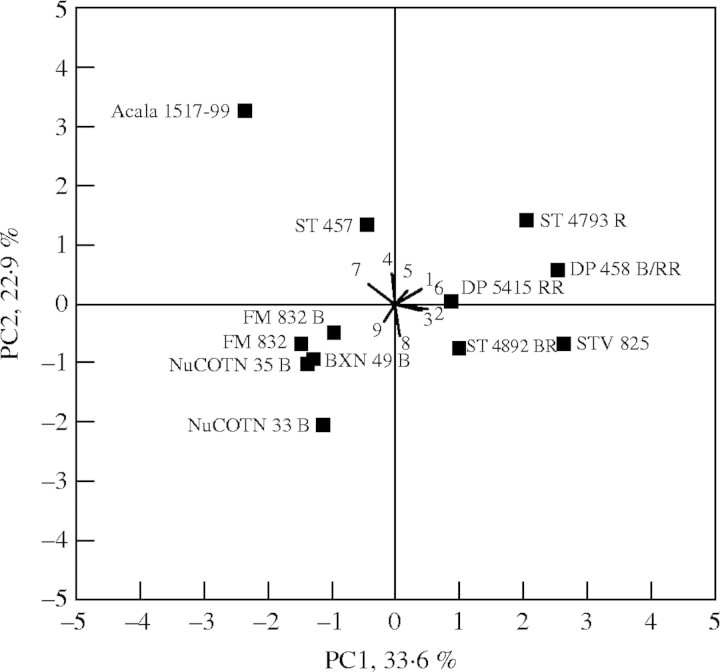
PCA is a multivariate technique for examining relationships among several quantitative variables and is especially a valuable analytical technique in exploratory data analysis (Johnson, 1998). The PCA identified the pollen parameters that best separated the cultivars for their tolerance to temperature. The first three principal component vectors (PC1, PC2 and PC3) accounted for 78 % of the total variation (Table 4). The PC1 eigenvector contrasted cultivars with high positive loadings for variables PTLmax, PG%max, PTL Tmax and PG Tmax (Table 4; Fig. 4). Cultivars with higher PG%max and PTLmax were placed on the right of the biplot while cultivars with low values were placed on the left of the biplot (Fig. 4). The PC2 had high positive loadings for PGTopt indicating the role of optimum temperature in separating sensitive from tolerant cultivars. The cultivars were divided into four groups based on the scores of the first two principal components (Fig. 4): group 1 cultivars as tolerant with positive scores for PC1 and PC2, group 2 as moderately tolerant with positive PC1 and negative PC2 scores, group 3 as moderately susceptible with negative PC1 and positive PC2 and finally group 4 as susceptible with negative PC1 and PC2 scores (Table 5).
Table 4.
Principal component analysis eigenvectors PC1, PC2 and PC3 of 12 cotton cultivars for maximum percentage pollen germination (PG%max), maximum pollen tube length (PTLmax) and their respective cardinal temperatures (Tmin, Topt and Tmax) and RI% and the variation accounted for by each eigenvector
| Principal component eigenvectors |
|||||
|---|---|---|---|---|---|
| Parameter |
PC1 |
PC2 |
PC3 |
||
| PG%max | 0·41 | 0·27 | −0·02 | ||
| PTLmax | 0·51 | −0·08 | 0·22 | ||
| PG% Tmax | 0·41 | −0·10 | −0·55 | ||
| PG% Topt | −0·05 | 0·52 | 0·51 | ||
| PG% Tmin | 0·19 | 0·24 | −0·39 | ||
| PTL Tmax | 0·38 | 0·25 | 0·28 | ||
| PTL Topt | −0·43 | 0·35 | −0·27 | ||
| PTL Tmin | 0·08 | −0·55 | 0·25 | ||
| RI% | −0·18 | −0·30 | 0·14 | ||
| % variation | 33·6 | 22·9 | 15·9 | ||
Fig. 4.
First and second principal component scores (PC1 and PC2) for the identification of cotton cultivar response to temperature. The eigenvectors for variables are indicated by thick lines radiating from the centre showing the direction (angle) and magnitude (length) for maximum pollen germination (PG%; 2) and maximum pollen tube length (PTL; 1), cardinal temperatures (Tmin, Topt and Tmax) for pollen germination percentage (PG%max) (3, 4, 5) and pollen tube length (PTLmax) (6, 7, 8) and cell membrane thermostability as RI (9).
Table 5.
Classification of 12 cotton cultivars based on the scores of first two principal components (PC1 and PC2)
| Tolerant (+PC1, +PC2) |
Moderately tolerant (+PC1, −PC2) |
Moderately susceptible (−PC1, +PC2) |
Susceptible (−PC1, −PC2) |
|---|---|---|---|
| DP 458 B/RR (2·54, 0·57) | STV 825 (2·64, −0·69) | ST 457 (−0·46, 1·35) | FM 832 B (−0·97, −0·49) |
| ST 4793 R (2·05, 1·41) | ST 4892 BR (0·99, −0·75) | Acala 1517–99 (−2·36, 3·26) | BXN 49B (−1·28, −0·96) |
| DP 5415 RR (0·89, 0·06) | NuCOTN 35 B (−1·40, −1·03) | ||
| NuCOTN 33 B (−1·45, −2·05) | |||
| FM 832 (−1·48, −0·67) |
The principal component scores were obtained from the principal component analysis. The PC1 had highest positive loadings for PG, PTL, PG Tmax and PTL Tmax and the PC2 vector had highest positive loading for PGTopt. The cultivars which had +ve scores for PC1 and PC2 were classified as tolerant, +PC1 and −PC2 scores as moderately tolerant, −PC1 and +PC2 as moderately susceptible and finally −PC1 and −PC2 as susceptible. Values in parenthesis are the PC1 and PC2 scores of the cultivar.
DISCUSSION
Temperature is among the most important environmental factors affecting plant reproductive processes such as pollen germination, pollen tube growth and fruit-set. In the present study, in vitro pollen germination and pollen tube growth of all cultivars were severely reduced under both high and low temperature conditions. Earlier studies on cotton pollen by Suy (1979) and Barrow (1983) have shown that high temperatures (>30 °C) inhibit in vivo pollen germination and pollen tube penetration, but neither cultivar differences nor response to temperature were studied. In the current study, all 12 cultivars had defined temperature optima, above and below the point of which pollen germination and pollen tube growth were reduced. The modified bilinear model best described the response of pollen germination and pollen tube growth to temperature (Figs 1 and 2).
Significant cultivar differences for pollen germination and pollen tube growth were observed in the present study (Figs 1 and 2 and Tables 2 and 3). The cotton pollen germination percentage was between 33 and 60 % with a mean of 44 %, much higher than that observed by Taylor (1972). Recently, Burke et al. (2004) recorded mean pollen germination of 71 % at 37 °C from flowers collected from greenhouse-grown cultivars (Gregg 65, PM 2156, PM 2326 and DP 90) that were not included in the present study. This high germination could be due to the fact that the media contained gibberellic acid and the effects of temperature × gibberellic acid on pollen germination need to be investigated. Germination percentages recorded in the current study are not uncommon for pollen germination on artificial medium devoid of any growth regulators or promoters (Herrero and Johnson, 1980; Kuo et al., 1981; Kakani et al., 2002). Pollen tube lengths similar to those recorded in the present study (Table 3) were reported for several crops when pollen was grown on artificial media, such as 1000–1800 µm for corn (Binelli et al., 1985), 450–1400 µm for peanuts (Kakani et al., 2002) and 20–60 µm for muskmelon (Maestro and Alvarez, 1988). Therefore, the observed differences in pollen germination and pollen tube length in the present study were a reflection of cultivar variability.
Cultivar differences for cardinal temperatures were recorded in the current study (Tables 2 and 3). Cultivar DP 458 B/RR had an average pollen germination of about 60 % and had a Tmax of 46 °C. The conventional cultivar, STV 825, also had a high Tmax of 46 °C and the average pollen germination was 53 % (Table 2). The average cardinal temperatures for pollen germination and pollen tube growth were 14 °C (Tmin), 31 °C (Topt) and 43 °C (Tmax). Values obtained for cotton were similar to those reported for peanut (Tmin − 14, Topt − 30–34, and Tmax − 43 °C; Kakani et al., 2002) and snake melon (Cucumis melo) (Tmin − 10, Topt − 30 and Tmax − 48 °C; Matlob and Kelly, 1973). However, the differences in cardinal temperatures did not reflect the tolerance or susceptibility of a cultivar to high temperatures because the cultivars which had a higher optimum temperature did not always have a higher temperature maximum or vice versa. Cultivars that had higher Topt also had a higher pollen germination percentage and maintained a higher pollen germination even at high temperatures. Similar pollen behaviour was observed in snake melon (Matlob and Kelly, 1973), corn (Binelli et al., 1985) and peanuts (Kakani et al., 2002). Recent studies with Brassica napus have suggested that reduced pollen germination rather than pollen viability under high temperature is the major cause of low pollen fertility (Young et al., 2004). Prasad et al. (1999b) in peanuts and Aloni et al. (2001) in bell pepper established a high correlation between in vitro pollen germination and fruit-set/seed-set under high-temperature conditions; this suggests that pollen germination could be a useful tool for testing cultivar tolerance to high temperature. Therefore, the ability of pollen to germinate and grow well at temperatures above 30 °C could be used as a tool to identify high-temperature tolerance in cotton cultivars. Further studies will be required to determine the minimum number of germinated pollen grains required to have effective fertilization.
In the current study, the membrane thermostability expressed as relative injury ranged between 35 and 73 %, but had a poor correlation with pollen parameters (Fig. 3). Recently, ur Rahman et al. (2004) also concluded that membrane thermostability is not a useful parameter for discriminating high-temperature tolerance of cotton cultivars under ambient temperatures. In cotton, heat tolerance does not correlate with degree of cell membrane lipid saturation (Rikin et al., 1993), suggesting factors other than membrane stability may be limiting reproductive growth and development at high temperature. However, the genotypic differences for pollen germination and pollen tube growth identified in this study could be due to the variation in their pollen carbohydrate concentration. Studies have shown that carbohydrates are responsible for pollen development and, especially, pollen cytoplasmic carbohydrates and sucrose are involved in protecting pollen viability during exposure and dispersal (Pacini et al., 1996) and for pollen germination, simple sugars are the primary substrates (Stanley, 1971). In pepper plants, exposure to high temperature (32/26 °C) for 8 d resulted in pollen germination of 6 % and shorter pollen tubes compared with maximum pollen germination of 25 % obtained at normal temperature (28/22 °C) (Aloni et al., 2001). This was attributed to a decrease in sucrose utilization by pollen grains under high temperature, even though the pollen grains accumulated more starch and sugars than under normal temperature conditions. In contrast, a decrease in starch and sugar concentration was recorded in tomato pollen grown under high temperature (32/26 °C) conditions (Pressman et al., 2002). Therefore, under-utilization or unavailability of carbohydrates hinders pollen germination on exposure to high temperatures. Future studies need to study the genotypic differences or pollen carbohydrate concentration and its role in determining the temperature tolerance of cotton pollen.
The PCA is perhaps the most useful statistical tool for screening multivariate data with significantly high correlations (Johnson, 1998). The first three principal components, PC1, PC2 and PC3 from PCA, explained about 72 % of the total cultivar pollen variability in response to temperature. The cluster analysis applied to the principal components divided the cultivars into four distinct groups (Fig. 4; Table 5). The PC1 eigenvectors for variables PG%max and PTLmax have high positive loadings, while variables PTL Topt and PG% Topt have high negative loadings. The PC1 vectors indicated that cultivars with high optimum temperature do not necessarily have high pollen germination or long pollen tubes. But, tolerance to high temperatures will result only from successful fertilization of the megagametophyte that requires both pollen germination and pollen tube elongation. Cultivars that had higher PG%max maintained higher germination percentage at above optimum temperatures compared with those that had lower PG%max. Cultivars ST 4793 R, DP 458 B/RR and DP 5415 RR, with higher pollen germination and longer pollen tubes and with high PG Topt, were classified as tolerant, and cultivars FM 832, FM 832 B, NuCOTN 33 B were classified as susceptible to high temperature.
In conclusion, the cultivars with higher PG%max, PTLmax and an optimum temperature >32 °C for maximum pollen germination in vitro on a simple defined medium can be used for screening cultivars to high-temperature tolerance. However, for accurate yield predictions, future studies should quantify boll retention under high temperature and investigate the relationship between pollen germination, boll number and air temperatures under controlled conditions with high levels of solar radiation. Studies will also be required to validate the performance of high temperature-tolerant cultivars identified by these in vitro methods in high-temperature environments.
Supplementary Material
Acknowledgments
This research was funded by the National Aeronautical and Space Administration through the Remote Sensing Technology Center at Mississippi State University. We thank Drs Harry F. Hodges, Jack C. McCarthy and Donald T. Krizek for their critical comments and suggestions on the manuscript. We thank D. Brand and K. Gourley for technical support. This is contribution no. J10494 from Department of Plant and Soil Sciences, Mississippi State University, Mississippi Agricultural and Forestry Experiment Station.
LITERATURE CITED
- Aloni B, Peet M, Pharr M, Karni L. 2001. The effect of high temperature and high atmospheric CO2 on carbohydrate changes in bell pepper (Capsicum annuum) pollen in relation to its germination. Physiologia Plantarum 112: 505–512. [DOI] [PubMed] [Google Scholar]
- Ashraf M, Saeed MM, Qureshi MJ. 1994. Tolerance to high temperature in cotton (Gossypium hirsutum L.) at initial growth stages. Environmental and Experimental Botany 34: 275–283. [Google Scholar]
- Barrow JR. 1983. Comparisons among pollen viability measurement methods in cotton. Crop Science 23: 734–736. [Google Scholar]
- Binelli G, De Manincor EV, Ottaviano E. 1985. Temperature effects on pollen germination and pollen tube growth in maize. Genetica Agraria 39: 269–281. [Google Scholar]
- Burke JJ. 2003. Sprinkler-induced flower losses and yield reductions in cotton (Gossypium hirsutum L.). Agronomy Journal 95: 709–714. [Google Scholar]
- Burke JJ, Velten J, Oliver MJ. 2004.In vitro analysis of cotton pollen germination. Agronomy Journal 96: 359–368. [Google Scholar]
- Cotton Farming. 2002. Cotton farming's 2002 guide to seed. [Online] Available at http://www.cottonfarming.com/home/2002_seedguide.html (verified on 25 February 2005). [Google Scholar]
- Craufurd PQ, Prasad PVV, Kakani VG, Wheeler TR, Nigam SN. 2003. Heat tolerance in groundnut. Field Crops Research 80: 63–77. [Google Scholar]
- Cross RH, McKay SAB, McHughen AG, Bonham-Smith PC. 2003. Heat-stress effects on reproduction and seed set in Linum usitatissimum L. (flax). Plant, Cell and Environment 26: 1013–1020. [Google Scholar]
- Dai A, Wigley TML, Boville BA, Kiehl JT, Buja LE. 2001. Climates of the 20th and 21st centuries simulated by the NCAR climate system model. Journal of Climate 14: 485–519. [Google Scholar]
- Economic Research Service. 2003.Agricultural resources and environmental indicators, 2003. Agriculture Handbook, No. 722. Washington, DC: USDA-ERS. [Google Scholar]
- Hall AE. 1992. Breeding for heat tolerance. Plant Breeding Reviews 10: 129–167. [Google Scholar]
- Herrero MP, Johnson RR. 1980. High temperature stress and pollen viability of maize. Crop Science 20: 796–800. [Google Scholar]
- Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA. 2001.Climate change 2001: the scientific basis. Contribution of Working Group I of the Third Assessment Report of the Intergovernmental Panel on Climate Change. New York, NY: Cambridge University Press. [Google Scholar]
- Johnson DE. 1998.Applied multivariate methods for data analysis. New York: Duxbury Press. [Google Scholar]
- Kakani VG, Prasad PVV, Craufurd PQ, Wheeler TR. 2002. Response of in vitro pollen germination and pollen tube growth of groundnut (Arachis hypogaea L.) genotypes to temperature. Plant, Cell and Environment 25: 1651–1661. [Google Scholar]
- Kuo CG, Peng JS, Tsay JS. 1981. Effect of high temperature on pollen grain germination, pollen tube growth and seed yield in Chinese cabbage. HortScience 16: 67–68. [Google Scholar]
- Maestro MC, Alvarez J. 1988. The effects of temperature on pollination and pollen tube growth in muskmelon Cucumis melo L. Scientia Horticulturae 36: 173–181. [Google Scholar]
- Malik MN, Chaudhry FI, Makhdum MI. 1999. Cell membrane thermostability as a measure of heat-tolerance in cotton. Pakistan Journal of Scientific and Industrial Research 42: 44–46. [Google Scholar]
- Martineau JR, Specht JE, Williams JH, Sullivan CY. 1979. Temperature tolerance in soybeans. I. Evaluation of a technique for assessing cellular membrane thermostability. Crop Science 19: 75–78. [Google Scholar]
- Matlob AN, Kelly WC. 1973. Effect of high temperature on pollen tube growth of snake melon and cucumber. Journal of American Society of Horticultural Science 98: 296–300. [Google Scholar]
- Matsui T, Omasa K, Horie T. 1997. High temperature-induced spikelet sterility of japonica rice at flowering in relation to air temperature, humidity and wind velocity conditions. Japanese Journal of Crop Science 66: 449–455. [Google Scholar]
- Mitchell JC, Petolino JF. 1988. Heat stress effects on isolated reproductive organs of maize. Journal of Plant Physiology 133: 625–628. [Google Scholar]
- Omanga PA, Summerfield RJ, Qi A. 1995. Flowering of pigeonpea (Cajanus cajan L.) in Kenya: responses of early maturing genotypes to location and date of sowing. Field Crops Research 41: 25–34. [Google Scholar]
- Pacini E. 1996. Types and meaning of pollen carbohydrate reserves. Sexual Plant Reproduction 22: 362–366. [Google Scholar]
- Peet MM, Sato S, Gardener R. 1998. Comparing heat stress effects on male-fertile and male-sterile tomatoes. Plant, Cell and Environment 21: 225–231. [Google Scholar]
- Prasad PVV, Craufurd PQ, Summerfield RJ. 1999a. Sensitivity of peanut to timing of heat stress during reproductive development. Crop Science 39: 1352–1357. [Google Scholar]
- Prasad PVV, Craufurd PQ, Summerfield RJ. 1999b Fruit number in relation to pollen production and viability in groundnut exposed to short episodes of heat stress. Annals of Botany 84: 381–386. [Google Scholar]
- Prasad PVV, Boote KJ, Allen LH, Thomas JMG. 2002. Effects of elevated temperature and carbon dioxide on seed-set and yield of kidney bean (Phaseolus vulgaris L.). Global Change Biology 8: 710–721. [Google Scholar]
- Prasad PVV, Boote KJ, Allen LH, Thomas JMG. 2003. Super-optimal temperatures are detrimental to peanut (Arachis hypogaea L.) reproductive processes and yield at both ambient and elevated carbon dioxide. Global Change Biology 9: 1775–1787. [Google Scholar]
- Pressman E, Peet MM, Pharr DM. 2002. The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers. Annals of Botany 90: 631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pundir NS. 1972. Experimental embryology of Gossypium arboretum L. and G. hirsutum and their reciprocal crosses. Botanical Gazette 133: 7–26. [Google Scholar]
- ur Rahman H, Malik SA, Saleem M. 2004. Heat tolerance of upland cotton during the fruiting stage evaluated using cellular membrane thermostability. Field Crops Research 85: 149–158. [Google Scholar]
- Reddy VR, Reddy KR, Hodges HF. 1991. Temperature effects on growth and development of cotton during the fruiting period. Agronomy Journal 83: 211–217. [Google Scholar]
- Reddy KR, Hodges HF, Reddy VR. 1992. Temperature effects on cotton fruit retention. Agronomy Journal 84: 26–30. [Google Scholar]
- Reddy KR, Hodges HF, McKinion JM. 1993. A temperature model for cotton phenology. Biotronics 22: 47–59. [Google Scholar]
- Reddy KR, Hodges HF, McKinion JM. 1995. Carbon dioxide and temperature effects on pima cotton growth. Agriculture, Ecosystems and Environment 54: 17–29. [Google Scholar]
- Rikin A, Dillworth JW, Bergman DK. 1993. Correlation between circadian rhythm of resistance to extreme temperature and changes in fatty acid composition in cotton seedlings. Plant Physiology 101: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Garay B, Barrow JR. 1988. Pollen selection for heat tolerance in cotton. Crop Science 28: 857–859. [Google Scholar]
- Saini HS, Aspinall D. 1982. Sterility in wheat (Triticum aestivum L.) induced by water deficit or high temperature, possible mediation by abscisic acid. Australian Journal of Plant Physiology 9: 529–537. [Google Scholar]
- Sari-Gorla M, Pe ME, Rossini L. 1994. Detection of QTLs controlling pollen germination and growth in maize. Heredity 72: 332–335. [Google Scholar]
- SAS Institute. 1997.SAS/STAT user's guide, Version 8·2. Cary, NC: SAS Institute. [Google Scholar]
- Sato S, Peet MM, Thomas JF. 2002. Determining critical pre- and post-anthesis periods and physiological processes in Lycopersicon esculentum Mill. exposed to moderately elevated temperatures. Journal of Experimental Botany 53: 1187–1195. [DOI] [PubMed] [Google Scholar]
- Stanley RG. 1971. Pollen chemistry and tube growth. In: Heslop-Harrison J, ed. Pollen: development and physiology. London: Butterworths, 131–155. [Google Scholar]
- Suy TB. 1979. Contribution of l'etude de la croissance des tubes polliniques chez Gossypium hirsutum L. en function des conditions du milieu. Coton et Fibres Tropicales 34: 295–300. [Google Scholar]
- Taylor NB. 1972. Germination of cotton (Gossypium hirsutum L.) pollen on an artificial medium. Crop Science 12: 243–244. [Google Scholar]
- Tollenaar M, Daynard TB, Hunter RB. 1979. Effect of temperature on rate of leaf appearance and flowering date in maize. Crop Science 19: 363–366. [Google Scholar]
- Weaver ML, Timm H. 1988. Influence of temperature and water status on pollen viability in bean. Journal of American Society of Horticultural Science 113: 13–15. [Google Scholar]
- Yan WK, Wallace DH. 1998. Simulation and prediction of plant phenology for five crops based on photoperiod by temperature interaction. Annals of Botany 81: 705–716. [Google Scholar]
- Young LW, Wilen RW, Bonham-Smith PC. 2004. High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. Journal of Experimental Botany 55: 485–495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.